Abstract
Background
Fish is a frequent elicitor of severe IgE-mediated allergic reactions. Beside avoidance, there is currently no allergen-specific therapy available. Hypoallergenic variants of the major fish allergen, parvalbumin, for specific immunotherapy based on mutation of the 2 calcium-binding sites have been developed.
Objectives
This study sought to establish a mouse model of fish allergy resembling human disease and to investigate whether mouse and rabbit IgG antibodies induced by immunization with a hypoallergenic mutant of the major carp allergen protect against allergic symptoms in sensitized mice.
Methods
C3H/HeJ mice were sensitized with recombinant wildtype Cyp c 1 or carp extract by intragastric gavage. Antibody, cellular immune responses, and epitope specificity in sensitized mice were investigated by ELISA, rat basophil leukemia assay, T-cell proliferation experiments using recombinant wildtype Cyp c 1, and overlapping peptides spanning the Cyp c 1 sequence. Anti-hypoallergenic Cyp c 1 mutant mouse and rabbit sera were tested for their ability to inhibit IgE recognition of Cyp c 1, Cyp c 1–specific basophil degranulation, and Cyp c 1–induced allergic symptoms in the mouse model.
Results
A mouse model of fish allergy mimicking human disease regarding IgE epitope recognition and symptoms as close as possible was established. Administration of antisera generated in mice and rabbits by immunization with a hypoallergenic Cyp c 1 mutant inhibited IgE binding to Cyp c 1, Cyp c 1–induced basophil degranulation, and allergic symptoms caused by allergen challenge in sensitized mice.
Conclusions
Antibodies induced by immunization with a hypoallergenic Cyp c 1 mutant protect against allergic reactions in a murine model of fish allergy.
Keywords: Blocking antibodies, fish allergy, hypoallergenic parvalbumin mutant, specific immunotherapy
Fish represents an important elicitor of food allergy causing severe allergic reactions that are often life-threatening.1 The prevalence of fish allergy ranges from 0.2% to 10% depending on the population and is particularly high in countries with high fish consumption.2,3 Whereas many food allergies are diseases of early childhood that are often outgrown, allergy to fish often persists through adulthood.4
Allergen-specific immunotherapy (SIT) is highly effective for respiratory forms of allergy and insect venom allergy.5 There are also several approaches pursued for SIT of food allergy including oral, sublingual, epicutaneous, and subcutaneous administration of allergens or modified allergens.6,7 A recent review of clinical studies in oral SIT for food allergy indicated that outcomes of treatment may be different for different allergens.8 Despite the variability of SIT regarding clinical outcome for different food allergens, studies performed for different allergens suggest that besides alterations at the cellular level, an induction of allergen-specific IgG antibodies may be important for the success of SIT in food allergy.9,10
At present, SIT is not available for fish allergy although parvalbumin, a protein containing calcium-binding sites, has been characterized as a cross-reactive allergen in many fish species and recombinant fish parvalbumins mimicking the immunological properties of the corresponding natural allergens have been produced.4,11 Based on the observation that the depletion of calcium leads to a substantial loss of IgE reactivity of fish parvalbumins,12 we have developed a recombinantly expressed hypoallergenic variant of the fish allergen Cyp c 1 from carp by mutation of the calcium-binding sites in the protein as a candidate molecule for SIT of fish allergy.13 We recently also demonstrated that the strategy of introducing point mutations into the calcium-binding sites of fish parvalbumins can be used to reduce the allergenic activity of the major allergens from a variety of fish species.14
In this study we aimed to establish a murine model of fish allergy that mimics fish allergy in patients as closely as possible. For this purpose, mice were orally sensitized with the major fish allergen Cyp c 1 and the development, epitope-specificity, and biological activity of specific IgE antibodies were determined by ELISA, basophil degranulation experiments as well as by in vivo provocation testing and assessment of allergic symptoms. To investigate whether IgG antibodies induced by immunization with the recombinant Cyp c 1 mutant (ie, mCyp c 1) can protect against fish allergy, we performed passive immunization of mice who are allergic to fish with mCyp c 1–specific rabbit and mouse antisera before oral provocation. The results obtained demonstrate that mCyp c 1–specific antibodies can protect against fish allergy and thus indicate that blocking antibodies might represent a major mechanism in SIT with mCyp c 1.
Methods
Recombinant allergens, synthetic peptides
Recombinant wildtype Cyp c 1 (rCyp c 1) and recombinant Phl p 1 (rPhl p 1) were obtained from Biomay AG (Vienna, Austria). A recombinant grass pollen hypoallergen (hP62) consisting of Phl p 2– and Phl p 6–derived fragments was purified as described and used as the negative control.15 mCyp c 1 was expressed in Escherichia coli and purified as described.13 Overlapping peptides spanning the Cyp c 1 sequence were synthesized on a peptide synthesizer Apex 396 (AAPPTec, Louisville, Ky) as described.16 The biochemical characteristics and positions of the peptides within the amino acid sequence of Cyp c 1 are summarized in Table I.
Table I.
Sequences, MWs, and pIs of synthetic peptides spanning the Cyp c 1 sequence
| Peptide | aa Sequence | MW (Da) | pI |
|---|---|---|---|
| P1 | MAFAGILNDADITAALQGCQ | 2023.3 | 3.56 |
| P2 | LQGCQAADSFDYKSFFAKVG | 2182.4 | 5.95 |
| P3 | FAKVGLSAKTPDDIKKAFAV | 2106.4 | 9.53 |
| P4 | KAFAVIDQDKSGFIEEDELK | 2282.5 | 4.3 |
| P5 | EDELKLFLQNFSAGARALTD | 2238.4 | 4.32 |
| P6 | RALTDAETKAFLKAGDSDGD | 2081.2 | 4.36 |
| P7 | GDSDGDGKIGVDEFAALVKA | 1964.1 | 4.04 |
The 4 aspartic acids (D) that were exchanged to alanines in the Cyp c 1 mutant are underlined.
aa, Amino acid; MW, molecular weight; pI, isoelectric point.
Rabbit antisera, sera from patients who are allergic to fish
For the generation of mCyp c 1–specific rabbit antisera, 2 female New Zealand rabbits were immunized at Charles River Laboratories (Sulzfeld, Germany) 4 times subcutaneously with 200 μg mCyp c 1 using Freund adjuvant (first immunization: complete Freund adjuvant; second to fourth immunizations: incomplete Freund adjuvant). An antiserum obtained by immunizing a rabbit with rPhl p 1 in the same way served as a control. Rabbit antisera were heat inactivated at 56°C for 30 minutes before administration to mice.
Sera from the patients with fish allergy have been described elsewhere.14,17 Sensitization to fish allergens was determined by measuring specific IgE antibodies using the ImmunoCAP System (f3, cod and/or f355, rCyp c 1) with a cutoff of 0.35 kUA/L (Thermofisher/Phadia, Uppsala, Sweden). These patients had experienced at least 1 of the typical clinical symptoms (dermatitis, urticaria, angioedema, diarrhea, rhinitis, asthma, or a systemic anaphylactic reaction) that could be clearly attributed to consumption of fish. The use of anonymized patients’ sera for serological testing was approved by the ethics committee of the Medical University of Vienna.
Preparation of fish allergen extracts for immunoblotting and mouse immunization
Allergen extracts were prepared from fresh filets of carp, cod, mackerel, salmon, sardine, swordfish, and tuna as described elsewhere12 with modifications. Ten-gram aliquots of each fish were frozen in liquid nitrogen, crushed to powder, and dissolved in 50 mL ice-cold PBS. Extraction of the fish powder was performed overnight at 4°C. After centrifugation at 14,000 rpm and 4°C, supernatants were filtered through a 0.22-μm filter and protein concentrations were determined by Bradford protein assay (Bio-Rad, Hercules, Calif). For mouse sensitization, carp extract was lyophilized and freshly dissolved in 0.2-mol bicarbonate buffer (pH 9) before use. For immunoblot experiments, aliquots of 80 μg of total protein of each fish extract were used. Extracts, 1 μg purified rCyp c 1 (positive control) and the grass pollen allergen rPhl p 6 (negative control) were separated by 16% SDS-PAGE18 and blotted on a nitrocellulose membrane (Whatman Protran nitrocellulose membrane; Sigma-Aldrich, St Louis, Mo).19 After blocking, blotted proteins were incubated with mCyp c 1–specific rabbit serum diluted 1:1000 overnight at 4°C. Then membranes were washed and bound IgG antibodies were detected with 125I-labeled goat anti-rabbit IgG (PerkinElmer, Waltham, Mass) and visualized by autoradiography.20
Development of a mouse model of fish allergy
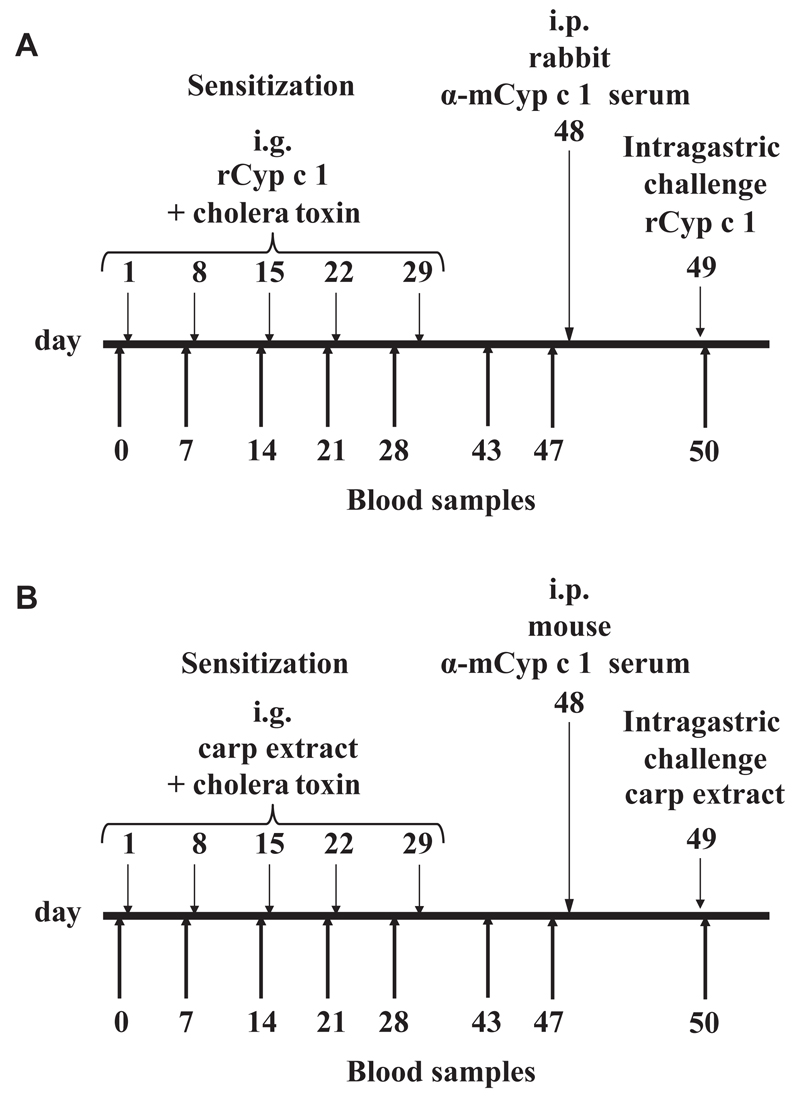
Six- to 8-week-old, female C3H/HeJ mice were obtained from Harlan (San Pietro Al Natisone, Italy) and Jackson Laboratories (Bar Harbor, Me). All experiments were approved by the local review board of the Medical University of Vienna and were performed in accordance with national and international guidelines of laboratory animal care. The mouse model of fish allergy was established according to the protocol in Fig 1, A. Mice (n = 24) were sensitized 5 times in weekly intervals with 100 μg rCyp c 1 and 20 μg cholera toxin (Sigma-Aldrich) in 200 μL 0.2-mol bicarbonate buffer (pH 9) by intragastric gavage. After sensitization, mice were randomized in 2 groups of 12 mice each with comparable Cyp c 1–specific IgE antibody levels. On day 48, the therapy group received 500 μL of heat-inactivated mCyp c 1–specific rabbit antiserum and the control group received 500 μL of heat-inactivated Phl p 1–specific antiserum intraperitoneally (i.p.). Blood samples were taken on the day before each sensitization and before and after rabbit serum application. Mice were then challenged by intragastric gavage on day 49 with 100 μg rCyp c 1. Symptoms were recorded by 2 independent observers according to the symptom score published by Li et al21 (0: no symptoms; 1: scratching and rubbing around the nose and head; 2: puffiness around the eyes and mouth, diarrhea, pilar erecti, reduced activity and/or decreased activity with increased respiratory rate; 3: wheezing, labored respiration; 4: no activity after prodding or tremor and convulsion; 5: death). For control purposes, naive mice were also included in the study.
Fig 1.
Sensitization and treatment protocol for a mouse model of fish allergy. A, Mice were sensitized to rCyp c 1 5 times by intragastric gavage as indicated, treated by the intraperitoneal (i.p.) application of the mCyp c 1–specific rabbit antiserum or control antiserum and challenged with rCyp c 1. B, Mice were sensitized by 5 intragastric (i.g.) gavages of carp extract, treated by mouse mCyp c 1–specific or control antiserum and were challenged with carp extract.
For a second set of experiments, C3H/HeJ mice were vaccinated with mCyp c 1 using a protocol similar to subcutaneous SIT in humans. The vaccine was produced by adsorption of mCyp c 1 to aluminium hydroxide (Alu-Gel-S; Serva, Ingelheim, Germany) and 20 μg mCyp c 1 were administered to mice (n = 5) subcutaneously 6 times in monthly intervals. Likewise, a second group of mice (n = 5) was vaccinated with 20 μg of a control protein (hP62) adsorbed to aluminium hydroxide 6 times in monthly intervals.
Immune sera specific for the respective antigens were collected after each immunization, pooled, and administered to mice sensitized with 500 μg carp extract and 20 μg cholera toxin according to the established protocol (Fig 1, B).
In another set of experiments investigating the specificity of the protective antisera, groups of carp extract–sensitized C3H/HeJ mice (n = 8) were randomized into 3 groups with comparable Cyp c 1–specific IgE levels. Following the established model described above, 600 μL of heat-inactivated mCyp c 1– or control antigen–specific mouse antisera were administered intraperitoneally to each mouse. An additional control group of sensitized mice received no treatment with any antiserum. One day later, all groups of mice were challenged with 10 mg carp extract by intragastric gavage and again symptoms were recorded. Results shown are representative for 2 independently performed experiments.
Measurement of rCyp c 1– and peptide-specific antibodies in mouse and human sera
Mouse IgM, IgA, IgG1, IgG2a, IgG3 specific for rCyp c 1 were determined by ELISA as described elsewhere.22 ELISA plates (Nunc Maxisorp, Roskilde, Denmark) were coated with rCyp c 1 (5 μg/mL). Mouse sera were diluted 1:50 (for IgM, IgA, IgG2a, and IgG3) and 1:500 (for IgG1). IgM, IgA, IgG1, IgG2a, and IgG3 were detected with monoclonal rat anti-mouse IgM, IgA, IgG1, IgG2a, and IgG3 antibodies, respectively (1:1000 dilution; GE Healthcare, Fairfield, Conn) followed by a horseradish peroxidase (HRP)-labeled goat anti-rat IgG (minimal cross-reactivity) antibody (1:2000 dilution; BioLegend, San Diego, Calif).
To measure human and mouse IgE specific for rCyp c 1 and Cyp c 1 peptides, ELISA plates (Nunc Maxisorp) were coated with rCyp c 1 or the individual peptides (5 μg/mL). Mouse and human sera were diluted 1:10. Human IgE was detected with a HRP-labeled goat anti-human IgE antibody (1:2500 dilution; KPL, Gaithersburg, Md), mouse IgE with a rat anti-mouse IgE antibody (1:1000 dilution; GE Healthcare) and HRP-labeled goat anti-rat IgG (1:2000 dilution; BioLegend).
rCyp c 1–specific rabbit IgG antibodies were measured in mouse sera, which were obtained before and after i.p. application of mCyp c 1–specific or rPhl p 1–specific rabbit antisera to the mice. For this purpose, ELISA plates were coated with rCyp c 1 (5 μg/mL). Mouse sera were diluted 1:50,000 and rabbit IgG was detected with HRP-labeled anti-rabbit IgG from donkey (1:2500 dilution; GE Healthcare). All measurements were performed in duplicates. OD values shown are mean values of the different mouse groups.
Titration and epitope mapping of mCyp c 1–induced rabbit and mouse IgG antiserum and inhibition of human IgE binding to rCyp c 1
ELISA plate-bound rCyp c 1 (5 μg/mL) was incubated with serial dilutions of the mCyp c 1–specific rabbit antiserum (diluted 1:100-1:819,200). Rabbit IgM was detected with a HRP-labeled anti-rabbit IgM from goat (1:2000 dilution; Rockland Immunochemicals, Limerick, Pa), rabbit IgA with a HRP-labeled anti-rabbit IgA from goat (1:2000 dilution; Thermo Fisher Scientific, Waltham, Mass), and rabbit IgG with a HRP-labeled anti-rabbit IgG antibody from donkey (dilution 1:2000; GE Healthcare). For the epitope mapping of rabbit anti-mCyp c 1 antibodies, ELISA plates were coated with rCyp c 1 or Cyp c 1 peptides (5 μg/mL) and exposed to the 1:50,000 diluted mCyp c 1–specific rabbit antiserum. For epitope mapping of IgG1 and IgG2a in mouse sera, a 1:250 serum dilution for IgG1 and a 1:50 dilution for IgG2a were used. IgG1 and IgG2a were detected as described above.
For IgE inhibition experiments, ELISA plate-bound rCyp c 1 (1 μg/mL) was preincubated with serial dilutions of the mCyp c 1–specific rabbit antiserum (1:20-1:100,000) or, for control purposes, with the preimmune serum (1:20). After washing, 1:10 diluted patients’ sera were added. Human IgE was detected with a HRP-labeled goat anti-human IgE antibody (1:2500; KPL). All measurements were performed as duplicates. The OD values shown are mean values with a deviation of <5%.
RBL cell degranulation experiments
Rat basophil leukemia (RBL) cells were grown in 96-well cell-culture plates (Costar, Corning, Tewksbury, Mass) for 20 hours at 37°C and 5% CO2. Aliquots of 5 μL of a serum pool from sensitized mice were added and the cells were incubated at 37°C, 5% CO2 for 2 hours and washed 2 times with Tyrode buffer (Sigma-Aldrich). Degranulation of RBL cells was induced by adding 100 μL of rCyp c 1 (0.3 μg/mL) in Tyrode buffer and incubation for 30 minutes at 37°C, 5% CO2. In the inhibition experiments, rCyp c 1 (0.3 μg/mL) was added together with 10% vol/vol mCyp c 1–specific heat-inactivated rabbit or mouse antiserum or control antiserum or buffer. Beta-hexosaminidase in culture supernatants was detected with 0.16 mmol 4-methylumbelliferyl-N-acetyl-β-d-glucosaminide (Sigma-Aldrich) and fluorescence was measured at excitation and emission wavelengths of 360 to 465 nm λex-λem using a microplate reader (VICTOR Plate Reader; Perkin Elmer). Results are reported as percentages of total β-hexosaminidase released after cell lysis by addition of 10% Triton X-100 (Merck Millipore, Darmstadt, Germany). All measurements were performed in triplicates and standard deviations are given for triplicate determinations.
Proliferation assays
Spleens were removed from mice on day 62 under aseptic conditions and homogenized. Single-cell suspensions were filtered through a 70-μm nylon cell strainer to remove remaining tissue. Erythrocytes were removed by adding ice-cold red blood cell lysing buffer (Sigma-Aldrich). Cells (2 × 106 cells/mL) were cultivated in 96-well round-bottom plates (Nunclon Delta Surface, Roskilde, Denmark) in the presence of rCyp c 1 or mCyp c 1 (2 μg/well) or synthetic Cyp c 1 peptides (0.36 μg/well). Concanavalin A (0.5 μg/well) (Sigma-Aldrich) was used as a positive control and medium as a negative control. The plates were incubated at 37°C, 5% CO2. On day 5, 0.5 μCi 3H thymidine ([methyl-3H] thymidine; Perkin Elmer) per well was added to cells. After 18 hours, cells were harvested and thymidine incorporation was measured in a beta counter (Beta scintillation liquid, Wallac Micro Beta TriLux Luminescence Counter; Perkin Elmer).
Statistical analysis
Differences between mouse groups were determined using a Mann-Whitney U test. P values of <.05 were considered significant (*) and P <.01 as highly significant (**). GraphPad Prism software (GraphPad Software, San Diego, Calif) was used for statistical analysis.
Results
Development of a mouse model of fish allergy
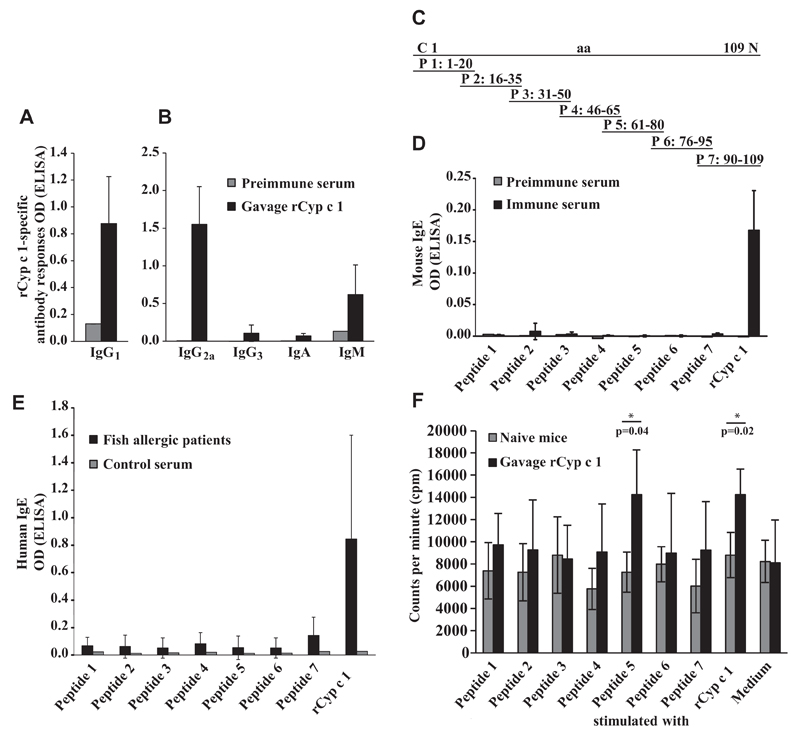
To develop a mouse model of IgE-associated fish allergy that resembles the human situation as closely as possible, C3H/HeJ mice, which have been described as being susceptible to the induction of food allergy,23 were sensitized by repeated intragastric gavage with the major fish allergen, Cyp c 1, from carp, which shows extensive IgE cross-reactivity with parvalbumins from various fish species. The application of rCyp c 1 together with the mucosal adjuvant cholera toxin resulted in the induction of a Cyp c 1–specific IgE response that was accompanied by a specific IgG1, IgG2a, and IgM response (Figs 1, A and 2, A-D). A slight induction of Cyp c 1–specific IgG3 and IgA responses was observed (Fig 2, B).
Fig 2.
Cyp c 1–specific immune responses in rCyp c 1-sensitized mice. A and B, Induction of Cyp c 1–specific IgG1 and IgG2a, IgG3, IgA, and IgM as measured by ELISA. C, Position of synthetic Cyp c 1–derived peptides 1 to 7 in the Cyp c 1 molecule. aa, Amino acids. D and E, Epitope mapping of mouse IgE and allergic patients’ IgE with Cyp c 1 peptides. Shown are mean antibody levels (y-axes: OD values ± SDs). F, Proliferation of splenocytes from Cyp c 1–sensitized and naive mice in response to Cyp c 1 peptides, rCyp c 1, and medium alone. Results are displayed as mean counts per minute ± SDs for the 2 mouse groups. Asterisks (*) indicate significant differences (P < .05) between mouse groups.
The analysis of the epitope specificity of this IgE response using 7 overlapping Cyp c 1 peptides gave similar results (Fig 2, C and D) as experiments performed with sera from patients with fish allergy (Fig 2, E). Cyp c 1–specific mouse IgE antibodies showed no relevant IgE reactivity to any of 7 Cyp c 1 peptides but only to the complete Cyp c 1 allergen, indicating that the murine IgE antibodies were not directed to linear/sequential epitopes but to the intact conformation of Cyp c 1 (Fig 2, D). Likewise, the IgE epitope mapping of sera of patients who are allergic to fish (n = 10) revealed that the patients IgE responses were mainly directed to the complete Cyp c 1 allergen molecule and not to the Cyp c 1 peptides (Fig 2, E). The mapping of the Cyp c 1–specific T-cell response performed with the overlapping synthetic Cyp c 1 peptides in cultivated splenocytes from sensitized mice identified peptide 5 spanning amino acids 61 to 80 of Cyp c 1 as the major T-cell epitope in the murine model (Fig 2, F).
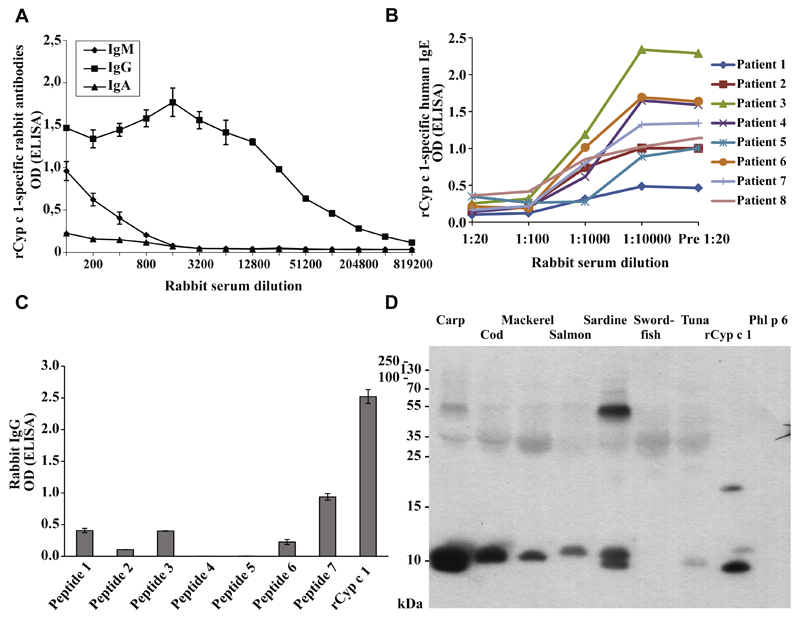
Anti-mCyp c 1 rabbit antiserum inhibits the binding of patients’ IgE to Cyp c 1 and cross-reacts with parvalbumins from many fish species
We have engineered a recombinant hypoallergenic derivative of the major carp allergen Cyp c 1 (mCyp c 1) by mutation of the 2 active calcium binding sites of the molecule (Table I).13,14 To investigate whether IgG antibodies induced by immunization with mCyp c 1 protect against fish allergy, we produced mCyp c 1–specific rabbit antisera. Rabbit anti-mCyp c 1 IgG antibodies reacted with rCyp c 1 even at high dilutions (1:819,200) (Fig 3, A). In fact, the majority of Cyp c 1–specific antibodies belonged to the IgG class, as Cyp c 1–specific IgM and IgA was only detectable up to a dilution of 1:800, whereas Cyp c 1–specific IgG could be detected up to a dilution of approximately 1:400,000 (Fig 3, A). Furthermore, rabbit anti-mCyp c 1 antibodies at a dilution of 1:100 inhibited almost completely the binding of fish allergic patients’ IgE to rCyp c 1 as shown in IgE-inhibition ELISA experiments (Fig 3, B). Epitope mapping studies performed with synthetic Cyp c 1 peptides showed that anti-mCyp c 1–specific IgG antibodies reacted also with sequential Cyp c 1 peptide epitopes (peptides 7>1 = 3>6) (Fig 3, C).
Fig 3.
Characterization of rabbit antiserum induced by immunization with mCyp c 1. A, Titration of rabbit antiserum induced by immunization with mCyp c 1 for IgM, IgA, and IgG reactivity to rCyp c 1. Mean OD values of triplicates ± SD corresponding to bound IgM, IgA, and IgG (y-axis) are displayed for different serum dilutions (x-axis). B, Inhibition of IgE binding to rCyp c 1 by mCyp c 1–induced IgG in patients who are allergic to fish. For these patients, IgE binding (patients 1-8) (y-axis: mean OD values) to rCyp c 1, which was preincubated with various dilutions of serum from a rabbit obtained before (Pre) or after immunization with mCyp c 1 (x-axis), is shown. C, Epitope mapping of mCyp c 1–induced rabbit IgG antibodies. IgG reactivity (y-axis: mean OD values ± SDs) of mCyp c 1–specific IgG to rCyp c 1 and Cyp c 1 peptides 1 to 7 (x-axis). D, Cross-reactivity of mCyp c 1–induced rabbit IgG antibodies to nitrocellulose-blotted fish allergen extracts. Bound mCyp c 1–specific rabbit IgG antibodies were detected with 125I-labeled anti-rabbit IgG antibodies and visualized by auto-radiography. Molecular weights (kDa) are indicated on the left margin. Phl p 6, Recombinant Phl p 6.
Furthermore, mCyp c 1–specific IgG antibodies cross-reacted with natural parvalbumins from 6 commonly consumed fish species (carp, cod, mackerel, salmon, sardine, and tuna) (Fig 3, D). Only parvalbumin from swordfish did not react with mCyp c 1–induced IgG antibodies (Fig 3, D).
mCyp c 1-specific antisera block IgE binding to Cyp c 1, Cyp c 1–induced basophil degranulation, and allergic reactions in the murine model of fish allergy
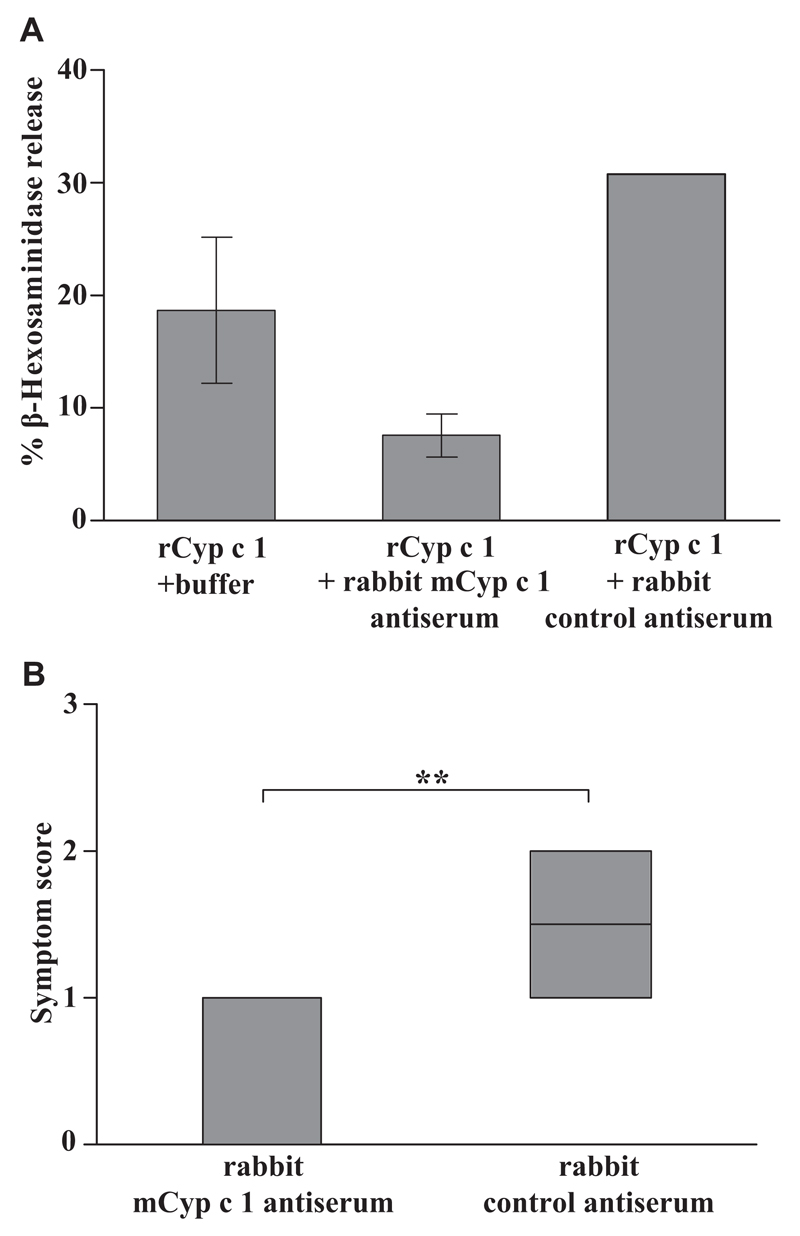
In a first ex vivo experiment, we investigated whether mCyp c 1–specific rabbit antiserum can suppress the Cyp c 1–induced degranulation of basophils loaded with IgE from rCyp c 1–sensitized mice (Fig 4, A). We found that rCyp c 1 in the presence of mCyp c 1–specific rabbit antiserum did not induce relevant basophil degranulation, whereas rCyp c 1 without mCyp c 1–specific IgG antibodies or in the presence of control antibodies induced basophil degranulation (Fig 4, A).
Fig 4.
mCyp c 1–specific rabbit antiserum inhibits rCyp c 1–induced basophil degranulation in vitro and Cyp c 1–induced allergic symptoms in vivo. A, Rat basophil leukemia cells were loaded with pooled sera from rCyp c 1–sensitized mice in the presence or absence of mCyp c 1–specific rabbit antiserum or control antiserum (x-axis). The percentage of total β-hexosaminidase release is displayed on the y-axis (triplicates ± SD). B, Significant (**P < .01) suppression of allergic symptoms in Cyp c 1–sensitized mice by mCyp c 1–specific rabbit antiserum after challenge with Cyp c 1. Allergic symptoms (y-axis: mean symptom scores ± SD) in Cyp c 1–sensitized mice having received mCyp c 1–specific or control antiserum (x-axis).
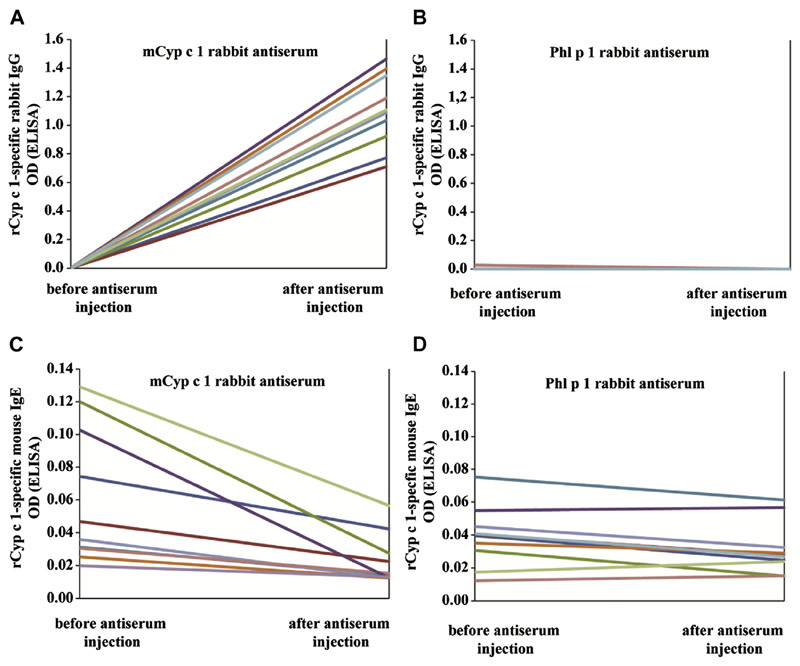
Next, we injected mCyp c 1–specific rabbit antiserum in 1 group of rCyp c 1–sensitized mice, whereas the other rCyp c 1–sensitized mouse group received rabbit antiserum specific for the unrelated grass pollen allergen Phl p 1. We then analyzed blood samples collected 1 day before and 2 days after treatment for the presence of the injected rabbit IgG antibodies. Cyp c 1–specific rabbit IgG antibodies were only detected in mice who had received mCyp c 1–specific antiserum (see Fig E1, A in this article’s Online Repository at www.jacionline.org) but not in mice who had been treated with Phl p 1–specific antiserum (see Fig E1, B). The latter group was found to contain Phl p 1–specific rabbit IgG (data not shown). The binding of mouse IgE to Cyp c 1 was reduced in mice that had been treated with anti-mCyp c 1–specific antiserum (see Fig E1, C) but not in mice that had been treated with Phl p 1–specific antiserum (see Fig E1, D) when Cyp c 1–specific IgE reactivity was compared before and after injection of rabbit antibodies.
Next, the 2 groups of Cyp c 1–sensitized mice that had received mCyp c 1–specific or Phl p 1–specific antiserum were challenged by intragastric application of 100 μg rCyp c 1. On being challenged, mice that had received the Phl p 1–specific antiserum (control group) developed allergic symptoms including scratching and rubbing around nose and mouth, reduced activity, and swelling around the eyes and mouth, whereas mice that had been treated with mCyp c 1–specific antiserum (therapy group) showed either no or only mild symptoms in response to oral allergen challenge (Fig 4, B). Thus a significant reduction of allergic symptoms was found in the group of mice that had been treated with mCyp c 1–specific antiserum when compared with the group treated with the Phl p 1–specific antiserum (Fig 4, B).
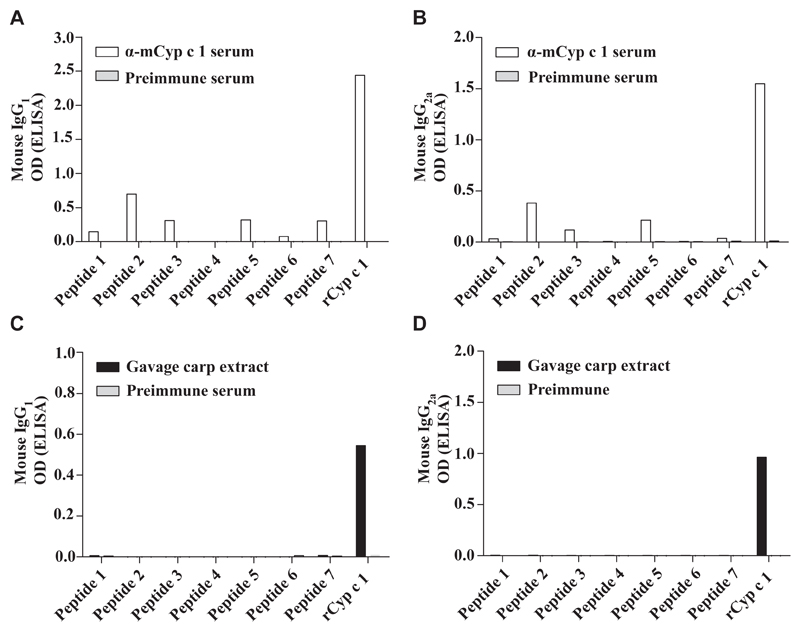
Next we established an active vaccination protocol in C3H/HeJ mice similar to subcutaneous SIT performed in patients with allergy. The vaccine was formulated by adsorption of mCyp c 1 to aluminium hydroxide and injected subcutaneously in monthly intervals. Already after 2 immunizations, high levels of Cyp c 1–specific IgG1 antibodies were induced in the vaccinated group (n = 5) but not in the control group having received an unrelated antigen (hP62) (data not shown). Epitope mapping studies revealed that vaccination with mCyp c 1 induced IgG1 and IgG2a antibodies that were specific not only for rCyp c 1 but also for linear peptides (peptides 1, 2, 3, 5, 6, 7) (Fig 5, A and B). Next, we sensitized groups of C3H/HeJ mice (n = 8) according to the established protocol. In this experiment, carp extract was used for sensitization to more closely mimic the sensitization process in humans (Fig 1, B). Allergic sensitization by intragastric gavage of carp extract induced a Cyp c 1–specific IgE as well as an endogenous Cyp c 1–specific IgG1 and IgG2a response in these mice. In contrast to the mCyp c 1–induced IgG antibodies, this IgG response was mainly directed to the folded protein (Fig 5, C and D).
Fig 5.
Epitope mapping of mouse IgG antibodies induced by gavage with carp extract and vaccination with mCyp c 1. IgG1 (A and C) and IgG2a (B and D) antibody levels (y-axes: OD values) specific for Cyp c 1–derived peptides 1 to 7 and rCyp c 1 (x-axes) were measured: A and B, after immunization with mCyp c 1 (α-mCyp c 1 serum, white bars; preimmune serum, gray bars); C and D, after sensitization (day 43) with carp extract (gavage carp extract, black bars; preimmune serum, gray bars).
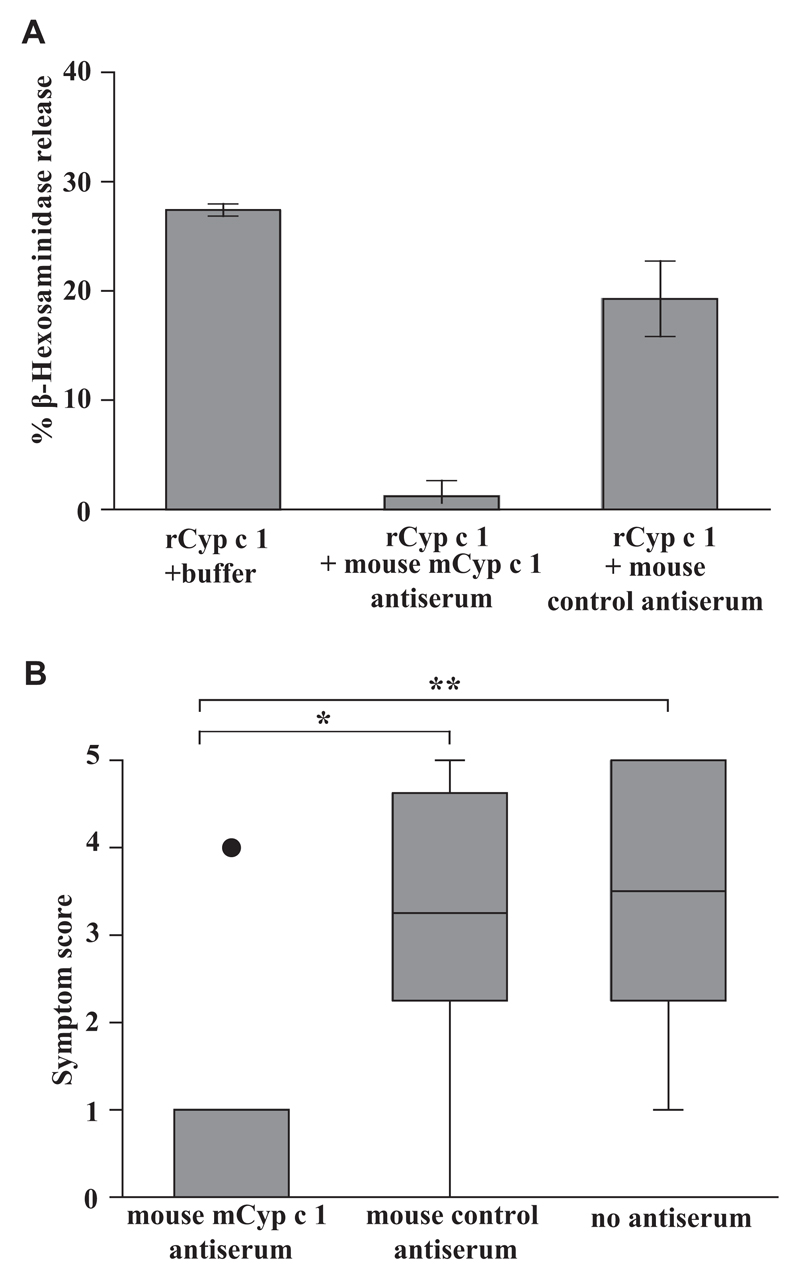
We then investigated the effect of vaccine-induced IgG antibodies on basophil activation and allergic symptoms. Mouse serum from mCyp c 1–immunized mice was able to suppress Cyp c 1–induced degranulation of RBL cells that were loaded with Cyp c 1–specific IgE up to 95.6% (Fig 6, A). Next we transferred mouse serum of mCyp c 1–vaccinated mice, or the mouse anti-hP62 control serum, or no serum to carp extract–sensitized mice. On being challenged with 10 mg carp extract, a significant reduction of allergic symptoms was again observed in the mouse group having received Cyp c 1–specific mouse antiserum compared with the control groups (Fig 6, B). Mice from both control groups that were either treated with an irrelevant antiserum or had received no treatment developed allergic symptoms of equal severity on being challenged.
Fig 6.
Suppression of Cyp c 1–induced basophil degranulation and allergic symptoms with a mCyp c 1–specific mouse antiserum. A, Rat basophil leukemia cells loaded with pooled sera from rCyp c 1–sensitized mice (day 43) were challenged with rCyp c 1 in the presence or absence of mCyp c 1–specific mouse antiserum or control hP62 (a recombinant grass pollen hypoallergen)–specific antiserum (x-axis). The percentage of total ß-hexosaminidase release is displayed on the y-axis (triplicates ± SD). B, Allergic symptoms (y-axis: mean symptom scores ± SD) of carp extract-sensitized mice treated with either mouse α-mCyp c 1 serum or mouse α-hP62 serum or of untreated mice (x-axis) after challenge with carp extract (*P < .05; **P < .01).
Discussion
Since the original demonstration by Prausnitz and Kuestner24 that allergic sensitization to fish can be transferred passively with serum, treatment of fish allergy and other life-threatening food allergies represents a major challenge.25,26 Due to the high allergenic activity of fish, which can induce severe allergic reactions, SIT is not available as a treatment option for patients who are allergic to fish. To establish an in vivo model for fish allergy that closely mimics fish allergy in patients and hence would allow studying approaches for SIT of fish allergy, we sensitized mice by the oral route using the recombinant major fish allergen, rCyp c 1, and carp extract. Mice sensitized by oral administration of rCyp c 1 or carp extract developed an IgE antibody response similar to the human disease. The IgE response of carp extract–sensitized mice was mainly directed to the single major allergen Cyp c 1 (data not shown). In addition, IgE antibodies mainly recognized conformational epitopes of Cyp c 1 similar to those of patients who are allergic to fish. We attribute the IgE recognition of mainly conformational epitopes on Cyp c 1 to the extremely high stability of the protein. The Cyp c 1 mutant, which is unable to bind calcium and shows an altered secondary structure, induced an IgE and IgG antibody response also to linear epitopes when it was adsorbed to aluminium hydroxide and injected via the subcutaneous route.13 Importantly, IgE antibodies induced by rCyp c 1 and carp extract also induced basophil activation and allergic reactions when sensitized mice were orally challenged. Epitope mapping studies performed with overlapping peptides of Cyp c 1 identified peptide 5 comprising amino acids 61 to 80 in the Cyp c 1 sequence as a major T-cell epitope that may be useful information for approaches studying T-cell epitope-targeting strategies in the murine model. Besides cellular mechanisms, the induction of allergen-specific blocking IgG antibodies has been identified as a major mechanism of SIT for other food allergen sources such as peanut allergy and egg allergy,9,10 so we decided to evaluate the therapeutic potential of mCyp c 1, a recombinant hypoallergenic variant of Cyp c 1 that is currently evaluated as a candidate for subcutaneous immunotherapy of fish allergy in the European Union–funded FAST (Food Allergy Specific Immunotherapy) project.7 Within the FAST project, mCyp c 1 has been formulated for subcutaneous injection immunotherapy because it has been shown that immunization of mice and rabbits with mCyp c 1 induces a robust induction of IgG antibodies that recognize the wildtype allergen. In fact, we found that IgG antibodies induced by immunization of rabbits with mCyp c 1 not only recognized the Cyp c 1 wildtype allergen, but they also inhibited IgE antibodies to Cyp c 1 in patients with allergy. Moreover, mCyp c 1–induced rabbit antibodies cross-reacted with parvalbumins from several fish species and inhibited the degranulation of basophils that were loaded with IgE antibodies from Cyp c 1-sensitized mice.
We were therefore interested to study whether mCyp c 1–specific antibodies can also suppress symptoms of fish allergy in vivo using the fish allergy model. For this purpose, we followed the classical experiments performed by Dunbar27 that basically inspired Noon28 to conduct the first SIT study for the treatment of grass pollen allergy. In fact, Dunbar27 had already shown that rabbit antisera raised against natural allergen preparations were able to suppress allergic inflammation in patients using conjunctival provocation testing and a similar experiment was then also performed with sera from immunotherapy-treated patients’ sera by Cooke et al.29
We therefore treated sensitized mice with mCyp c 1–specific rabbit antiserum containing Cyp c 1–specific IgG antibodies. We could show their presence in the circulation of mice and that they inhibited the binding of IgE from sensitized mice to Cyp c 1 by ELISA. More importantly, we found that mice that had received mCyp c 1–specific antisera were protected against allergic reactions induced by oral allergen challenge. Data from a mouse model of respiratory allergy suggest a duration of the blocking activity of passively applied allergen-specific rabbit antisera of up to 3 weeks after administration.30 For patients who are allergic to fish even longer effects may be expected due to the fact that human IgG antibodies have longer half-lives than mouse IgG antibodies do. The same result was obtained using sera from mice that had received immunotherapy by subcutaneous injection of aluminium hydroxide–adsorbed mCyp c 1 by 6 monthly injections as it is also performed in patients under maintenance immunotherapy treatment. It cannot be excluded that the IgG antibodies induced by sensitization to carp extract show some protective effect, but it seems to be incomplete such as in patients with allergy who usually also produce allergen-specific IgG antibodies but nevertheless have symptoms. We assume that the protective effect of the IgG response induced by mCyp c 1 is due to its polyclonal nature recognizing more epitopes on Cyp c 1 and perhaps due to higher avidity. We can also exclude a potential protective effect of other serum components than allergen-specific IgG antibodies in our passive transfer experiments, as control groups having received either a mouse antiserum specific for an irrelevant antigen or no treatment were both unprotected against allergic symptoms.
The strength of this experimental design of using the transfer of subcutaneous allergen-specific immunotherapy–induced antisera is that the protective effect can be directly attributed to mCyp c 1–specific antibodies. On the other hand, the weakness of this approach is that it does not allow for studying the role of complex regulatory mechanisms such as the influence of certain antigen presenting cells, the generation of regulatory T-cell populations, and/or cytokine effects. The latter might be analyzed by the transfer of cells or by direct vaccination of mice who are allergic to fish with mCyp c 1. However, we observed that in the murine model, immunization with mCyp c 1 also induced Cyp c 1–specific IgE antibody responses that superimpose the transient IgE response induced by oral gavage, making an unambiguous analysis in an active vaccination model in the mouse impossible. Nevertheless, we think that the obtained results are important because they indicate that antibodies induced by immunization with mCyp c 1 can suppress allergic reactions caused by the major fish allergen and hence may represent an important mechanism of SIT with mCyp c 1. Because the allergenic activity of mCyp c 1 has been reduced compared with the wildtype allergen, it may be possible to vaccinate patients who are allergic to fish with fewer and higher doses to induce protective IgG antibodies directly by active vaccination. Ultimately, it may be possible to establish safe and convenient subcutaneous SIT protocols for the treatment of fish allergy that are based on mCyp c 1.
Extended Data
Fig E1.
Reduced allergen-specific IgE reactivity of Cyp c 1–sensitized mice that had been treated with mCyp c 1–specific rabbit antiserum. A and B, Presence of Cyp c 1–specific rabbit IgG in mice before and after injection of mCyp c 1–specific antiserum or Phl p 1–specific antiserum. C and D, Cyp c 1–specific IgE reactivity of mice before and after injection of mCyp c 1–specific antiserum or Phl p 1–specific antiserum. Antibody reactivities are shown for each mouse (y-axes: OD values) before and after antiserum injection (y-axes).
Clinical implications.
IgG antibodies induced by vaccination with a hypoallergenic Cyp c 1 mutant may protect against fish allergy.
Acknowledgments
Supported by the FAST (Food Allergy Specific Immunotherapy) project 201871 of the Seventh Framework Program for Research of the European Union and by the Austrian Science Fund, Projects P23350-B11 and F4605.
Abbreviations used
- ELISA
Enzyme-linked immunosorbent assay
- hP62
Recombinant grass pollen hypoallergen
- HRP
Horseradish peroxidase
- i.p.
Intraperitoneal
- mCyp c 1
Recombinant Cyp c 1 mutant
- OD
Optical density
- RBL
Rat basophil leukemia
- rCyp c 1
Recombinant wildtype Cyp c 1
- rPhl p 1
Recombinant Phl p 1
- SIT
Allergen-specific immunotherapy
Footnotes
Disclosure of potential conflict of interest: A. Gstoettner has received travel support from FAST Project of the European Union and is employed by Sandoz. R. van Ree’s institution has received a grant from the European Commission. R. Valenta has received research support from Biomay AG, Vienna, Austria, Thermofisher, Uppsala, Sweden, and Fresenius Medical Care, Bad Homburg, Germany, and serves as consultant for these companies. B. Linhart has received research support from the FAST Project 201871 of the European Union and the Austrian Science Fund. The rest of the authors declare they have no relevant conflicts of interest.
References
- 1.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 2.Burney PG, Potts J, Kummeling I, Mills EN, Clausen M, Dubakiene R, et al. The prevalence and distribution of food sensitization in European adults. Allergy. 2014;69:365–71. doi: 10.1111/all.12341. [DOI] [PubMed] [Google Scholar]
- 3.Chiang WC, Kidon MI, Liew WK, Goh A, Tang JP, Chay OM. The changing face of food hypersensitivity in an Asian community. Clin Exp Allergy. 2007;37:1055–61. doi: 10.1111/j.1365-2222.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharp MF, Lopata AL. Fish allergy: in review. Clin Rev Allergy Immunol. 2014;46:258–71. doi: 10.1007/s12016-013-8363-1. [DOI] [PubMed] [Google Scholar]
- 5.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 6.Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133:318–23. doi: 10.1016/j.jaci.2013.12.1040. [DOI] [PubMed] [Google Scholar]
- 7.Zuidmeer-Jongejan L, Fernandez-Rivas M, Poulsen LK, Neubauer A, Asturias J, Blom L, et al. FAST: towards safe and effective subcutaneous immunotherapy of persistent life-threatening food allergies. Clin Transl Allergy. 2012;2:5. doi: 10.1186/2045-7022-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato S, Yanagida N, Ogura K, Imai T, Utsunomiya T, Iikura K, et al. Clinical studies in oral allergen-specific immunotherapy: differences among allergens. Int Arch Allergy Immunol. 2014;164:1–9. doi: 10.1159/000361025. [DOI] [PubMed] [Google Scholar]
- 9.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, for the Consortium of Food Allergy Research et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–43. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131:128–34, e1-3. doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swoboda I, Bugajska-Schretter A, Verdino P, Keller W, Sperr WR, Valent P, et al. Recombinant carp parvalbumin, the major cross-reactive fish allergen: a tool for diagnosis and therapy of fish allergy. J Immunol. 2002;168:4576–84. doi: 10.4049/jimmunol.168.9.4576. [DOI] [PubMed] [Google Scholar]
- 12.Bugajska-Schretter A, Grote M, Vangelista L, Valent P, Sperr WR, Rumpold H, et al. Purification, biochemical, and immunological characterisation of a major food allergen: different immunoglobulin E recognition of the apo- and calcium-bound forms of carp parvalbumin. Gut. 2000;46:661–9. doi: 10.1136/gut.46.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swoboda I, Bugajska-Schretter A, Linhart B, Verdino P, Keller W, Schulmeister U, et al. A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J Immunol. 2007;178:6290–6. doi: 10.4049/jimmunol.178.10.6290. [DOI] [PubMed] [Google Scholar]
- 14.Swoboda I, Balic N, Klug C, Focke M, Weber M, Spitzauer S, et al. A general strategy for the generation of hypoallergenic molecules for the immunotherapy of fish allergy. J Allergy Clin Immunol. 2013;132:979–81.e1. doi: 10.1016/j.jaci.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Linhart B, Mothes-Luksch N, Vrtala S, Kneidinger M, Valent P, Valenta R. A hypoallergenic hybrid molecule with increased immunogenicity consisting of derivatives of the major grass pollen allergens, Phl p 2 and Phl p 6. Biol Chem. 2008;389:925–33. doi: 10.1515/BC.2008.105. [DOI] [PubMed] [Google Scholar]
- 16.Linhart B, Narayanan M, Focke-Tejkl M, Wrba F, Vrtala S, Valenta R. Prophylactic and therapeutic vaccination with carrier-bound Bet v 1 peptides lacking allergen-specific T cell epitopes reduces Bet v 1-specific T cell responses via blocking antibodies in a murine model for birch pollen allergy. Clin Exp Allergy. 2014;44:278–87. doi: 10.1111/cea.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douladiris N, Linhart B, Swoboda I, Gstoettner A, Vassilopoulou E, Stolz F, et al. In vivo allergenic activity of a hypoallergenic mutant of the major fish allergen Cyp c 1 evaluated by means of skin testing. J Allergy Clin Immunol. 2015;136:493–5. doi: 10.1016/j.jaci.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, et al. Profilins constitute a novel family of functional plant pan-allergens. J Exp Med. 1992;175:377–85. doi: 10.1084/jem.175.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 22.Linhart B, Bigenzahn S, Hartl A, Lupinek C, Thalhamer J, Valenta R, et al. Costimulation blockade inhibits allergic sensitization but does not affect established allergy in a murine model of grass pollen allergy. J Immunol. 2007;178:3924–31. doi: 10.4049/jimmunol.178.6.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morafo V, Srivastva K, Huang CK, Kleiner G, Lee SY, Sampson HA, et al. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and Balb/c mice. J Allergy Clin Immunol. 2003;111:1122–8. doi: 10.1067/mai.2003.1463. [DOI] [PubMed] [Google Scholar]
- 24.Prausnitz C, Küstner H. Studien über die Überempfindlichkeit. Zentralbl Bakteriol. 1921;86:160–9. [Google Scholar]
- 25.Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. J Allergy Clin Immunol. 2012;129:906–20. doi: 10.1016/j.jaci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90:256–62. doi: 10.1016/0091-6749(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 27.Dunbar WP. Zur Ursache und spez. Heilung des Heufiebers. Oldenbourg; Munich: 1903. [Google Scholar]
- 28.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–3. [Google Scholar]
- 29.Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy (hay fever) J Exp Med. 1935;62:733–50. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flicker S, Linhart B, Wild C, Wiedermann U, Valenta R. Passive immunization with allergen-specific IgG antibodies for treatment and prevention of allergy. Immunobiology. 2013;218:884–91. doi: 10.1016/j.imbio.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]