Abstract
Renal-limited vasculitis (RLV) is a type of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis that presents with crescentic glomerulonephritis with no other organ involvement. Although several studies reported patients with crescentic glomerulonephritis who were dual positive for proteinase 3 (PR3)-ANCA and myeloperoxidase (MPO)-ANCA or ANCA and anti-glomerular basement membrane (GBM) antibody, patients positive for all three antibodies, i.e., triple-positive patients, were rarely reported. We herein report the case of a male with pauci-immune type crescentic glomerulonephritis positive for MPO-ANCA, PR3-ANCA, and anti-GBM antibody. Renal biopsy led to the definitive diagnosis of RLV with pauci-immune-type crescentic glomerulonephritis. Fluorescence immunostaining showed no linear deposition of IgG on GBM, indicating no involvement of anti-GBM associated diseases. Intensive therapy, including prednisolone, plasma exchange, and intravenous cyclophosphamide, was effective. We report the case of triple-positive patient with crescentic glomerulonephritis, who was successfully treated with glucocorticoid, plasma exchange, and cyclophosphamide, suggesting that treatment for RLV in the patient with serological triple antibodies positivity in the absence of linear IgG deposition could benefit from the combination therapy regimen for plasma exchange and primary induction of remission against microscopic polyangiitis.
Keywords: MPO-ANCA, PR3-ANCA, Anti-GBM antibody, Case report
Background
Renal-limited vasculitis (RLV) is a type of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis that presents with only a renal manifestation; no other organs, including lungs, are involved. RLV is also suggested to be a subtype of microscopic polyangiitis (MPA) or granulomatosis with polyangiitis (GPA) based on the presence of myeloperoxidase (MPO)-ANCA and proteinase3 (PR3)-ANCA. RLV is also characterized as an anti-GBM-associated disease with no pulmonary manifestation [1]. The incidence rates of MPA are higher than that of Wegener’s granulomatosis, and RLV is the most frequent cause of progressive glomerulonephritis in Japan, accounting for 42.1% of all cases [2, 3]. In one study in Japan, MPO-ANCA titer was positive in 88.1% of all RLV patients, whereas PR3-ANCA titer was positive in only 7.4% of the cases [2]. Another report showed that p-ANCA was most frequently observed in patients with RLV, whereas c-ANCA positivity was highest in patients with lung and sinus involvement in a study in the USA [4].
However, little is known about the triple presence of these antibodies. Here, we describe a patient with crescentic glomerulonephritis who was positive for MPO-ANCA, PR3-ANCA, and anti-GBM antibody.
Case report
A 68-year-old male was admitted to a local hospital with fever and cough on the suspicion of pneumonia. The patient had a history of bile duct stricture and obstruction, and was positive for p-ANCA (indirect immunofluorescence assay: IFA) and speckled-type anti-nuclear antibody at 1 year before admission, but was negative for c-ANCA (IFA). He also had a history of chronic obstructive pulmonary disease and early gastric cancer, which was removed by endoscopic mucosal resection. The patient was started on antibiotics for pneumonia; however, because of the deterioration of renal functions, he was transferred to our hospital. On admission, his height and body weight were 174 cm and 71.5 kg, respectively. He had a body temperature of 37.1 °C, a heart rate of 98/min, a blood pressure of 128/83 mmHg, and SpO2 of 100% in room air. Both lung sounds were clear except for diminished breath sounds over the right upper lobe. There was no rash on the body, and the physical examination was otherwise unremarkable. There was no significant change of body weight. Laboratory results were as follows: serum creatinine, 3.18 mg/dl; CRP, 12.8 mg/dl; MPO-ANCA (Chemiluminescent enzyme immunoassay; CLEIA), 19.1 U/ml; MPO-ANCA (Fluorescence enzyme immunoassay; FEIA), 25.2 U/mL; PR3-ANCA (CLEIA), 30.5 U/ml; PR3-ANCA (FEIA), negative; anti-GBM antibody, 5.1 U/ml; Anti-nuclear antibody, 1:1280 positive; anti-SS-A antibody, 129 U/ml and anti-SS-B antibody, 190 U/ml. Urinalysis showed 3+ occult blood, 2+ protein, and proteinuria at 1.9 g/gCr. Other laboratory results are shown in Table 1. Abdominal ultrasound showed normal renal size and corticomedullary differentiation in both kidneys. Chest radiograph was normal, and computed tomography of the chest and abdomen revealed a cyst in the left kidney and localized consolidation in right upper lung (Fig. 1), which was consistent with pneumonia but not alveolar haemorrhage. The size of pulmonary consolidation was reducing by the simultaneous treatment with antibiotics and prednisolone. Birmingham Vasculitis Activity Score (BVAS) [5] was 12 on admission, and clinical score and grade in the evidence-based clinical practice guidelines for rapidly progressive glomerulonephritis (RPGN) 2014 in Japan [6] were 4 and II, respectively.
Table 1.
Laboratory data
| Parameter | Patient value | Reference |
|---|---|---|
| Haemoglobin (g/dL) | 8.7 | 11.3–16.3 |
| White-cell count (/μL) | 7570 | 3200–9400 |
| Neutrophil count (%) | 82.6 | 46–62 |
| Eosinophil count (%) | 0.9 | 3–5 |
| Platelet count (/μL) | 359,000 | 96,000–348,000 |
| APTT (s) | 44.4 | 24–35 |
| Creatinine (mg/dL) | 3.19 | 0.65–1.06 |
| eGFR (ml min−1 1.73 m2−1) | 16.3 | ≥60 |
| BUN (mg/dL) | 33.9 | 8.1–22 |
| Albumin (g/dL) | 2.7 | 3.9–5.1 |
| CRP (mg/dL) | 12.8 | <0.2 |
| MPO-ANCA (U/mL; CLEIA) | 19.1 | <3.5 |
| MPO-ANCA (U/mL; FEIA) | 25.2 | <3.5 |
| PR3-ANCA (U/mL; CLEIA) | 30.5 | <3.5 |
| PR3-ANCA (U/mL; FEIA) | negative | <2 |
| anti-GBM antibody (U/mL) | 5.1 | <3.0 |
| Speckled-type anti-nuclear antibody (titer) | 1:1280 | <1:40 |
| anti-SS-A/Ro antibody (U/mL) | 129 | <10 |
| anti-SS-B/La antibody (U/mL) | 190 | <15 |
| Urinary occult blood | 3+ | Negative |
| Urinary RBC (/HPF) | 50–100 | <1–4 |
| Urinary RBC shape | Dysmorphic | None |
| Urinary protein | 3+ | Negative |
| Urinary protein-creatinine ratio (g/gCre) | 1.9 | <0.15 |
APTT activated partial thromboplastin time, eGFR estimated glomerular filtration rate, BUN blood urea nitrogen, CRP C-reactive protein, RBC red blood cells, CLEIA chemiluminescent enzyme immunoassay, FEIA fluorescence enzyme immunoassay
Fig. 1.
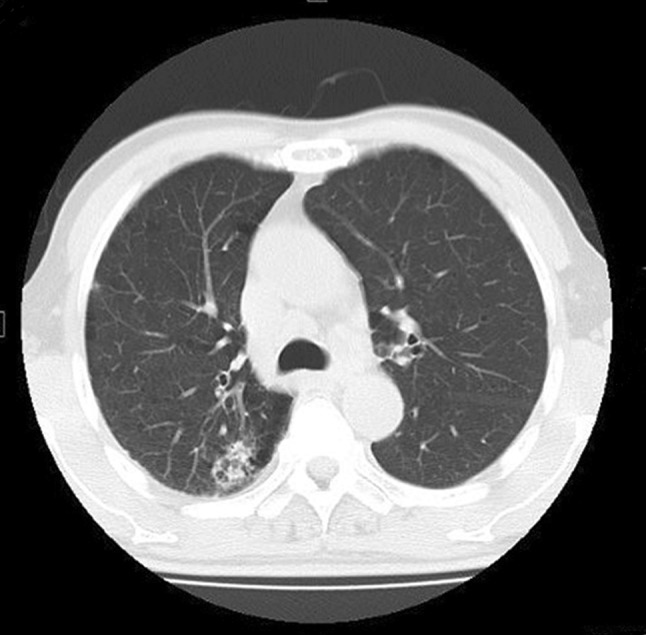
Computed tomography (CT) of the chest. A CT image shows a localized consolidation in right upper lung
As renal functions of the patient deteriorated following admission, with serum creatinine level rising from 3.18 mg/dl on admission to 4.10 mg/dl on day 2, we preceded the treatment without renal biopsy. He was administered intravenous high-dose methylprednisolone therapy (500 mg/day) for 3 days, followed by oral prednisolone at 40 mg/day. Plasma exchange was consecutively performed five times to remove anti-GBM antibody with the consideration of anti-GBM-antibody-associated diseases in differential diagnosis. As suggested in the previous studies, PE can decrease the titer of anti-GBM antibody [7], and can help renal survival in the patient with ANCA-related vasculitis [8]. We used 3360 mL of fresh frozen plasma (FFP) for 4 times and 3000 mL of 5% albumin for one time.
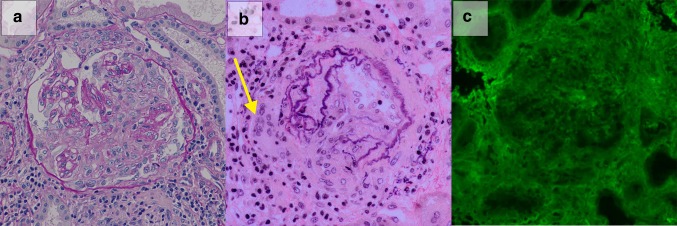
Renal biopsy was performed on day 7, and biopsy specimen included 30 glomeruli. As seen in Fig. 2, global sclerosis, segmental sclerosis, cellular crescents, and fibrocellular crescents were found in 10, 2, 5, and 6 glomeruli, respectively (Fig. 2a). Some of glomeruli exhibiting cellular crescents contained necrotic lesions of capillaries. There were prominent interstitial fibrosis, tubular atrophy, and infiltration of inflammatory cells, including lymphocytes and plasma cells, in 70% of the interstitium, suggesting that these changes were secondary to glomerular injury. Arterioles showed moderate sclerotic changes and the destruction of elastic fibers. Granulomatous change with multinucleated giant cell formation was detected around adventitia of small arteries (Fig. 2b). Immunofluorescence showed the absence of IgG, IgA, IgM, C3, C3c, and C1q deposition, and was consistent with pauci-immune-type crescentic glomerulonephritis (Fig. 2c). Fibrinogen deposition was observed in one crescent.There was no dense deposit observed by electron microscopy. Thus, the patient was diagnosed with RLV.
Fig. 2.
Renal biopsy specimen included 30 glomeruli, including 5 cellular crescents and 6 fibrocellular crescents. a Representative glomerulus containing cellular crescents with necrotizing lesion is shown (Periodic acid Schiff staining ×200). b Small artery exhibited granulomatous reaction with multinucleated giant cell formation (A arrow) and the destruction of elastic fibers (Elastica van Gieson staining ×200). c There was no staining of the IgG on immunofluorescence staining (×200)
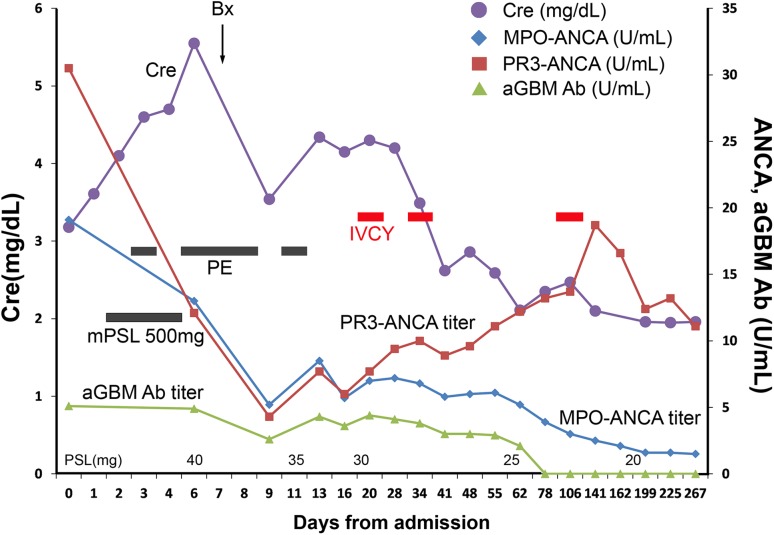
Treatment was initiated with intermittent intravenous high-dose cyclophosphamide therapy (IVCY; 500 mg at 375 mg/m2 × 75%) for MPO-ANCA-associated vasculitis on day 20. At day 34, the second pulse of IVCY (650 mg at 500 mg/ m2 × 75%) was administered. Oral glucocorticoid therapy was gradually tapered. Laboratory findings related to renal functions, i.e., serum creatinine level and protein–creatinine ratio, showed gradual improvement with glucocorticoid and IVCY treatment (Fig. 3). At 109 days after admission, the third cycle of IVCY at 650 mg was administered. Serum levels of PR3-ANCA (CLEIA), MPO-ANCA (CLEIA), and anti-GBM antibody were improved. Bile duct stricture was not changed during the course. The patient did not show any signs of recurrence during follow-up at 12 months after admission at our hospital.
Fig. 3.
Clinical course. The y-axis of the left side shows the level of serum creatinine; the right side shows the titer of ANCA and anti-GBM antibody (aGBM Ab). After the treatment of intravenous high-dose methylprednisolone (mPSL) (days 2–4) followed by oral prednisolone (PSL), plasma exchange (PE: days 3,6,7,8,11), and intravenous high-dose cyclophosphamide therapy (IVCY: days 20,34), laboratory data were gradually improved. Bx, renal biopsy
Discussion
In the present case, not only MPO-ANCA (CLEIA) but also PR3-ANCA (CLEIA) and anti-GBM antibody were elevated. Although several rapidly progressive glomerulonephritis cases with dual positivity for either MPO-ANCA/anti-GBM or PR3-ANCA/anti-GBM were previously reported [9], patients who were positive for all three antibodies, such as our patient, have rarely been reported. One previous paper reporting a patient positive for all three autoantibodies did not present a clinical feature in detail [10]. In the case of double-positive patients who were positive for both MPO-ANCA and PR3-ANCA on ELISA, no specific patterns of clinical presentation or outcome were observed [11]. One study reported that patients with ANCA-associated vasculitis complicating infection were more often double positive with higher MPO-ANCA and lower PR3-ANCA titers on ELISA [12].
A previous study reviewing patients who were double positive for ANCA and anti-GBM antibody reported that although 32% of anti-GBM antibody-positive patients were ANCA-positive, only 5% of ANCA-positive patients were also positive for anti-GBM antibodies [13]. However, the appearance of antibodies may not necessarily be simultaneous and may exhibit a time-lag. The previous studies reported patients who became positive for anti-GBM antibody several years after the detection of p-ANCA [14, 15]. Based on pathological findings, including both chronic and active glomerular lesions at the time of renal biopsy, we speculated that the patient had most likely developed vasculitis about 1 year before admission, at a time when p-ANCA was already positive, as the urinalysis showed 2+ protein and 3+ occult blood at that time. Epidemiologically, patients who are double positive for ANCA and anti-GBM antibody are almost 20 years older than those who are positive only for anti-GBM antibody, suggesting that ANCA but not anti-GBM antibody may be the fundamental cause of the disease [16], consistent with the present case. Furthermore, we assumed that anti-GBM antibody was not the main cause of renal injury in this patient because of the absence of linear IgG deposition.
Although a close relationship between the production of ANCA and anti-GBM antibody has been suggested, the underlying mechanism remains unclear. One of the postulated hypotheses involves unmasking of hidden antigens on GBM by endogenous oxidants activated by ANCA, which elicits anti-GBM antibody production [17]. Prognosis of double-positive patients is still controversial. Findings of studies comparing outcomes of double-positive patients with those positive only for anti-GBM antibody revealed that prognosis ranged from good [18, 19] and comparable [20] to worse [13] in patients who were serologically double positive for these antibodies. The presence of additional antibodies should be assessed in patients who are positive for one antibody. A previous report showed that when rats are injected with anti-GBM antibody to develop glomerulonephritis, glomerulonephritis was more severe in rats immunized with MPO-ANCA than those that received control serum [21]. These findings underlie the significance of the assessment of more than one antibody in these patients.
In addition, PR3-ANCA can be induced in patients with subacute bacterial endocarditis, tuberculosis, and ulcerative colitis without vasculitis [22]. Furthermore, PR3-ANCA is recently reported to be a biomarker for primary sclerotic cholangitis (PSC) [23]. Because our patient had a history of bile duct obstruction, PSC might have led to increased PR3-ANCA levels. Although granulomatous changes on small arteries were detected, some reports showed that granulomatous changes can be seen in the cases with MPA or RLV [24]. In addition, GPA cannot be completely excluded in this case, considering a pulmonary lesion which was disappearing by the simultaneous treatment with antibiotic and prednisolone. However, PR3-ANCA titer did not correlate with the extent of glomerulonephritis, and therefore, we speculated that PR3-ANCA was not the main cause of renal failure in this patient. The course of PSC was not changed during the treatment. The cause of elevated PR3-ANCA titer (CLEIA) remains unclear and will be the focus of future studies. The PR3-ANCA (CLEIA) keeps high titer during follow-up, although MPO-ANCA (CLEIA) and anti-GBM antibody titer became normal, which might be the indicator for relapse [25].
Taken together, the main cause of renal failure in our patient was considered to be MPO-ANCA. The present case showed successful recovery of renal function along with the decrease of proteinuria and MPO-ANCA titer, suggesting that standard regimen for induction of remission on MPA, glucocorticoid, and cyclophosphamide therapy in BSR and BHPR guideline [5], along with plasma exchange, could be beneficial to the patients with crescentic glomerulonephritis with triple-positive antibodies. The standard induction therapy for AAV is combination of glucocorticoids and intravenous pulse cyclophosphamide or rituximab in Western countries [5]. However, initiation therapy in this patient younger than 70 years, whose clinical grade was II, was recommended for pulse methylprednisolone and oral corticosteroid according to the evidence-based clinical practice guidelines for RPGN 2014 in Japan [6]. Patients with severe renal dysfunction may be treated with adjuvant cyclophosphamide and plasma exchange [6]. Anti-glomerular antibody-related glomerulonephritis will be treated with corticosteroids, cyclophosphamide, and plasma exchange [6, 7]. The objective of plasma exchange on AAV patients is to reduce ANCA titers and other molecules, such as cytokines, which are relevant to progression of diseases [26]. Plasma exchange can be effective on the reduction of the titer of MPO-ANCA and cytokines.
In summary, we present a patient with crescentic glomerulonephritis and serological positivity for MPO-ANCA, PR3-ANCA, and anti-GBM antibody who was successfully treated with methylprednisolone, plasma exchange, and cyclophosphamide, suggesting that RLV patients with seropositivity for triple antibodies in the absence of linear IgG deposition along the GBM can be treated with the combination therapy for primary induction of remission on MPA, which include treatment with glucocorticoids, cyclophosphamide, and plasma exchange.
Acknowledgements
We gratefully acknowledge Y. Mizukami, A. Morita, and M. Ozone in the Department of Nephrology, Graduate School of Medicine, Kyoto University for secretarial assistance.
Abbreviations
- ANCA
Anti-neutrophil cytoplasmic antibody
- PR3-ANCA
Proteinase 3 anti-neutrophil cytoplasmic antibody
- MPO-ANCA
Myeloperoxidase anti-neutrophil cytoplasmic antibody
- GBM
Glomerular basement membrane
- RLV
Renal-limited vasculitis
- MPA
Microscopic polyangiitis
- RPGN
Rapidly progressive glomerulonephritis
- APTT
Activated partial thromboplastin time
- IVCY
Intravenous high-dose cyclophosphamide therapy
- PSC
Primary sclerotic cholangitis
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethics and consent to participate
All relevant ethics for the case report and its publication were obtained.
Consent to publish
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- 1.Weidner S, Geuss S, Hafezi-Rachti S, Wonka A, Ruprecht HD. ANCA-associated vasculitis with renal involvement: an outcome analysis. Nephrol Dial Transplant. 2004;19:1403–1411. doi: 10.1093/ndt/gfh161. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi S, Fujimoto S, Takahashi K, Suzuki K. Anti-neutrophil cytoplasmic antibody associated vasculitis, large vessel vasculitis and Kawasaki Disease in Japan. Kidney Blood Press Res. 2010;33:442–455. doi: 10.1159/000320383. [DOI] [PubMed] [Google Scholar]
- 3.Koyama A, Yamagata K, Makino H, Arimura Y, Wada T, Nitta K, et al. A nationwide survey of rapidly progressive glomerulonephritis in Japan: etiology, prognosis and treatment diversity. Clin Exp Nephrol. 2009;13:633–650. doi: 10.1007/s10157-009-0201-7. [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135:921–930. [PMC free article] [PubMed] [Google Scholar]
- 5.Ntatsaki E, Carruthers D, Chakravarty K, D’Cruz D, Harper L, Jayne D, et al. BSR and BHPR guideline for the management of adults with ANCA-associated vasculitis. Rheumatology. 2014;53:2306–2309. doi: 10.1093/rheumatology/ket445. [DOI] [PubMed] [Google Scholar]
- 6.Arimura Y, Muso E, Fujimoto S, Hasegawa M, Kaname S, Usui J, et al. Evidence-based clinical practice guidelines for rapidly progressive glomerulonephritis 2014. Clin Exp Nephrol. 2016;20:322–341. doi: 10.1007/s10157-015-1218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013;28:145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 7.Mavani GP, Pommier M, Win S, Michelis MF, Rosenstock J. Presence of anti-glomerular basement membrane antibodies and myeloperoxidase anti-neutrophilic cytoplasmic antibodies in a case of rapidly progressive glomerulonephritis. Front Med. 2015;2:53. doi: 10.3389/fmed.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janye DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–2188. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 8.Hellmark T, Niles JL, Collins AB, Muccluskey RT, Brunmark C. Comparison of anti-GBM antibodies in sera with or without ANCA. J Am Soc Nephrol. 1997;8:376–385. doi: 10.1681/ASN.V83376. [DOI] [PubMed] [Google Scholar]
- 9.Chou J, Randall K, Gatenby P. Clinical outcomes of patients with dual positivity for proteinase 3 and myeloperoxidase specific antineutrophil cytoplasmic antibodies. J Clin. Cell Immunol. 2015;6:3. [Google Scholar]
- 10.Bonaci-Nikolic B, Andrejevic S, Pavlovic M, Dimcric Z, Ivanovic B, Nikolic M. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenge. Clin Rheumatol. 2010;29:893–904. doi: 10.1007/s10067-010-1424-4. [DOI] [PubMed] [Google Scholar]
- 11.Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2014;66:1535–1540. doi: 10.1111/j.1523-1755.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 12.Serratrice J, Chiche L, Dussol B, Granel B, Daniel L, Jego-Desplat S, et al. Sequential development of perinuclear ANCA-associated vasculitis and anti-glomerular basement membrane glomerulonephritis. Am J Kidney Dis. 2014;43:e14.15. doi: 10.1053/j.ajkd.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Olson SW, Arbogast CB, Baker TP, Owshalimpur D, Oliver DK, Abbott KC, et al. Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol. 2011;22:1946–1952. doi: 10.1681/ASN.2010090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alchi B, Griffiths M, Sivalingam M, Jayne D, Farrington K. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant. 2015;30:814–821. doi: 10.1093/ndt/gfu399. [DOI] [PubMed] [Google Scholar]
- 15.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 16.Bosch X, Mirapeix E, Font J, Borrellas X, Rodríguez R, López-Soto A, et al. Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in anti-glomerular basement membrane disease. Clin Nephrol. 1991;36:107–113. [PubMed] [Google Scholar]
- 17.Segelmark M, Hellmark T, Wieslander J. The prognostic significance in Goodpasture’s disease of specificity, titre and affinity of anti-glomerular basement membrane antibodies. Nephron Clin Pract. 2003;94:c59–c68. doi: 10.1159/000072022. [DOI] [PubMed] [Google Scholar]
- 18.Rutgers A, Slot M, van Paassen P, van Breda Vriesman P, Heeringa P, Tervaert JW. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis. 2005;46:253–262. doi: 10.1053/j.ajkd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Heeringa P, Brouwer E, Klok PA, Huitema MG, van den Born J, Weening JJ, et al. Autoantibodies to myeloperoxidase aggravate mild anti-glomerular-basement-membrane-mediated glomerular injury in the rat. Am J Pathol. 1996;149:1695–1706. [PMC free article] [PubMed] [Google Scholar]
- 20.Csernok E, Lamprecht P, Gross WL. Diagnostic significance of ANCA in vasculitis. Nat Clin Pract Rheumatol. 2006;2:174–175. doi: 10.1038/ncprheum0159. [DOI] [PubMed] [Google Scholar]
- 21.Stinton LM, Bentow C, Mahler M, Norman GL, Eksteen B, Mason AL, et al. PR3-ANCA: a promising biomarker in primary sclerosing cholangitis (PSC) PLoS One. 2014;9:e112877. doi: 10.1371/journal.pone.0112877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 2002;61:80–89. doi: 10.1046/j.1523-1755.2002.00089.x. [DOI] [PubMed] [Google Scholar]
- 23.Kemna MJ, Damoiseaux J, Austen J, Winkens B, Peters J, van Paassen P, et al. ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol. 2015;26:536–542. doi: 10.1681/ASN.2013111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tesar V, Jelinková E, Masek Z, Jirsa M, Jr, Zabka J, Bartůnková J. Influence of plasma exchange on serum levels of cytokines and adhesion molecules in ANCA-positive renal vasculitis. Blood Purif. 1998;16:72–80. doi: 10.1159/000014316. [DOI] [PubMed] [Google Scholar]