Abstract
Early work in pressure overloaded (PO) myocardium shows that integrins mediate focal adhesion complex formation by recruiting the adaptor protein p130Cas (Cas) and nonreceptor tyrosine kinase c-Src. To explore c-Src role in Cas-associated changes during PO, we used a feline right ventricular in vivo PO model and a three-dimensional (3D) collagen-embedded adult cardiomyocyte in vitro model that utilizes a Gly-Arg-Gly-Asp-Ser (RGD) peptide for integrin stimulation. Cas showed slow electrophoretic mobility (band-shifting), recruitment to the cytoskeleton, and tyrosine phosphorylation at 165, 249, and 410 sites in both 48 h PO myocardium and 1 h RGD-stimulated cardiomyocytes. Adenoviral mediated expression of kinase inactive (negative) c-Src mutant with intact scaffold domains (KN-Src) in cardiomyocytes did not block the RGD stimulated changes in Cas. Furthermore, expression of KN-Src or kinase active c-Src mutant with intact scaffold function (A-Src) in two-dimensionally (2D) cultured cardiomyocytes was sufficient to cause Cas band-shifting, although tyrosine phosphorylation required A-Src. These data indicate that c-Src’s adaptor function, but not its kinase function, is required for a serine/threonine specific phosphorylation(s) responsible for Cas band-shifting. To explore this possibility, Chinese hamster ovary cells that stably express Cas were infected with either β-gal or KN-Src adenoviruses and used for Cas immunoprecipitation combined with mass spectrometry analysis. In the KN-Src expressing cells, Cas showed phosphorylation at the serine-639 (human numbering) site. A polyclonal antibody raised against phospho-serine-639 detected Cas phosphorylation in 24–48 h PO myocardium. Our studies indicate that c-Src’s adaptor function mediates serine-639 phosphorylation of Cas during integrin activation in PO myocardium.
Keywords: HYPERTROPHY, INTEGRINS, Cas, c-Src, FOCAL ADHESIONS
P130Cas or Cas (Crk-associated substrate, also known as breast cancer anti-estrogen resistance-1 [BCAR1]) is a focal adhesion adapter protein that facilitates adhesion-related signaling [Nikonova et al., 2014]. Focal adhesion complexes (FAC) are specialized cell-matrix contact sites where integrins link extracellular matrix and the actin CSK. As part of the cardiomyocyte costamere, FAC contributes to transducing mechanical load signal into biochemical signal overload that contributes to myocardial remodeling during pressure overload (PO) [Samarel, 2014]. Therefore, in addition to their adhesive roles, specific integrin subtypes serve as mechanosensors by forming the FAC consisting of several signaling molecules such as nonreceptor tyrosine kinases (NTKs) and adaptor proteins [Janostiak et al., 2014; Samarel, 2014]. Our earlier work demonstrates FAC formation with the recruitment of several signaling molecules including NTKs (c-Src, Fak, and Bmx) and adaptor proteins (Cas, Nck, and Shc) to the actin-rich CSK fractions in 24–48 h PO myocardium [Kuppuswamy et al., 1997; Laser et al., 2000; Willey et al., 2003; Willey et al., 2008]. Under these conditions, many of the CSK-bound proteins were also tyrosine phosphorylated, indicating the activation of associated tyrosine kinases and the enhancement of the adaptor’s function. The observation during 48 h RVPO was partially mimicked in a cell culture model where isolated cardiomyocytes were plated three dimensionally (3D) in type I collagen and stimulated with Arg-Gly-Asp (RGD) peptide [Laser et al., 2000; Willey et al., 2003].
Cas is a key molecule involved in the assembly of signaling proteins at the focal adhesion during integrin activation. Although Cas has no catalytic activity, it has several distinct domains that enable it to serve as an adaptor molecule: the SH3 domain in the N-terminus of Cas for binding of protein partners with proline-rich sequence, several tyrosine residues in the substrate domain whose phosphorylation by NTKs can serve as docking sites for proteins with SH2 domains, a dedicated tyrosine site in the C-terminus whose phosphorylation serves as a c-Src binding site, several proline rich motifs in the C-terminus for the binding of proteins with their SH3 domains [O’Neill et al., 2000]. Whereas several studies show tyrosine phosphorylation by NTKs at critical Cas sites during integrin activation, only a few serine site-specific phosphorylations of Cas have been reported [Briknarova et al., 2005; Makkinje et al., 2009]. For example, Cas and Fak have been shown to undergo serine phosphorylation during mitosis which was observed with the dissociation of Fak/Cas/c-Src complex [Yamashiro et al., 1995]. Similarly, cells overexpressing BCAR3 (breast cancer anti-estrogen resistance-3) exhibit Cas phosphorylation at serine residues 139, 437, and 639 that alters intracellular localization of Cas. Importantly, Cas serine phosphorylation was demonstrated in an integrin-dependent manner in fibroblasts when they freshly adhere to fibronectin and the phosphorylated serine residue in Cas has been shown to serve as a docking site for 14-3-3 adaptor proteins [Schlaepfer et al., 1997; Garcia-Guzman et al., 1999; Pozuelo Rubio et al., 2004]. Since Cas and NTKs are the major components of the FAC in PO myocardium [Laser et al., 2000; Willey et al., 2008], we explored whether Cas undergoes phosphorylation during PO and whether NTKs, in particular c-Src, contribute to serine phosphorylation of Cas. Our studies demonstrate that Cas undergoes serine-639 phosphorylation during an early (24–48 h) period of PO and that this phosphorylation requires the adaptor function of c-Src.
METHODS
ANIMAL MODELS
Feline right ventricular pressure-overload (RVPO) models have been described previously [Cooper et al., 1973; Rozich et al., 1995]. Briefly, adult 12 months old male intact cats weighing 2.8–3.5 kg were subjected to partial occlusion of the pulmonary artery by external suture banding. The pulmonary arterial pressure was at least doubled while systemic pressure remained constant. Thus, the normally loaded left ventricle (LV) served as an internal control for the pressure-overloaded right ventricle (RV) from the same animal. For additional controls, cats were sham operated by thoracotomy and pericardiotomy but did not have arterial occlusion. The care of the animals and all experiments were conducted in accordance with the US National Institutes of Health guidelines for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee of the Medical University of South Carolina (IACUC).
ADULT CARDIOMYOCYTE CELL CULTURE
Adult ventricular feline cardiomyocytes were isolated using enzymatic digestion via a hanging heart preparation and cultured by the protocols described previously [Kent et al., 1989]. Freshly isolated cardiomyocytes were plated on laminin-coated tissue culture plates at a density of 7.5 × 104 cells/ml media. After allowing 4 h for attachment, media was changed with serum-free Medium 199 (Gibco-BRL, Grand Island, NY) containing 200 units/ml penicillin and 200 μg/ml streptomycin (Gibco-BRL).
We have shown in our earlier studies that the focal adhesion complex formation, which was observed in 24–48 h PO myocardium in vivo and accompanied with the secretion of ECM proteins (such as fibronectin and vitronectin), can be mimicked in vitro in isolated adult cardiomyocytes by three dimensionally embedding cells with collagen type-I and stimulating with 9 mM synthetic RGD motif (GRGDS) peptides for 1 h [Laser et al., 2000; Willey et al., 2003]. Therefore, for the present work, we adopted the same protocol to embed freshly isolated cardiomyocytes three-dimensionally in type I collagen in the presence or absence of 9 mM RGD peptide for 1 h. For two-dimensional (2D) studies, adult cardiomyocytes cultured on laminin-coated plates were directly stimulated with 9 mM RGD peptide for 1 h [Laser et al., 2000; Willey et al., 2003].
GENERATION OF C-SRC ADENOVIRAL CONSTRUCTS
Adenoviral constructs were generated using He’s pAdEasy-1 system [He et al., 1998] and as described previously [Willey et al., 2003]. Briefly, dominant negative c-Src cDNA (K295R, Y527F) and kinase active c-Src cDNA (Y527F) were obtained from Upstate Biotechnology as a cloning vector and subcloned into the cloning vector, pSP72 (Gibco-BRL). Restriction fragments were gel purified (Qiagen, Valencia, CA) and ligated into the adenoviral shuttle vector, pAdTrack-CMV. Positive clones obtained on kanamycin were selected and screened by PCR using insert specific primers followed by propagation and DNA sequencing. The pAdTrack-CMV shuttle vectors were linearized using PmeI (New England Biolabs, Ipswich, MA), gel purified, and recombined with the adenoviral genome plasmid with ampicillin resistance, pAdEasy-1, in competent BJ5183 cells and plated on kanamycin plates. Positive clones were selected by an initial PCR screen for the insert and propagated for restriction enzyme analysis. Selected clones were transformed into DH5a cells, maxiprepped, and linearized with PacI. The adenoviral plasmids were transfected into HEK293 cells for packaging of the replication-defective adenovirus. After two plaque purifications, the virus titers were calculated.
PREPARATION OF TISSUE AND CELL LYSATES
Triton-X-100 soluble and insoluble fractions from fresh tissue samples were prepared from ventricular tissue samples as described before [Kuppuswamy et al., 1997; Willey et al., 2008]. Briefly, 100 mg of ventricular tissue sample was homogenized in 2 ml ice-cold Triton-X-100 extraction buffer (100 mM Tris-HCl, pH 7.4, 10 mM EGTA, 2% Triton X-100) and protease and phosphatase inhibitor cocktails (protease inhibitor cocktail I and phosphatase inhibitor cocktail II, Sigma-Aldrich, St. Louis, MO) were added at the time of lysate preparation. After initial homogenization and centrifugation at 15,000g, the supernatant was preserved for a subsequent high-speed spin. The pellet (insoluble material) was reextracted with extraction buffer to remove any remaining detergent-soluble proteins, pelleted, re-suspended in 0.5 ml of 1X SDS sample buffer and boiled to obtain CSK fractions. The supernatant from the previous centrifugation step was spun again at a higher speed (100,000 g) for 2.5 h at 4°C. The pellet was solubilized in 1 ml of 1X SDS-sample buffer to obtain membrane skeleton proteins whereas the supernatant from the high-speed spin was mixed with an equal volume of 2X SDS sample buffer to obtain a soluble fraction.
To prepare cardiomyocyte lysates, cultured cardiomyocytes were washed with ice cold PBS and the soluble (Sol) and CSK fractions were prepared as described before (19). Briefly, the cells were scraped in Triton X-100 extraction buffer (30 mM Tris-HCl, pH 7.4, 2% Triton X-100 containing protease and phosphatase inhibitors) and centrifuged at 15,000g to obtain supernatant and pellet, representing detergent soluble and insoluble CSK fractions, respectively. The soluble fraction was mixed with an equal volume of 2X SDS sample buffer and boiled. The insoluble pellet was boiled in SDS sample buffer to obtain the CSK fraction. In the case of 3D cultured cardiomyocytes, cells were recovered from collagen gels by collagenase treatment, soluble and insoluble samples were prepared as described previously [Laser et al., 2000; Willey et al., 2003; Willey et al., 2008].
CHINESE HAMSTER OVARY (CHO) STABLE CELL LINE CULTURE AND PREPARATION OF CELL LYSATES
Chinese Hamster Ovary/CHO-K1 cells were grown in Ham’s F12K medium (Gibco-BRL) containing 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 10% fetal bovine serum, 50 units/ml penicillin and 50 units/ml streptomycin. Cells were passaged two days prior to the start of the experiments. Cells were incubated at 37°C with 5% CO2. Stable cell lines were created by transfecting CHO cells with rat Cas cDNA in pcDNA6V5/His vector harboring blasticidin resistance gene. For transfections, Lipofectamine 2000 (Invitrogen, Grand Island, NY) was used per manufacturer’s instructions. Stable transformants had ~20-fold increases in Cas levels. When needed, cells were infected with 50 moi (multiplicity of infection) of adenoviral constructs for the expression of KN-Src.
After appropriate treatments, cultured cells were isolated with 2% Triton X-100 buffer with protease and phosphatase inhibitors, scraped and syringed three times with 1 ml 26 G3/8 needle to disperse cells (BD Biosciences, San Jose, CA). Then the samples were centrifuged at 4°C for 10 min. The resulting supernatant was mixed with 2X Laemmlli’s SDS buffer for Western blot or used for immunoprecipitation.
IMMUNOPRECIPITATION OF CAS FROM CHO CELL LYSATES FOR MASS SPECTROPHOTOMETRY
CHO cell supernatant extracted from ten 150 mm culture plates for each condition was precleared with agarose-beads for 30 min at 4°C. The solution was centrifuged at 800g for 10 min. The resulting supernatant (~15 mg protein) was rotated with 20 μg of anti-Cas antibody coupled with protein A beads at 4°C overnight. The beads were then washed with the Triton X-100 buffer three times, boiled with 1X SDS sample buffer and part of the samples were used for gel electrophoresis and Western blotted as described earlier. For mass spectrometry, parallel gels were run and stained with Coomassie blue and the band corresponding to the size of Cas was excised for further processing as described previously [Chinnakkannu et al., 2010].
GENERATION OF PHOSPHOSERINE-639 CAS POLYCLONAL ANTIBODY
Custom polyclonal antibody using a synthetic peptide Cys-KASSIQSRPLPS(p)PPKFT that corresponds to rat/mouse serine-643 (or human serine-639) phosphorylated Cas was generated and purified using phospho (immunogen)- and nonphospho- Cas peptides by Antagene Inc, Sunnyvale, CA. The antibody was further characterized in our lab using the phosphorylated Cas peptide (immunogen). Neutralization of purified antibody with the Cas phospho-peptide resulted in a complete loss of detection of phosphorylated Cas (data not shown).
WESTERN BLOTTING
Protein samples (~20 μg) prepared in SDS sample buffer were used for SDS–PAGE (polyacrylamide gel electrophoresis) and transferred to Immobilon-P membranes (Millipore, Billerica, MA). After blocking the membrane for 1 h using 5% milk in TBST (10 mM Tris, 0.1 M NaCl, 0.1% Tween 20, pH 7.4), blots were incubated overnight at 4°C with primary antibodies (1:1000 dilution in TBST). The following primary antibodies were obtained from commercial sources: polyclonal antibodies for phospho-Cas165, phospho-Cas249, and phospho-Cas410 (All obtained from Santa Cruz Biotechnology, Dallas, TX); monoclonal phosphotyrosine Src 416 and monoclonal nonphospho Src 416 antibodies (Cell Signaling Technology, Danvers, MA); monoclonal antibody for c-Src (Upstate Biotechnology Inc., Lake Placid, NY); polyclonal anti-actin antibody (Sigma-Aldrich); polyclonal antibody for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Research Diagnostics Inc., Flanders, NJ). After incubation with the primary antibodies, blots were washed five times for 5 min in 1x TBST and incubated in appropriate secondary antibodies (1:10,000 dilution in TBST) for 1 h. After incubation, blots were washed for five times, 5 min each in 1x TBST and proteins were detected by enhanced chemiluminescence (Renaissance Bioscience, Vancouver, BC, Canada). To normalize protein concentration, GAPDH (Triton-soluble fraction) and actin (Triton-insoluble fractions) levels were measured by Western blot.
For Western blot quantitation, scanned images were processed using Adobe Photoshop (Version 10.0.1) and saved as JPEG grey image files. Files were opened using NIH-ImageJ and the Grey Mean Values for the Cas band and the selected background areas were measured. The pixel densities were inverted by subtracting the values from 255. The inverted values were corrected for background values and then used for the statistical evaluation.
STATISTICS
Values were presented as mean ± SEM. Differences were analyzed between groups using one-way analysis of variance (ANOVA) followed by a Bonferroni t-test to determine the statistical significance of the respective experiments. A value of P < 0.05 was considered significant.
MASS SPECTROMETRIC ANALYSIS
The mass spectrometry analyses for the detection of phosphorylated Cas were performed using a previously applied protocol [Chinnakkannu et al., 2010]. The gel plugs corresponding to Cas from the immunoprecipitation experiments were pooled, washed with 50 mM NH4CO3 for 10 min, and de-stained twice using 25 mM NH4CO3 in 50% acetonitrile for 15 min. The plugs were then dehydrated with 100% acetonitrile for 15 min. After drying them in a SpeedVac, the plugs were digested overnight with proteomics-grade trypsin (Sigma-Aldrich) at 37°C overnight. After transferring the supernatant to a clean Eppendorf tube, any residual peptides were reextracted from the plugs with 25 mM NH4CO3 for 20 min and followed with three washes of 5% formic acid and 50% acetonitrile for 20 min each. The supernatants were pooled and dried in a SpeedVac and resolubilized in 0.2% formic acid and separated by nanoflow reversed-phase capillary HPLC (LC Packings). The column effluent was used for the MUSC mass spectrometry analysis by directing the sample into the nanospray source of a Finnigan LTQ XL linear ion-trap mass spectrometry (ThermoFisher Scientific, Waltham, MA). The collected data were analyzed with the aid of the TurboSequest unit of the Bioworks 3.3 software (ThermoFisher Scientific) as before [Chinnakkannu et al., 2010]. The mass spectrometry data showed 22% coverage of Cas with the detection of 35 trypsin digested Cas peptides.
RESULTS
CAS IS RECRUITED TO THE CSK AND TYROSINE PHOSPHORYLATED IN PO MYOCARDIUM AND IN RGD-STIMULATED ADULT CARDIOMYOCYTES
To explore Cas-associated changes in PO myocardium, we analyzed whether Cas undergoes CSK recruitment and phosphorylation at different time intervals of RVPO, and whether these processes are mediated through integrins. Our earlier work shows integrin activation and CSK recruitment of Cas and NTKs in 48 h PO myocardium that returned to baseline (control) levels by 1 wk [Laser et al., 2000]. Therefore, ventricular tissue samples from both sham-operated control and pressure overloaded felines (48 h and 1 wk) were analyzed after preparing Triton X-100 soluble and insoluble fractions (Fig. 1A). The blots were quantitated and the RV/LV ratios of corresponding fractions were calculated for statistical evaluation (Fig. 1A). In control, Cas is predominantly present in the soluble fraction, and there were no observable differences between LV and RV (RV/LV ratio is close to 1). Similarly, at 48 h RVPO samples, there was no significant difference in Cas levels in the soluble fractions of the pressure overloaded RV when compared to normally loaded LV samples (Fig. 1A). However, a significant amount of Cas was recruited to the CSK (low-spin insoluble fraction) of 48 PO RV samples. Furthermore, both in the soluble and CSK fractions of 48 h PO, Cas exhibited retarded electrophoretic mobility in SDS–PAGE (band-shifting) indicating post-translational modifications,suchas phosphorylation. When the RVPO was extended for 1 wk, most of the Cas-associated changes returned to basal conditions, although Cas level in the RV sample of soluble fractions remained significantly elevated (Fig. 1A).
Fig. 1.
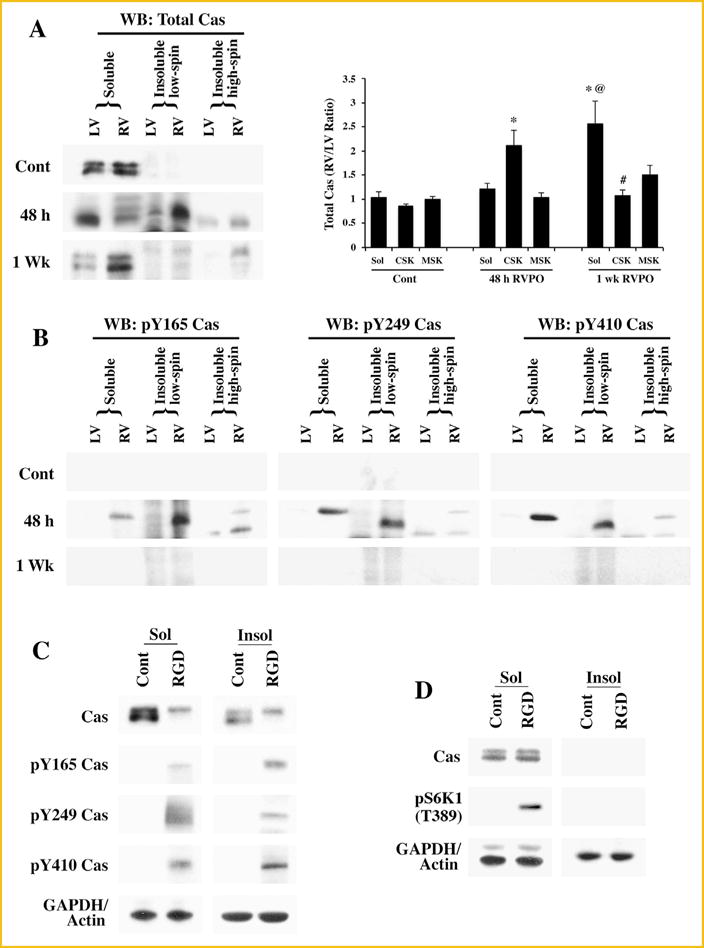
Subcellular localization and tyrosine phosphorylation of Cas: (A) Adult felines underwent pulmonary artery banding to produce RVPO and were sacrificed after the indicated time points. In addition to Sham operated animals that served as controls (Contl; LV or RV), the left ventricles (LV) in each experimental animal also served as internal normally loaded controls. The LV and RV samples from Sham control and PO samples for 48 h and 1 wk were processed to obtain Triton X-100 soluble (Sol) and insoluble (low spin and high spin) protein samples. Prior to the Western blot analyses on Cas, protein concentrations between LV and RV pairs for the soluble, insoluble low spin pellet (cytoskeleton) and the insoluble high spin pellet (membrane skeleton) were adjusted based on the GAPDH levels for the soluble samples and actin levels for the insoluble samples. The figure shows Western blot analyses for total Cas (left panel). For quantitation (right panel), Sham controls (n = 4), 48 h (n = 5) and 1 wk (n = 4) RVPO feline groups were scanned and RV to LV ratios for each fraction were determined and plotted. Values are the mean ± SEM. *P < 0.05 compared to control CSK; @P < 0.05 compared to Cont Sol or 48 h RVPO Sol; #P< 0.05 compared to 48 h CSK. (B) Triton X-100 soluble and insoluble fractions were used for Western blot with specific antibodies to detect tyrosine phosphorylated Cas at the Y165, Y249, and Y410 sites. Results were confirmed in at least two additional independent experiments (n = 3). (C) Adult cardiomyocytes were embedded within a 3D collagen matrix (3D) in the presence or absence of 9 mM RGD peptide for 1 h. After RGD treatment, cells were harvested for subcellular fractionation (Triton X-100 soluble and insoluble) and the protein samples were used for Western blot analyses of total and tyrosine phosphorylated Cas with specific antibodies as described in (A and B). Results were confirmed in two other independent experiments (n = 3). (D) Adult cardiomyocytes were cultured 2D on laminin-coated plates for 24 h and then stimulated with ± 9 mM RGD peptide for 1 h. Cells were harvested for subcellular fractionation (Triton X-100 soluble and insoluble) and the protein samples were used for Western blot analyses of total and tyrosine phosphorylated Cas with specific antibodies as described in (A and B). Results were confirmed in two other independent experiments (n = 3).
As Cas exhibited band-shifting in the soluble and CSK fractions of 48 h PO RV, we next analyzed whether these changes were accompanied with Cas phosphorylation. The band-shifting pattern of Cas in CSK and soluble fractions were not identical in 48 h PO myocardium, indicating additional modifications of Cas in these fractions during PO. Western blot using phospho-specific antibodies showed that Cas was phosphorylated at Y165, Y249, and Y410 at 48 h PO (Fig. 1B). However, at 1 wk of RVPO, Cas phosphorylation at these tyrosine sites was nearly absent, even though Cas level was more in the RV soluble fractions. These data are consistent with our earlier studies showing FAC formation and activation at 48 h PO myocardium [Laser et al., 2000].
Next, we performed in vitro experiments to demonstrate that Cas associated changes in PO myocardium can be reproduced during integrin activation. We have previously demonstrated the presence of Cas in the insoluble fractions during RGD stimulation of cardiomyocytes in a 3D cell culture model [Laser et al., 2000; Willey et al., 2003]. Since phosphorylation of Cas is associated with its enhanced adaptor function, we analyzed the phosphorylation status of Cas. Adult feline cardiomyocytes were embedded in type I collagen matrix in the presence or absence of 9 mM RGD peptide for 1 h at 37°C. In this 3D model, subfractionation of the Triton X-100 lysed homogenate by high speed centrifugation does not yield any membrane skeletal (MSK) pellet, unlike the ventricular tissue samples that produced a pellet corresponding to MSK. Therefore, many of the MSK proteins in the 3D culture system were found to sediment with the CSK fraction during low-speed centrifugation itself. Soluble and insoluble fractions were analyzed for Cas band-shifting and phosphorylation at specific sites (Fig. 1C). Similar to the findings in 48 h RVPO animals, Cas exhibited a band-shifting in the RGD treated soluble fractions. In addition, Cas was recruited to the insoluble fraction where it displays a similar band-shifting pattern. Analysis of Cas phosphorylation using phospho-specific antibodies showed phosphorylation at Y165, Y249, and Y410 similar to the observation in 48 h RVPO.
To analyze whether integrin clustering seen during RGD stimulation of cardiomyocytes in 3D collagen model was necessary for changes observed in Cas, freshly isolated adult feline cardiomyocytes were cultured two-dimensionally (2D) on laminin coated culture dishes (Fig. 1D). Medium was changed after 4 h, allowing time for the cells to adhere to the laminin and cells were left in the tissue culture incubator overnight. Nine millimolar RGD peptide was then added to the treatment groups and maintained in a tissue culture incubator for 1 h. Cells were harvested and subfractionated into Triton X-100 soluble and insoluble fractions. Our previous studies showed no RGD-stimulated recruitment of Cas or other focal adhesion proteins to CSK [Willey et al., 2003] when 2D cultured cardiomyocytes were used. Similarly, RGD stimulation of 2D cultured cardiomyocytes showed neither CSK recruitment nor band-shifting of Cas (Fig. 1D). Furthermore, analysis with phospho-specific antibodies exhibited absence of Cas phosphorylation at Y165, Y249, and Y410 sites (Data not shown). We have previously shown that integrin activation in 2D cultured cardiomyocytes leads to S6K1 activation, although FAC formation is absent under these conditions. As an indirect measure of integrin activation in 2D cultured cardiomyocytes, we measured S6K1 activation (Fig. 1D). These data indicate that integrin activation alone (as in the RGD stimulated adult cardiomyocytes in 2D model) is sufficient for S6K1 activation in the soluble fraction. However, CSK recruitment and phosphorylation of Cas requires the 3D model. Together, these data suggest that integrin-mediated FAC formation is necessary for Cas associated changes, observed in PO myocardium.
MUTANT C-SRC EXPRESSION AND CAS-ASSOCIATED CHANGES IN ADULT CARDIOMYOCYTES
Previous studies show that during integrin activation, recruitment of c-Src to the focal adhesion site leads to an open configuration where c-Src functions both as a kinase and as an adaptor molecule [Burnham et al., 2000]. In addition to its kinase function, c-Src’s adaptor function is also critical for the assembly of signaling proteins including Cas. Therefore, we explored whether Cas associated changes during integrin activation requires c-Src’s kinase and/or scaffold (adaptor) function. For this, we developed two adenoviral constructs (detailed in Methods) for the expression of A-Src (single point mutation to obtain open configuration with preserved kinase activity and adaptor functions) and KN-Src (double mutation to obtain open configuration with no kinase activity, but with intact adaptor function). We validated these two adenoviral constructs in adult cardiomyocytes (Fig. 2A). Both the viral constructs expressed c-Src several fold higher than the baseline expression of c-Src. Next we used Y416 phosphorylation state specific antibodies to detect active c-Src. Active c-Src expressing cells showed the presence of Y416 phosphorylated c-Src and non-Y416 phosphorylated c-Src was nearly absent. As expected, expression of KN-Src showed an opposite trend where the expressed c-Src was in the nonY416-phosphorylated, kinase inactive state.
Fig. 2.
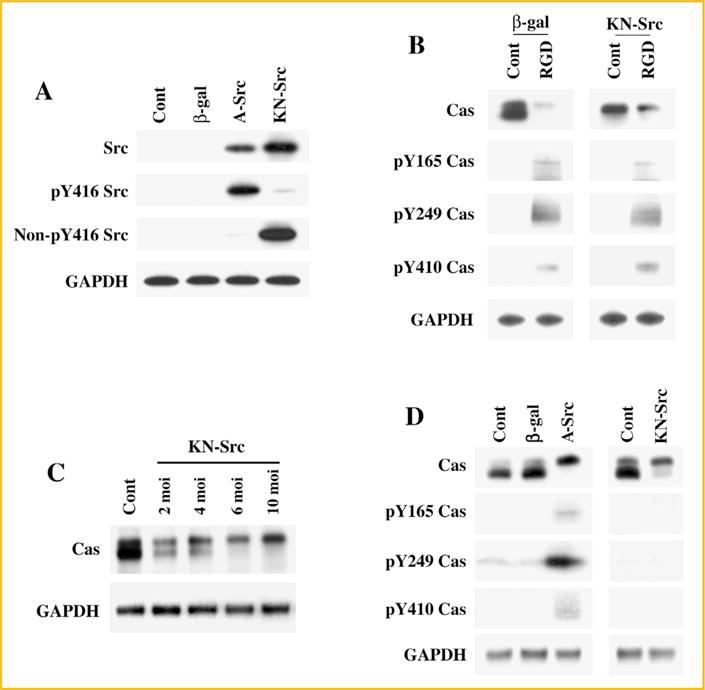
Cas associated changes in c-Src mutant expressing cells: (A) Adult cardiomyocytes were plated on laminin coated plates and infected with β-gal, A-Src, or DN-Src adenoviruses. After 36 h post-infection, cells were extracted to obtain Triton X-100 soluble proteins and used subsequently for Western blot analyses using anti-Src, anti-phospho Y416 Src (active state), anti-non-phospho Y416 Src (inactive state), and anti-GAPDH antibodies. Results were confirmed in two other independent experiments (n = 2). (B) Adult cardiomyocytes expressing β-gal or DN-Src adenoviruses were used for RGD stimulation in the 3D environment as described in Fig. 1C. After RGD treatment, cells were harvested for subcellular fractionation (Triton X-100 soluble and insoluble) and the protein samples were used for Western blot analyses of total and tyrosine phosphorylated Cas with specific antibodies as described in Fig. 1A. Results were confirmed in two other independent experiments (n = 2). (C) Adult cardiomyocytes cultured 2D on laminin coated plates were infected for 36 h with various doses of KN-Src (2, 4, 6, 10 moi) or β-gal (10 moi, Contl) adenoviruses. Cells were extracted with Triton X-100 buffer and the soluble samples were used for Western blot with anti-Cas and anti-GAPDH antibodies (n = 2). (D) Adult cardiomyocytes cultured 2D on laminin coated plates were infected for 36 h with 10 moi of β-gal, A-Src, or KN-Src adenoviruses. Uninfected cells also served as a control (Contl) for these experiments. Cells were extracted with Triton X-100 buffer and the soluble samples were used for Western blot analyses with anti-Cas anti-phosphotyrosine Cas (Y165, Y249, and Y410) and anti-GAPDH antibodies. Results were confirmed with independent experiments (n = 3).
Next, we used the KN-Src expressing cardiomyocytes in the 3D system to explore whether Cas associated changes during integrin activation requires c-Src’s catalytic activity. Cells expressing β-gal served as controls. Similar to the data shown in the previous experiments (Fig. 1C), RGD stimulation in β-gal expressing control cells caused both band-shifting and tyrosine phosphorylation of Cas (Fig. 2B). Interestingly, KN-Src expressing control cells, even in the absence of RGD treatment, showed Cas band-shifting, although tyrosine phosphorylation at the Y165, Y249, and Y410 sites was absent. Stimulation of KN-Src expressing cells with RGD showed both Cas band-shifting and tyrosine phosphorylation. These data reveal that c-Src adaptor function, which is available during integrin activation and FAC formation, is critical for Cas band-shifting. Furthermore, the RGD-stimulated tyrosine phosphorylation of Cas was unaffected by the exogenously expressed KN-Src.
Since our data indicates that the expression of kinase inactive c-Src with its intact SH2 and SH3 domains causes Cas band-shifting, we next tested if this could be observed in cardiomyocytes cultured 2D and infected with KN-Src adenovirus, prior to using them for the 3D experiments. For this, we infected cardiomyocytes with varying levels (moi) of KN-Src adenovirus. Our studies show a dose response of Cas band-shifting to the adenoviral infection levels in 2D cultured cardiomyocytes (Fig. 2C). Finally, we also tested whether the expression of active c-Src mutant (A-Src), which also has an open configuration with intact SH2 and SH3 domains, causes band-shifting similar to KN-Src. For this, uninfected, β-gal adenovirus infected controls were compared with A-Src or KN-Src adenoviral infected cardiomyocytes (Fig. 2D). These data analyzed in the detergent soluble fractions showed that the expression of either A-Src or KN-Src mutants resulted in Cas band-shifting. Furthermore, the Cas band-shifting in KN-Src expressing cardiomyocytes was not accompanied by tyrosine phosphorylation at the 165, 249, and 410 sites of Cas whereas A-Src expression was observed with both band-shifting and low levels of tyrosine phosphorylation at these sites, especially at the Y249 site. These studies indicated that the presence of an open configuration of c-Src, with or without kinase activity, is sufficient to mediate certain unique phosphorylation of Cas resulting in Cas band-shifting. Furthermore, although our earlier work [Willey et al., 2003] and present in vitro studies show that the band-shifting, tyrosine phosphorylation, and cytoskeletal recruitment of Cas require integrin activation by RGD in a 3D environment, expression of c-Src mutants possessing an open configuration with or without its catalytic function in 2D-cultured cardiomyocytes was found to be sufficient to cause Cas band-shifting. Therefore, we used the simple 2D cell culture system to characterize Cas band-shifting observed during in vivo PO.
MUTANT C-SRC EXPRESSION AND CAS-ASSOCIATED CHANGES IN CHO CELLS
The ability of a mutant c-Src with an open configuration to modify Cas with band-shifting was tested in another cell type. For this, we used CHO cells, since these cells could be used to generate a stable cell line for high expression and detection of post-translational modifications of Cas. CHO cells were infected with 10 moi of KN-Src or A-Src adenoviruses. Following a 36 h infection period, cells were extracted with Triton X-100 buffer and the soluble fractions were used for Western blot analyses. Similar to our observations in adult cardiomyocytes, CHO cells exhibited Cas band shifting upon expression of either KN-Src or A-Src (Fig. 3A). Furthermore, comparing the data between KN-Src and A-Src expressing cells, the band shifting proceeds independent of tyrosine phosphorylation of Cas at the 165, 249, and 410 sites.
Fig. 3.
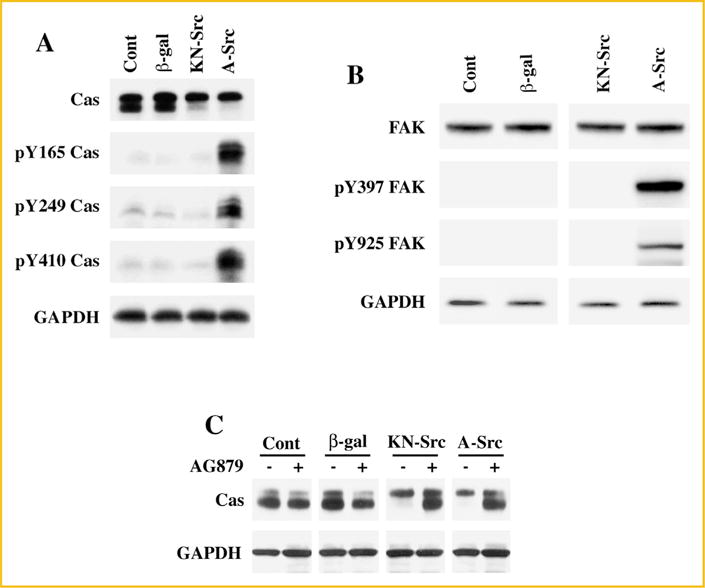
Cas band-shifting and phosphorylation in A-Src and DN-Src expressing CHO cells: (A) CHO cells cultured on six-well tissue culture plates were infected with β-gal, DN-Src, and A-Src adenoviruses for 36 h. Cells were extracted with Triton X-100 buffer and the soluble fractions for Western blot analyses with anti-Cas, anti-phosphotyrosine Cas (Y165, Y249, and Y410) and anti-GAPDH antibodies. Results were confirmed in two other independent experiments. (B) Soluble protein fractions from (A) were used for Western blot analyses using anti-Fak, anti-pYFak (Y397 and Y925) and anti-GAPDH antibodies. Results were confirmed in two other independent experiments. (C) CHO cells cultured on six-well tissue culture plates were infected with β-gal, DN-Src, and A-Src adenoviruses. After 36 h post infection, cells were treated with ± 10 nM AG879 for 1 h. Cells were then extracted with Triton X-100 buffer and the soluble fractions were used for Western blot analyses with anti-Cas anti-GAPDH antibodies. Results were confirmed in two other independent experiments (n = 3).
Tyrosine phosphorylation of Cas is primarily mediated by c-Src and focal adhesion kinase (Fak). c-Src mediates critical phosphorylation at the Y397 site of Fak, which results in a subsequent Y925 phosphorylation and activation of Fak [Janostiak et al., 2011]. Therefore, we tested the phosphorylation state of Fak during the expression of c-Src mutants in order to explore the possible role of Fak in Cas band-shifting. Western blot analyses reveal that the expression of A-Src but not KN-Src results in the tyrosine phosphorylation of Fak at the 397 and 925 sites, critical for its activation (Fig. 3B). Therefore, absence of phosphorylation at these sites of Fak in KN-Src expressing cells indicate that the Cas-associated changes showing band shifting on SDS–PAGE proceeds independent of catalytic activities of c-Src and Fak. Finally, to explore whether receptor or nonreceptor tyrosine kinases contribute either directly or indirectly to Cas band-shifting, we infected CHO cells with adenoviral vectors to express KN-Src or A-Src. After 36 h post infection, cells were treated with 10 nM AG879 (a tyrosine kinase-specific inhibitor) for 1 h and then cells were processed for Western blot analysis (Fig. 3C). These studies show that pretreatment with AG879 blocks Cas band-shifting caused by the mutant c-Src expression. Together, these studies demonstrate that c-Src in its open configuration with intact scaffold function mediates a unique modification in Cas which could be observed as a retarded electrophoretic mobility during SDS–PAGE separation. Furthermore, this modification in Cas does not require the catalytic activities of c-Src or Fak and occurs independent of tyrosine phosphorylation at the 165, 249, and 410 sites, although other tyrosine kinases appear to be involved in this modification.
IDENTIFICATION OF CAS PHOSPHORYLATION AT THE SERINE-639 SITE IN CHO CELLS EXPRESSING KN-SRC
A stable cell line overexpressing rat Cas was generated using CHO cells (see Methods). Stably expressing cells were infected with KN-Src or β-Gal adenovirus and used for immunoprecipitation with agarose-coupled anti-Cas antibody and the precipitated proteins were resolved on SDS–PAGE. The immunoprecipitation of Cas was confirmed by Western blot (Fig. 4A). The gel was stained with Coomassie blue and the band corresponding to Cas was processed for trypsin digestion followed by mass spectrometric analysis. While the trypsin-digested protein bands from both β-Gal and KN-Src expressing cells readily showed the presence of Cas, only KN-Src expressing cells generated the following phosphorylated peptide fragment: ASSIQSRPLPS*PPK (MH+ = 1544.783) (Fig. 4B). Further search on the database revealed that the identified serine phosphorylation site corresponds to serine-639 of human Cas (or S643 of Rat/mouse) (Fig. 4C). Careful analyses revealed no other phosphorylated peptide fragments detected in KN-Src or β-gal expressing CHO cells. Therefore, serine-639 appears to be the major site of phosphorylation mediated by the scaffold function of c-Src.
Fig. 4.
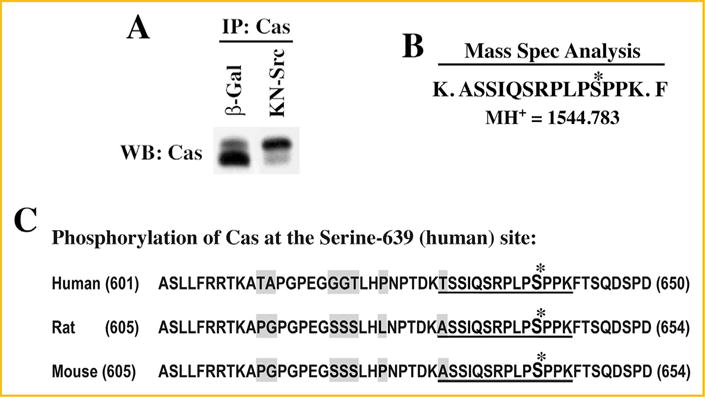
Mass spectrometry analysis of Cas phosphorylation: CHO cells were transfected with pcDNA6V5/His vector containing Cas cDNA (Rat) and selected for stably expressing cells with blasticidin resistance. (A) CHO cells stably overexpressing Cas were infected with β-gal and DN-Src adenoviruses for 36 h. Triton X-100 soluble samples were prepared and used for immunoprecipitation with anti-Cas antibody immobilized on agarose beads as detailed under Methods. A small part of the immunoprecipitated proteins were run on SDS–PAGE and used for Western blotting with anti-Cas antibody. (B) The remaining part of the immunoprecipitate run on SDS–PAGE was stained with Coomassie blue, and the region corresponding to the size (130 kDa) of Cas were cut, digested with trypsin and used for mass spectrometric analysis. A peptide with the molecular mass of 1,544.783 that corresponds to amino acids -ASSIQSRPLPSPPK- with phosphate group on the 4th serine residue was detected in KN-Src expressing cell extracts but not in β-gal control extracts. (C) Cas amino acid sequence flanking the phosphorylated serine residue is shown for human, rat, and mouse. The phosphorylated residue corresponds to serine-639 (human) and serine-643 (rat and mouse). The phosphorylated residue and the flanking amino acids identified in the mass spectrometry analysis are indicated with *mark and underline, respectively. The non-conserved residues among the species are highlighted in gray.
CAS IS PHOSPHORYLATED AT SERINE-639 IN PRESSURE OVERLOADED MYOCARDIUM
Based on the serine-639 phosphorylation of Cas, a polyclonal antibody using a synthetic peptide Cys-KASSIQSRPLP(p)SPPKFT that corresponds to human serine-639 of Cas, was generated and purified commercially by Antagene Inc. This new phospho antibody was used to characterize Cas serine-639 phosphorylation during in vivo pressure overload. Samples from 24 to 48 h RVPO, compared to normally loaded LV or sham-operated control (LV or RV), showed that Cas, recruited predominantly to CSK, was phosphorylated at the serine-639 site (Fig. 5).
Fig. 5.
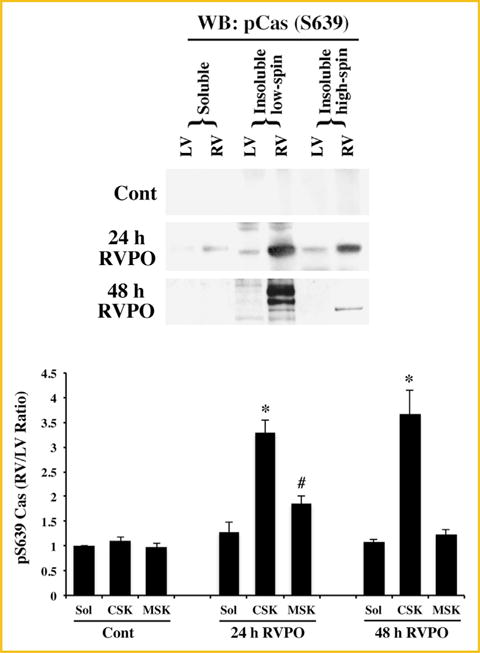
Serine-639 phosphorylation of Cas in PO myocardium: Adult felines underwent either sham surgery or pulmonary artery banding to induce RVPO for 24 and 48 h. and were sacrificed at the indicated time points. The LV and RV samples from Sham control and PO samples were processed to obtain Triton X-100 soluble and insoluble protein samples and normalized for protein concentration as in Fig. 1A. The samples were run on SDS–PAGE and Western blotted with a polyclonal anti-phospho serine-639 Cas antibody. For quantitation (bottom panel), Sham controls (n = 3), 24 h (n = 4), and 48 h (n = 3) RVPO feline groups were scanned and RV to LV ratios for each fraction were determined and plotted. Values are the mean ± SEM. *P < 0.05 compared to control CSK; #P < 0.05 compared to control MSK.
DISCUSSION
Adhesive interactions between cells and extracellular matrix proteins play a vital role in biological processes such as cell proliferation, differentiation, and survival. Integrins comprise a major family of cell surface receptors that mediate these interactions. Integrin engagement triggers adhesion-dependent intracellular signaling cascades that result in the activation of non-receptor tyrosine kinases and tyrosine phosphorylation of several intracellular signaling proteins. One of the important proteins phosphorylated following integrin ligation in several different cell types is the adaptor protein Cas that is shown to be crucial for cell migration, proliferation, and growth. Similarly, Cas has been shown to be critical for cardiovascular development and normal functioning of the heart [Honda et al., 1998].
Signaling pathways mediated by Cas is important for the hypertrophic response, since integrins, which are mechanosensors transmitting the mechanical load into biochemical responses, mediate their effects primarily through adaptor proteins, such as Cas. In this study, an RVPO model was used to study changes in Cas including cellular redistribution and phosphorylation in pressure overloaded RV at various time points and compared them with normally loaded LV. These studies clearly demonstrate that Cas is not only significantly increased in 48 h PO myocardium but also redistributed to the detergent insoluble CSK fraction. Furthermore, Cas also exhibited altered electrophoretic mobility in SDS–PAGE, which is characteristic of Cas phosphorylation [Mayer et al., 1995; Sakai et al., 1997]. Phospho-specific antibodies for Y165, Y249, and Y410 of Cas showed that Cas is in fact tyrosine phosphorylated at these sites.
In several cell types, tyrosine phosphorylation of Cas has been shown to occur primarily through the activation of c-Src and Fak [Ruest et al., 2001]. Our earlier observation in PO myocardium and in 3D collagen models, demonstrate that the recruitment of c-Src and Fak to the detergent insoluble CSK fraction is accompanied by the recruitment of Cas to this compartment. The present study in a 3D collagen model shows Cas-associated changes, including CSK recruitment, band-shifting and tyrosine phosphorylation (Fig. 1C). However, these changes were not observed when RGD stimulation is performed in a monolayer, two-dimensional culture (2D) (Fig. 1D and data not shown). Our earlier studies [Willey et al., 2003] and present work (Fig. 1) both in PO myocardium in vivo and RGD stimulated adult cardiomyocytes cultured in a 3D collagen environment in vitro show that Cas is recruited to the CSK along with NTKs and tyrosine phosphorylated at the 165, 249, and 410 sites. Since these changes were accompanied with Cas band-shifting in SDS–PAGE, we explored whether the tyrosine phosphorylation or other unique serine/threonine phosphorylation was responsible for the band-shifting. In this context, both c-Src and Fak have kinase and adaptor functions that might be important for Cas binding, phosphorylation and band-shifting.
To explore the importance of c-Src in Cas phosphorylation, adenoviral constructs were used to express either kinase negative c-Src (KN-Src) or active c-Src (A-Src). The kinase negative construct has double mutations: K295R mutation in the kinase domain affecting catalytic activity, and Y527F mutation in the c-terminal negative regulatory site that converts c-Src into an open configuration, thus allowing its SH2 and SH3 domain to function as adaptor domains. On the other hand, A-Src has a single mutation in the c-terminal tail (Y527F) that allows the molecule to undergo an open conformational change providing constitutive kinase activation and adaptor functions. Our studies with the expression of c-Src mutants in adult cardiomyocytes cultured in 3D and 2D environments reveal several important observations as follows: (i) Cas tyrosine phosphorylation at the Y165, Y249, and Y410 sites and its band shifting were unaffected in KN-Src expressing cardiomyocytes, stimulated with RGD peptide in a 3D environment, (ii) in both 3D and 2D environment, KN-Src expressing cardiomyocytes exhibit Cas band shifting, (iii) expression of KN-Src alone was sufficient to cause band shifting even in the absence of tyrosine phosphorylation at Y165, Y249, and Y410 sites, and (iv) expression of A-Src in 2D cultured cardiomyocytes results in Cas band-shifting accompanied with tyrosine phosphorylation primarily at the Y249 site. These studies clearly demonstrate that: (i) specific integrin stimulation can cause Cas band-shifting and tyrosine phosphorylation, (ii) c-Src’s adaptor (scaffolding) function but not catalytic activity is critical for the band-shifting of Cas, and (iii) phosphorylation site(s) other than Y165, Y249, and Y410 is responsible for the band-shifting. Our additional studies using CHO cells indicate that Cas associated changes observed in cardiomyocytes following KN-Src and A-Src expression could be reproduced in CHO cells and that Cas band-shifting in K-Src expressing cells proceeds independent of Fak activation. Together, these studies strongly indicate that a distinct kinase other than c-Src and Fak catalyzes phosphorylation at one or more unique sites in Cas that might lead to Cas band-shifting in SDS–PAGE. Furthermore, it is possible that this unique phosphorylation includes serine/threonine site(s).
To explore whether Cas undergoes serine/threonine phosphorylation in KN-Src expressing cells, we first generated CHO cells stably-expressing Cas (rat). These cells were then infected with KN-Src or β-gal control viruses. Immunoprecipitation combined with mass spectrometry analysis revealed that Cas from KN-Src expressing CHO cells generated a trypsin digested peptide fragment with a mass value of 1544.783 where the phosphorylated residue was identified as the fourth serine (Fig. 4A and B). The phosphorylated residue in Cas corresponds to serine 643 in rat and mouse and serine-639 in human (Fig. 4C). Therefore, these data reveal that the presence of c-Src in an open configuration with its scaffold function in the cell can cause serine-639 phosphorylation of Cas (maintained human sequence-based number for uniformity) and that this can proceed independent of c-Src’s kinase function. Finally, to explore whether serine-639 phosphorylation occurs during PO, we generated a serine-639 phosphorylation state specific polyclonal rabbit antibody. Our studies performed in 24– 48 h PO myocardium clearly show that Cas, which undergoes CSK recruitment and band-shifting during PO (Fig. 1) is phosphorylated at the serine-639 site (Fig. 5). Since our earlier work demonstrates that PO causes integrin activation and CSK recruitment of c-Src, together these data show that CSK-bound c-Src with its open configuration and adaptor function recruits a pivotal serine/threonine kinase for the serine-639 phosphorylation of CSK-bound c-Src.
Cas serves as a focal adhesion adaptor protein where the RPLPSPP motif has been shown to serve as the proline rich motif for the interaction with c-Src’s SH3 domain. The RPLPSPP domain lies adjacent to the carboxy terminus FAT domain of Cas where several NSP family proteins including BCAR3 are known to bind and enhance the interaction between c-Src and Cas [Makkinje et al., 2012]. BCAR3 overexpression in epithelial cells has been shown to enhance Cas phosphorylation that results in slow migration of Cas on SDS–PAGE (band-shifting) [Makkinje et al., 2009]. Furthermore, these studies reveal that Cas undergoes serine phosphorylation at the 139, 437, and 639 sites and that phosphorylation at all three sites contribute to the slow migratory trend of Cas on SDS–PAGE. Therefore, in the present study, it is possible that the expression of the c-Src mutant with open configuration recruits BCAR3 and serine/threonine kinase(s) for Cas phosphorylation at these sites, although we detected only serine-639 phosphorylation in the mass spectrometry analysis. Since our experiments with mass spectrometry showed only 22% recovery of trypsin-digested Cas peptides, it is possible that the present work missed other sites of serine/threonine phosphorylation of Cas that might also contribute to band-shifting during KN-Src expression.
Our studies with AG879 showed increased levels of unphosphorylated (fast migratory) Cas in KN-Src expressing cells (Fig. 3C). These data suggest the possible involvement of a tyrosine kinase that might function upstream of a putative serine/threonine kinase(s) responsible for the serine-639 phosphorylation and band-shifting. However, in these experiments, the slow migratory band (phosphorylated Cas) due to KN-Src expression was largely unaffected while the fast migratory band (unphosphorylated Cas) showed up during AG879 treatment. These data suggest other possibilities, including stabilization of unphosphorylated Cas when tyrosine kinase activities were blocked by AG879 treatment. Further studies are needed to understand how the catalytic function of tyrosine kinases contributes to either band-shifting and/or stabilization (if any) of Cas.
c-Src/Cas interaction has been shown to be critical for cell transformation and FAC formation [Linder, 2007]. Phosphorylation at the serine-639 site in the RPLPSPP motif of Cas was recently characterized in several cell types [Makkinje et al., 2009, 2012]. Although a previous work shows that mutations in the serine-639 did not affect Cas binding to c-Src in in vitro pull down experiments [Makkinje et al., 2012], it is not clear whether serine-639 phosphorylation plays a critical role in the c-Src/Cas/BCAR3 signaling axis. Since our earlier work demonstrates that PO causes integrin activation and CSK recruitment of c-Src and Cas, together these data show that CSK-bound c-Src with its open configuration and adaptor function recruits Cas and one or more pivotal kinases for the serine-639 phosphorylation of CSK-bound c-Src. In the adult heart, hypertrophying cardiomyocytes undergo focal adhesion turnover to accommodate ventricular remodeling as part of adaptive and maladaptive changes. Cas binding to c-Src and the associated phosphorylation might be a critical event for the formation and turnover of FAC in the hypertrophying heart. Our present work indicates that serine-639 phosphorylation of Cas, which is observed during early PO, requires the scaffold function of c-Src for which the catalytic activities of c-Src or Fak are not needed. The further, critical question is what role serine-639 phosphorylation of Cas may have in transducing cardiac load into a hypertrophic response.
Acknowledgments
We thank Dorea Pleasant for careful reading of the manuscript. This study was supported partly by the NIH Program Project Grant HL-48788.
Grant sponsor: Program Project; Grant number: HL-48788.
Footnotes
Conflict of Interest: None.
References
- Briknarova K, Nasertorabi F, Havert ML, Eggleston E, Hoyt DW, Li C, Olson AJ, Vuori K, Ely KR. The serine-rich domain from Crk-associated substrate (p130cas) is a four-helix bundle. J Biol Chem. 2005;280:21908–21914. doi: 10.1074/jbc.M501258200. [DOI] [PubMed] [Google Scholar]
- Burnham MR, Bruce-Staskal PJ, Harte MT, Weidow CL, Ma A, Weed SA, Bouton AH. Regulation of c-SRC activity and function by the adapter protein CAS. Mol Cell Biol. 2000;20:5865–5878. doi: 10.1128/mcb.20.16.5865-5878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnakkannu P, Samanna V, Cheng G, Ablonczy Z, Baicu CF, Bethard JR, Menick DR, Kuppuswamy D, Cooper G., 4th Site-specific microtubule-associated protein 4 dephosphorylation causes microtubule network densification in pressure overload cardiac hypertrophy. J Biol Chem. 2010;285:21837–21848. doi: 10.1074/jbc.M110.120709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G, 4th, Satava RM, Jr, Harrison CE, Coleman HN., 3rd Mechanisms for the abnormal energetics of pressure-induced hypertrophy of cat myocardium. Circ Res. 1973;33:213–223. doi: 10.1161/01.res.33.2.213. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Dolfi F, Russello M, Vuori K. Cell adhesion regulates the interaction between the docking protein p130(Cas) and the 14-3-3 proteins. J Biol Chem. 1999;274:5762–5768. doi: 10.1074/jbc.274.9.5762. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src- induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Janostiak R, Pataki AC, Brabek J, Rosel D. Mechanosensors in integrin signaling: The emerging role of p130Cas. Eur J Cell Biol. 2014;93:445–454. doi: 10.1016/j.ejcb.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Janostiak R, Tolde O, Bruhova Z, Novotny M, Hanks SK, Rosel D, Brabek J. Tyrosine phosphorylation within the SH3 domain regulates CAS subcellular localization, cell migration, and invasiveness. Mol Biol Cell. 2011;22:4256–4267. doi: 10.1091/mbc.E11-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent RL, Mann DL, Urabe Y, Hisano R, Hewett KW, Loughnane M, Cooper G., 4th Contractile function of isolated feline cardiocytes in response to viscous loading. Am J Physiol. 1989;257:H1717–H1727. doi: 10.1152/ajpheart.1989.257.5.H1717. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy D, Kerr C, Narishige T, Kasi VS, Menick DR, Cooper G. Association of tyrosine-phosphorylated c-Src with the cytoskeleton of hypertrophying myocardium. J Biol Chem. 1997;272:4500–4508. doi: 10.1074/jbc.272.7.4500. [DOI] [PubMed] [Google Scholar]
- Laser M, Willey CD, Jiang W, Cooper G, 4th, Menick DR, Zile MR, Kuppuswamy D. Integrin activation and focal complex formation in cardiac hypertrophy. J Biol Chem. 2000;275:35624–35630. doi: 10.1074/jbc.M006124200. [DOI] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Makkinje A, Near RI, Infusini G, Vanden Borre P, Bloom A, Cai D, Costello CE, Lerner A. AND-34/BCAR3 regulates adhesion-dependent p130Cas serine phosphorylation and breast cancer cell growth pattern. Cell Signal. 2009;21:1423–1435. doi: 10.1016/j.cellsig.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkinje A, Vanden Borre P, Near RI, Patel PS, Lerner A. Breast cancer anti-estrogen resistance 3 (BCAR3) protein augments binding of the c-Src SH3 domain to Crk-associated substrate (p130cas) J Biol Chem. 2012;287:27703–27714. doi: 10.1074/jbc.M112.389981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BJ, Hirai H, Sakai R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr Biol. 1995;5:296–305. doi: 10.1016/s0960-9822(95)00060-1. [DOI] [PubMed] [Google Scholar]
- Nikonova AS, Gaponova AV, Kudinov AE, Golemis EA. CAS proteins in health and disease: An update. IUBMB Life. 2014;66:387–395. doi: 10.1002/iub.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- Pozuelo Rubio M, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N, Mackintosh C. 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem J. 2004;379:395–408. doi: 10.1042/BJ20031797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozich JD, Barnes MA, Schmid PG, Zile MR, McDermott PJ, Cooper G., 4th Load effects on gene expression during cardiac hypertrophy. J Mol Cell Cardiol. 1995;27:485–499. doi: 10.1016/s0022-2828(08)80044-2. [DOI] [PubMed] [Google Scholar]
- Ruest PJ, Shin NY, Polte TR, Zhang X, Hanks SK. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol Cell Biol. 2001;21:7641–7652. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R, Nakamoto T, Ozawa K, Aizawa S, Hirai H. Characterization of the kinase activity essential for tyrosine phosphorylation of p130Cas in fibroblasts. Oncogene. 1997;14:1419–1426. doi: 10.1038/sj.onc.1200954. [DOI] [PubMed] [Google Scholar]
- Samarel AM. Focal adhesion signaling in heart failure. Pflugers Archiv: Eur J Physiol. 2014;466:1101–1111. doi: 10.1007/s00424-014-1456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: Involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey CD, Balasubramanian S, Rodriguez Rosas MC, Ross RS, Kuppuswamy D. Focal complex formation in adult cardiomyocytes is accompanied by the activation of beta3 integrin and c-Src. J Mol Cell Cardiol. 2003;35:671–683. doi: 10.1016/s0022-2828(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Willey CD, Palanisamy AP, Johnston RK, Mani SK, Shiraishi H, Tuxworth WJ, Zile MR, Balasubramanian S, Kuppuswamy D. STAT3 activation in press ure-overloaded feline myocardium: role for integrins and the tyrosine kinase BMX. Int J Biol Sci. 2008;4:184–199. doi: 10.7150/ijbs.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Yamakita Y, Yoshida K, Takiguchi K, Matsumura F. Characterization of the COOH terminus of non-muscle caldesmon mutants lacking mitosis-specific phosphorylation sites. J Biol Chem. 1995;270:4023–4030. doi: 10.1074/jbc.270.8.4023. [DOI] [PubMed] [Google Scholar]


