Abstract
Renal cell carcinoma has the ability to metastasize to any organ; about 16 % of affected patients present initially with metastasis. However, it is rare for this tumor to present with metastasis from an unidentified primary. The current use of immunohistochemistry and molecular genetics has enabled clinicians to reach a precise diagnosis. It has been hypothesized that the treatment protocol for metastatic renal cell carcinoma can be applied to cases with undetectable primary. In this paper, a novel case of metastatic renal cell carcinoma presenting with lymphadenopathy with no evidence of a primary renal lesion is reported from Kuwait cancer center.
Keywords: mRCC, Metastatic renal cell carcinoma, Clear cell carcinoma, Cancer of unknown primary, Pazopanib
Introduction
Renal cell carcinoma (RCC) is the most prevalent renal neoplasm, constituting 80–85 % of primary kidney cancers. The global incidence of RCC was estimated to be 304, 200 cases in 2012 [1]. Its incidence varies tremendously from one region to another. It is well renowned internationally as well as in Kuwait, that the rates are higher among males; in 2012, kidney cancer was the eighth most common cancer among Kuwaiti males accounting for 3.5 % of all malignancies [2]. RCC is known for its predilection to metastasize to any organ, most commonly the lungs, lymph nodes (LNs), bone, and liver [3]. At the time of diagnosis, approximately 16 % of the cases present with metastatic RCC (mRCC) [4]. However, it is extremely rare to diagnose mRCC with an occult primary.
Upon reviewing the literature, a total of 12 cases of metastatic kidney cancer had no evidence of a renal mass [5–12]. In this case report, a case of mRCC with an initial presentation of generalized lymphadenopathy with an undetectable primary will be presented.
Case description
A previously healthy 77 year-old Kuwaiti gentleman presented with a slowly progressive left-sided neck mass in July 2015. The patient’s ECOG performance status was 0. A left-sided supraclavicular LN was palpable; otherwise, the examination was normal. The examination was consistent with the MRI head and neck that showed an ill-defined solid mass; it was about 4.7 × 3 × 3.3 cm in left supraclavicular region along the left carotid sheath displacing the left common artery medially and infiltrating the surrounding fat planes. Incidentally, another bulky left submandibular gland was noted. Subsequently, a CT scan was done confirming the previous finding in addition to the presence of generalized lymphadenopathy in the following locations: posterior lateral neck, bilateral axillas, para-aortic, retrocrural, bilateral external iliac and inguinal. Brucellosis and TB as infective causes of the lymphadenopathy were ruled out.
Fine needle aspiration cytology (FNAC) of the left supraclavicular lesion showed aggregates of papillaroid structures and tumor cells (Fig. 1) which were highly suggestive of metastatic clear cell carcinoma, signifying that a primary of RCC cannot be ruled out. As there was no evidence of a renal mass in the CT, an FDG PET CT scan was done and it showed hypermetabolic activity in the aforementioned LNs with no obvious primary site observed. Tumor markers (CEA, Ca19.9, PSA) as well as both upper and lower GI scopes were within normal limits.
Fig. 1.
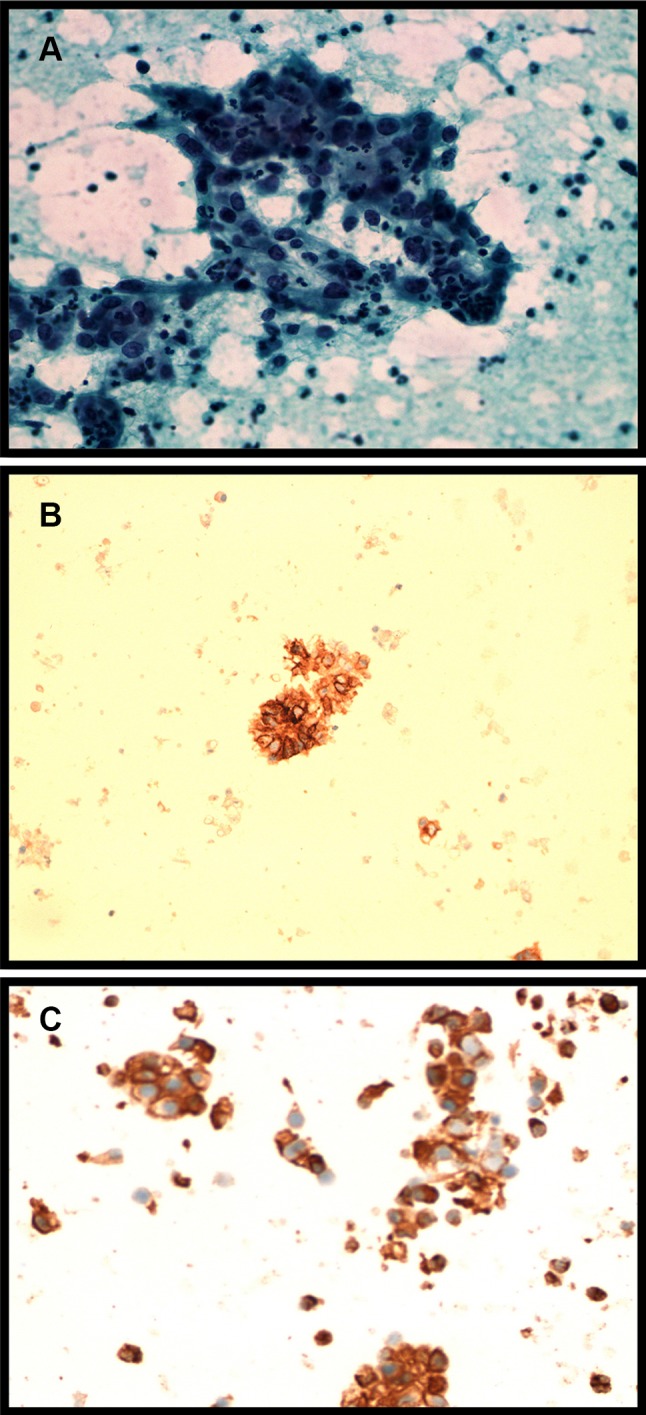
a Smears show aggregates of tumor cells that have round nuclei with anisonucleosis, prominent nucleoli, and abundant finely vacuolated cytoplasm. b Positive staining for vimentin. c Cells showing positive expression of CD10
Tissue biopsy from left supraclavicular node was done in London. Histopathology was consistent with either clear cell myoepithelium or RCC. The cells strongly expressed low molecular weight cytokeratin (CAM 5.2) and CD10 and were strongly positive for PAX8 and vimentin, but negative for CK7, CK 20, S100, and CD117. Moreover, there was focal definite expression of CD 56, RCC marker, Glut1, CAIX on the papillary subsection of cells. Full immunohistochemical profile on the sample excluded the possibility of metastatic gastric, salivary, myoepithelial carcinomas or lung adenocarcinoma. Further genetic profiling revealed three genomic alterations in the LN sample: CDKN2A/B, PBRM1, and SLIT2.
The best probable diagnosis of this patient was that of mRCC with no detected primary. Therefore, the patient was started on pazopanib in Oct 2015. In November, he developed thrombocytopenia for which his dose was reduced to half. The patient continues to be under regular follow up. He has shown no progression of the disease and no evidence of a renal lesion has been detected yet.
Discussion
Renal cell carcinoma (RCC) has the propensity to metastasize distally to any organ either through hematogenous or lymphatic spread. It has been estimated that 16 % of RCC patients present with distant metastases at the time of diagnosis [4]. However, presenting with mRCC with an undetectable primary is infrequent. A total of 12 cases were documented based on our literature review [5–12]; none of which had diffuse lymphadenopathy metastasizing from an unidentifiable renal primary as presented in our case. In such a challenging situation, immunohistochemical profiling as well as the promising field of molecular genetics aid in reaching a diagnosis. In the case above, the patient presented with lymphadenopathy where benign causes were initially excluded. Fine needle aspiration cytology suggested the diagnosis of clear cell carcinoma (Fig. 1). Due to the rarity of this presentation, full immunohistochemical profiling is the approach for clarifying this ambiguity. The positivity of expression of low molecular weight CAM 5.2, PAX 8, CD 10, vimentin, CAIX and RCC marker and the negativity of CK 7, CK 20, TTF1 and S100 highly supports the proposed diagnosis of mRCC [13, 14]. The genomic alterations detected further favored the diagnosis; recent data implicated PBRM1 to be associated with clear cell RCC16 as well as alterations in CDKN2A [15–17]. Afterwards, upon reviewing numerous radiological images, no solid renal mass was detected.
It still remains unclear how mRCC occurs with an unidentifiable primary lesion; however, there have been several possible explanations [5, 6]. The mass could be occult, hence undetectable by imaging modalities. Moreover, few documented cases of RCC have reported spontaneous regression of the lesion without therapy, which is another possibility. Finally, patients may develop metastasis from RCC of an ectopic renal tissue.
Based on the overall diagnostic evaluation of the reported patient, he was treated as mRCC with pazopanib, a multi-targeted tyrosine kinase inhibitor [18].
The patient showed good tolerance and response to the administered therapy with no sign of disease progression.
To conclude, despite the rarity of mRCC presenting as cancer with an unknown primary lesion, it is an entity that should be considered upon evaluating suspicious patients. To aid in the diagnosis of such an ambiguous presentation, immunohistochemistry and molecular profiling are extremely advantageous. Although no current guidelines are available at the time being, tyrosine kinase inhibitors, namely pazopanib and sunitinib, can be considered as a first line treatment option for mRCC with an undetected primary.
Compliance with ethical standards
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee at which the study was conducted and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the individual participant included in the study.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray, F. GLOBOCAN 2012 v1.1, cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon: International Agency for Research on Cancer; 2014. [DOI] [PubMed]
- 2.Kuwait Cancer Registry; Annual report. Kuwait: Ministry of Health; 2012.
- 3.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population based analysis. Ann Oncol. 2012;23(4):973–980. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, epidemiology, and end results program. Cancer of the kidney and renal pelvis, n.p., n.d. Web; 2016.
- 5.Choi YR, Han HS, Lee OJ, et al. Metastatic renal cell carcinoma in a supraclavicular lymph node with no known primary: a case report. Cancer Res Treat. 2012;44(3):215–218. doi: 10.4143/crt.2012.44.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar RM, Aziz T, Jamshaid H, Gill J, Kapoor A. Metastatic renal cell carcinoma without evidence of a primary renal tumour. Curr Oncol. 2014;21(3):e521–e524. doi: 10.3747/co.21.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia S, Ng S, Hodder SC. Metastatic cutaneous head and neck renal cell carcinoma with no known primary: case report. Br J Oral Maxillofac Surg. 2010;48:214–215. doi: 10.1016/j.bjoms.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Wayne M, Wang W, Bratcher J, Cumani B, Kasmin F, Cooperman A. Renal cell cancer without a renal primary. World J Surg Oncol. 2010;8:18. doi: 10.1186/1477-7819-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costantino C, Thomas GV, Ryan C, Coakley FV, Troxell ML. Metastatic renal cell carcinoma without evidence of a renal primary. Int Urol Nephrol. 2016;48(1):73–77. doi: 10.1007/s11255-015-1145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heary RF, Agarwal N, Barrese JC, Barry MT, Baisre A. Metastatic renal cell carcinoma, with a radiographically occult primary tumor, presenting in the operative site of a thoracic meningioma: long-term follow-up: case report. J Neurosurg Spine. 2014;21(4):628–633. doi: 10.3171/2014.6.SPINE13448. [DOI] [PubMed] [Google Scholar]
- 11.Wei EY, Chen YB, Hsieh JJ. Genomic characterisation of two cancers of unknown primary cases supports a kidney cancer origin. BMJ Case Rep. 2015. [DOI] [PMC free article] [PubMed]
- 12.Sorscher SM, Greco FA. Papillary renal carcinoma presenting as a cancer of unknown primary (CUP) and diagnosed through gene expression profiling. Case Rep Oncol. 2012;5(2):229–232. doi: 10.1159/000339130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen SS, Truong LD, Scarpelli M, Lopez-beltran A. Role of immunohistochemistry in diagnosing renal neoplasms: when is it really useful? Arch Pathol Lab Med. 2012;136(4):410–417. doi: 10.5858/arpa.2011-0472-RA. [DOI] [PubMed] [Google Scholar]
- 14.Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135(1):92–109. doi: 10.5858/2010-0478-RAR.1. [DOI] [PubMed] [Google Scholar]
- 15.Pawłowski R, Mühl SM, Sulser T, Krek W, Moch H, Schraml P. Loss of PBRM1 expression is associated with renal cell carcinoma progression. Int J Cancer. 2013;132(2):E11–E17. doi: 10.1002/ijc.27822. [DOI] [PubMed] [Google Scholar]
- 16.Schraml P, Struckmann K, Bednar R, Fu W, Gasser T, Wilber K, Kononen J, Sauter G, Mihatsch MJ, Moch H. CDKN2A mutation analysis, protein expression, and deletion mapping of chromosome 9p in conventional clear-cell renal carcinomas. Am J Pathol. 2001;158(2):593–601. doi: 10.1016/S0002-9440(10)64001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Mokadem I, Fitzpatrick J, Rai B, et al. Significance of chromosome 9p status in renal cell carcinoma: a systematic review and quality of the reported studies. Biomed Res Int. 2014;2014:521380. doi: 10.1155/2014/521380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]


