Abstract
Cancer is among the leading causes of morbidity and mortality worldwide. Many of the chemotherapeutic agents used in cancer treatment exhibit cell toxicity and display teratogenic effect on nontumor cells. Therefore, the search for alternative compounds which are effective against tumor cells but reduce toxicity against nontumor ones is of great importance in the progress or development of cancer treatments. In this sense, scientific knowledge about relevant aspects of nutrition intimately involved in the development and progression of cancer progresses rapidly. Phytochemicals, considered as bioactive ingredients present in plant products, have shown promising effects as potential therapeutic/preventive agents on cancer in several in vitro and in vivo assays. However, despite their bioactive properties, phytochemicals are still not commonly used in clinical practice due to several reasons, mainly attributed to their poor bioavailability. In this sense, new formulation strategies are proposed as carriers to improve their bioefficacy, highlighting the use of lipid-based delivery systems. Here, we review the potential antitumoral activity of the bioactive compounds derived from plants and the current studies carried out in animal and human models. Furthermore, their association with lipids as a formulation strategy to enhance their efficacy in vivo is also reported. The development of high effective bioactive supplements for cancer treatment based on the improvement of their bioavailability goes through this association.
1. Introduction
The conventional treatments against cancer are nowadays replaced by new approaches such as hormone therapy, biological therapy, and stem cell transplantation. In addition to these proposals, new chemical compounds are tested, focusing on founding antitumoral agents with high specificity response and low toxic side effects and warding off resistance development. In this sense, phytochemicals (Phy) have received increasing attention due to their high potency and low toxicity compared with common chemotherapeutic agents [1] and with pharmacological properties acting through specific molecular targets [2–4]. Thus, Phy are considered as nonnutritive compounds found in plants and safe for human intake [2] and with promising applications since their consumption is integrated within diet components.
However, despite their promising benefits in vitro, results from several studies highlight a low Phy bioactivity in vivo [5], mainly attributed to their poor water solubility, rapid metabolism, and short half-live and even causing gastrointestinal irritation. These factors lead to low and variable oral bioavailability and nonreproducible absorption, which gives rise to therapeutic concentrations that are difficult to achieve, high intra- and intersubject variability, and lack of dose proportionality [6], offering significant limitations or challenges to the cancer therapy with Phy.
Therefore, to increase the Phy applicability, developing formulation strategies that overcome limited oral bioavailability of Phy is needed. In this sense, the association of Phy to delivery systems or carriers composed of diverse materials has been proposed [7]. Particularly, in the last decade, association with lipids, usually referred to as lipid-based delivery systems, gained much interest as they are nontoxic, biodegradable, and highly biocompatible and show great versatility. In this respect, lipid formulations can be modified in various ways to meet a wide range of product stability requirements (molecular weight and physicochemical properties), disease conditions and route of administration, and existing commercial formulations for topical, oral, pulmonary, or parenteral product delivery [8, 9].
In these frameworks, the present work summarizes the existing dietary Phy with promising anticarcinogenic properties and Phy-based therapies that are being currently evaluated in vitro, in vivo, and in clinical trials as efficient approaches for the prevention and treatment of cancer and their bioavailability. Likewise, it also summarizes the delivery systems currently used to enhance the clinical use of Phy by increasing their oral bioavailability and by promoting their safe and targeted activity, mainly emphasizing the lipid-based delivery systems.
2. Dietary Phytochemicals Possessing Anticancer Properties
In the last years, several studies have amply demonstrated that tumor development could be highly associated with diet habits [10, 11]. In this sense, current researches on new approaches for cancer treatment are focused on the study of three axes: dietary patterns, specific foods, and safe and bioavailable dietary compounds [12]. Among the latter, Phy derived from diet might be considered as promising preventive and therapeutic alternative agents against cancer.
According to their chemical structure, Phy can be mainly classified into four groups: polyphenols, terpenes, organosulfur compounds, and phytosterols. The following provides a description of Phy belonging to the mentioned structural categories that have shown potential anticancer properties in in vitro studies, as the first step to evaluate their enhanced activities, and in in vivo models, as the second step of efficacy evaluation and determination of molecular action and targets. Phy tested in preclinical and clinical studies conducted with human cancer patients to validate their in vivo therapeutic effect are also listed.
2.1. Polyphenols
Antitumor benefits of polyphenols have been widely described. Polyphenols constitute one of the major constituents of plants and are abundant in our diet. The occurrence in plant matrix is very variable, going from simple phenolic molecules to complex associations (highly polymerized compounds). They are usually classified into different groups according to their structure and number of rings, highlighting phenolic acids, flavonoids, stilbenes, and curcuminoids, which are described below and compiled in Table 1.
Table 1.
Polyphenols studied in experimental in vitro tests, in vivo models, and clinical trials.
| Polyphenols | Phytochemical | Main source | Cancer targets in vivo and in vitro | Clinical trials | References cancer targets/clinical trials | Chemical structure |
|---|---|---|---|---|---|---|
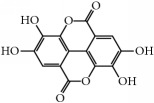
| Phenolic acids | Ellagic acid | Pomegranate, berries, grapes | Prostate | Prostate Follicular lymphoma |
[161–165]/ [166, 167] |
|
| Pancreas | ||||||
| Bladder | ||||||
| Breast | ||||||
| Colon | ||||||
|
| ||||||
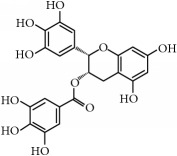
| Flavonoids | (−)-Epigallocatechin-3-gallate (EGCG) | Green tea (Camellia sinensis) |
Prostate | Prostate Papilloma cervical Breast Prostate |
[168–174]/ [175–178] |
|
| Renal carcinoma | ||||||
| Breast | ||||||
| Laryngeal carcinoma | ||||||
| Non-small cell lung | ||||||
| Colon | ||||||
| Pancreas | ||||||
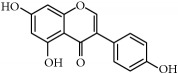
| Genistein | Soybean | Bone marrow | Prostate | [179–183]/ [184–187] |
|
|
| Prostate | Bone | |||||
| Breast | Endometrial | |||||
| Cervical | Breast | |||||
| Colon | Bladder | |||||
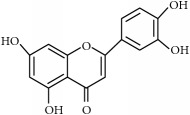
| Luteolin | Cabbages, celery, broccoli, onion leaves, parsley | Hepatocellular carcinoma | — | [188–193] |
|
|
| Oral squamous carcinoma | ||||||
| Prostate | ||||||
| Breast | ||||||
| Thyroid | ||||||
| Colorectal | ||||||
| Cervical | ||||||
| Lung | ||||||
| Silymarin | Thistle (Silybum marianum) |
Prostate | Upper gastrointestinal Leukemia |
[194–198]/ [199, 200] |
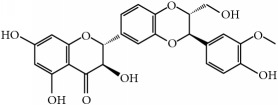
|
|
| Breast | ||||||
| Ovary | ||||||
| Colon | ||||||
| Lung | ||||||
| Bladder | ||||||
| Skin | ||||||
| Prostate | ||||||
| Quercetin | Capers, lovage leaves, apple | Pancreas | Large bowel | [201–206]/ [207–209] |
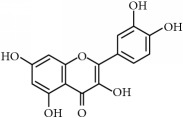
|
|
| Breast | Ovary | |||||
| Cervical | Pancreas | |||||
| Colon | Prostate | |||||
| Prostate | Thrombotic | |||||
| Lung | Colorectal | |||||
|
| ||||||
| Stilbenes | Resveratrol | Grape, berries | Breast | [38, 39, 210–217]/ [218–222] |
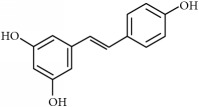
|
|
| Colorectal | ||||||
| Hepatic melanoma | ||||||
| Lung | Colorectal | |||||
| Pancreas | Colon | |||||
| Prostate | Gastrointestinal tumors | |||||
| Skin | ||||||
| Bladder | ||||||
| Ovarian | ||||||
|
| ||||||
| Curcuminoids | Curcumin | Curcuma longa L. | Pancreas | Pancreas Colorectal Colon Liver Pancreas Breast Head and neck |
[40, 41, 223–230]/ [96, 231–238] |
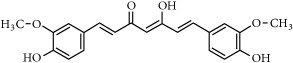
|
| Prostate | ||||||
| Ovarian | ||||||
| Melanoma | ||||||
| Head and neck squamous cell carcinoma | ||||||
| Leukemia | ||||||
| Hepatoma | ||||||
| Gastric | ||||||
| Glioblastoma | ||||||
| Lung | ||||||
| Breast | ||||||
| Cervical | ||||||
| Colorectal | ||||||
Clinical trials carried out considering phytochemicals as dietary complements or drugs (therapy) in cancer patients.
For the experimental studies, in vivo studies are in italic characters.
Chemical structures were obtained by using ChemDraw Professional 15.0 software.
(i) Phenolic acids represent 30% of total dietary polyphenols [13] and they are the major constituents of phenolic compounds. They usually include hydroxybenzoic acids and hydroxycinnamic acids [14], where one of the positions of the aromatic benzoic o cinnamic ring is occupied by a hydroxyl group and the remaining four positions are available for other chemical groups. One of the most studied phenolic compounds is the ellagic acid, as described in Table 1.
(ii) Flavonoids. Although they are not considered as essential dietary factors, they represent 60% of dietary polyphenols [2, 13] and are starting to be considered the key between prevention and treatment of chronical diseases and diet. Chemically, the flavonoid skeleton consists of two phenyl rings joined by a linear three-carbon bridge [15]. Table 1 summarizes those studied against cancer. Genistein, (−)-epigallocatechin-3-gallate (EGCG), and quercetin are the flavonoids more frequently tested in clinical trials against tumors. Genistein have been extensively studied as prospective antitumor molecules in the treatment of prostate cancer. Meanwhile, EGCG has also been largely studied in experimental studies against different types of tumors, even in clinical trials, particularly against prostate or cervical injuries. Quercetin was tested, in addition, against tumors related to the digestive tract, such as bowel, colon, or pancreas.
Within flavonoids, proanthocyanidins are also underlined as effective naturally occurring compounds in grape seeds or pine bark with antitumorigenic effects. They take the form of oligomers or polymers (+) catechin and (−) epicatechin, and the carried-out in vivo studies have remarked the preventive and effective action against UV-induced skin tumors but also showed the inhibition of lung metastasis and mammary and prostate cancer [16]. Concerning clinical studies, the is just one concluded trial which studied the positive chemoprevention proanthocyanidin effect on breast cancer [17].
(iii) Stilbenes constitute a large family within polyphenols and have numerous implications in plant disease resistance and human health (including antitumoral activity). Stilbenes have a 1,2-diphenylethylene core and belong to a small group of phenylpropanoids and only a few plants spices can synthetize them. They are produced in response to a biotic or abiotic stress [18]. The most largely studied is resveratrol, which is produced in plants in response to mechanical injuries. It is reported to be efficient against gastrointestinal tumors in clinical trials, and in vivo tests were carried out in breast, ovarian, lung, or skin tumors (Table 1).
(iv) Curcuminoids are derived from curcumin, and they are obtained from turmeric (Curcuma longa). Curcumin belongs to diarylheptanoid series and is characterized by 1,3-diketones and two methoxylated phenols [19]. Curcumin is largely used as medicinal and food ingredient in Asia, especially in India. Within cancer therapies, it has been tested in several in vivo tumor models and even in clinical trials (Table 1).
2.2. Terpenes
Another important group of phytochemicals is that constituted by terpenoids or terpenes, which is the most abundant and structurally diverse group synthetized by plants. Terpenes show a wide range of physiological functions, many of them related to the plant defense system, and they are often components of essential oils and resins [20]. Terpenes are synthesized from two to five carbon building blocks based upon the isoprene unit. Depending on the number of blocks, they can be classified as monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes (C40), and polyterpenes [21]. Their potential antitumor properties have been described in several works [22], as shown in Table 2.
Table 2.
Terpenes, organosulfur, and phytosterols commonly studied in cancer therapy.
| Family | Phytochemical | Main source | Cancer targets in vivo and in vitro | Clinical trials | References cancer targets/clinical trials | Chemical structure |
|---|---|---|---|---|---|---|
| Terpenes | ||||||
|
| ||||||
| Carotenoids | Lycopene (tetraterpene) | Tomato (Lycopersicon esculentum) | Prostate | Prostate | [239–242]/ [243, 244] |
|
| Colon | ||||||
| Breast | ||||||
| Lung | ||||||
| Cervical | ||||||
| Breast | ||||||
| Laryngeal | ||||||
| Liver carcinoma | ||||||
| Astaxanthin | Green microalgae (Haematococcus pluvialis) | Hepatic | — | [245–251] |
|
|
| Oral carcinoma | ||||||
| Fibrosarcoma | ||||||
| Skin | ||||||
| Bladder | ||||||
| Colon | ||||||
| β-Elemene | Ginger, celery | Laryngeal | Glioma | [252–256]/ [257] |
|
|
| Non-small cell lung | ||||||
| Gastric cancer | ||||||
| Prostate | ||||||
| Brain | ||||||
| Breast | ||||||
| Cervical | ||||||
| Colon | ||||||
| Ovarian | ||||||
| Melanoma | ||||||
| Glioblastoma | ||||||
| Noncarotenoid | Carnosol (diterpene) | Sage (Salvia carnosa), Rosemary (Rosmarinus officinalis) | Colon | — | [258–263] |
|
| Prostate | ||||||
| Skin | ||||||
| Breast | ||||||
| Ovarian | ||||||
| Intestinal | ||||||
| Melanoma | ||||||
|
| ||||||
| Organosulfur | ||||||
|
| ||||||
| Thiosulfinates | Sulforaphane | Brassica vegetables | Skin | Breast | [42, 95, 264–267]/ [268] |
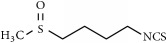
|
| Gastrointestinal-colon | ||||||
| Prostate | ||||||
| Pancreas | ||||||
| Breast | ||||||
| Bladder | ||||||
| Ovary | ||||||
| Mammary | ||||||
| Diallyl disulfide | Allyl vegetables | Gastric | — | [43, 44, 269–273] |
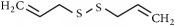
|
|
| Breast | ||||||
| Leukemia | ||||||
| Neuroblastoma | ||||||
| Prostate | ||||||
| Colon | ||||||
| Thyroid | ||||||
|
| ||||||
| Phytosterols | ||||||
|
| ||||||
| Phytosterols | β-Sitosterol | Vegetal oils | Colon | — | [274–278] |
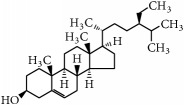
|
| Breast | ||||||
| Stomach | ||||||
| Prostate | ||||||
| Fibrosarcoma | ||||||
Clinical trials carried out considering phytochemicals as dietary complements or drugs (therapy) in cancer patients.
For the experimental studies, in vivo studies are in italic characters.
Chemical structures were obtained by using ChemDraw Professional 15.0 software.
(i) Carotenoids are the most abundant tetraterpenes, and in natural samples they could be found free or esterified by fatty acids, the degree of esterification being related to the hydroxyl groups. They also are characterized by the presence of 11 or 12 conjugated carbon double bounds [23]. All of them represent variants or degradation derivatives of β-carotene, which is found in carrot (Daucus carota). Antitumor activity of the acyclic tetraterpene lycopene has been largely studied in both in vivo and clinical trials, especially conducted with prostate tumors (Table 2). Besides lycopene, astaxanthin may exert antitumor activity through its antioxidant and immunomodulatory characteristics in tumors such as colon and hepatic carcinomas, as shown in Table 2.
(ii) Noncarotenoids are not derived from carotenes. This group of terpenes includes carnosol, a phenolic diterpene largely studied in cancer and associated with bioactivity of rosemary (Table 2). For carnosol, there are in vivo positive studies against colon, prostate, and skin tumors and no clinical studies proposed.
2.3. Organosulfur Compounds
Organosulfur compounds are Phy with one or more carbon-sulfur bonds in their structure and a thioketal-linked glucose molecule (S-glycosides). They are classified into two groups: glucosinolates and thiosulfinates [24]. Glucosinolates are sulfur-containing plant secondary metabolites that usually exist in cruciferous plants and are hydrolyzed by specific enzymes (myrosinases) to release biologically active sulfurated aglycones, known as isothiocyanates [2, 25]. Glucosinolates and their hydrolysis products exhibit direct and indirect antioxidant effects by scavenging harmful radicals and modulation of detoxification enzymes, such as glutathione S-transferase [26]. Thus, consumption of cruciferous plants, such as cabbage and broccoli, is believed to promote health and to reduce the risk of cancer development [27]. Among isothiocyanates, sulforaphane, produced from the glucosinolate glucoraphanin, has been largely studied as chemopreventive agent in different tumors in vivo, and it is the unique organosulfur compound that has been tested in a clinical trial as antitumorigenic agent [28] (Table 2).
Thiosulfinates (allyl sulfides), such as diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS), are mainly present in garlic and onion (Allium family) [25]. Among them, DADS, an oil-soluble organosulfur compound, has been described as the major one responsible for therapeutic properties against prostate and colon in in vitro models and gastric, breast, and leukemia in in vivo models (Table 2).
2.4. Phytosterols
Phytosterols are lipid-like compounds and essential for maintaining permeability and fluidity on cell plant permeability. Vegetable oils are the main source of dietary phytosterols. They occur in various structural forms (as steryl glucosides, acetylated steryl glucosides, esters, or alcohols) [29], each of them existing in different compartments of the plant cell. There are approximately 200 phytosterols, among which β-sitosterol, campesterol, and sitostanol are the major ones [30].
β-Sitosterol is the most abundant phytosterol and although it is well known for its cholesterol lowering action [31], several in vitro and in vivo evidences suggest it possesses preventive effects against cancer (Table 2). Campesterol and sitostanol, however, have not shown any effect on tumor growth [32].
Within terpenes, triterpenoids (squalene) play a determinant role as they are considered common precursors of steroids, including phytosterols. Triterpenoids exist in free form or combined with sugar into glycosides. The free form shares the same chemical properties as phytosterol so long as they can be dissolved in organic solvents but insoluble in water [33]. In the last years, triterpenoids have demonstrated antitumor efficacy against breast, leukemia, multiple myeloma, and non-small cell lung carcinomas, specially affecting cell proliferation [34, 35]. Some triterpenes are already tested in Phase I clinical trials [36], with beneficial effects, even if some authors defend their combination with other triterpenoids, Phy, or synthetic drugs.
In general, in vitro and in vivo assays conducted with dietary Phy (Tables 1 and 2) showed tumorigenesis inhibition or potential chemopreventive effects. However, a high variability in anticancer effects was observed among different patients during clinical trials, which is one of the major limitations of the Phy-based therapy in the clinical practice.
3. In Vivo and Clinical Bioactivity of Phytochemicals
Although Phy hold part of their biological activity in vivo, as said above, their activity in this context is lower than observed for the same compound in the in vitro evaluation phase. An obvious reason for the “loss” of activity is the lack of pharmacokinetic optimization or compatibility [37]. One of the main factors that influences pharmacokinetics of the tested bioactive compound is its tissue bioavailability, which is defined by the Food and Drug Administration as “the rate and extent to which the active ingredient or active moiety is absorbed from a drug product, reach plasma and body tissues and becomes available at the site of action in an unchanged form”. Thus, bioavailability should be considered when the efficacy of dietary Phy is evaluated in vivo in animal models and/or human clinical trials. The impact of bioavailability is especially pronounced when the bioactive compound is intended for oral use, whereby gastrointestinal (GI) absorption constitutes the primary barrier between an active ingredient and systemic circulation. In the present review, we focus on oral bioavailability as the major pharmacokinetic aspect for the clinical application of orally delivered dietary Phy with high bioefficacy as anticancer agents. In this respect, factors affecting GI absorption and oral bioavailability of main dietary Phy will be addressed.
3.1. Oral Bioavailability of Dietary Phytochemicals
Oral route is generally considered the easiest and most convenient method for the delivery of drugs and dietary bioactive compounds due to properties such as noninvasiveness, cost-effectiveness, and being less prone to side effects, such as injection-site reactions [9]. In fact, although in some of the in vivo studies and clinical trials listed in Tables 1 and 2 Phy were administered by intraperitoneal or intratumoral injection and topical route [38–44], in most of the cases, they were orally administered (by gavage, diet supplementation, water suspension, or capsules).
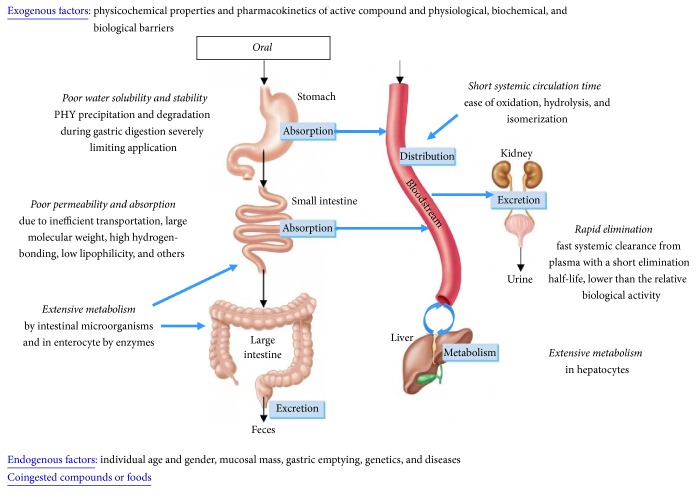
However, as commented above, the suitability of this administration route depends on the oral bioavailability of the active ingredient, which, as summarized in Figure 1, is the result of the synergistic effect of the following factors:
Physicochemical properties of Phy, which determine their water solubility and stability inside the GI tract
Physiological barriers, including the chemical (e.g., pH) and biological environment (e.g., microbiota) inside the GI tract, which also have a significant influence on Phy stability during digestion and absorption [45]
Biochemical barriers (including biodistribution), biological barrier (GI wall permeability), and pharmacokinetics (metabolism and clearance) of the active ingredient
Endogenous factors, as the individual age and gender, mucosal mass, gastric emptying, genetics, and diseases [46]
Amount of coingested compounds or foods
A compound which can exist in a stable form to survive the GI environment and that has optimum physicochemical properties to penetrate the GI wall is most likely to possess acceptable oral bioavailability. Most of Phy, however, have shown physicochemical properties that lead to a poor water solubility and stability in the GI environment and poor permeability. These include complex structure, size, high molecular weight, high lipophilicity, compound H-bonding to solvent, intramolecular H-bonding, intermolecular H-bonding, crystal packing, crystallinity, polymorphic forms, ionic charge status, isoelectric point (pI), and salt form [47]. In addition to physicochemical properties limiting their GI absorption, Phy are usually subjected to extensive metabolism in the enterocyte and hepatocyte and/or quickly eliminated in the urine [48]. All these factors result in a poor and variable bioavailability, which leads to therapeutic concentrations that are difficult to achieve, nonreproducible absorption, variable efficacy intra- and intersubject during clinical trials, and lack of dose proportionality. This explains the lower in vivo bioactivity and nonreproducible data obtained in previous studies (Table 2) [6, 49]. Bioavailability studies of the major dietary Phy are described below.
Figure 1.
Determinant factors of the oral bioavailability of bioactive compounds, including phytochemicals.
3.1.1. Bioavailability Studies of the Major Dietary Phytochemicals
(i) Polyphenols. Most of the studies focus on bioavailability related to levels of the polyphenol present in blood or urine [50], but few of them determine the bioavailability in target tissues, which can be more determinant for affirming their application for a specific illness. After intestinal hydrolysis, polyphenols are conjugated by glucuronidation (addition of glucuronic acid), methylation (addition of a methyl group), or sulfurylation (addition of a sulfo-group), which often facilitate their urinary elimination. Thus, they are well absorbed on tissues where they are metabolized (bowel and liver) [51, 52], but their bioavailability in target tissues is low because of their rapid clearance from the body.
Nevertheless, there is a study that reveals that once sulfate and glucuronide conjugates of resveratrol are circulating in plasma (with an expected low bioavailability), their subsequent hydrolysis releases free resveratrol which can be captured by those cells with specific membrane receptors, increasing thus its bioavailability in specific tissues [53].
These conjugations may also depend on factors described in Section 3.1 such as age and gender, genetics and diseases, and protein-binding in tissues and blood. Moreover, independently of the mechanistic processing of flavonoids, some authors have also described the preventive efficacy of flavonoids (resveratrol) as dependent on the type of diet. In this sense, it has been demonstrated that low doses of resveratrol were able to reduce colon tumor progression better than high doses in subjects exposed to a high fat diet. [54].
(ii) Terpenes. Clinical relevancies of terpenes depend on their presence in target organs. Terpenes have a high lipophilic behavior, and therefore they depend on their solubility in the aqueous phase of the gut lumen. Thus, it has been observed that bioavailability of terpenes is related upon their incorporation to a lipid phase either during digestion or during food processing, making the presence of a quantity of fat necessary for their absorption [20]. Lycopene, one of the major carotenoids described for its anticarcinogenic potential, has been demonstrated to enhance its bioavailability when they are integrated in a chylomicron [55].
(iii) Organosulfur Compounds. Studies related to organosulfur compounds are frequently carried out in combination with other Phy or drugs. Indeed, few experimental data determine their bioavailability, and urine levels after uptake of Brussels or broccoli sprouts [56] are the unique parameter usually measured.
But as they are increasingly consumed due to their potential antitumoral effects, a new variety with genetic variations has been proposed increasing thus the expression of transcription factors involved in glucosinolate biosynthesis. The resulting broccoli could deliver a larger amount of glucoraphanin (active sulforaphane) in plasma and urine [57], although it has not been evaluated in specific organs levels.
(iv) Phytosterols. Phytosterol structure is similar to that of cholesterol but each phytosterol has an additional side chain, which confers dissimilarities in their absorption. Low bioavailability of phytosterols is reported in human plasma after intake. Before absorption starts, the esters are split in the duodenum, increasing their hydrophobicity and reducing their absorption at the same time. In addition, it has been described that they poorly reesterify in the enterocytes, explaining their poor absorption and their subsequently low concentration in the blood circulation [58, 59].
4. Use of Lipid-Based Delivery Systems to Increase the Clinical Efficacy of Antitumor Phytochemicals Administered Orally
The development of crystalline solid formulations by modifying physicochemical properties, as salt formation and micronization (particle size reduction), was initially adopted to amend the poor water solubility of Phy [60]. However, the low wettability and handling difficulties of reduced size formulations as well as the aggregation of nanocrystals inside the body and the impossibility of salt formation from neutral compounds limit the use of these approaches [61]. Amorphous formulations, including solid solutions (active compound immobilized in polymer) and self-dispersing solid solutions (with surfactants), have been also applied; however, the questionable physical stability of product (possibility of crystallization of drug or polymer) limited their use [62].
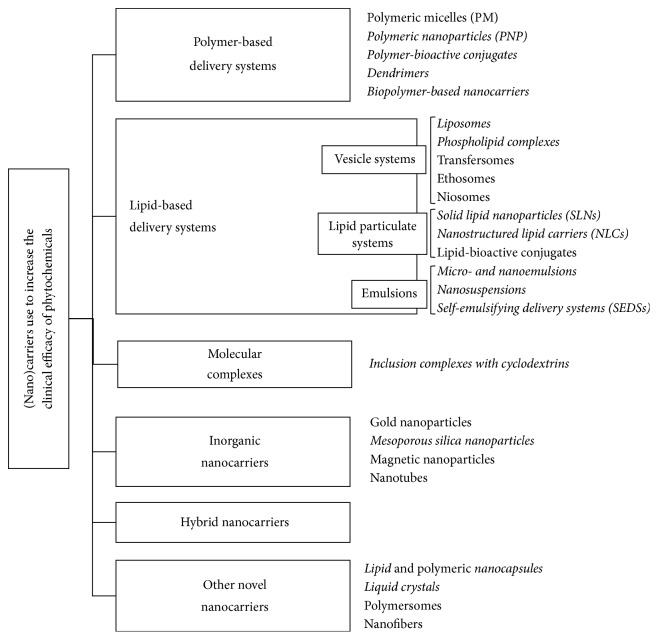
Over the last years, new formulation strategies to increase the clinical efficacy of poor water-soluble active compounds have been developed. Figure 2 shows the new ones, specifically those developed for oral administration of active compounds (in italic). In addition, polymer-based delivery systems (PBDS) have also been popularly adopted to increase the clinical efficacy of some Phy, as observed in Table 4 [63, 64]. To a lesser extent, inclusion complexes with cyclodextrins and its derivatives as well as inorganic, hybrid, and other novel nanocarriers are being currently used (Table 4).
Figure 2.
Types of (nano)carriers used to increase bioefficacy of phytochemicals. Those developed for oral administration of active compounds are in italic characters.
Table 4.
Overview of nonlipid formulations, which have been designed to administer phytochemicals by oral route.
| Active ingredient | Lipid-based formulation | Effect of formulation | Ref. | |
|---|---|---|---|---|
| Type | Subcategory | |||
| Curcumin | PBDS | PLGAa-NPs | Overcome multidrug resistance and increased oral bioavailability in vivo. | [320] |
| Silymarin | In vitro sustained release and enhanced cytotoxicity. | [321] | ||
| Curcumin | Hydroxypropyl cellulose NPs | Temperature-dependent release in vitro. | [322] | |
| Puerarin | Dendrimers | Increased in vitro oral bioavailability and reduced side effects. | [323, 324] | |
| Curcumin | ||||
| Resveratrol | ||||
| Genistein | ||||
| Podophyllotoxin | ||||
| Curcumin | Hyaluronic acid conjugate | Improved water solubility, stability, and antitumoral activity in vitro. | [325] | |
| Alginate conjugate | Higher water solubility, stability, and cytotoxicity in vitro. | [326] | ||
|
| ||||
| Rutin | CD inclusion complexes | α-CD, β-CD, HP-β-CD, and DM-β-CDb | Improved water solubility and stability, increasing the oral bioavailability and bioefficacy. | [327] |
| 3-EGCG | [328] | |||
|
| ||||
| Silymarin (Silybum marianum) | Inorganic nanocarriers | Porous silica nanoparticles (PSN) | Sustained release and enhanced oral bioavailability in vivo. | [321] |
| Silybin meglumine | [329] | |||
|
| ||||
| Resveratrol | Hybrid nanocarriers | TCCc- liposomes | Improved absorption and oral bioavailability and reduced side effects in vivo In vitro controlled release and in vivo enhanced targeting and reduced side effects Overcome multidrug resistance. Enhanced in vitro and in vivo antitumor activity. |
[330] |
| DQA-PEG1930-DSPEa liposomes | [153] | |||
| Vincristine | Dextran-sulfate-SLNs | [331] | ||
| PLGA-PEG-R7a NPs | [332] | |||
| Tripterine | CPPa-NLCs | [118] | ||
|
| ||||
| Silymarin | Other novel nanocarriers | Liquid crystalline nanocarrier | Sustained release. Improved water solubility, oral bioavailability, and biological activity (active targeting-liver) in vivo. |
[333] |
| Quercetin | Folate-modified lipid nanocapsules | [334] | ||
| Tetrandrine | Lipid nanocapsules | [335] | ||
aPLGA: poly(lactic-co-glycolic acid); PEG: polyethylene glycol; R7 is a cell-penetrating peptide; DQA: dequalinium; DSPE: polyethylene glycol-distearoylphosphatidylethanolamine; R7 is a cell-penetrating peptide (CPP).
b α/β-CD: alpha/beta-cyclodextrin; HP-β-CD: hydroxypropyl-β-cyclodextrin; DM-β-CD: dimethyl-β-cyclodextrin.
cTCC: N-trimethyl chitosan chloride-coated.
Furthermore, it is worth mentioning that, in recent years, an increased interest has been focused on the incorporation of poorly water-soluble compounds into lipid-based delivery systems (LBDS). Association with lipid-based delivery systems has been shown to be one the most powerful strategies for the formulation of poorly water-soluble active compounds [8, 9], as they show several advantages compared to other carriers, including
higher degree of biodegradability and biocompatibility;
higher degree of versatility: lipid formulations can be modified in various ways to suit the stability requirements (molecular weight and physicochemical properties) and toxicity and efficacy of the active agent as well as the route of administration and cost;
high and enhanced loading capacity;
pharmaceutical stability;
release of the active compound in controlled and targeted way;
simple preparation methods and easy scale production;
low risk of side effects (nontoxic).
The present work reviews the novel LBDS (vesicle and lipid particulate systems and emulsions) as recorded in Figure 2, describing the formulation approaches and mechanism of action. Furthermore, the LBDS combined with Phy in vitro and in vivo studies are also listed.
4.1. Formulation Approaches for Oral Lipid-Based Delivery Systems
LBDS can be obtained by blending excipients such as pure triglyceride oils, mixed glycerides, lipophilic surfactants, hydrophilic surfactants, and water-soluble cosolvents, which determine the absorption process [65]. Thus, in order to maximize the success in lipid-Phy formulation development and commercialization, it is precise to consider the following aspects:
Screening and preselection of lipid excipients, mainly considering their solubility, dissolution/dispersion properties, digestibility, and absorption. Other factors are irritancy, toxicity, purity, chemical stability (regulatory issues), capsule compatibility, melting point (depending on the fatty acid composition), and cost
Identification of the suitable formulation technique for the intended dosage form. Often solid form, developed mainly by adsorption on solid carriers [66], spray drying [67], lyophilization [68], melt extrusion [69], and nanoparticle technology [62], is preferred over liquid and semisolid forms, which offer low stability, irreversible drug/excipient precipitation, large volume of dose, and difficulty of handling and portability
Testing the formulation in appropriate animal models to predict the in vivo behavior (bioavailability, pharmacokinetics, and intestinal lymphatic absorption)
Optimization of the formulation based on the Phy loading and dissolution profile.
4.2. Mode of Action of Oral Lipid-Based Delivery Systems
The goal of any oral LBDS is to enhance the GI absorption and oral bioavailability of the active compound. Their mode of action involves the alteration of the following physiological effects.
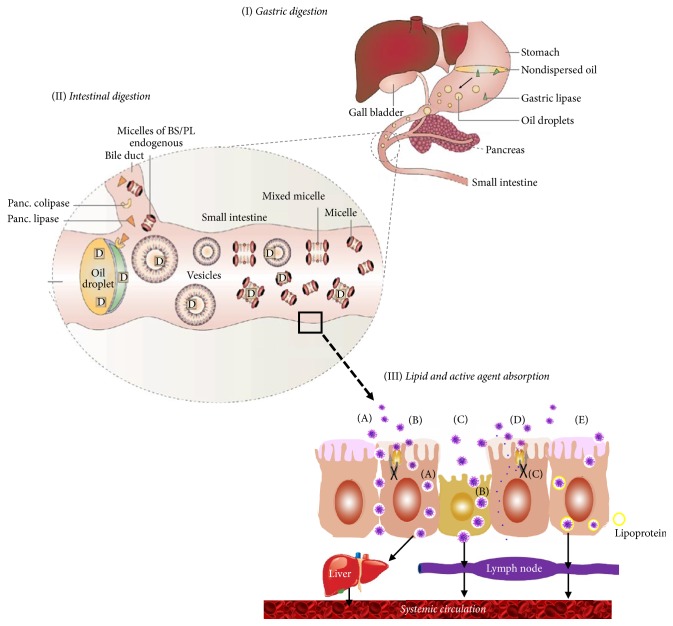
(I) After oral administration of the lipid-Phy formulation and once in the aqueous environment of the stomach, gastric lipase initiates the digestion of formulation lipids. Simultaneously, peristaltic movements of the stomach facilitate dispersion of lipid excipients into small droplets (Figure 3(I)). This accelerates the solubilization process of Phy in the lipid base and keeps the Phy in solution for prolonged period, avoiding its precipitation and protecting it from the low pH in stomach and the enzymatic and/or chemical degradation within the GI tract [1, 5, 6, 70].
Figure 3.
Mode of action of lipid-based delivery systems designed for the efficient oral administration of phytochemicals. (A) Allowing paracellular transport by opening tight junction; (B) facilitating transcellular absorption due to increased membrane fluidity; (C) promotion of phagocytosis via specialized microfold cells (M cells) of Peyer's patches; (D) increased intracellular concentration and residence time by surfactants due to inhibition of P-gp and/or CYP450; (E) lipid stimulation of lipoprotein/chylomicron production.
(II) Once in the small intestine, lipid excipients stimulate bile flow and pancreatic juices excretion [71]. Pancreatic lipase hydrolyzes triglycerols (TG) into free fatty acids (FFA), monoglyceride (MG), and diglyceride (DG), which, along with bile salts and phospholipids (PL) from gallbladder, form vesicles, micelles, and mixed micelles (Figure 3(II)). These colloidal structures favor solubilization and transportation of Phy until absorption area protecting it from microbiota metabolism and enzymatic degradation, prolonging its residence time, and leading to the uniform distribution of Phy in the GI tract, which minimizes irritation of gut wall due to direct contact with Phy [1, 72].
(III) Formation of colloidal systems (vesicles, micelles, and mixed micelles) that significantly enhances the intestinal absorption of lipid digestion products and Phy as follows:
(i) Changing Phy uptake by interacting with transport processes of enterocyte. These include mucoadhesion (interaction with mucin to increase membrane fluidity), paracellular transport by modulating tight junctions, and promotion of receptor-mediated transport processes (endocytosis, transcytosis, and phagocytosis) via M cells of Peyer's patches and other mucosa-associated lymphoid tissues (MALT) (Figure 3(III)(A)–(C)).
(ii) Inhibiting efflux transporter P-glycoprotein (P-gp) and metabolism by cytochrome P450 (CYP450) or cytochrome 3A (CYP-3A) isozymes (Figure 3(III)(D)). This increases the intracellular concentration and residence time of Phy in enterocyte.
(iii) Enhancing Phy transport to the systemic circulation via intestinal lymphatic system [73–75]. Lipid metabolites stimulate lipoprotein/chylomicron production, which react with Phy molecules enhancing its intestinal lymphatic transport (Figure 3(III)(E)). This avoids the first-pass hepatic metabolism, which provides resistance to metabolic processes, leading to changes in Phy disposition and, finally, in its pharmacokinetic properties [70, 75].
All of this leads to an enhanced absorption, oral bioavailability, and bioefficacy of Phy, which should allow applying an accurate oral dosage to obtain reproducible results in clinical assays (reduced inter- and intrasubject variability) and enhance, thus, the clinical use of Phy therapy.
(IV) In addition of increasing water solubility, absorption, and oral bioavailability, lipid-based delivery systems have been shown to
reduce the effect of coingested food on pharmacokinetics of the bioactive molecule [70];
increase Phy pharmaceutical stability and lengthen its systemic circulation time [76];
release Phy slowly over an extended duration (days or months) after a single administration (sustained release) [77];
enhance penetration into tumoral matrices, promoting more reliable Phy access, and enhance blood-brain barrier permeability [78, 79];
modulate the biodistribution of incorporated molecules, which leads to targeted effects and, hence, reduced side effects [1];
overcome multidrug resistance [80];
enhance efficiency of codelivery of active ingredients and therapeutic agents [81].
4.3. Types of the Main Oral Lipid-Based Delivery Systems and Their Applications
4.3.1. Vesicle Systems
As indicated in Figure 2, lipid-based delivery systems can be classified in three categories, including vesicle systems, lipid particulate systems, and emulsions. Among the vesicle systems, liposomes and phospholipid complexes are the most frequently used.
(i) Liposomes. Liposomes are the most common and well-investigated nanocarriers for targeted drug/active delivery. The use of liposomes to deliver phytochemicals began in the 1980s as an approach to overcome limitations of clinical application of these compounds [1]. Conventional liposomes consist in small spherical vesicles, which present a simple bilayer membrane enclosing aqueous spaces. The lipids mainly used are phospholipids, so that, in an aqueous medium, the hydrophobic tails tend to gather together, while the hydrophilic heads are exposed towards water, thereby forming the round-shape vesicles. Amphiphilic nature of these systems makes them capable of encapsulating from hydrophilic agents, which can be located within the aqueous core, to hydrophobic substances, which can be embedded into the inner fatty acid layers [82–85].
Liposomes are highly biocompatible and possess self-assembly capacity. They are considered pharmacologically inactive with minimal toxicity [82–85], although they are not as immunologically inert as previously suggested [86]. Likewise, conventional liposomes have been shown to increase oral bioavailability and bioefficacy of loaded agents by
improving their water solubility and stability;
avoiding their early precipitation and intestinal and hepatic degradation;
leading to drug concentration in tumoral tissues. This is because liposomes are preferentially delivered and passively accumulate here due to the high interstitial pressure, enhanced vascular permeability and retention, and the lack of functional lymphatic drainage of solid tumors (passive targeting effect) [87, 88];
minimizing side effects.
However, conventional liposomes show some disadvantages that limit their applicability. These include poor stability in the systemic circulation and high recognition by reticuloendothelial system (RES), which leads to short circulation time (short shelf life) and low encapsulation efficacy expulsion of loaded molecules by intermembrane transfer [89].
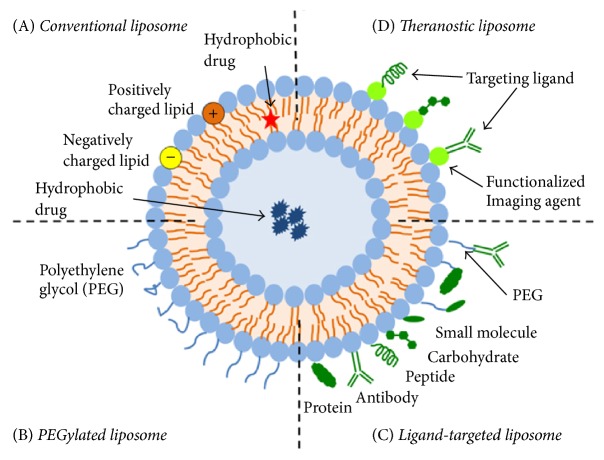
Over the last years, structural and physicochemical properties of liposomes have been modified to develop different types of liposomal delivery systems, called nanostructured liposomes, which do not show the drawbacks of the conventional ones [90] (Figure 4). Among them, we find the PEGylated liposomes, which are modified by adding polyethylene glycol (PEG) to the surface. This confers steric stabilization and, hence, higher stability in vivo. Structural modification can also consist in the attachment of different types of ligands (e.g., antibodies, peptides, and carbohydrates) to the surface or to the terminal end of the attached PEG chains. These systems, which are called ligand-targeted liposomes, are used for specific (active or physicochemical) targeting [91, 92]. Finally, to develop more efficient drug delivery systems, multifunctional liposomal formulations, also called theranostic liposomes, have been recently developed. These carriers usually consist of the nanoparticle, the therapeutic agent, an imaging component, and one or more targeting ligands which enhance their accumulation in pathological sites and promotes organelle-specific delivery. In this sense, theranostic liposomes can be used as therapeutic and diagnostic tool at the same time [87, 91].
Figure 4.
Schematic representation of the different types of liposomal drug delivery systems: (A) conventional liposome; (B) PEGylated liposome; (C) ligand-targeted liposome; (D) theranostic liposome (reprinted from Frontiers in Pharmacology, 6, article 286, 1–12. Advances and Challenges of Liposome Assisted Drug Delivery, by Sercombe et al. [87], with permission from the authors).
The stability in vitro and in vivo of nanostructured liposomes as well as the release profile of the loaded agent is determined by the liposome surface charge, particle size, lipid composition, and number of lamellae and the nature of polymers and ligands attached to their surface [85, 93].
Nanostructured liposomes have been adopted in recent years for the efficient oral delivery of several Phy with poor water solubility and stability in the gastric environment (Table 5). Thus, for instance, vinorelbine, a chemotherapeutic obtained by semisynthesis from alkaloids extracted from the rosy periwinkle (Catharanthus roseus), has been loaded into a cholesterol-polyethylene glycol (cho-PEG) coated liposome with the purpose of increasing circulating half-life and reducing severe side effects of this agent [94]. Likewise, N-trimethyl chitosan chloride- (TMC-) coated liposomes for the oral delivery of curcumin were found to be a promising strategy to reduce toxicity and increase therapeutic index [88].
Table 5.
Overview of lipid-based delivery systems to administer phytochemicals by oral route.
| Active ingredient | Lipid-based formulation | Effect of formulation | Ref. |
|---|---|---|---|
| Vinorelbine | Liposomes | Reduced side effects and increased circulation half-life. | [94] |
| Improved therapeutic effect in vivo. | |||
| Gypenoside | Activated in vitro immune response in macrophages. | [335] | |
| Curcumin | Improved pharmacokinetics and oral bioavailability in vivo. | [329, 336] | |
| 3-EGCG | Enhanced in vitro antitumor activity. | [337] | |
| Brucine | Improved absorption and oral bioavailability, enhanced targeting, and reduced side effects in vivo. | [338, 339] | |
|
| |||
| Quercetin | Phytosome | Enhanced membrane permeability, sustained and controlled release. Enhanced absorption, oral bioavailability, and bioefficacy. |
|
| Kaempferol | [101] | ||
| Isorhamnetin | |||
| Silybin | [98, 99] | ||
| 3-EGCG | [102] | ||
| Quercetin | [100] | ||
|
| |||
| β-Elemene | Microemulsions | Increased water solubility and permeability and improved oral bioavailability. | [131] |
| Hydroxysafflor yellow A | [130] | ||
| Puerarin | [127, 340] | ||
|
| |||
| Baicalin | SEDS | Enhanced stability, oral bioavailability, and targeting effects in vitro and in vivo. | [134] |
|
| |||
| Curcumin | SMEDS | Enhanced stability, oral bioavailability, and targeting effects in vitro and in vivo. | [341] |
| Indirubin | [88] | ||
| Hydroxysafflor yellow A | [143, 144] | ||
| Gentiopicrin | [341] | ||
| Lutein | [342] | ||
| Apigenin | [343] | ||
| Nobiletin | [137] | ||
| Oridonin | [139] | ||
| Silymarin | [140] | ||
| Puerarin | [344] | ||
| Hesperidin | [345] | ||
| Berberine hydrochloride (BBH) | [346] | ||
|
| |||
| Morin | SNEDS | Enhanced stability, oral bioavailability, and targeting effects in vitro and in vivo. | [145] |
| Curcumin | [347] | ||
| Lutein | [146] | ||
| Oleanolic acid | [348] | ||
| Vinpocetine | [349] | ||
|
| |||
| Puerarin | SLNs | Improved absorption and oral bioavailability and reduced side effects (irritation of GI mucous membrane) in vivo. | [120, 121] |
| Triptolide | [350] | ||
| Cantharidin | [351] | ||
| Resveratrol | [352] | ||
|
| |||
| Silymarin | NLCs | Increased absorption and oral bioavailability in vivo. Enhanced in vitro and in vivo antitumor activity. |
[123] |
| Tripterine | [124] | ||
| Curcumin | |||
Moreover, brucine, an alkaloid isolated from Strychnos nux-vomica L. (Loganiaceae), produced impressive dose-dependent antitumor effects by causing apoptosis. However, brucine was characterized by a narrow therapeutic index, and high doses of brucine cause severe central nervous system toxicity. Brucine-loaded stealth liposomes enhanced antitumor activity and decreased distribution to the brain [95], which, therefore, considerably improved its therapeutic index.
(ii) Phospholipid-Phytochemical Complexes (Phytosomes®). Several plant bioactive compounds and extracts, mainly constituted by polyphenols and terpenoids, are conjugated with naturally occurring phospholipids, as phosphatidylcholine (PC), in a ratio of 1 : 1 or 1 : 2 (w : w). This formulation strategy leads to the formation of the patented complexes called Phytosomes. Like liposomes, structure of these complexes consists in spherical vesicles with a bilayer membrane of phospholipids, in which the hydrophilic heads are exposed towards the aqueous medium, while the hydrophobic tails remain together in the inner layer. Unlike liposomes, the active agent is not located within the aqueous core, but it binds to the polar end of phospholipid through weak chemical bonds, and the nonpolar portion of the phospholipid remains free [96, 97]. Phy-loaded phytosomes are highly biocompatible and bioavailable as compared to unloaded Phy. Incorporation into phytosomes increases the enterocyte cell membrane permeability of Phy and, hence, the amount reaching the systemic circulation. Likewise, phytosomes offer a controlled and sustained Phy release pattern, which leads to a longer action time and, therefore, to the need of a reduced Phy dose [96, 97].
Silybin was the first bioactive compound marketed as Phytosome formulation. Phospholipid complexation significantly increased the water solubility and liver protection of silybin, which resulted in an increase of its oral bioavailability and pharmacological activity [98]. In a comparative pharmacokinetic study using an equimolar dose of silybin and its complex, the plasma Cmax of silybin after four hours was <35 ng/mL, whereas, for the silybin complex, it was 112 ng/mL [99]. Similarly, quercetin loaded-phytosome showed a water solubility 12-fold higher than free-form quercetin. However, complexation did not affect its antioxidant activity [100].
Ginkgo biloba L. and green tea extracts have been also loaded into phytosomes. Ginkgo biloba L. phytosome was supplied via oral to rats and, then, the pharmacokinetic profile of the major flavonoids of the extract (quercetin, kaempferol, and isorhamnetin) was evaluated by measuring their plasma Cmax, AUC0, and Tmax. Pharmacokinetic parameters of the three flavonoids were significantly improved after formulation, demonstrating that complexation with phospholipids leads to a large increase in Phy oral bioavailability [101]. Likewise, phytosome of green tea extract, principally represented by (−)-epigallocatechin 3-O-gallate, showed an enhanced absorption of catechins as compared to unloaded green tea catechins [102].
4.3.2. Lipid Particulate Systems: SLNs and NLCs
Generally, there are two types of lipid nanoparticles (LNPs), solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) [103]. Both SLNs and NLCs have spherical shape and their average size usually ranges from 40 to 1000 nm. LNPs can be produced by several techniques such as high-pressure or high-speed homogenization, supercritical fluid extraction of emulsions, solvent emulsification/evaporation, spray drying, and ultrasonication [104–106].
LNPs are composed of a lipid solid matrix lipid and surfactants that provide stability [107]. SLN matrix is constituted by biocompatible, biodegradable, and GRAS solid lipids, which are solid at room and body temperature (e.g., highly purified triglycerides, partial glycerides, fatty acids, and steroids) [108]. The matrix of NCLs is also solid at room/body temperature; however, unlike SLNs, often it is composed of a mixture of solid and liquid lipids [101]. Figure 5 shows a scheme of these formulations, where structural differences between both LNPs are observed.
Figure 5.
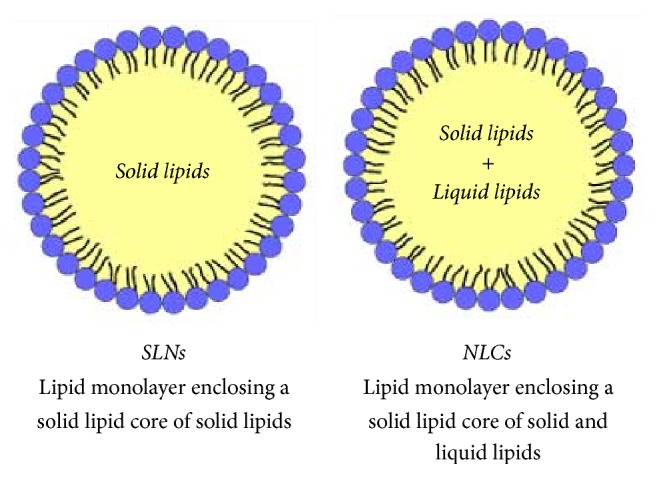
Structure of solid lipid nanoparticles (SLNs) versus nanostructured lipid carriers (NLCs).
In the last years, a great attention has been paid to LNPs as an interesting and cost-effective alternative to polymeric nanoparticles, liposomes, and emulsions. LNPs are cheaper and safer than polymeric carriers, as their production is an organic solvent-free process [103]. Likewise, compared to conventional liposomes, nanoparticle solid matrix allows a higher control release and specific delivery of the loaded agent, which minimizes side effects [109]. LNPs show other benefits as compared to other systems, including ease of preparation and high scale production and sterilization [110, 111], excellent physical stability, and chemical versatility. Moreover, incorporation into the nanoparticle matrix can protect molecules from light, moisture, chemical degradation, and oxidation [109] and favor their penetration through mucus barrier due to nanosize [103, 112–114].
(i) Solid Lipid Nanoparticles (SLNs). Despite all these advantages, applicability of SLNs presents several limitations such as the growth of matrix lipid particles, high water content, ease of gelation, and unpredictable polymorphic transitions, resulting in poor loading capacity [115–117]. In general, drug molecules stay in between the fatty acid chains or as amorphous clusters in crystal imperfections within SLN matrix. However, during SLN storage time, a transition of lipids to a low-energetic form can occur, giving rise to a perfect crystalline structure with very little space for the drug molecules. This promote the expulsion of encapsulated molecules, especially when SLN matrix is composed of a highly purified lipid, which results in a nanoparticle low incorporation capacity and a changing release profile with storage time [103, 113].
(ii) Nanostructured Lipid Complexes (NLCs). To overcome SLNs drawbacks, NLCs have been developed as alternative carrier systems. The presence of liquid lipids (oil) in the solid matrix makes more imperfections to accommodate more active molecules than SLNs, which reduces the active molecule expulsion and enhances the nanoparticle loading capacity. Furthermore, the release and delivery of the active compound can be easily modulated by changing the lipid composition of matrix [113]. NLCs present a lower water content than SLNs and no significant differences regarding biotoxicity have been observed [118].
Table 5 shows in vitro/in vivo studies where SLNs and NLCs have been applied for the efficient oral delivery of antitumor Phy, mainly flavonoids, with limited therapeutic potential [119]. Thus, for instance, Luo et al. [120, 121] investigated the effect of loading puerarin, an isoflavonoid derived from Radix Puerariae, into SLNs, including pharmacokinetics, tissue distribution, and relative bioavailability in rats. When incorporated into the SLNs, puerarin was rapidly absorbed and its relative oral bioavailability was improved more than 3-fold as compared with that of the puerarin suspensions. In addition, SLNs produced increased tissue concentrations in puerarin target organs, particularly heart and brain. Likewise, triptolide, a diterpenoid epoxide isolated from Tripterygium wilfordii with anti-inflammatory, anticystogenesis, and anticancer effects, showed enhanced clinical efficacy and minimized side effects (irritation of the gastrointestinal tract) after encapsulation into SLN [122]. This was attributed to the solubilization of triptolide during GI digestion by the SLN matrix and colloidal mixed micelles (Figure 4), avoiding its precipitation and degradation as well as the GI irritation caused by insolubilized crystals. Moreover, SLNs minimize direct contact of triptolide with the mucosal surface and lead to a gradual release, avoiding high local and irritating concentrations.
Several Phy have been also loaded into NLCs in studies focused on improving water solubility, enhancing GI absorption and oral bioavailability, controlling release, increasing stability, and lengthening circulation time by reducing the recognition by the reticuloendothelial system (RES) (Table 5). The flavonoid silymarin has been used clinically to treat several hepatic disorders without a high efficiency. To improve oral absorption, silymarin-loaded NLCs were developed [123]. These formulations showed fast in vitro lipid digestion, suggesting that NLCs may facilitate the rapid silymarin absorption, and gave rise to relative silymarin bioavailability 2.54- and 3.10-fold greater than that produced by marketed LEGALON® and solid dispersion pellets, respectively. The ability of NLCs to enhance absorption was confirmed in other studies using tripterine, a triterpenoid from the Celastraceae family, extracted from the Chinese herbal plant Tripterygium wilfordii [124]. More recently, various novel and complex NLCs have emerged as carrier designed to achieve specific functions. For example, cell penetrating peptide- (CPP-) coated NLCs loaded with tripterine noticeably enhanced antitumor activity in vitro in prostate tumor cells, as well as in prostate tumor-bearing mice [124]. Ionic complex loaded NLCs enhanced the encapsulation efficiency, improved lipophilicity, and produced sustained release in vivo [95].
4.3.3. Emulsions
(i) Microemulsions and Nanoemulsions. Microemulsions (MEs) are optically isotropic systems with special features, including an average particle size that ranges from 10 to 100 nm; spontaneous formation, that is, without any energy input; thermodynamic stability; optical transparence or slight opalescence; and low viscosity and allergenicity. All this makes them very attractive delivery systems [125].
MEs are constituted by an oil phase, an aqueous phase, a surfactant, and, probably, a cosurfactant [126]. When there are similar amounts of oil and water, a bicontinuous ME is usually formed, in which both phases form continuous domains separated by surfactant-stabilized interfaces. Otherwise, when amounts of oil and water are not similar, MEs with droplet-like structure are formed, which can be water-in-oil (w/o) or oil-in-water (o/w) MEs depending on the major compound.
Like other promising carriers, MEs have been shown to improve oral delivery of bioactive compound by (i) enhancing stability and permeability, (ii) allowing a controlled and sustained release, and (iii) improving GI absorption and oral bioavailability via the lymphatic transport pathway [1, 127]. In this respect, it has been found that this absorption pathway can be significantly favored by w/o MEs as compared to o/w MEs. In addition, due to their special features, MEs offer further advantages, such as ease of preparation, high capacity to solubilize hydrophilic, and lipophilic compounds and long-term stability.
Despite their numerous advantages, MEs present some limitations. They are sensitive to changes of environmental conditions, such as temperature, ionic strength, and composition (adding/removing molecules to/from the aqueous continuous phase), which may compromise their stability. In addition, MEs formation requires the use of relatively large amounts of synthetic surfactants to achieve an efficient loading capacity, especially when using triglycerides as dispersed oil phase [126].
Nanoemulsions (NEs), often also called miniemulsions, are systems with droplet-like structure. They are formed by an oil phase, an aqueous phase, and a mixture of surfactants and cosurfactants stabilizing droplets, whose average size is significantly (10-fold or so) smaller than that of droplets present in conventional emulsions [126]. Like MEs, they are optically transparent and show low viscosity. Moreover, although NEs do not form spontaneously and have been shown to be thermodynamically unstable, they show high kinetic stability, which can be for several years. As compared to MEs, these systems are much less sensitive to changes of environmental conditions and require lower amounts of synthetic surfactants to be formed due to their higher loading capacity [126].
Application of MEs and NEs as carriers for the efficient oral administration of Phy is shown in Table 5. Hydroxysafflor yellow A (HSYA) is a flavonoid derived and isolated from the safflower plant (Carthamus tinctorius L.) that has been shown to possess antioxidant and anti-inflammatory actions, antiplatelet aggregation, and antitumor properties as well as antimyocardial injury effects [128, 129]. Unlike other flavonoids, water solubility of HSYA is high; however, it has very poor permeability, which limits its GI absorption, oral bioavailability, and bioefficacy. Qi et al. [130] developed a HSYA-loaded ME (w/o), which showed a bioavailability ca. 19-folds higher than that of the unloaded compound. MEs have been also used to deliver poor water-soluble and stable Phy, such as elemenes (sesquiterpene). Elemene-loaded emulsions have been used clinically as antitumor agents. However, due to their poor stability and water solubility, the oral bioavailability of these emulsions was only 18.8%. An o/w elemene-loaded ME was then prepared [131]. This showed high entrapment efficiency of 99.81% and significantly higher stability than a normal emulsion, which led to a relative bioavailability 1.63-fold greater than that of the conventional emulsion (Table 5).
(ii) Self-Emulsifying Delivery Systems. A further and very successful approach to overcome problems associated with poor water solubility of Phy is self-emulsifying delivery systems (SEDSs), self-microemulsifying delivery systems (SMEDSs), and self-nanoemulsifying delivery systems (SNEDSs). These systems consist in isotropic mixtures, which include a large variety of liquid or waxy excipients available, ranging from oils through biological lipids (natural/synthetic oil) and hydrophobic and hydrophilic surfactants to water-soluble cosolvents, generally regarded as safe (GRAS) status [132]. Moreover, additives like α-tocopherol, β-carotene, and propyl gallate can be added to prevent the oxidation of SEDSs-Phy formulations [133].
Unlike all the previously described lipid formulations, these systems have a unique property: they remain in a preformulation state until ingestion. Upon dilution in aqueous physiological fluids of GI tract and with the gentle agitation provided by peristaltic movements, SEDSs are able to spread readily and self-emulsify spontaneously, forming fine o/w emulsions (50 nm > droplet size > 250 nm), that keep the active agent in solubilized form [134–136]. SEDDS formulations (oil, 40–80% (HLB < 12), 20–60%) commonly give rise to opaque dispersions with particle sizes >250 nm, while SMEDS formulations (oil, 40–80% (HLB > 11), 20–40%; hydrophilic cosolvents, 0–40%) disperse into smaller droplets with particle sizes between 50 and 250 nm, leading to optically clear or slightly opalescent microemulsions. SNEDS formulations (oil, <20% (HLB > 11), 20–50%; hydrophilic cosolvents, 20–50%) further disperse in GI fluids, giving rise to nanoemulsions with a droplet size less than 50 nm and completely transparent [9, 62].
The reduction in emulsion particle size of these formulations once in the GI tract increases the surface area of particles, which, in turn, provides higher interfacial surface area and a very low interfacial tension. This provides SEDSs with a high capacity to solubilize the loaded Phy in the GI tract and to enhance its release and absorption and oral bioavailability [136–138]. It should be noted that droplet size of o/w emulsions formed after self-emulsification inside the body and, hence, capacity of SEDSs to act as efficient Phy carriers is highly determined by the excipient combination used in the formulation of these systems. Therefore, selection of excipients is a quite challenging task that should be considered.
Besides improving oral bioavailability of poor water-soluble Phy, SEDSs show multiple advantages. Among them are the following:
Formulation surfactants increasing the intestinal permeability, which decreases surface tension and facilitates formulation contact with intestinal mucus [139]
SEDSs protecting loaded Phy against enzymatic degradation and avoid its first-pass hepatic metabolism
SEDSs providing higher loading capacity than conventional lipid solutions
Thermodynamic stability
Ease of manufacture and scale-up. These advantages make SEDS unique when compared to other drug delivery systems like solid dispersions, liposomes, nanoparticles, and so forth [140–142]
Ease of administration and versatility of dosage form, in either liquid or solid form. Liquid dosage forms can be administered in soft or hard gelatin capsules but these have shown some drawbacks, such as high production costs, low drug compatibility and stability, drug leakage and precipitation, capsule ageing, and need of a large quantity of surfactants (30–60%), which can induce GI irritation. These disadvantages are overcome by formulating SEDS as solid forms by extrusion/spheronization methods [72].
The delivery of poorly water-soluble Phy using SEDSs has been extensively studied during the past decade and many of these studies are summarized in Table 5. Thus, for instance, the self-double emulsifying formulation of Hydroxysafflor yellow A (HSYA) was developed using phospholipid dissolved in Labrafac™, Lipophile WL1349, Tween 80, and oleic acid. The formulation results in 20-fold increase in Cmax and 35-fold rise in AUC value of Phy as compared to the aqueous solution [143, 144]. The SMEDS of gentiopicrin obtained from the roots of gentians was formulated using phospholipids in Labrasol as oil phase and Cremophor EL and Transcutol P as other excipients. The SMEDS of gentiopicrin with phospholipids enhanced the relative bioavailability of Phy to 703.62% as compared to gentiopicrin alone. Similarly, the phospholipid complex of morin (MPC) was developed as SNEDS using Labrafil M1944 CS, Cremophor RH 40, and Transcutol P as excipients which exhibited a significant increase in Cmax, Tmax, and relative oral bioavailability (6.23-fold) as compared to the morin suspension [145]. Likewise, lutein formulated as SNEDDS demonstrated having immediate dissolution (within 5 min) as compared to commercial product of lutein (Eyelac®) where there is no dissolution within specific time [146]. Many other studies have been carried out to enhance oral bioavailability and therapeutic effect of other plant active compounds, including apigenin, berberine hydrochloride (BBH), puerarin, hesperidin, quercetin, curcumin, baicalin, oleanolic acid, vinpocetine, nobiletin, oridonin, and silymarin.
5. Other Approaches to Increase Bioefficacy of Antitumor Phytochemicals
5.1. Oral Codelivery of Phytochemicals and Chemotherapeutic Drugs
Combined cancer therapy consisting in (i) the combined application of some of the most common types of cancer treatment, including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy or (ii) the coadministration of different chemotherapy drugs, is often more effective. The rationale for combination chemotherapy is to use drugs that work by different mechanisms, thereby decreasing the likelihood that resistant cancer cells will develop. Moreover, when drugs with different effects are combined, each drug can be used at its optimal dose, without intolerable side effects [147].
Following the same rationale, it is believed that codelivery of antitumor drugs and plant bioactive compounds could improve therapeutic effects by targeting diverse molecular targets, reducing toxicity, overcoming drug resistance, and facilitating the use of lower and safer doses [1]. Thus, as observed in Table 3, there are many in vitro and in vivo studies as well as some clinical trials focused on demonstrating the potential synergistic effect when codelivering phytochemicals, mainly polyphenols, and first line chemotherapeutic agents [148, 149].
Table 3.
Phytochemicals combined with first-line antitumor drugs and their study in clinical trials. Nanocarriers used to enhance bioefficacy of codelivery are also shown.
| Phytochemical | Codelivered antitumor agent | In vitro/in vivo | Clinical trial | Phase of study | Ref. |
|---|---|---|---|---|---|
| Ellagic acid | 5-Fluorouracil | Colon | — | — | [279] |
| Vinorelbine | — | Hormone refractory prostate cancer | Completed | [167] | |
| α-Difluoromethylornithine | Colon | — | — | [280] | |
|
| |||||
| (−)-Epigallocatechin-3-gallate (EGCG) | Tamoxifen + sulindac | Lung | — | — | [281] |
| Sulindac | Intestinal | — | — | [281] | |
|
| |||||
| Genistein | Tamoxifen | Breast | — | — | [282] |
| Gemcitabine hydrochloride | Pancreas | Breast | Completed | [283–285] | |
| Osteosarcoma | |||||
| Decitabine | — | Pediatric solid tumors, leukemia | Recruiting | [286] | |
| Decitabine | — | Non-small cell lung | Completed | [287] | |
| Interleukin-2 (high-dose) | — | Kidney cancer | Completed | [288] | |
| Melanoma | |||||
| 5-Fluorouracil | Colon | — | — | [289] | |
| Docetaxel | Prostate | — | — | [290] | |
| Lung | |||||
| Breast | |||||
| Pancreas | |||||
| Doxorubicin | Prostate | — | — | [290] | |
| Lung | |||||
| Breast | |||||
| Pancreas | |||||
| Cisplatin | Ovarian | — | — | [290, 291] | |
| Prostate | |||||
| Lung | |||||
| Breast | |||||
| Pancreas | |||||
| Erlotinib | — | Pancreas | Completed | [292] | |
| Erlotinib + gemcitabine | Pancreas | Pancreas | Completed | [293, 294] | |
|
| |||||
| Luteolin | Celecoxib | Breast | — | — | [295] |
|
| |||||
| Quercetin | Docetaxel | Prostate | — | — | [296] |
| 5-Fluorouracil | Esophageal | — | — | [297–299] | |
| Colorectal | |||||
| Liver | |||||
| Sulindac | Colorectal | Colon | Completed | [300] | |
| Tamoxifen | Breast | — | — | [301] | |
| Paclitaxel | Liver | — | — | [302] | |
|
| |||||
| Resveratrol | Rapamycin | Breast | — | — | [303] |
| Doxorubicin | Breast | — | — | [304] | |
| Temozolomide | Glioma | — | — | [305] | |
| 5-Fluorouracil | Colon | [306] | |||
| Mitomycin | Colorectal | — | — | [307] | |
|
| |||||
| Curcumin | Irinotecan | Colorectal | Colorectal | Active | [308, 309] |
| Folfox | Colon | Active | [310] | ||
| Sulindac | Lung | Colorectal | Completed | [224, 311] | |
| Capecitabine | Rectal | Active | [311] | ||
| 5-Fluorouracil | Colorectal | — | — | [312] | |
| Dasatinib | Colon | — | — | [313] | |
| Paclitaxel | Breast | [314] | |||
| Celecoxib | Colon | [315] | |||
| Gemcitabine | Lung | — | — | [316] | |
| Genistein | Prostate | — | — | [317] | |
|
| |||||
| Lycopene | Docetaxel | Prostate | Adenocarcinoma of the prostate | Active | [318, 319] |
Codelivery strategy is, however, usually limited by low water solubility, poor oral bioavailability, undesirable pharmacokinetic characteristics, and side effects [1]. In this sense, incorporation of two or more molecules (Phy + Phy or Phy + drug) in one nanocarrier seems to be a promising way to increase the bioefficacy of codelivery method. It has demonstrated to (i) improve water solubility and oral bioavailability; (ii) suppress drug resistance, by inhibiting transporter mediated efflux; (iii) delay adaptation processes; (iv) retard cells mutations; (v) produce synergistic therapeutic effect through the simultaneous delivery of multiple agents to the action site; and (vi) minimize side effects [1, 150].
In this sense, few Phy described in Table 3 have been coencapsulated or coloaded in one oral nanocarrier. Quercetin + tamoxifen was administrated through PLGA nanoparticles, while quercetin + paclitaxel was administrated through CQ-PM and curcumin + genistein through NLC.
5.2. Parenteral and Topical Administration of Phytochemicals as Alternative to the Oral Route
To overcome limitations in the oral administration of poor water-soluble Phy, parental (intravenous and intraperitoneal) and topical (transdermal, nasal, and ocular) administration routes can be used to increase dose precision and clinical efficacy.
Likewise, in recent years, topical delivery of bioactive compounds has also drawn great attention owing to its advantages over other administration routes and outstanding contribution in improving local action [151] or systemic absorption, which can minimize the first-pass effect [152]. Nevertheless, this application also shows several barriers that limit its use, including low skin permeation, short biological half-life, presystemic metabolism, or systemic toxicity [1].
On the other hand, and to get over limitations of parenteral and topical administration routes, application of nanocarriers has demonstrated to be also an efficient formulation strategy. Table 6 shows and overviews the lipid and nonlipid formulations specifically designed to parenteral and topical Phy administration. In case of the parenteral route, most of the investigations have focused on utilizing carriers to enhance antitumor efficiency through passive targeting or active targeting [153, 154], controlling drug release at the tumor site to minimize side effects [155, 156], or overcoming multidrug resistance [157]. Parenteral nanocarriers include either lipid formulations (liposomes, SLNs, and NCLs) or polymer formulations (polymeric NPs and polymer-bioactive conjugates). For topical application, the incorporation of active compounds into nanocarriers aims to enhance skin permeation and stability, lengthen systemic circulating time, and minimize metabolic degradation and systemic toxicity. Thus, for instance, MEs provide a safe, effective, and noninvasive means to topically deliver Phy such as quercetin [158], genistein [159], and chlorogenic acid and resveratrol [160]. Other nanocarriers used for the topical delivery of Phy include liposomes, ethosomes, NLCs, polymeric NPs, and polymer-bioactive conjugates (Table 6).
Table 6.
Overview of lipid and nonlipid formulations, which have been designed to administer phytochemicals by parental and topical routes.
| Phytochemical | Lipid-based formulation | Effect of formulation | Admin. route | Ref. | |
|---|---|---|---|---|---|
| Type | Subcategory | ||||
| Curcumin | LBDS | NLCs | Enhanced stability and brain targeting in vivo. | Intraperitoneal | [353] |
| Baicalein | LBDS | Tocol-NLCs | [354] | ||
| β-Elemene | NLCs | Less irritating and toxic and enhanced bioavailability and antitumor efficacy in vivo. | [355] | ||
| Bufadienolides | Reduced toxicity and improved pharmacokinetic profile in vivo. | Intravenous | [356] | ||
| Breviscapine | Ionic-complex-based NLCs | Sustained-release and protection against liver enzyme degradation in vivo. | [357] | ||
| Berberine | DQA-PEG2000-DSPEa liposomes | Overcome multidrug resistance in vivo. | [358] | ||
|
| |||||
| Quercetin | LBDS | MEs | Transdermal | [158] | |
| Genistein | Increased permeation and skin retention. | [159] | |||
| Chlorogenic acid | Efficient systemic distribution in vivo. | [160] | |||
| Resveratrol | |||||
| Curcumin | PEGa liposomes | Increased stability and anti-inflammatory effects in vivo | [359] | ||
| Bufadienolides | Poloxamer-liposomes | Reduced toxicity and enhanced antitumor efficacy in vivo. | [360] | ||
| Ligustrazine phosp. | Ethosomes | Enhanced skin permeation in vitro and bioactivity in vivo. | [152] | ||
| Apigenin | Enhanced anti-inflammatory effects in vivo. | [361] | |||
| Curcumin | NLCs | Enhanced antitumor activity and brain targeting in vitro. | Intranasal | [362] | |
| Tetrandrine | Charged SLNs | Reduced irritation of eye mucous membrane in vivo. | Ocular | [363] | |
|
| |||||
| 3-ECGC | Inorganic carriers | Gold NPs | Enhanced efficacy and reduced toxicity in vivo. | Intratumoral injection | [155] |
|
| |||||
| Curcumin | PBDS | Dextran sulfate-chitosan NPs | Controlled release and targeted effect against tumor cells in vitro. | Intravenous | [364] |
| Curcumin | Chitosan/PBCAb NPs | In vivo anticancer effect on hepatic tumor cells. | [365] | ||
| Trans-resveratrol | Chitosan-NPs | Higher in vivo liver targeting effect and in vitro cytotoxicity on hepatic cancer cells. | [366–368] | ||
| Oridonin | Galactosylated chitosan NPs | Enhanced targeting and binding to the specific site of action (liver). | |||
|
| |||||
| Artemisinin | PBDS | Polymeric micelles Targeted polymeric micelles |
Achieving site-specific cell targeting and enhancing intracellular drug accumulation. | Intraperitoneal | [369] |
| Resveratrol | Transferrin modified PEG-PLAc conjugate | Cellular uptake, in vivo biodistribution, and antitumor activity. Targeted therapy of glioma. | [370] | ||
| Bufalin | Biotinylated chitosan NPs | Enhanced targeting and binding to the specific site of action breast carcinoma. | [371] | ||
|
| |||||
| Quercetin | PBDS | Lecithin-chitosan NPs | In vitro and in vivo enhanced skin permeation. | Topical | [371] |
aPEG: polyethylene glycol; DQA: dequalinium; DSPE: polyethylene glycol-distearoylphosphatidylethanolamine.
bPBCA: poly(butyl cyanoacrylate).
cPLA: polylactic acid.
6. Conclusions
Phy are molecules obtained from natural plant species and in the last decades have shown their positive benefits in human health, in prevention and treatment.
In the framework of cancer, polyphenols are the most studied group of phytochemicals, in both the in vitro/in vivo studies and clinical trials, with promising expectative, including the lack of side effects. Regarding terpenes, phytosterols, and organosulfur phytochemicals, they show hopeful results in breast, colon, and prostate models, although there are few clinical trials that started to confirm their effects in human models, compared with polyphenols.
The bioavailability of these compounds still adheres to measure urine levels as a routine parameter, but many authors defend the use of carriers to improve their availability in plasma and in targeted organs. This need is reflected in the development of new delivery mechanisms, where lipid-based delivery systems are part of a strategy to increase the water solubility and stability, prevent the rapid systemic clearance, prevent the intestinal and hepatic metabolism, enhance the bioavailability, and enhance the cancer cell targeting. The importance of measuring tissue levels of the chemopreventive agents would help to better understand the mode of action of the nanoparticles and phytochemicals and to avoid toxicity of both.
Acknowledgments
This work has been supported by Ministerio de Economía y Competitividad del Gobierno de España (MINECO, Plan Nacional I+D+i AGL2013-48943-C2-2-R), Gobierno Regional de la Comunidad de Madrid (P2013/ABI-2728, ALIBIRD-CM), and EU Structural Funds. Marta Corzo-Martínez also thanks Ministerio de Economía y Competitividad (Spain) for her Juan de la Cierva contract.
Conflicts of Interest
All the authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Lamia Mouhid and Marta Corzo-Martínez contributed equally to the manuscript.
References
- 1.Liu Y., Feng N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM) Advances in Colloid and Interface Science. 2015;221:60–76. doi: 10.1016/j.cis.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 2.González-Vallinas M., González-Castejón M., Rodríguez-Casado A., Ramírez de Molina A. Dietary phytochemicals in cancer prevention and therapy: a complementary approach with promising perspectives. Nutrition Reviews. 2013;71(9):585–599. doi: 10.1111/nure.12051. [DOI] [PubMed] [Google Scholar]
- 3.Lee K. W., Bode A. M., Dong Z. Molecular targets of phytochemicals for cancer prevention. Nature Reviews Cancer. 2011;11(3):211–218. doi: 10.1038/nrc3017. [DOI] [PubMed] [Google Scholar]
- 4.Liu R. H. Potential synergy of phytochemicals in cancer prevention: mechanism of action. The Journal of Nutrition. 2004;134(12):3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 5.Bilia A. R., Isacchi B., Righeschi C., Guccione C., Bergonzi M. C. Flavonoids loaded in nanocarriers: an opportunity to increase oral bioavailability and bioefficacy. Food and Nutrition Sciences. 2014;5(13):1212–1327. doi: 10.4236/fns.2014.513132. [DOI] [Google Scholar]
- 6.Porter C. J. H., Charman W. N. In vitro assessment of oral lipid based formulations. Advanced Drug Delivery Reviews. 2001;50(supplement 1):S127–S147. doi: 10.1016/s0169-409x(01)00182-x. [DOI] [PubMed] [Google Scholar]
- 7.Aqil F., Munagala R., Jeyabalan J., Vadhanam M. V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Letters. 2013;334(1):133–141. doi: 10.1016/j.canlet.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalepu S., Manthina M., Padavala V. Oral lipid-based drug delivery systems—an overview. Acta Pharmaceutica Sinica B. 2013;3(6):361–372. doi: 10.1016/j.apsb.2013.10.001. [DOI] [Google Scholar]
- 9.Shrestha H., Bala R., Arora S. Lipid-based drug delivery systems. Journal of Pharmaceutics. 2014;2014:10. doi: 10.1155/2014/801820.801820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson T. M., Ferrucci L. M., Tangrea J. A., Schatzkin A. Anonymous Seminars in Oncology. Amsterdam, Netherlands: Elsevier; 2010. Epidemiological and clinical studies of nutrition; pp. 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norat T., Scoccianti C., Boutron-Ruault M.-C., et al. European code against cancer 4th edition: diet and cancer. Cancer Epidemiology. 2015;39(supplement 1):S56–S66. doi: 10.1016/j.canep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Umar A., Dunn B. K., Greenwald P. Future directions in cancer prevention. Nature Reviews Cancer. 2012;12(12):835–848. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 13.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Molecular Nutrition and Food Research. 2008;52(5):507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 14.Huang W.-Y., Cai Y.-Z., Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutrition and Cancer. 2010;62(1):1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 15.Iwashina T. The structure and distribution of the flavonoids in plants. Journal of Plant Research. 2000;113(1111):287–299. doi: 10.1007/PL00013940. [DOI] [Google Scholar]
- 16.Nandakumar V., Singh T., Katiyar S. K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Letters. 2008;269(2):378–387. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.City of Hope Medical Center. IH636 Grape Seed Extract in Preventing Breast Cancer in Postmenopausal Women at Risk of Developing Breast Cancer. NCT00100893; 2015. [Google Scholar]
- 18.Chong J., Poutaraud A., Hugueney P. Metabolism and roles of stilbenes in plants. Plant Science. 2009;177(3):143–155. doi: 10.1016/j.plantsci.2009.05.012. [DOI] [Google Scholar]
- 19.Portes E., Gardrat C., Castellan A. A comparative study on the antioxidant properties of tetrahydrocurcuminoids and curcuminoids. Tetrahedron. 2007;63(37):9092–9099. doi: 10.1016/j.tet.2007.06.085. [DOI] [Google Scholar]
- 20.Tiwari B. K., Brunton N. P., Brennan C. Handbook of Plant Food Phytochemicals: Sources, Stability and Extraction. John Wiley & Sons; 2013. [Google Scholar]
- 21.Rabi T., Bishayee A. Terpenoids and breast cancer chemoprevention. Breast Cancer Research and Treatment. 2009;115(2):223–239. doi: 10.1007/s10549-008-0118-y. [DOI] [PubMed] [Google Scholar]
- 22.Huang M., Lu J.-J., Huang M.-Q., Bao J.-L., Chen X.-P., Wang Y.-T. Terpenoids: natural products for cancer therapy. Expert Opinion on Investigational Drugs. 2012;21(12):1801–1818. doi: 10.1517/13543784.2012.727395. [DOI] [PubMed] [Google Scholar]
- 23.Breitmaier E. Terpenes, Flavors, Fragrances, Pharmaca, Pheromones. New York, NY, USA: John Wiley & Sons; 2006. [Google Scholar]
- 24.Patra A. K., Saxena J. Dietary phytochemicals as rumen modifiers: a review of the effects on microbial populations. International Journal of General and Molecular Microbiology. 2009;96(4):363–375. doi: 10.1007/s10482-009-9364-1. [DOI] [PubMed] [Google Scholar]
- 25.Dewick P. M. Medicinal Natural Products: A Biosynthetic Approach. John Wiley & Sons; 2002. [Google Scholar]
- 26.Holst B., Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Natural Product Reports. 2004;21(3):425–447. doi: 10.1039/b204039p. [DOI] [PubMed] [Google Scholar]
- 27.Higdon J. V., Delage B., Williams D. E., Dashwood R. H. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacological Research. 2007;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuentes F., Paredes-Gonzalez X., Kong A. T. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: antioxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Current Pharmacology Reports. 2015;1(3):179–196. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreau R. A., Whitaker B. D., Hicks K. B. Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Progress in Lipid Research. 2002;41(6):457–500. doi: 10.1016/s0163-7827(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 30.Smith J., Charter E. Functional Food Product Development. John Wiley & Sons; 2011. [Google Scholar]
- 31.Varady K. A., Houweling A. H., Jones P. J. H. Effect of plant sterols and exercise training on cholesterol absorption and synthesis in previously sedentary hypercholesterolemic subjects. Translational Research. 2007;149(1):22–30. doi: 10.1016/j.trsl.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Downie A., Fink C., Awad A. B. Effect of phytosterols on MDA-MB-231 human breast cancer cell growth. The FASEB Journal. 1999;13, article A333 [Google Scholar]
- 33.Yan X.-J., Gong L.-H., Zheng F.-Y., Cheng K.-J., Chen Z.-S., Shi Z. Triterpenoids as reversal agents for anticancer drug resistance treatment. Drug Discovery Today. 2014;19(4):482–488. doi: 10.1016/j.drudis.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Bishayee A., Ahmed S., Brankov N., Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Frontiers in Bioscience. 2011;16(3):980–996. doi: 10.2741/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liby K. T., Yore M. M., Sporn M. B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nature Reviews Cancer. 2007;7(5):357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 36.Yadav V. R., Prasad S., Sung B., Kannappan R., Aggarwal B. B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins. 2010;2(10):2428–2466. doi: 10.3390/toxins2102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoner C. L., Cleton A., Johnson K., et al. Integrated oral bioavailability projection using in vitro screening data as a selection tool in drug discovery. International Journal of Pharmaceutics. 2004;269(1):241–249. doi: 10.1016/j.ijpharm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Lee-Chang C., Bodogai M., Martin-Montalvo A., et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. Journal of Immunology. 2013;191(8):4141–4151. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan L., Wang W., He G., et al. Resveratrol inhibits ovarian tumor growth in an in vivo mouse model. Cancer. 2016;122(5):722–729. doi: 10.1002/cncr.29793. [DOI] [PubMed] [Google Scholar]
- 40.Lin Y. G., Kunnumakkara A. B., Nair A., et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clinical Cancer Research. 2007;13(11):3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 41.Odot J., Albert P., Carlier A., Tarpin M., Devy J., Madoulet C. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. International Journal of Cancer. 2004;111(3):381–387. doi: 10.1002/ijc.20160. [DOI] [PubMed] [Google Scholar]
- 42.Castro N. P., Rangel C. M., Salomon D., Saylor K., Kim Y. S. Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Research. 2015;75(15, article 912) doi: 10.1158/1538-7445.am2015-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang H., Kong Y., Guo J., et al. Diallyl disulfide suppresses proliferation and induces apoptosis in human gastric cancer through Wnt-1 signaling pathway by up-regulation of miR-200b and miR-22. Cancer Letters. 2013;340(1):72–81. doi: 10.1016/j.canlet.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa H., Tsuta K., Kiuchi K., et al. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001;22(6):891–897. doi: 10.1093/carcin/22.6.891. [DOI] [PubMed] [Google Scholar]
- 45.Jambhekar S. S., Foye W., Lemke T., Williams D. Physicochemical and biopharmaceutical properties of drug substnaces and pharmacokinetics. Foyes Principles of Medicinal Chemistry. 2008:61–105. [Google Scholar]
- 46.Holst B., Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Current Opinion in Biotechnology. 2008;19(2):73–82. doi: 10.1016/j.copbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Lipinski C. A. Drug-like properties and the causes of poor solubility and poor permeability. Journal of Pharmacological and Toxicological Methods. 2000;44(1):235–249. doi: 10.1016/S1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 48.Prabhu S., Ortega M., Ma C. Novel lipid-based formulations enhancing the in vitro dissolution and permeability characteristics of a poorly water-soluble model drug, piroxicam. International Journal of Pharmaceutics. 2005;301(1-2):209–216. doi: 10.1016/j.ijpharm.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 49.Robinson J. Introduction: semi-solid formulations of oral drug delivery. Bulletin Technique-Gattefosse. 1996:11–14. [Google Scholar]
- 50.D'Archivio M., Filesi C., Varì R., Scazzocchio B., Masella R. Bioavailability of the polyphenols: status and controversies. International Journal of Molecular Sciences. 2010;11(4):1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. The Journal of Nutrition. 2000;130(8):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 52.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. American Journal of Clinical Nutrition. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 53.Patel K. R., Andreadi C., Britton R. G., et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Science Translational Medicine. 2013;5(205):p. 205ra133. doi: 10.1126/scitranslmed.3005870. [DOI] [PubMed] [Google Scholar]
- 54.Cai H., Scott E., Kholghi A., et al. Cancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Science Translational Medicine. 2015;7(298) doi: 10.1126/scitranslmed.aaa7619.298ra117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y. J., Inbaraj B. S., Pu Y. S., Chen B. H. Development of lycopene micelle and lycopene chylomicron and a comparison of bioavailability. Nanotechnology. 2014;25(15) doi: 10.1088/0957-4484/25/15/155102.155102 [DOI] [PubMed] [Google Scholar]
- 56.Kensler T. W., Chen J.-G., Egner P. A., et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo Township, Qidong, People's Republic of China. Cancer Epidemiology Biomarkers and Prevention. 2005;14(11 I):2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 57.Sivapalan T., Melchini A., Traka M., Saha S., Mithen R. Investigating the bioavailability of phytochemicals and minerals from broccoli soups. Proceedings of the Nutrition Society. 2015;74, article E191 doi: 10.1017/s0029665115002177. [DOI] [Google Scholar]
- 58.Sato Y., Nishikawa K., Aikawa K., et al. Side-chain structure is critical for the transport of sterols from lysosomes to cytoplasm. Biochimica et Biophysica Acta—Lipids and Lipid Metabolism. 1995;1257(1):38–46. doi: 10.1016/0005-2760(95)00053-f. [DOI] [PubMed] [Google Scholar]
- 59.Rozner S., Garti N. The activity and absorption relationship of cholesterol and phytosterols. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2006;282-283:435–456. doi: 10.1016/j.colsurfa.2005.12.032. [DOI] [Google Scholar]
- 60.Khoo S.-M., Shackleford D. M., Porter C. J. H., Edwards G. A., Charman W. N. Intestinal lymphatic transport of halofantrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogs. Pharmaceutical Research. 2003;20(9):1460–1465. doi: 10.1023/A:1025718513246. [DOI] [PubMed] [Google Scholar]
- 61.Swenson E. S., Milisen W. B., Curatolo W. Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharmaceutical Research. 1994;11(8):1132–1142. doi: 10.1023/a:1018984731584. [DOI] [PubMed] [Google Scholar]
- 62.Gupta S., Kesarla R., Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharmaceutics. 2013;2013:16. doi: 10.1155/2013/848043.848043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aungst B. J. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. Journal of Pharmaceutical Sciences. 1993;82(10):979–987. doi: 10.1002/jps.2600821002. [DOI] [PubMed] [Google Scholar]
- 64.Patel B. D., Modi R. V., Thakkar N. A., Patel A. A., Thakkar P. H. Development and characterization of solid lipid nanoparticles for enhancement of oral bioavailability of Raloxifene. Journal of Pharmacy and Bioallied Sciences. 2012;4(5):14–16. doi: 10.4103/0975-7406.94121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pouton C. W. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and 'self-microemulsifying' drug delivery systems. European Journal of Pharmaceutical Sciences. 2000;11(2):S93–S98. doi: 10.1016/s0928-0987(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 66.Ito Y., Kusawake T., Ishida M., Tawa R., Shibata N., Takada K. Oral solid gentamicin preparation using emulsifier and adsorbent. Journal of Controlled Release. 2005;105(1-2):23–31. doi: 10.1016/j.jconrel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Nicolaos G., Crauste-Manciet S., Farinotti R., Brossard D. Improvement of cefpodoxime proxetil oral absorption in rats by an oil-in-water submicron emulsion. International Journal of Pharmaceutics. 2003;263(1-2):165–171. doi: 10.1016/S0378-5173(03)00365-X. [DOI] [PubMed] [Google Scholar]
- 68.El-Badry M., Fathy M. Enhancement of the dissolution and permeation rates of meloxicam by formation of its freeze-dried solid dispersions in polyvinylpyrrolidone K-30. Drug Development and Industrial Pharmacy. 2006;32(2):141–150. doi: 10.1080/03639040500465983. [DOI] [PubMed] [Google Scholar]
- 69.Verreck G., Brewster M. E. Melt extrusion-based dosage forms: excipients and processing conditions for pharmaceutical formulations. Bulletin Technique Gattefossé. 2004;97:85–95. [Google Scholar]
- 70.Humberstone A. J., Charman W. N. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Advanced Drug Delivery Reviews. 1997;25(1):103–128. doi: 10.1016/S0169-409X(96)00494-2. [DOI] [Google Scholar]
- 71.Singh B. N., Kim K. H. Drug delivery-oral route. Encyclopedia of Pharmaceutical Technology. 2002;1 [Google Scholar]
- 72.Chengaiah B., Alagusundaram M., Ramkanth S., Chetty C. M. Self emulsifying drug delivery system: a novel approach for drug delivery. Research Journal of Pharmacy and Technology. 2011;4(2):175–181. [Google Scholar]
- 73.Porter C. J. H., Trevaskis N. L., Charman W. N. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nature Reviews Drug Discovery. 2007;6(3):231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 74.Charman W. N., Porter C. J. H., Mithani S., Dressman J. B. Physicochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. Journal of Pharmaceutical Sciences. 1997;86(3):269–282. doi: 10.1021/js960085v. [DOI] [PubMed] [Google Scholar]
- 75.O'Driscoll C. M. Lipid-based formulations for intestinal lymphatic delivery. European Journal of Pharmaceutical Sciences. 2002;15(5):405–415. doi: 10.1016/S0928-0987(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 76.Gabizon A., Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(18):6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Natarajan J. V., Nugraha C., Ng X. W., Venkatraman S. Sustained-release from nanocarriers: a review. Journal of Controlled Release. 2014;193:122–138. doi: 10.1016/j.jconrel.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 78.Kakkar V., Mishra A. K., Chuttani K., Kaur I. P. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. International Journal of Pharmaceutics. 2013;448(2):354–359. doi: 10.1016/j.ijpharm.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 79.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Advanced Drug Delivery Reviews. 2001;47(1):65–81. doi: 10.1016/S0169-409X(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 80.Kunjachan S., Rychlik B., Storm G., Kiessling F., Lammers T. Multidrug resistance: physiological principles and nanomedical solutions. Advanced Drug Delivery Reviews. 2013;65(13-14):1852–1865. doi: 10.1016/j.addr.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barui S., Saha S., Mondal G., Haseena S., Chaudhuri A. Simultaneous delivery of doxorubicin and curcumin encapsulated in liposomes of pegylated RGDK-lipopeptide to tumor vasculature. Biomaterials. 2014;35(5):1643–1656. doi: 10.1016/j.biomaterials.2013.10.074. [DOI] [PubMed] [Google Scholar]
- 82.Koning G. A., Storm G. Targeted drug delivery systems for the intracellular delivery of macromolecular drugs. Drug Discovery Today. 2003;8(11):482–483. doi: 10.1016/S1359-6446(03)02699-0. [DOI] [PubMed] [Google Scholar]
- 83.Metselaar J. M., Storm G. Liposomes in the treatment of inflammatory disorders. Expert Opinion on Drug Delivery. 2005;2(3):465–476. doi: 10.1517/17425247.2.3.465. [DOI] [PubMed] [Google Scholar]
- 84.Ding B.-S., Dziubla T., Shuvaev V. V., Muro S., Muzykantov V. R. Advanced drug delivery systems that target the vascular endothelium. Molecular Interventions. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 85.Hua S., Wu S. Y. The use of lipid-based nanocarriers for targeted pain therapies. Frontiers in Pharmacology. 2013;4, article 143 doi: 10.3389/fphar.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szebeni J., Moghimi S. M. Liposome triggering of innate immune responses: a perspective on benefits and adverse reactions: biological recognition and interactions of liposomes. Journal of Liposome Research. 2009;19(2):85–90. doi: 10.1080/08982100902792855. [DOI] [PubMed] [Google Scholar]
- 87.Sercombe L., Veerati T., Moheimani F., Wu S. Y., Sood A. K., Hua S. Advances and challenges of liposome assisted drug delivery. Frontiers in Pharmacology. 2015;6, article 286 doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Z.-Q., Liu Y., Zhao J.-H., Wang L., Feng N.-P. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. International Journal of Nanomedicine. 2012;7:1115–1125. doi: 10.2147/ijn.s28761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dwivedi C., Sahu R., Tiwari S. P., Satapathy T., Roy A. Role of liposome in novel drug delivery system. Journal of Drug Delivery and Therapeutics. 2014;4:116–129. [Google Scholar]
- 90.Hu Y., Zeng F., Ju R., Lu W. Advances in liposomal drug delivery system in the field of chemotherapy. Clinical Oncology. 2016;1, article 1092 [Google Scholar]
- 91.Deshpande P. P., Biswas S., Torchilin V. P. Current trends in the use of liposomes for tumor targeting. Nanomedicine. 2013;8(9):1509–1528. doi: 10.2217/nnm.13.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanamala M., Wilson W. R., Yang M., Palmer B. D., Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials. 2016;85:152–167. doi: 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 93.Monteiro N., Martins A., Reis R. L., Neves N. M. Liposomes in tissue engineering and regenerative medicine. Journal of the Royal Society Interface. 2014;11(101) doi: 10.1098/rsif.2014.0459.20140459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li C., Cui J., Wang C., et al. Encapsulation of vinorelbine into cholesterol-polyethylene glycol coated vesicles: drug loading and pharmacokinetic studies. Journal of Pharmacy and Pharmacology. 2011;63(3):376–384. doi: 10.1111/j.2042-7158.2010.01227.x. [DOI] [PubMed] [Google Scholar]
- 95.Li S.-H., Fu J., Watkins D. N., Srivastava R. K., Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Molecular and Cellular Biochemistry. 2013;373(1-2):217–227. doi: 10.1007/s11010-012-1493-6. [DOI] [PubMed] [Google Scholar]
- 96.Sharma R. A., McLelland H. R., Hill K. A., et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clinical Cancer Research. 2001;7(7):1894–1900. [PubMed] [Google Scholar]
- 97.Fricker G., Kromp T., Wendel A., et al. Phospholipids and lipid-based formulations in oral drug delivery. Pharmaceutical Research. 2010;27(8):1469–1486. doi: 10.1007/s11095-010-0130-x. [DOI] [PubMed] [Google Scholar]
- 98.Gabetta B., Bombardelli E., Pifferi G. Complexes of flavanolignans with phospholipids, preparation thereof and associated pharmaceutical compositions. 1988.
- 99.Song Y., Zhuang J., Guo J., Xiao Y., Ping Q. Preparation and properties of a silybin-phospholipid complex. Die Pharmazie. 2008;63(1):35–42. doi: 10.1691/ph.2008.7132. [DOI] [PubMed] [Google Scholar]
- 100.Singh D., Rawat M. S. M., Semalty A., Semalty M. Quercetin-phospholipid complex: an amorphous pharmaceutical system in herbal drug delivery. Current Drug Discovery Technologies. 2012;9(1):17–24. doi: 10.2174/157016312799304507. [DOI] [PubMed] [Google Scholar]
- 101.Chen Z.-P., Sun J., Chen H.-X., et al. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia. 2010;81(8):1045–1052. doi: 10.1016/j.fitote.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 102.Pietta P., Simonetti P., Gardana C., Brusamolino A., Morazzoni P., Bombardelli E. Relationship between rate and extent of catechin absorption and plasma antioxidant status. Biochemistry and Molecular Biology International. 1998;46(5):895–903. doi: 10.1080/15216549800204442. [DOI] [PubMed] [Google Scholar]
- 103.Das S., Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blasi P., Giovagnoli S., Schoubben A., Ricci M., Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Advanced Drug Delivery Reviews. 2007;59(6):454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 105.Muchow M., Maincent P., Müller R. H. Lipid nanoparticles with a solid matrix (SLN®, NLC®, LDC®) for oral drug delivery. Drug Development and Industrial Pharmacy. 2008;34(12):1394–1405. doi: 10.1080/03639040802130061. [DOI] [PubMed] [Google Scholar]
- 106.Yuan H., Huang L.-F., Du Y.-Z., et al. Solid lipid nanoparticles prepared by solvent diffusion method in a nanoreactor system. Colloids and Surfaces B: Biointerfaces. 2008;61(2):132–137. doi: 10.1016/j.colsurfb.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 107.Attama A. A., Momoh M. A., Builders P. F. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage form Design and Development. Rijeka, Croatia: INTECH Open Access; 2012. [Google Scholar]
- 108.Irache J. M., Esparza I., Gamazo C., Agüeros M., Espuelas S. Nanomedicine: novel approaches in human and veterinary therapeutics. Veterinary Parasitology. 2011;180(1-2):47–71. doi: 10.1016/j.vetpar.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 109.Das S., Ng W. K., Tan R. B. H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? European Journal of Pharmaceutical Sciences. 2012;47(1):139–151. doi: 10.1016/j.ejps.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 110.Mehnert W., Mäder K. Solid lipid nanoparticles: production, characterization and applications. Advanced Drug Delivery Reviews. 2001;47(2-3):165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 111.Pardeshi C., Rajput P., Belgamwar V., et al. Solid lipid based nanocarriers: an overview. Acta Pharmaceutica. 2012;62(4):433–472. doi: 10.2478/v10007-012-0040-z. [DOI] [PubMed] [Google Scholar]
- 112.Gershkovich P., Hoffman A. Effect of a high-fat meal on absorption and disposition of lipophilic compounds: the importance of degree of association with triglyceride-rich lipoproteins. European Journal of Pharmaceutical Sciences. 2007;32(1):24–32. doi: 10.1016/j.ejps.2007.05.109. [DOI] [PubMed] [Google Scholar]
- 113.Müller R. H., Radtke M., Wissing S. A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews. 2002;54:S131–S155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 114.Saupe A., Gordon K. C., Rades T. Structural investigations on nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers by cryo-field emission scanning electron microscopy and Raman spectroscopy. International Journal of Pharmaceutics. 2006;314(1):56–62. doi: 10.1016/j.ijpharm.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 115.Corveleyn S., Remon J. P. Formulation and production of rapidly disintegrating tablets by lyophilisation using hydrochlorothiazide as a model drug. International Journal of Pharmaceutics. 1997;152(2):215–225. doi: 10.1016/S0378-5173(97)00092-6. [DOI] [Google Scholar]
- 116.Strickley R. G. Solubilizing excipients in oral and injectable formulations. Pharmaceutical Research. 2004;21(2):201–230. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 117.Tokumura T., Tsushima Y., Tatsuishi K., Kayano M., Machida Y., Nagai T. Enhancement of the oral bioavailability of cinnarizine in oleic acid in beagle dogs. Journal of Pharmaceutical Sciences. 1987;76(4):286–288. doi: 10.1002/jps.2600760404. [DOI] [PubMed] [Google Scholar]
- 118.Doktorovova S., Souto E. B., Silva A. M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—a systematic review of in vitro data. European Journal of Pharmaceutics and Biopharmaceutics. 2014;87(1):1–18. doi: 10.1016/j.ejpb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 119.Leonarduzzi G., Testa G., Sottero B., Gamba P., Poli G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Current Medicinal Chemistry. 2010;17(1):74–95. doi: 10.2174/092986710789957760. [DOI] [PubMed] [Google Scholar]
- 120.Luo C.-F., Yuan M., Chen M.-S., et al. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. International Journal of Pharmaceutics. 2011;410(1-2):138–144. doi: 10.1016/j.ijpharm.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 121.Luo C.-F., Hou N., Tian J., et al. Metabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticles. International Journal of Nanomedicine. 2013;8:933–940. doi: 10.2147/IJN.S39349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang M., Truscott J., Davie J. Loss of MEF2D expression inhibits differentiation and contributes to oncogenesis in rhabdomyosarcoma cells. Molecular Cancer. 2013;12(1, article 150) doi: 10.1186/1476-4598-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shangguan M., Lu Y., Qi J., et al. Binary lipids-based nanostructured lipid carriers for improved oral bioavailability of silymarin. Journal of Biomaterials Applications. 2014;28(6):887–896. doi: 10.1177/0885328213485141. [DOI] [PubMed] [Google Scholar]
- 124.Yuan L., Liu C. Y., Chen Y., Zhang Z. H., Zhou L., Qu D. Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model. International Journal of Nanomedicine. 2013;8:4339–4350. doi: 10.2147/IJN.S51621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heuschkel S., Goebel A., Neubert R. H. H. Microemulsions—modern colloidal carrier for dermal and transdermal drug delivery. Journal of Pharmaceutical Sciences. 2008;97(2):603–631. doi: 10.1002/jps.20995. [DOI] [PubMed] [Google Scholar]
- 126.McClements D. J. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8(6):1719–1729. doi: 10.1039/c2sm06903b. [DOI] [Google Scholar]
- 127.Tang T.-T., Hu X.-B., Liao D.-H., Liu X.-Y., Xiang D.-X. Mechanisms of microemulsion enhancing the oral bioavailability of puerarin: comparison between oil-in-water and water-in-oil microemulsions using the single-pass intestinal perfusion method and a chylomicron flow blocking approach. International Journal of Nanomedicine. 2013;8:4415–4426. doi: 10.2147/ijn.s51469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zang B.-X., Jin M., Si N., Zhang Y., Wu W., Piao Y.-Z. Antagonistic effect of hydroxysafflor yellow a on the platelet activating factor receptor. Yao Xue Xue Bao. 2002;37(9):696–699. [PubMed] [Google Scholar]
- 129.Zhu H., Wang Z., Ma C., et al. Neuroprotective effects of hydroxysafflor yellow A: in vivo and in vitro studies. Planta Medica. 2003;69(5):429–433. doi: 10.1055/s-2003-39714. [DOI] [PubMed] [Google Scholar]
- 130.Qi J., Zhuang J., Wu W., et al. Enhanced effect and mechanism of water-in-oil microemulsion as an oral delivery system of hydroxysafflor yellow A. International Journal of Nanomedicine. 2011;6:985–991. doi: 10.2147/IJN.S18821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeng Z., Zhou G., Wang X., et al. Preparation, characterization and relative bioavailability of oral elemene o/w microemulsion. International Journal of Nanomedicine. 2010;5(1):567–572. doi: 10.2147/ijn.s12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chansiri G., Lyons R. T., Patel M. V., Hem S. L. Effect of surface charge on the stability of oil/water emulsions during steam sterilization. Journal of Pharmaceutical Sciences. 1999;88(4):454–458. doi: 10.1021/js980293i. [DOI] [PubMed] [Google Scholar]
- 133.Jiang Y.-N., Mo H.-Y. Preparation of self-emulsifying soft capsule and its pharmacokinetic in rats for epimedium flavonoids. Journal of Chinese Medicinal Materials. 2010;33(5):767–771. [PubMed] [Google Scholar]
- 134.Luo X.-Q., Yang J.-Q. Prescription design and dissolution evaluation of self-emulsifying drug delivery systems of baicalin. Journal of Chinese Medicinal Materials. 2010;33(7):1157–1159. [PubMed] [Google Scholar]
- 135.Tang J.-L., Sun J., He Z.-G. Self-emulsifying drug delivery systems: strategy for improving oral delivery of poorly soluble drugs. Current Drug Therapy. 2007;2(1):85–93. doi: 10.2174/157488507779422400. [DOI] [Google Scholar]
- 136.Chouhan N., Mittal V., Kaushik D., Khatkar A., Raina M. Self Emulsifying Drug Delivery System (SEDDS) for phytoconstituents: a review. Current Drug Delivery. 2015;12(2):244–253. doi: 10.2174/1567201811666141021142606. [DOI] [PubMed] [Google Scholar]
- 137.Yao J., Lu Y., Zhou J. P. Preparation of nobiletin in self-microemulsifying systems and its intestinal permeability in rats. Journal of Pharmacy and Pharmaceutical Sciences. 2008;11(3):22–29. doi: 10.18433/j3ms3m. [DOI] [PubMed] [Google Scholar]
- 138.Mosharraf M., Nyström C. The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. International Journal of Pharmaceutics. 1995;122(1-2):35–47. doi: 10.1016/0378-5173(95)00033-F. [DOI] [Google Scholar]
- 139.Zhang P., Liu Y., Feng N., Xu J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. International Journal of Pharmaceutics. 2008;355(1-2):269–276. doi: 10.1016/j.ijpharm.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 140.Liu L., Pang X., Zhang W., Wang S. Formulation design and in vitro evaluation of silymarin-loaded self-microemulsifying drug delivery systems. Asian Journal of Pharmaceutical Sciences. 2007;2:150–160. [Google Scholar]
- 141.Tang J., Sun J., Cui F., He Z. Preparation of self-emulsifying drug delivery systems of Ginkgo biloba extracts and in vitro dissolution studies. Asian Journal of Traditional Medicines. 2006;1:138–141. [Google Scholar]
- 142.Kohli K., Chopra S., Dhar D., Arora S., Khar R. K. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discovery Today. 2010;15(21-22):958–965. doi: 10.1016/j.drudis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 143.Wang S., Sun M., Ping Q. Enhancing effect of Labrafac Lipophile WL 1349 on oral bioavailability of hydroxysafflor yellow A in rats. International Journal of Pharmaceutics. 2008;358(1-2):198–204. doi: 10.1016/j.ijpharm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 144.Lv L.-Z., Tong C.-Q., Lv Q., et al. Enhanced absorption of hydroxysafflor yellow A using a self-double-emulsifying drug delivery system: in vitro and in vivo studies. International Journal of Nanomedicine. 2012;7:4099–4107. doi: 10.2147/ijn.s33398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang J., Peng Q., Shi S., et al. Preparation, characterization, and in vivo evaluation of a self-nanoemulsifying drug delivery system (SNEDDS) loaded with morin-phospholipid complex. International Journal of Nanomedicine. 2011;6:3405–3414. doi: 10.2147/IJN.S25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoo J. H., Shanmugam S., Thapa P., et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Archives of Pharmacal Research. 2010;33(3):417–426. doi: 10.1007/s12272-010-0311-5. [DOI] [PubMed] [Google Scholar]
- 147.Tsouris V., Joo M. K., Kim S. H., Kwon I. C., Won Y.-Y. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnology Advances. 2014;32(5):1037–1050. doi: 10.1016/j.biotechadv.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 148.Brito A. F., Ribeiro M., Abrantes A. M., et al. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Current Medicinal Chemistry. 2015;22(26):3025–3039. doi: 10.2174/0929867322666150812145435. [DOI] [PubMed] [Google Scholar]
- 149.Kaminski B. M., Steinhilber D., Stein J. M., Ulrich S. Phytochemicals resveratrol and sulforaphane as potential agents for enhancing the anti-tumor activities of conventional cancer therapies. Current Pharmaceutical Biotechnology. 2012;13(1):137–146. doi: 10.2174/138920112798868746. [DOI] [PubMed] [Google Scholar]
- 150.Park K. True combination therapy using synergistic drug combination. Journal of Controlled Release. 2014;187, article 198 doi: 10.1016/j.jconrel.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 151.Gong C., Wu Q., Wang Y., et al. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials. 2013;34(27):6377–6387. doi: 10.1016/j.biomaterials.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 152.Shi J., Wang Y., Luo G. Ligustrazine phosphate ethosomes for treatment of Alzheimer's disease, in vitro and in animal model studies. AAPS PharmSciTech. 2012;13(2):485–492. doi: 10.1208/s12249-012-9767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu J., Liu J., Xu H., et al. Novel tumor-targeting, self-assembling peptide nanofiber as a carrier for effective curcumin delivery. International Journal of Nanomedicine. 2013;9(1):197–207. doi: 10.2147/IJN.S55875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X.-X., Li Y.-B., Yao H.-J., et al. The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials. 2011;32(24):5673–5687. doi: 10.1016/j.biomaterials.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 155.Chen C.-C., Hsieh D.-S., Huang K.-J., et al. Improving anticancer efficacy of (-)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells. Drug Design, Development and Therapy. 2014;8:459–474. doi: 10.2147/dddt.s58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Manju S., Sreenivasan K. Gold nanoparticles generated and stabilized by water soluble curcumin-polymer conjugate: blood compatibility evaluation and targeted drug delivery onto cancer cells. Journal of Colloid and Interface Science. 2012;368(1):144–151. doi: 10.1016/j.jcis.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 157.Moorthi C., Kathiresan K. Curcumin-piperine/curcumin-quercetin/curcumin- silibinin dual drug-loaded nanoparticulate combination therapy: a novel approach to target and treat multidrug-resistant cancers. Journal of Medical Hypotheses and Ideas. 2013;7(1):15–20. doi: 10.1016/j.jmhi.2012.10.005. [DOI] [Google Scholar]
- 158.Censi R., Martena V., Hoti E., Malaj L., Di Martino P. Permeation and skin retention of quercetin from microemulsions containing Transcutol® P. Drug Development and Industrial Pharmacy. 2012;38(9):1128–1133. doi: 10.3109/03639045.2011.641564. [DOI] [PubMed] [Google Scholar]
- 159.Kitagawa S., Inoue K., Teraoka R., Morita S.-Y. Enhanced skin delivery of genistein and other two isoflavones by microemulsion and prevention against UV irradiation-induced erythema formation. Chemical and Pharmaceutical Bulletin. 2010;58(3):398–401. doi: 10.1248/cpb.58.398. [DOI] [PubMed] [Google Scholar]
- 160.Yutani R., Kikuchi T., Teraoka R., Kitagawa S. Efficient delivery and distribution in skin of chlorogenic acid and resveratrol induced by microemulsion using sucrose laurate. Chemical and Pharmaceutical Bulletin. 2014;62(3):274–280. doi: 10.1248/cpb.c13-00820. [DOI] [PubMed] [Google Scholar]
- 161.Bell C., Hawthorne S. Ellagic acid, pomegranate and prostate cancer—a mini review. Journal of Pharmacy and Pharmacology. 2008;60(2):139–144. doi: 10.1211/jpp.60.2.0001. [DOI] [PubMed] [Google Scholar]
- 162.Zhao M., Tang S.-N., Marsh J. L., Shankar S., Srivastava R. K. Ellagic acid inhibits human pancreatic cancer growth in Balb c nude mice. Cancer Letters. 2013;337(2):210–217. doi: 10.1016/j.canlet.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 163.Qiu Z., Zhou B., Jin L., et al. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food and Chemical Toxicology. 2013;59:428–437. doi: 10.1016/j.fct.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 164.Wang N., Wang Z.-Y., Mo S.-L., et al. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Research and Treatment. 2012;134(3):943–955. doi: 10.1007/s10549-012-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Molina A. R., Vargas T., Molina S., et al. The ellagic acid derivative 4,4′-Di-O-methylellagic acid efficiently inhibits colon cancer cell growth through a mechanism involving WNT16. Journal of Pharmacology and Experimental Therapeutics. 2015;353(2):433–444. doi: 10.1124/jpet.114.221796. [DOI] [PubMed] [Google Scholar]
- 166.Oslo University Hospital. Dietary Intervention in Follicular Lymphoma (KLYMF) 2008. ( NCT00455416). [Google Scholar]
- 167.Falsaperla M., Morgia G., Tartarone A., Ardito R., Romano G. Support ellagic acid therapy in patients with hormone refractory prostate cancer (HRPC) on standard chemotherapy using vinorelbine and estramustine phosphate. European Urology. 2005;47(4):449–454. doi: 10.1016/j.eururo.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 168.Wang X., Hao M.-W., Dong K., Lin F., Ren J.-H., Zhang H.-Z. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Archives of Pharmacal Research. 2009;32(9):1263–1269. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- 169.Milligan S. A., Burke P., Coleman D. T., et al. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clinical Cancer Research. 2009;15(15):4885–4894. doi: 10.1158/1078-0432.CCR-09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hwang J.-T., Ha J., Park I.-J., et al. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Letters. 2007;247(1-2):115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 171.Paschka A. G., Butler R., Young C. Y.-F. Induction of apoptosis in prostate cancer cell lines by the green tea component, (-)-epigallocatechin-3-gallate. Cancer Letters. 1998;130(1-2):1–7. doi: 10.1016/S0304-3835(98)00084-6. [DOI] [PubMed] [Google Scholar]
- 172.Gu B., Ding Q., Xia G., Fang Z. EGCG inhibits growth and induces apoptosis in renal cell carcinoma through TFPI-2 overexpression. Oncology Reports. 2009;21(3):635–640. doi: 10.3892/or_00000266. [DOI] [PubMed] [Google Scholar]
- 173.Mittal A., Pate M. S., Wylie R. C., Tollefsbol T. O., Katiyar S. K. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. International Journal of Oncology. 2004;24(3):703–710. [PubMed] [Google Scholar]
- 174.Hoffmann J., Junker H., Schmieder A., et al. EGCG downregulates IL-1RI expression and suppresses IL-1-induced tumorigenic factors in human pancreatic adenocarcinoma cells. Biochemical Pharmacology. 2011;82(9):1153–1162. doi: 10.1016/j.bcp.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 175.Bettuzzi S., Brausi M., Rizzi F., Castagnetti G., Peracchia G., Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Research. 2006;66(2):1234–1240. doi: 10.1158/0008-5472.can-05-1145. [DOI] [PubMed] [Google Scholar]
- 176.Ahn W.-S., Yoo J., Huh S.-W., et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. European Journal of Cancer Prevention. 2003;12(5):383–390. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 177.Shandong Cancer Hospital and Institute, Zhao H. X., Shandong Cancer Hospital and Institute . Study of Epigallocatechin-3-Gallate (EGCG) for Skin Prevention in Patients With Breast Cancer Receiving Adjuvant Radiotherapy. 2015. ( NCT02580279). [Google Scholar]
- 178.Case Comprehensive Cancer Center. Defined Green Tea Catechin Extract in Treating Patients With Localized Prostate Cancer Undergoing Surgery. 2012. ( NCT01340599). [Google Scholar]
- 179.Pugalendhi P., Manoharan S., Panjamurthy K., Balakrishnan S., Nirmal M. R. Antigenotoxic effect of genistein against 7,12-dimethylbenz[a]anthracene induced genotoxicity in bone marrow cells of female Wistar rats. Pharmacological Reports. 2009;61(2):296–303. doi: 10.1016/S1734-1140(09)70035-0. [DOI] [PubMed] [Google Scholar]
- 180.Farina H. G., Pomies M., Alonso D. F., Gomez D. E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncology Reports. 2006;16(4):885–891. [PubMed] [Google Scholar]
- 181.Shao Z.-M., Wu J., Shen Z.-Z., Barsky S. H. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Research. 1998;58(21):4851–4857. [PubMed] [Google Scholar]
- 182.Kim S.-H., Kim S.-H., Lee S.-C., Song Y.-S. Involvement of both extrinsic and intrinsic apoptotic pathways in apoptosis induced by genistein in human cervical cancer cells. Annals of the New York Academy of Sciences. 2009;1171:196–201. doi: 10.1111/j.1749-6632.2009.04902.x. [DOI] [PubMed] [Google Scholar]
- 183.Nakamura Y., Yogosawa S., Izutani Y., Watanabe H., Otsuji E., Sakai T. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Molecular Cancer. 2009;8, article 100:1476–4598. doi: 10.1186/1476-4598-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Northwestern University. Genistein in Treating Patients With Prostate Cancer. 2015. ( NCT01126879). [Google Scholar]
- 185.Masonic Cancer Center. Genistein in Treating Patients Undergoing External-Beam Radiation Therapy for Bone Metastases. 2011. ( NCT00769990). [Google Scholar]
- 186.UNC Lineberger Comprehensive Cancer Center. National Cancer Institute (NCI) Information provided by (Responsible Party):. genistein in preventing breast or endometrial cancer in healthy postmenopausal women. 2013;( NCT00099008)
- 187.University of Wisconsin M. Genistein in Patients Who Are Undergoing Surgery for Bladder Cancer. NCT00118040; 2010. [Google Scholar]
- 188.Yoo D. R., Jang Y. H., Jeon Y. K., et al. Proteomic identification of anti-cancer proteins in luteolin-treated human hepatoma Huh-7 cells. Cancer Letters. 2009;282(1):48–54. doi: 10.1016/j.canlet.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 189.Lin Y., Shi R., Wang X., Shen H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Current Cancer Drug Targets. 2008;8(7):634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yin F., Giuliano A. E., Van Herle A. J. Growth inhibitory effects of flavonoids in human thyroid cancer cell lines. Thyroid. 1999;9(4):369–376. doi: 10.1089/thy.1999.9.369. [DOI] [PubMed] [Google Scholar]
- 191.Yang S.-F., Yang W.-E., Chang H.-R., Chu S.-C., Hsieh Y.-S. Luteolin induces apoptosis in oral squamous cancer cells. Journal of Dental Research. 2008;87(4):401–406. doi: 10.1177/154405910808700413. [DOI] [PubMed] [Google Scholar]
- 192.Shi R.-X., Ong C.-N., Shen H.-M. Luteolin sensitizes tumor necrosis factor-α-induced apoptosis in human tumor cells. Oncogene. 2004;23(46):7712–7721. doi: 10.1038/sj.onc.1208046. [DOI] [PubMed] [Google Scholar]
- 193.Park S.-H., Park H. S., Lee J. H., et al. Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in NCI-H460 lung carcinoma cells. Food and Chemical Toxicology. 2013;56:100–109. doi: 10.1016/j.fct.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 194.Ramasamy K., Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Letters. 2008;269(2):352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Agarwal R., Agarwal C., Ichikawa H., Singh R. P., Aggarwal B. B. Anticancer potential of silymarin: from bench to bed side. Anticancer Research. 2006;26(6):4457–4498. [PubMed] [Google Scholar]
- 196.Zi X., Feyes D. K., Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cipl/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clinical Cancer Research. 1998;4(4):1055–1064. [PubMed] [Google Scholar]
- 197.Singh R. P., Agarwal R. Flavonoid antioxidant silymarin and skin cancer. Antioxidants and Redox Signaling. 2002;4(4):655–663. doi: 10.1089/15230860260220166. [DOI] [PubMed] [Google Scholar]
- 198.Deep G., Singh R. P., Agarwal C., Kroll D. J., Agarwal R. Silymarin and silibinin cause G1 and G2–M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25(7):1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 199.Tehran University of Medical Sciences. Evaluation of Effects of Silymarin on Cisplatin Induced Nephrotoxicity in Upper Gastrointestinal Adenocarcinoma, NCT01829178. 2025.
- 200.Herbert Irving Comprehensive Cancer Center. Silymarin (Milk Thistle Extract) in Treating Patients With Acute Lymphoblastic Leukemia Who Are Receiving Chemotherapy. NCT00055718; 2013. [Google Scholar]
- 201.Johnson J., De Mejia E. G. Dietary factors and pancreatic cancer: the role of food bioactive compounds. Molecular Nutrition and Food Research. 2011;55(1):58–73. doi: 10.1002/mnfr.201000420. [DOI] [PubMed] [Google Scholar]
- 202.Gibellini L., Pinti M., Nasi M., Montagna J. P., De Biasi S., Roat E. Quercetin and cancer chemoprevention. Evidence-Based Complementary and Alternative Medicine. 2011;2011:15. doi: 10.1093/ecam/neq053.591356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vidya Priyadarsini R., Senthil Murugan R., Maitreyi S., Ramalingam K., Karunagaran D., Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. European Journal of Pharmacology. 2010;649(1–3):84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 204.Shan B.-E., Wang M.-X., Li R.-Q. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/β-catenin signaling pathway. Cancer Investigation. 2009;27(6):604–612. doi: 10.1080/07357900802337191. [DOI] [PubMed] [Google Scholar]
- 205.Yang F., Song L., Wang H., Wang J., Xu Z., Xing N. Quercetin in prostate cancer: chemotherapeutic and chemopreventive effects, mechanisms and clinical application potential (review) Oncology Reports. 2015;33(6):2659–2668. doi: 10.3892/or.2015.3886. [DOI] [PubMed] [Google Scholar]
- 206.Zheng S.-Y., Li Y., Jiang D., Zhao J., Ge J.-F. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Molecular Medicine Reports. 2012;5(3):822–826. doi: 10.3892/mmr.2011.726. [DOI] [PubMed] [Google Scholar]
- 207.Bischoff S. C., University of Hohenheim, University Hospital Tuebingen, Quercegen Pharmaceuticals Prostate Cancer Prevention Trial with Quercetin and Genistein (QUERGEN), NCT01538316, 2012.
- 208.Zwicker J., Dana-Farber Cancer Institute, Quercegen Pharmaceuticals, National Heart, Lung, Blood Institute (NHLBI) Cancer Associated Thrombosis and Isoquercetin (CAT IQ) 2015. ( NCT02195232). [Google Scholar]
- 209.Ferry D. R., Smith A., Malkhandi J., et al. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clinical Cancer Research. 1996;2(4):659–668. [PubMed] [Google Scholar]
- 210.Carter L. G., D'Orazio J. A., Pearson K. J. Resveratrol and cancer: focus on in vivo evidence. Endocrine-Related Cancer. 2014;21(3):R209–R225. doi: 10.1530/erc-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Alfaras I., Emìlia Juan M., Planas J. M. Trans-resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats. Journal of Agricultural and Food Chemistry. 2010;58(13):8104–8110. doi: 10.1021/jf100702x. [DOI] [PubMed] [Google Scholar]
- 212.Salado C., Olaso E., Gallot N., et al. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. Journal of Translational Medicine. 2011;9, article 59 doi: 10.1186/1479-5876-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Roy S. K., Chen Q., Fu J., Shankar S., Srivastava R. K. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025166.e25166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sheth S., Jajoo S., Kaur T., et al. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0051655.e51655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kalra N., Roy P., Prasad S., Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sciences. 2008;82(7-8):348–358. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 216.Wu M.-L., Li H., Yu L.-J., et al. Short-term resveratrol exposure causes in vitro and in vivo growth inhibition and apoptosis of bladder cancer cells. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0089806.e89806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stakleff K. S., Sloan T., Blanco D., Marcanthony S., Booth T., Bishayee A. Resveratrol exerts differential effects in vitro and in vivo against ovarian cancer cells. Asian Pacific Journal of Cancer Prevention. 2012;13(4):1333–1340. doi: 10.7314/APJCP.2012.13.4.1333. [DOI] [PubMed] [Google Scholar]
- 218.Chao Family Comprehensive Cancer Center. University of California, Irvine, University of California I, University of California, Los Angeles. Resveratrol for Patients with Colon Cancer. NCT00256334. 2014; 2016.
- 219.GlaxoSmithKline. Sirtris. A Clinical Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of SRT501 in Subjects with Colorectal Cancer and Hepatic Metastases. NCT00920803. 2011, 2016.
- 220.National Cancer Institute (NCI) Resveratrol in Treating Patients with Colorectal Cancer That Can Be Removed by Surgery. 2011/2016. ( NCT00433576). [Google Scholar]
- 221.University of Wisconsin. A Biological Study of Resveratrol's Effects on Notch-1 Signaling in Subjects with Low Grade Gastrointestinal Tumors. NCT01476592. 2015; 2016.
- 222.Patel K. R., Brown V. A., Jones D. J. L., et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Research. 2010;70(19):7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wright L. E., Frye J. B., Gorti B., Timmermann B. N., Funk J. L. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Current Pharmaceutical Design. 2013;19(34):6218–6225. doi: 10.2174/1381612811319340013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cheng K.-W., Wong C. C., Mattheolabakis G., Xie G., Huang L., Rigas B. Curcumin enhances the lung cancer chemopreventive efficacy of phospho-sulindac by improving its pharmacokinetics. International Journal of Oncology. 2013;43(3):895–902. doi: 10.3892/ijo.2013.1995F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Madden K., Flowers L., Salani R., et al. Proteomics-based approach to elucidate the mechanism of antitumor effect of curcumin in cervical cancer. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;80(1):9–18. doi: 10.1016/j.plefa.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 226.Guo L.-D., Chen X.-J., Hu Y.-H., Yu Z.-J., Wang D., Liu J.-Z. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytotherapy Research. 2013;27(3):422–430. doi: 10.1002/ptr.4731. [DOI] [PubMed] [Google Scholar]
- 227.Bimonte S., Barbieri A., Palma G., Luciano A., Rea D., Arra C. Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. BioMed Research International. 2013;2013:8. doi: 10.1155/2013/810423.810423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wilken R., Veena M. S., Wang M. B., Srivatsan E. S. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Molecular Cancer. 2011;10, article 12 doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cui S.-X., Qu X.-J., Xie Y.-Y., et al. Curcumin inhibits telomerase activity in human cancer cell lines. International Journal of Molecular Medicine. 2006;18(2):227–231. [PubMed] [Google Scholar]
- 230.Aoki H., Takada Y., Kondo S., Sawaya R., Aggarwal B. B., Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of akt and extracellular signal-regulated kinase signaling pathways. Molecular Pharmacology. 2007;72(1):29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 231.Anderson Cancer Center M. D. Sabinsa Corporation. Trial of Curcumin in Advanced Pancreatic Cancer. NCT00094445. 2014, 2016.
- 232.Dhillon N., Aggarwal B. B., Newman R. A., et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clinical Cancer Research. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 233.University of Rochester, Ryan J. Curcumin for the Prevention of Radiation-induced Dermatitis in Breast Cancer Patients. NCT01042938. 2012; 2016.
- 234.Nathan C. A., Louisiana State University Health Sciences Center Shreveport, Feist-Weiller Cancer Center, National Cancer Institute (NCI) Curcumin Biomarker Trial in Head and Neck Cancer. NCT01160302. 2014, 2016.
- 235. Curcumin Biomarkers, University of North Carolina, Chapel Hill, NC, USA, NCT01333917, 2013; 2016.
- 236.Tata Memorial Hospital. Pilot Study of Curcumin Formulation and Ashwagandha Extract in Advanced Osteosarcoma (OSCAT) NCT00689195. 2011; 2016.
- 237.Cruz-Correa M., Shoskes D. A., Sanchez P., et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clinical Gastroenterology and Hepatology. 2006;4(8):1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 238.Garcea G., Jones D. J. L., Singh R., et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. British Journal of Cancer. 2004;90(5):1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tang F.-Y., Pai M.-H., Kuo Y.-H., Wang X.-D. Concomitant consumption of lycopene and fish oil inhibits tumor growth and progression in a mouse xenograft model of colon cancer. Molecular Nutrition and Food Research. 2012;56(10):1520–1531. doi: 10.1002/mnfr.201200098. [DOI] [PubMed] [Google Scholar]
- 240.Uppala P. T., Dissmore T., Lau B. H. S., Andacht T., Rajaram S. Selective inhibition of cell proliferation by lycopene in MCF-7 breast cancer cells in vitro: a proteomic analysis. Phytotherapy Research. 2013;27(4):595–601. doi: 10.1002/ptr.4764. [DOI] [PubMed] [Google Scholar]
- 241.Palozza P., Simone R. E., Catalano A., Mele M. C. Tomato lycopene and lung cancer prevention: from experimental to human studies. Cancers. 2011;3(2):2333–2357. doi: 10.3390/cancers3022333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Teodoro A. J., Oliveira F. L., Martins N. B., Maia G. D. A., Martucci R. B., Borojevic R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell International. 2012;12, article 36 doi: 10.1186/1475-2867-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Clark P. E., Hall M. C., Borden L. S., Jr., et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67(6):1257–1261. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 244.clinicaltrials.gov. Clinical trials database. 2015.
- 245.Kurihara H., Koda H., Asami S., Kiso Y., Tanaka T. Contribution of the antioxidative property of astaxanthin to its protective effect on the promotion of cancer metastasis in mice treated with restraint stress. Life Sciences. 2002;70(21):2509–2520. doi: 10.1016/S0024-3205(02)01522-9. [DOI] [PubMed] [Google Scholar]
- 246.Tanaka T., Morishita Y., Suzui M., Kojima T., Okumura A., Mori H. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis. 1994;15(1):15–19. doi: 10.1093/carcin/15.1.15. [DOI] [PubMed] [Google Scholar]
- 247.Tanaka T., Makita H., Ohnishi M., Mori H., Satoh K., Hara A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Research. 1995;55(18):4059–4064. [PubMed] [Google Scholar]
- 248.Jyonouchi H., Sun S., Iijima K., Gross M. D. Antitumor activity of astaxanthin and its mode of action. Nutrition and Cancer. 2000;36(1):59–65. doi: 10.1207/s15327914nc3601_9. [DOI] [PubMed] [Google Scholar]
- 249.Rao A. R., Sindhuja H. N., Dharmesh S. M., Sankar K. U., Sarada R., Ravishankar G. A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga haematococcus pluvialis. Journal of Agricultural and Food Chemistry. 2013;61(16):3842–3851. doi: 10.1021/jf304609j. [DOI] [PubMed] [Google Scholar]
- 250.Tanaka T., Kawamori T., Ohnishi M., et al. Suppression of azoxymethane-induced rat colon carcinogenesis by dietary administration of naturally occurring xanthophylls astaxanthin and canthaxanthin during the postinitiation phase. Carcinogenesis. 1995;16(12):2957–2963. doi: 10.1093/carcin/16.12.2957. [DOI] [PubMed] [Google Scholar]
- 251.Palozza P., Torelli C., Boninsegna A., et al. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Letters. 2009;283(1):108–117. doi: 10.1016/j.canlet.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 252.Li Q. Q., Wang G., Huang F., Banda M., Reed E. Antineoplastic effect of β-elemene on prostate cancer cells and other types of solid tumour cells. Journal of Pharmacy and Pharmacology. 2010;62(8):1018–1027. doi: 10.1111/j.2042-7158.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 253.Li X., Wang G., Zhao J., et al. Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cellular and Molecular Life Sciences. 2005;62(7-8):894–904. doi: 10.1007/s00018-005-5027-1. [DOI] [PubMed] [Google Scholar]
- 254.Yao Y.-Q., Ding X., Jia Y.-C., Huang C.-X., Wang Y.-Z., Xu Y.-H. Anti-tumor effect of β-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Letters. 2008;264(1):127–134. doi: 10.1016/j.canlet.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 255.Chen W., Lu Y., Wu J., Gao M., Wang A., Xu B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemotherapy and Pharmacology. 2011;67(4):799–808. doi: 10.1007/s00280-010-1378-x. [DOI] [PubMed] [Google Scholar]
- 256.Liu J., Zhang Y., Qu J., et al. β-elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer. 2011;11, article 183 doi: 10.1186/1471-2407-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chen Z., Sun Yat-sen University A Study on β-elemene as Maintain Treatment for Complete Remission Patients of Newly Diagnosed Malignant Gliomas Following Standard Treatment (β-elemene) NCT02629757. 2015.
- 258.Moran A. E., Carothers A. M., Weyant M. J., Redston M., Bertagnolli M. M. Carnosol inhibits β-catenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/+ (Min/+) mouse. Cancer Research. 2005;65(3):1097–1104. [PubMed] [Google Scholar]
- 259.Johnson J. J., Syed D. N., Suh Y., et al. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prevention Research. 2010;3(9):1112–1123. doi: 10.1158/1940-6207.capr-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Huang M.-T., Ho C.-T., Yuan Wang Z., et al. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Research. 1994;54(3):701–708. [PubMed] [Google Scholar]
- 261.Iratni R., Al Dhaheri Y., Attoub S., Karuventevida N., Arafat K. P0174 Anti-metastatic and anti-tumour growth effects of carnosol on breast cancer through autophagy and apoptosis. European Journal of Cancer. 2014;50(supplement 4):p. e59. doi: 10.1016/j.ejca.2014.03.218. [DOI] [Google Scholar]
- 262.Vergara D., Simeone P., Bettini S., et al. Antitumor activity of the dietary diterpene carnosol against a panel of human cancer cell lines. Food and Function. 2014;5(6):1261–1269. doi: 10.1039/c4fo00023d. [DOI] [PubMed] [Google Scholar]
- 263.Huang S.-C., Ho C.-T., Lin-Shiau S.-Y., Lin J.-K. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappaB and c-Jun. Biochemical Pharmacology. 2005;69(2):221–232. doi: 10.1016/j.bcp.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 264.Gills J. J., Jeffery E. H., Matusheski N. V., Moon R. C., Lantvit D. D., Pezzuto J. M. Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Letters. 2006;236(1):72–79. doi: 10.1016/j.canlet.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 265.Shen G., Tin O. K., Hu R., et al. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in Apc Min/+ mouse. Cancer Research. 2007;67(20):9937–9944. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- 266.Singh S. V., Warin R., Xiao D., et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Research. 2009;69(5):2117–2125. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Abbaoui B., Riedl K. M., Ralston R. A., et al. Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: characterization, metabolism, and interconversion. Molecular Nutrition and Food Research. 2012;56(11):1675–1687. doi: 10.1002/mnfr.201200276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Cornblatt B. S., Ye L., Dinkova-Kostova A. T., et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28(7):1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 269.Yang J.-S., Chen G.-W., Hsia T.-C., et al. Diallyl disulfide induces apoptosis in human colon cancer cell line (COLO 205) through the induction of reactive oxygen species, endoplasmic reticulum stress, caspases casade and mitochondrial-dependent pathways. Food and Chemical Toxicology. 2009;47(1):171–179. doi: 10.1016/j.fct.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 270.Filomeni G., Aquilano K., Rotilio G., Ciriolo M. R. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Research. 2003;63(18):5940–5949. [PubMed] [Google Scholar]
- 271.Xiao D., Choi S., Johnson D. E., et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23(33):5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 272.Yang J.-S., Kok L.-F., Lin Y.-H., et al. Diallyl disulfide inhibits WEHI-3 leukemia cells in vivo. Anticancer Research. 2006;26(1):219–225. [PubMed] [Google Scholar]
- 273.Shin H. A., Cha Y. Y., Park M. S., Kim J. M., Lim Y. C. Diallyl sulfide induces growth inhibition and apoptosis of anaplastic thyroid cancer cells by mitochondrial signaling pathway. Oral Oncology. 2010;46(4):e15–e18. doi: 10.1016/j.oraloncology.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 274.Baskar A. A., Ignacimuthu S., Paulraj G. M., Al Numair K. S. Chemopreventive potential of β-Sitosterol in experimental colon cancer model—an in vitro and in vivo study. BMC Complementary and Alternative Medicine. 2010;10, article 24 doi: 10.1186/1472-6882-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Awad A. B., Chinnam M., Fink C. S., Bradford P. G. β-sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine. 2007;14(11):747–754. doi: 10.1016/j.phymed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 276.Zhao Y., Chang S. K. C., Qu G., Li T., Cui H. β-Sitosterol inhibits cell growth and induces apoptosis in SGC-7901 human stomach cancer cells. Journal of Agricultural and Food Chemistry. 2009;57(12):5211–5218. doi: 10.1021/jf803878n. [DOI] [PubMed] [Google Scholar]
- 277.Von Holtz R. L., Fink C. S., Awad A. B. β-sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutrition and Cancer. 1998;32(1):8–12. doi: 10.1080/01635589809514709. [DOI] [PubMed] [Google Scholar]
- 278.Moon D.-O., Lee K.-J., Choi Y. H., Kim G.-Y. β-Sitosterol-induced-apoptosis is mediated by the activation of ERK and the downregulation of Akt in MCA-102 murine fibrosarcoma cells. International Immunopharmacology. 2007;7(8):1044–1053. doi: 10.1016/j.intimp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 279.González-Sarrías A., Tomé-Carneiro J., Bellesia A., Tomás-Barberán F. A., Espín J. C. The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food and Function. 2015;6(5):1460–1469. doi: 10.1039/c5fo00120j. [DOI] [PubMed] [Google Scholar]
- 280.Rao C. V., Tokumo K., Rigotty J., Zang E., Kelloff G., Reddy B. S. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, alpha-difluoromethylornithine, 16 alpha-fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer Research. 1991;51:4528–4534. [PubMed] [Google Scholar]
- 281.Fujiki H., Suganuma M., Kurusu M., et al. New TNF-α releasing inhibitors as cancer preventive agents from traditional herbal medicine and combination cancer prevention study with EGCG and sulindac or tamoxifen. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2003;523-524:119–125. doi: 10.1016/s0027-5107(02)00327-5. [DOI] [PubMed] [Google Scholar]
- 282.Ju Y. H., Doerge D. R., Allred K. F., Allred C. D., Helferich W. G. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Research. 2002;62(9):2474–2477. [PubMed] [Google Scholar]
- 283.Banerjee S., Zhang Y., Ali S., et al. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Research. 2005;65(19):9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 284.Barbara Ann Karmanos Cancer Institute. Gemcitabine Hydrochloride and Genistein in Treating Women with Stage IV Breast Cancer. 2015. ( NCT00244933). [Google Scholar]
- 285.Zhang B., Shi Z.-L., Liu B., Yan X.-B., Feng J., Tao H.-M. Enhanced anticancer effect of gemcitabine by genistein in osteosarcoma: the role of Akt and nuclear factor-κB. Anti-Cancer Drugs. 2010;21(3):288–296. doi: 10.1097/cad.0b013e328334da17. [DOI] [PubMed] [Google Scholar]
- 286.Bittencourt H. St. Justine's Hospital. Phase I/II a study of decitabine in combination with genistein in pediatric relapsed or refractory malignancies. 2016;( NCT02499861)
- 287.Uman Pharma. Genistein and Interleukin-2 in Treating Patients with Metastatic Melanoma or Kidney Cancer. NCT01628471. 2015, 2015.
- 288.Kuzel T. Northwestern University, National Cancer Institute (NCI). Genistein and Interleukin-2 in treating patients with metastatic melanoma or kidney cancer. 2015;( NCT00276835)
- 289.Hwang J.-T., Ha J., Park O. J. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochemical and Biophysical Research Communications. 2005;332(2):433–440. doi: 10.1016/j.bbrc.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 290.Li Y., Ahmed F., Ali S., Philip P. A., Kucuk O., Sarkar F. H. Inactivation of nuclear factor κB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Research. 2005;65(15):6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 291.Solomon L. A., Ali S., Banerjee S., Munkarah A. R., Morris R. T., Sarkar F. H. Sensitization of ovarian cancer cells to cisplatin by genistein: the role of NF-kappaB. Journal of Ovarian Research. 2008;1(1, article 9) doi: 10.1186/1757-2215-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.El-Rayes B. F., Philip P. A., Sarkar F. H., et al. A phase II study of isoflavones, erlotinib, and gemcitabine in advanced pancreatic cancer. Investigational New Drugs. 2011;29(4):694–699. doi: 10.1007/s10637-010-9386-6. [DOI] [PubMed] [Google Scholar]
- 293.El-Rayes B. F., Ali S., Ali I. F., Philip P. A., Abbruzzese J., Sarkar F. H. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-κB. Cancer Research. 2006;66(21):10553–10559. doi: 10.1158/0008-5472.can-06-2333. [DOI] [PubMed] [Google Scholar]
- 294.Barbara Ann Karmanos Cancer Institute. Genistein, gemcitabine, and erlotinib in treating patients with locally advanced or metastatic pancreatic cancer. 2014;( NCT00376948)
- 295.Jeon Y.-W., Suh Y. J. Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncology Reports. 2013;29(2):819–825. doi: 10.3892/or.2012.2158. [DOI] [PubMed] [Google Scholar]
- 296.Wang P., Henning S., Heber D., Vadgama J. Enhanced inhibition of PC-3 xenograft prostate tumor growth by combination of green tea and quercetin with docetaxel. Cancer Research. 2015;75, article 5345 [Google Scholar]
- 297.Chuang-Xin L., Wen-Yu W., Yao C., Xiao-Yan L., Yun Z. Quercetin enhances the effects of 5-fluorouracil-mediated growth inhibition and apoptosis of esophageal cancer cells by inhibiting NF-κB. Oncology Letters. 2012;4(4):775–778. doi: 10.3892/ol.2012.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Xavier C. P. R., Lima C. F., Rohde M., Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemotherapy and Pharmacology. 2011;68(6):1449–1457. doi: 10.1007/s00280-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 299.Dai W., Gao Q., Qiu J., Yuan J., Wu G., Shen G. Quercetin induces apoptosis and enhances 5-FU therapeutic efficacy in hepatocellular carcinoma. Tumor Biology. 2016;37(5):6307–6313. doi: 10.1007/s13277-015-4501-0. [DOI] [PubMed] [Google Scholar]
- 300.University of Medicine and Dentistry of New Jersey. Sulindac and plant compounds in preventing colon cancer. Compounds in Preventing Colon Cancer. 2011;( NCT00003365)
- 301.Jain A. K., Thanki K., Jain S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Molecular Pharmaceutics. 2013;10(9):3459–3474. doi: 10.1021/mp400311j. [DOI] [PubMed] [Google Scholar]
- 302.Wang X., Chen Y., Dahmani F. Z., Yin L., Zhou J., Yao J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials. 2014;35(26):7654–7665. doi: 10.1016/j.biomaterials.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 303.He X., Wang Y., Zhu J., Orloff M., Eng C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Letters. 2011;301(2):168–176. doi: 10.1016/j.canlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 304.Kim T. H., Shin Y. J., Won A. J., et al. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochimica et Biophysica Acta (BBA)—General Subjects. 2014;1840(1):615–625. doi: 10.1016/j.bbagen.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 305.Lin C.-J., Lee C.-C., Shih Y.-L., et al. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radical Biology and Medicine. 2012;52(2):377–391. doi: 10.1016/j.freeradbiomed.2011.10.487. [DOI] [PubMed] [Google Scholar]
- 306.Chan J. Y., Meng S. P., Clement M.-V., Pervaiz S., Shao C. L. Resveratrol displays converse dose-related effects on 5-fluorouracilevoked colon cancer cell apoptosis: the roles of caspase-6 and p53. Cancer Biology and Therapy. 2008;7(8):1305–1312. doi: 10.4161/cbt.7.8.6302. [DOI] [PubMed] [Google Scholar]
- 307.Ali I., Braun D. P. Resveratrol enhances mitomycin C-mediated suppression of human colorectal cancer cell proliferation by up-regulation of p21WAF1/CIP1. Anticancer Research. 2014;34(10):5439–5446. [PubMed] [Google Scholar]
- 308.Zhu D.-J., Chen X.-W., Wang J.-Z., Ju Y.-L., Yang M.-Z. O., Zhang W.-J. Proteomic analysis identifies proteins associated with curcumin-enhancing efficacy of irinotecan-induced apoptosis of colorectal cancer LOVO cell. International Journal of Clinical and Experimental Pathology. 2014;7(1, article 7) [PMC free article] [PubMed] [Google Scholar]
- 309.UNC Lineberger Comprehensive Cancer Center. A Prospective Evaluation of the Effect of Curcumin on Dose Limiting Toxicity and Pharmacokinetics of Irinotecan in Patients with Solid Tumors. NCT01859858. 2016, 2015.
- 310.University of Leicester. Combining Curcumin with FOLFOX Chemotherapy in Patients with Inoperable Colorectal Cancer (CUFOX) NCT01490996. 2016, 2015.
- 311.M.D. Anderson Cancer Center. Curcumin with pre-operative capecitabine and radiation therapy followed by surgery for rectal cancer. 2015;( NCT00745134)
- 312.Shakibaei M., Mobasheri A., Lueders C., Busch F., Shayan P., Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0057218.e57218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nautiyal J., Banerjee S., Kanwar S. S., et al. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. International Journal of Cancer. 2011;128(4):951–961. doi: 10.1002/ijc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Aggarwal B. B., Shishodia S., Takada Y., et al. Curcumin suppresses the paclitaxel-induced nuclear factor-κB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clinical Cancer Research. 2005;11(20):7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 315.Lev-Ari S., Strier L., Kazanov D., et al. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clinical Cancer Research. 2005;11(18):6738–6744. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 316.Rocks N., Bekaert S., Coia I., et al. Curcumin-cyclodextrin complexes potentiate gemcitabine effects in an orthotopic mouse model of lung cancer. British Journal of Cancer. 2012;107(7):1083–1092. doi: 10.1038/bjc.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Aditya N. P., Shim M., Lee I., Lee Y., Im M.-H., Ko S. Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. Journal of Agricultural and Food Chemistry. 2013;61(8):1878–1883. doi: 10.1021/jf305143k. [DOI] [PubMed] [Google Scholar]
- 318.Tang Y., Parmakhtiar B., Simoneau A. R., et al. Lycopene enhances docetaxel's effect in castration-resistant prostate cancer associated with insulin-like growth factor I receptor levels. Neoplasia. 2011;13(2):108–119. doi: 10.1593/neo.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Medical University of South Carolina. Docetaxel and Lycopene in Metastatic Prostate Cancer. 2015. ( NCT01949519). [Google Scholar]
- 320.Snima K. S., Arunkumar P., Jayakumar R., Lakshmanan V.-K. Silymarin encapsulated poly (D, L-lactic-co-glycolic acid) nanoparticles: a prospective candidate for prostate cancer therapy. Journal of Biomedical Nanotechnology. 2014;10(4):559–570. doi: 10.1166/jbn.2014.1735. [DOI] [PubMed] [Google Scholar]
- 321.Bielska D., Karewicz A., Kamiński K., et al. Self-organized thermo-responsive hydroxypropyl cellulose nanoparticles for curcumin delivery. European Polymer Journal. 2013;49(9):2485–2494. doi: 10.1016/j.eurpolymj.2013.02.012. [DOI] [Google Scholar]
- 322.Abderrezak A., Bourassa P., Mandeville J.-S., Sedaghat-Herati R., Tajmir-Riahi H.-A. Dendrimers bind antioxidant polyphenols and cisplatin drug. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033102.e33102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wang L., Xu X., Zhang Y., et al. Encapsulation of curcumin within poly(amidoamine) dendrimers for delivery to cancer cells. Journal of Materials Science: Materials in Medicine. 2013;24(9):2137–2144. doi: 10.1007/s10856-013-4969-3. [DOI] [PubMed] [Google Scholar]
- 324.Manju S., Sreenivasan K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. Journal of Colloid and Interface Science. 2011;359(1):318–325. doi: 10.1016/j.jcis.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 325.Dey S., Sreenivasan K. Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin. Carbohydrate Polymers. 2014;99:499–507. doi: 10.1016/j.carbpol.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 326.Miyake K., Arima H., Hirayama F., et al. Improvement of solubility and oral bioavailability of rutin by complexation with 2-hydroxypropyl-β-cyclodextrin. Pharmaceutical Development and Technology. 2000;5(3):399–407. doi: 10.1081/PDT-100100556. [DOI] [PubMed] [Google Scholar]
- 327.Folch-Cano C., Guerrero J., Speisky H., Jullian C., Olea-Azar C. NMR and molecular fluorescence spectroscopic study of the structure and thermodynamic parameters of EGCG/β-cyclodextrin inclusion complexes with potential antioxidant activity. Journal of Inclusion Phenomena and Macrocyclic Chemistry. 2014;78(1–4):287–298. doi: 10.1007/s10847-013-0297-y. [DOI] [Google Scholar]
- 328.Cao X., Deng W.-W., Fu M., et al. In vitro release and in vitro-in vivo correlation for silybin meglumine incorporated into Hollow-type mesoporous silica nanoparticles. International Journal of Nanomedicine. 2012;7:753–762. doi: 10.2147/IJN.S28348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Chen H., Wu J., Sun M., et al. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. Journal of Liposome Research. 2012;22(2):100–109. doi: 10.3109/08982104.2011.621127. [DOI] [PubMed] [Google Scholar]
- 330.Aboutaleb E., Atyabi F., Khoshayand M. R., et al. Improved brain delivery of vincristine using dextran sulfate complex solid lipid nanoparticles: optimization and in vivo evaluation. Journal of Biomedical Materials Research—Part A. 2014;102(7):2125–2136. doi: 10.1002/jbm.a.34890. [DOI] [PubMed] [Google Scholar]
- 331.Wang Y., Dou L., He H., Zhang Y., Shen Q. Multifunctional nanoparticles as nanocarrier for vincristine sulfate delivery to overcome tumor multidrug resistance. Molecular Pharmaceutics. 2014;11(3):885–894. doi: 10.1021/mp400547u. [DOI] [PubMed] [Google Scholar]
- 332.Lian R., Lu Y., Qi J., et al. Silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices: physical characterization and enhanced oral bioavailability. AAPS PharmSciTech. 2011;12(4):1234–1240. doi: 10.1208/s12249-011-9666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ding B., Chen P., Kong Y., et al. Preparation and evaluation of folate-modified lipid nanocapsules for quercetin delivery. Journal of Drug Targeting. 2014;22(1):67–75. doi: 10.3109/1061186X.2013.839685. [DOI] [PubMed] [Google Scholar]
- 334.Zhao Y.-Q., Wang L.-P., Ma C., Zhao K., Liu Y., Feng N.-P. Preparation and characterization of tetrandrine-phospholipid complex loaded lipid nanocapsules as potential oral carriers. International Journal of Nanomedicine. 2013;8:4169–4181. doi: 10.2147/IJN.S50557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yu Y., Lu Y., Bo R., et al. The preparation of gypenosides liposomes and its effects on the peritoneal macrophages function in vitro. International Journal of Pharmaceutics. 2014;460(1-2):248–254. doi: 10.1016/j.ijpharm.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 336.Karewicz A., Bielska D., Loboda A., et al. Curcumin-containing liposomes stabilized by thin layers of chitosan derivatives. Colloids and Surfaces B: Biointerfaces. 2013;109:307–316. doi: 10.1016/j.colsurfb.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 337.Luo X., Guan R., Chen X., Tao M., Ma J., Zhao J. Optimization on condition of epigallocatechin-3-gallate (EGCG) nanoliposomes by response surface methodology and cellular uptake studies in Caco-2 cells. Nanoscale Research Letters. 2014;9(1):1–9. doi: 10.1186/1556-276X-9-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Li J., Chen J., Cai B.-C., Yang T. Preparation, characterization and tissue distribution of brucine stealth liposomes with different lipid composition. Pharmaceutical Development and Technology. 2013;18(4):772–778. doi: 10.3109/10837450.2011.598165. [DOI] [PubMed] [Google Scholar]
- 339.Chen J., Yan G.-J., Hu R.-R., et al. Improved pharmacokinetics and reduced toxicity of brucine after encapsulation into stealth liposomes: role of phosphatidylcholine. International Journal of Nanomedicine. 2012;7:3567–3577. doi: 10.2147/ijn.s32860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wu H., Lu C., Zhou A., Min Z., Zhang Y. Enhanced oral bioavailability of puerarin using microemulsion vehicle. Drug Development and Industrial Pharmacy. 2009;35(2):138–144. doi: 10.1080/03639040801973495. [DOI] [PubMed] [Google Scholar]
- 341.Chouhan N., Mittal V., Kaushik D., Khatkar A., Raina M. Self emulsifying drug delivery system (SEDDS) for phytoconstituents: a review. Current Drug Delivery. 2015;12(2):244–253. doi: 10.2174/1567201811666141021142606. [DOI] [PubMed] [Google Scholar]
- 342.Bongkyu Y., Hee Y. J., Srinivasan S. Oral pharmaceutical composition containing lutein using self microemulsion system. 2011.
- 343.Zhao L., Zhang L., Meng L., Wang J., Zhai G. Design and evaluation of a self-microemulsifying drug delivery system for apigenin. Drug Development and Industrial Pharmacy. 2013;39(5):662–669. doi: 10.3109/03639045.2012.687378. [DOI] [PubMed] [Google Scholar]
- 344.Zhang Y., Wang R., Wu J., Shen Q. Characterization and evaluation of self-microemulsifying sustained-release pellet formulation of puerarin for oral delivery. International Journal of Pharmaceutics. 2012;427(2):337–344. doi: 10.1016/j.ijpharm.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 345.Xiaoyan F., Jun L., Wen W. Study on the absorption kinetics of hesperidin self-microemulsion in rat's intestine. Acta Universitatis Medicinalis Anhui. 2011;8, article 010 [Google Scholar]
- 346.Zhu J.-X., Tang D., Feng L., et al. Development of self-microemulsifying drug delivery system for oral bioavailability enhancement of berberine hydrochloride. Drug Development and Industrial Pharmacy. 2013;39(3):499–506. doi: 10.3109/03639045.2012.683875. [DOI] [PubMed] [Google Scholar]
- 347.Zhao Y., Wang C., Chow A. H. L., et al. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: formulation and bioavailability studies. International Journal of Pharmaceutics. 2010;383(1-2):170–177. doi: 10.1016/j.ijpharm.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 348.Xi J., Chang Q., Chan C. K., et al. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech. 2009;10(1):172–182. doi: 10.1208/s12249-009-9190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Mantri S. K., Pashikanti S., Murthy K. V. R. Development and characterization of self-nanoemulsifying drug delivery systems (SNEDDS) of atorvastatin calcium. Current Drug Delivery. 2012;9(2):182–196. doi: 10.2174/156720112800234594. [DOI] [PubMed] [Google Scholar]
- 350.Zhang C., Gu C., Peng F., et al. Preparation and optimization of triptolide-loaded solid lipid nanoparticles for oral delivery with reduced gastric irritation. Molecules. 2013;18(11):13340–13356. doi: 10.3390/molecules181113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Dang Y.-J., Zhu C.-Y. Oral bioavailability of cantharidin-loaded solid lipid nanoparticles. Chinese Medicine. 2013;8, article 1 doi: 10.1186/1749-8546-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Neves A. R., Lúcio M., Martins S., Lima J. L. C., Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. International Journal of Nanomedicine. 2013;8:177–187. doi: 10.2147/IJN.S37840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Chen Y., Pan L., Jiang M., Li D., Jin L. Nanostructured lipid carriers enhance the bioavailability and brain cancer inhibitory efficacy of curcumin both in vitro and in vivo. Drug Delivery. 2016;23(4):1383–1392. doi: 10.3109/10717544.2015.1049719. [DOI] [PubMed] [Google Scholar]
- 354.Tsai M., Wu P., Huang Y., et al. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. International Journal of Pharmaceutics. 2012;423:461–470. doi: 10.1016/j.ijpharm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 355.Shi F., Yang G., Ren J., Guo T., Du Y., Feng N. Formulation design, preparation, and in vitro and in vivo characterizations of β-elemene-loaded nanostructured lipid carriers. International Journal of Nanomedicine. 2013;8:2533–2541. doi: 10.2147/IJN.S46578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Li F., Weng Y., Wang L., He H., Yang J., Tang X. The efficacy and safety of bufadienolides-loaded nanostructured lipid carriers. International Journal of Pharmaceutics. 2010;393(1-2):204–212. doi: 10.1016/j.ijpharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 357.Li M., Zheng Y., Shan F.-Y., Zhou J., Gong T., Zhang Z.-R. Development of ionic-complex-based nanostructured lipid carriers to improve the pharmacokinetic profiles of breviscapine. Acta Pharmacologica Sinica. 2013;34(8):1108–1115. doi: 10.1038/aps.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ma X., Zhou J., Zhang C.-X., et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34(18):4452–4465. doi: 10.1016/j.biomaterials.2013.02.066. [DOI] [PubMed] [Google Scholar]
- 359.Zhao Y.-Z., Lu C.-T., Zhang Y., et al. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. International Journal of Pharmaceutics. 2013;454(1):302–309. doi: 10.1016/j.ijpharm.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 360.Hu K., Zhu L., Liang H., Hu F., Feng J. Improved antitumor efficacy and reduced toxicity of liposomes containing bufadienolides. Archives of Pharmacal Research. 2011;34(9):1487–1494. doi: 10.1007/s12272-011-0910-9. [DOI] [PubMed] [Google Scholar]
- 361.Shen L.-N., Zhang Y.-T., Wang Q., Xu L., Feng N.-P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. International Journal of Pharmaceutics. 2014;460(1-2):280–288. doi: 10.1016/j.ijpharm.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 362.Madane R. G., Mahajan H. S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: design, characterization, and in vivo study. Drug Delivery. 2016;23(4):1326–1334. doi: 10.3109/10717544.2014.975382. [DOI] [PubMed] [Google Scholar]
- 363.Li J., Guo X., Liu Z., et al. Preparation and evaluation of charged solid lipid nanoparticles of tetrandrine for ocular drug delivery system: pharmacokinetics, cytotoxicity and cellular uptake studies. Drug Development and Industrial Pharmacy. 2014;40(7):980–987. doi: 10.3109/03639045.2013.795582. [DOI] [PubMed] [Google Scholar]
- 364.Anitha A., Deepagan V. G., Rani V. V. D., Menon D., Nair S. V., Jayakumar R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohydrate Polymers. 2011;84(3):1158–1164. doi: 10.1016/j.carbpol.2011.01.005. [DOI] [Google Scholar]
- 365.Duan J., Zhang Y., Han S., et al. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. International Journal of Pharmaceutics. 2010;400(1-2):211–220. doi: 10.1016/j.ijpharm.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 366.Bu L., Gan L.-C., Guo X.-Q., et al. Trans-resveratrol loaded chitosan nanoparticles modified with biotin and avidin to target hepatic carcinoma. International Journal of Pharmaceutics. 2013;452(1-2):355–362. doi: 10.1016/j.ijpharm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 367.Yao Q., Gan L., Hou S., et al. Development and biodistribution of trans-resveratrol loaded chitosan nanoparticles with free amino groups. Latin American Journal of Pharmacy. 2012;31(7):1038–1042. [Google Scholar]
- 368.Zheng D., Duan C., Zhang D., et al. Galactosylated chitosan nanoparticles for hepatocyte-targeted delivery of oridonin. International Journal of Pharmaceutics. 2012;436(1-2):379–386. doi: 10.1016/j.ijpharm.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 369.Wang Z., Yu Y., Ma J., et al. LyP-1 modification to enhance delivery of Artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Molecular Pharmaceutics. 2012;9(9):2646–2657. doi: 10.1021/mp3002107. [DOI] [PubMed] [Google Scholar]
- 370.Guo W., Li A., Jia Z., Yuan Y., Dai H., Li H. Transferrin modified PEG-PLA-resveratrol conjugates: in vitro and in vivo studies for glioma. European Journal of Pharmacology. 2013;718(1–3):41–47. doi: 10.1016/j.ejphar.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 371.Tian X., Yin H., Zhang S., et al. Bufalin loaded biotinylated chitosan nanoparticles: an efficient drug delivery system for targeted chemotherapy against breast carcinoma. European Journal of Pharmaceutics and Biopharmaceutics. 2014;87(3):445–453. doi: 10.1016/j.ejpb.2014.05.010. [DOI] [PubMed] [Google Scholar]