Abstract
Few studies have examined relationships between circadian rhythms and unipolar major depressive disorder. Further, no study to date has examined circadian markers as predictors of response to depression treatment. In the present study, we examined associations between circadian timing and its alignment with sleep and depression severity in 30 adults with major depressive disorder who completed a randomized controlled trial of two weeks of time in bed (TIB) restriction administered adjunctive to fluoxetine, with a focus on sex differences. Thirty adults with major depressive disorder received 8 weeks of fluoxetine 20–40 mgs and were randomized to 8h TIB or 6h TIB for the first 2 weeks. Participants in the 6h TIB condition were further randomized to a delayed bedtime or advanced risetime group. Circadian measures included dim light melatonin onset (DLMO) and the difference between DLMO and midsleep point (i.e., phase angle difference). Depression was assessed using the Hamilton Rating Scale for Depression. For females, a phase delay after 2 weeks of fluoxetine and the experimental TIB manipulation was associated with a poorer response to fluoxetine, and depression severity was negatively correlated with phase angle difference, whereas males showed a positive correlation between depression severity and phase angle difference.
Keywords: depression, melatonin, circadian, phase angle difference, sleep, antidepressant, phase
1. Introduction
Circadian rhythm dysregulation is implicated in the pathogenesis and course of many affective disorders (Jones and Benca, 2015). However, most empirical investigations in this area have focused on seasonal affective disorder (Lewy et al., 2006). Emerging evidence from the last decade has provided preliminary support for early hypotheses linking circadian rhythms to unipolar major depressive disorder (MDD).
Several studies have established associations between an evening preference (e.g., evening chronotype), measured by self-report, and depression severity. In a cross-sectional study of 100 adults with MDD, participants with an evening chronotype had more severe suicidal ideation and greater functional impairment relative to those with morning or neither chronotypes (Gaspar-Barba et al., 2009). Further, in a naturalistic follow-up study of adults with major depressive disorder, a self-reported evening preference was predictive of more severe depression symptoms, and significantly greater odds of non-remission of depression (Chan et al., 2014).
Beyond chronotype, two recent studies have examined the role of misalignment between objective markers of the circadian pacemaker (e.g., dim light melatonin onset, or DLMO) and the timing of sleep in MDD (Emens et al., 2009; Hasler et al., 2010). In a cross-sectional study of 18 females with MDD who had persistent symptoms of depression despite antidepressant treatment, depression severity was negatively correlated with the amount of time between dim light melatonin onset (DLMO) and midsleep (i.e., phase angle difference; PAD), such that a shorter PAD (corresponding to a phase delay) was associated with more severe depression (Emens et al., 2009). In a comparison of circadian and sleep markers in 18 adults with depression and 19 healthy controls, the group with depression had a later mean sleep onset and a later mean midsleep point relative to the control group (Hasler et al., 2010). Although a delay in core body temperature minimum relative to midsleep and DLMO was correlated with depression severity, no relationship was observed between the DLMO-midsleep PAD in this study (Hasler et al., 2010). Although these studies have relatively small, homogenous samples, their findings suggest that further exploration of misalignment between the circadian and sleep timing systems in MDD is warranted. In particular, no studies to date have examined circadian measures as predictors of response to antidepressant therapy. This line of inquiry is critical to the development of personalized treatment approaches to enhance outcomes for individuals with MDD.
One of the most well-established sleep-based therapies for depression, sleep deprivation (Giedke and Schwarzler, 2002; Schilgen and Tölle, 1980) is hypothesized to improve depression by directly influencing the sleep homeostatic and circadian systems. Sleep deprivation treatments for MDD includes single-night total deprivation, single-night partial deprivation, and repeated partial sleep deprivation. In partial deprivation, an advanced wake time (i.e., wakefulness during the second half of the night) is generally more effective than a delayed bedtime (i.e., wakefulness during the first half of the night (Sack et al., 1988)). Our group recently completed a randomized controlled trial designed to compare depression remission rates and time to depression remission for two weeks of 6 hours’ time in bed (6h TIB) to 8h TIB delivered adjunctive to fluoxetine in adults with MDD (Arnedt et al., 2016). Participants completed the 2-week TIB condition protocol at home and were assigned to 8h TIB, 6h TIB with delay of bedtime (Late Bedtime), or 6h TIB with advance of rise time (Early Rise time). It was hypothesized that participants assigned to the Early Rise time condition would report the greatest symptom improvement relative to those assigned to the Late Bedtime or 8h TIB conditions. Contrary to hypotheses, participants assigned to the 8h TIB group had lower depression severity, greater remission rates, and earlier onset of remission relative to both 6h TIB groups (Arnedt et al., 2016).
In the present study, we conducted a secondary analysis of the parent randomized controlled trial to explore change in circadian timing from baseline to post-experimental TIB manipulation and to examine the role of circadian timing and its alignment with sleep as a predictor of depression severity and treatment response. We note that the parent trial was specifically designed to minimize the effects of the TIB schedule on the circadian system. To this end, participants were instructed to remain in dim light and to engage in only quiet, sedentary activities during the period of sleep deprivation. Thus, although we assessed whether circadian measures changed from baseline to post-experimental TIB manipulation, we did not have specific hypotheses regarding the effects of the different TIB schedules on circadian measures. However, based on previous findings, we hypothesized the following: 1. Baseline DLMO would be positively correlated with depression severity (i.e., later DLMO would be associated with more severe depression); 2. Baseline PAD (defined as DLMO – midsleep) would be negatively correlated with depression severity (i.e., a shorter PAD would be associated with more severe depression); 3. Delay in DLMO from baseline to post-experimental TIB manipulation (indicating a shift towards eveningness) would predict poorer response to fluoxetine across the 8-week study (i.e., more severe depression symptoms). Upon further considerations of the possible ways to analyze the data, associations between post-experimental TIB manipulation DLMO, PAD, and depression severity were analyzed, sex differences were examined, and quadratic (parabolic) analyses were added.
2. Methods
2.1 Participants
The parent trial was an 8-week randomized, controlled trial with 68 participants (Arnedt et al., 2016). All participants in the parent trial completed the dim light melatonin saliva sample collection procedure (except for the last 5 participants, who did not complete the procedure due to financial considerations); however, due to financial constraints, for this secondary analysis, saliva samples were assayed for melatonin in a subsample of 30 participants randomly selected from parent trial. The study was approved by the University of Michigan Medical School Institutional Review Board. Recruitment occurred from September 2009 to December 2012; participants were recruited through advertisements for the study and clinical referrals. Written informed consent was obtained from all participants. The eligibility criteria for the parent trial was as follows: (1) 18–65 years of age; (2) diagnosis of major depressive disorder per DSM-IV plus a score of ≥ 18 on the 17-item Hamilton Rating Scale for Depression (HAMD-17 (Hamilton, 1960)); and (3) habitual time in bed of 7–10 hours per night. Participants were excluded from participation for any of the following: (1) current or past history of DSM-IV diagnosis other than major depressive disorder or generalized anxiety disorder; (2) diagnosis of DSM-IV alcohol abuse in the past 6 months; (3) presence of medical conditions associated with depression or affecting sleep; (4) sleep disorders other than insomnia; (5) use of medication (prescription or over-the-counter) for sleep or depression; (6) use of fluoxetine in the past 6 months; (7) night shift work; (8) pregnancy or breastfeeding; (9) known contraindication to fluoxetine; or (10) blood biochemistry not within normal limits. All participants were free of antidepressant medications for ≥ 2 weeks (≥4 weeks for longer-acting antidepressants). Participants completed an in-laboratory screen to assess for eligibility, including overnight polysomnography to screen for sleep disorders (Ilber et al., 2007). A similar number of participants were enrolled in the study in the fall/winter (53.33%) and spring/summer (46.67%).
2.2 Study Design and Procedures
In the study preparatory phase, participants maintained a consistent 8-hour time in bed (TIB) schedule at home, based on their habitual sleep schedule, for 5–7 days. To assess for melatonin levels, saliva samples were collected in dim light at Baseline (for more detail, see Measures below). Through random assignment, participants were then allocated 1:1:1 to one of three TIB conditions for the first 2 weeks of the study (experimental phase): (1) 8h TIB (n = 10; 7 males, 3 females); (2) 6h TIB (n = 20), with a 2-hour delay of habitual bedtime (Late Bedtime, n = 10; 3 males, 7 females); or (3) 6h TIB with a 2-hour advance of habitual rise time (Early Rise time, n = 10; 4 males, 6 females). Participants began open-label fluoxetine 20 mgs daily the morning after their first experimental phase night; doses could be increased to 40 mgs after Week 4. Participants assigned to the 6h TIB conditions were instructed to remain in dim light conditions for the 2h period of sleep deprivation (e.g., a participant assigned to the Late Bedtime condition with a habitual bedtime of 10 pm would remain in dim light from 10 pm to 12 pm) during the first 2 weeks of the study to minimize direct effects on the circadian system. Participants wore a wrist actigraph with an integrated light meter (Philips Respironics Actiwatch-2) on their non-dominant wrist during the preparatory and experimental phases of the study; they were instructed to keep the face of the actigraph uncovered by clothing at all times. Actigraphy data were collected at a 60-second sampling rate. Actigraphy were scored according to established procedures (Ancoli-Israel et al., 2015) using Actiware® – Sleep software (Version 5.0) in conjunction with daily sleep/wake diaries. Participants were prohibited from using drugs and alcohol and napping during the preparatory and experimental phases of the study. They were allowed to use their habitual amount of caffeine prior to 12 pm.
Participants underwent overnight polysomnography in the laboratory for the first 3 nights of the study (respectively, screening/adaptation (8h TIB), Baseline (8h TIB), and first experimental night (6h or 8h TIB)) and again for 2 consecutive nights at the end of the experimental phase ((the final experimental night (6h or 8h TIB) and an 8h TIB recovery night; Week 2); polysomnography results are reported in the parent trial (Arnedt et al., 2016). On the final experimental night, participants again completed a saliva sample collection protocol in dim light to assess melatonin levels (for more detail, see Measures below).
Following completion of the 2-week experimental phase of the study, participants entered the 6-week follow-up phase of the study, during which they maintained self-selected sleep schedules, took fluoxetine daily, and completed mood assessments weekly through week 8 of the study.
2.3 Measures
Depression symptom severity was assessed using the HAMD-17. Total scores on this measure range from 0 to 46. Clinicians, trained to reliability and blinded to experimental assignment, rated participants’ symptoms at weekly intervals.
To determine dim light melatonin onset, saliva samples were collected in dim light conditions (< 30 lux at eye level (Lewy, 1999); in laboratory on the Baseline night and again on the final night of the experimental schedule (Week 2). Participants began the dim light saliva collection protocol approximately 5h prior to their assigned bedtime (7h prior for participants in the Late Bedtime condition at Week 2), accompanied by laboratory staff. Dim light conditions were maintained throughout the protocol. Participants were in dim light for 30 minutes prior to the first saliva sample collection. Samples were collected via Salivette (Sarstedt, Newton, NC) every 30 minutes, with the final sample collected at the assigned bedtime. Eleven samples in total were collected for all participants at Baseline and for participants in the 8h TIB and Early Rise time condition at Week 2. Fifteen samples were collected from participants assigned to the Late Bedtime condition at Week 2 due to the 2-hour delay in bedtime in this condition. Participants were allowed food or liquids except in the 10 minutes prior to sample collection. Participants were required to remain seated for 10 minutes prior to sample collection, during which time they brushed their teeth with water if they had consumed food or liquids. Salivettes were centrifuged immediately after collection to extract saliva, which was then frozen. Samples were shipped on dry ice to Solidphase Inc. (Portland, ME), where they were radioimmunoassayed for melatonin using Buhlmann direct kits (Buhlmann Laboratories, Switzerland). The sensitivity of the melatonin assay was 0.5 pg/ml.
2.4 Circadian Phase Calculations
To determine DLMO, linear interpolation of the clock time when the melatonin level exceeded the mean of three consecutive low daytime values plus twice the standard deviations of these points was calculated (also termed the “3k” method; (Voultsios et al., 1997)). Phase angle difference (PAD) was calculated as the difference (in hours and minutes) between DLMO and the midpoint of sleep from polysomnography (calculated as (sleep onset + [time of sleep offset − sleep onset)/2]); (Lewy et al., 2006)). Change in DLMO was calculated in minutes by subtracting the time of DLMO at Baseline from the time of DLMO at Week 2; negative values indicated an advance of DLMO. A change in DLMO between Baseline and Week 2 of ≥ 30 minutes was characterized as either a phase delay (later at Week 2 than Baseline) or phase advance (earlier at Week 2 than Baseline).
2.4 Statistical Analyses
Shapiro-Wilk tests were used to evaluate whether the data were normally distributed; all variables except change in PAD from Baseline to Week 2 were normally distributed. Analyses were conducted with and without data points identified as outliers; results were not changed by removal of outliers, and thus the final analyses included all available data points.
For participants assigned to the 6h TIB conditions, compliance with dim light conditions while at home during the 2-week experimental phase of the study was assessed using light data from wrist actigraphy. Epochs of exposure to ≥ 50 lux (Lewy, 1999) were coded as non-compliant. From the wrist actigraphy light data, we also calculated the average light intensity in lux during the nightly 2h sleep deprivation periods across the two-week experimental phase for participants assigned to the 6h TIB conditions. To determine whether light exposure during the two hours before bedtime or after wake time (two hours before the sleep deprivation period for the Late Bedtime group, and two hours after the sleep deprivation period for the Early Rise time group) was associated with circadian measures, Pearson product-moment correlation coefficients were calculated between log-transformed average lux values from the wrist actigraphy light data and DLMO at Baseline and Week 2, and change in DLMO from Baseline to Week 2.
Paired-sample t-tests evaluated differences in DLMO and PAD between Baseline and Week 2 for the entire sample and for females and males separately. One-way ANOVA tested differences in DLMO and PAD at Baseline and Week 2 by experimental sleep condition, and evaluated whether change in DLMO and PAD from Baseline to Week 2 differed by experimental sleep condition. Fisher’s Exact Test was used to assess whether the type of phase shift was different by experimental sleep condition or by sex.
Pearson product-moment correlation coefficients were calculated between the circadian variables and HAMD-17 at Baseline and Week 2, and change in circadian variables and Week 2 HAMD-17 for the entire sample and by males and females separately. Curve estimation models were fitted within scatterplots to test quadratic relationships between the circadian variables (except for change in PAD, which was not normally distributed) and HAMD-17 at Baseline and Week 2 for males and females separately.
The HAMD-17 assessments are hierarchical in structure, with weekly observations nested within person, therefore, linear mixed models were employed (Raudenbush & Bryk, 2002) using the mixed program in Stata 14 (StataCorp LP, College Station, TX) to estimate the unique within-person relationship for weekly HAMD-17 score using circadian phase shift type (delay or advance relative to no shift) and time (week of study, as a linear and quadratic function) as main effects and the interaction of circadian phase shift type * time. All models contained a random intercept per subject and a random slope associated with the HAMD-17 score. The analytic sample included two levels of data: Level 1 (weekly level) included 241 total HAMD-17 scores across 9 weeks and people (out of a possible 252 (i.e., 9 weeks * 28 people)); Level 2 (person level) included the 28 participants with Baseline HAMD-17 score and circadian phase shift type. Linear mixed models were fit for the entire sample and for males and females separately.
3. Results
Participant demographics are shown in Table 1. One participant (male) had no discernible onset in melatonin at baseline. Another participant (female) had undetectable melatonin levels at both time points. The average pg/ml concentration of DLMO was 1.10 (SD = 0.59) at Baseline and 1.20 (SD= 0.63) at Week 2.
Table 1.
Sample Demographic Characteristics
| n (%) or M ± SD | |
|---|---|
| Age | 25.73 ± 6.85 |
| Sex (M/F) | 14/16 |
| Race | |
| Caucasian | 24 (80%) |
| African American | 4 (13%) |
| Asian | 1 (3%) |
| Native American | 1 (3%) |
| Marital Status | |
| Married/partnered | 5 (17%) |
| Never married | 24 (80%) |
| Divorced/separated | 1 (3%) |
| Education (years) | 15.37 ± 1.63 |
| HAMD-17 Score at Study Entry | 20.20 ± 2.58 |
Note. N = 30.
Overall, non-compliance with dim light conditions during the nightly sleep deprivation periods for the 2-week experimental phase for participants assigned to the 6h TIB group ranged from 0% to 16.7% of all 60-second epochs, with an average of 4.33% of non-complaint epochs (SD = 5.07%). Rates of dim light non-compliance were not different between the Late Bedtime (M = 2.8%, SD = 2.57%) and Early Rise time (M = 5.78%, SD = 6.56%) groups (t (18) = −1.30, p = .210). Average light intensity during the sleep deprivation periods in participants assigned to the 6h TIB group was 12.47 lux (SD = 159.13 lux) across the 2-week experimental phase, with only 7.3% of all 60-second epochs above 30 lux (chosen as a conservative lux value unlikely to suppress melatonin (Lewy, 1999)). Correlations between average light exposure values before bedtime and after wake time and the circadian measures were not significant (see Supplemental Table 1).
Circadian parameters are shown in Table 2 by experimental group and sex. Approximately half of the sample experienced a phase shift between Baseline and Week 2; eight participants (28.57%) evidenced a phase advance, and 5 (17.86%) showed a phase delay between Baseline and Week 2. Paired-sample t-tests were non-significant for differences in DLMO and PAD between Baseline and Week 2 for the entire sample and when analyzed for males and females separately (all ps > 0.05). Fisher’s Exact Test was non-significant for differences in type of phase shift by sex (p = 1.0).
Table 2.
Circadian characteristics by group and sex
| 8h TIBa | Late Bedtimeb | Early Rise timec | All Participants | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | |||||
|
| ||||||||
| Malesa | Females n = 3 | Males n = 3 | Females n = 7 | Males n = 4 | Females n = 5 | Malesb | Females n = 15 | |
|
| ||||||||
| DLMO Baseline† | 21:36 (1:18) | 22:31 (1:42) | 21:13 (0:57) | 20:34 (1:38) | 22:10 (1:28) | 20:48 (1:16) | 21:41 (1:14) | 21:02 (1:37) |
| DLMO Week 2† | 21:18 (0:52) | 22:28 (0:54) | 21:11 (1:06) | 20:51 (2:26) | 20:50 (1:30) | 20:25 (1:21) | 21:08 (1:03) | 21:02 (1:56) |
| Midsleep Baseline† | 03:47 (0:40) | 05:11 (0:04) | 03:45 (0:35) | 03:51 (1:18) | 05:15 (1:40) | 03:34 (1:03) | 04:12 (1:10) | 03:59 (1:11) |
| Midsleep Week 2† | 03:46 (0:41) | 05:02 (1:06) | 04:42 (0:34) | 04:57 (1:19) | 03:45 (1:30) | 02:42 (1:10) | 03:58 (0:58) | 04:07 (1:37) |
| No Phase Shift | 3 (50.00) | 1 (33.30) | 3 (100.00) | 5 (71.40) | 1 (25.00) | 2 (33.30) | 7 (53.85) | 8 (53.33) |
| Phase Delay | 1 (16.70) | 1 (33.30) | 0 (0.00) | 1 (14.30) | 1 (25.00) | 1 (16.70) | 2 (15.38) | 3 (20.00) |
| Phase Advance | 2 (33.30) | 1 (33.30) | 0 (0.00) | 1 (14.30) | 2 (50.00) | 2 (33.30) | 4 (30.77) | 4 (26.67) |
| PAD Baseline‡ | 6:17 (1:04) | 6:40 (1:46) | 6:32 (0:28) | 7:17 (0:42) | 7:04 (0:39) | 6:50 (1:11) | 6:35 (0:52) | 7:00 (1:04) |
| PAD Week 2‡ | 6:28 (0:55) | 6:34 (0:52) | 7:30 (0:43) | 8:05 (1:15) | 6:55 (1:22) | 6:24 (1:02 | 6:49 (1:02) | 7:13 (1:20) |
Note. DLMO = dim light melatonin onset. PAD = phase angle difference.
n = 6 at Baseline, 7 at Week 2.
n = 13 at Baseline, 14 at week 2.
Data are shown in clock time, h:min.
Data are shown in h:min.
With respect to comparison of circadian measures by experimental group, there were no differences in DLMO by group at Baseline (F(2,25) = 1.51, p = 0.241) or Week 2 (F(2,26) = 1.14, p = 0.335), or PAD at Baseline (F(2,25) = 1.15, p = 0.334). One-way ANOVA showed a significant effect of group on PAD at Week 2 (F(2,26) = 5.46, p = 0.010). Bonferroni post hoc comparisons showed that the PAD at Week 2 was significantly longer in participants assigned to the Late Bedtime group (M = 7:55, SD = 1:07) relative to those in the 8h TIB group (M = 6:30, SD = 0:51, p = 0.017) and Early Rise time group (M = 6:38, SD = 1:09, p = .040). There was a trend for an effect of group on shift in minutes in DLMO from Baseline to Week 2 (F(2,25) = 3.25, p = 0.056). Bonferroni post hoc comparisons showed a delay on average for the Late Bedtime group (M = 10.9 minutes, SD = 50.80 minutes) relative to the Early Rise time group, which showed an advance on average (M = −48.56, SD = 54.53). However, Fisher’s Exact Test was non-significant for differences in the type of phase shift by experimental group (p = 0.326), even when the sample was constrained to only those participants assigned to a time in bed restriction (p = 0.139).
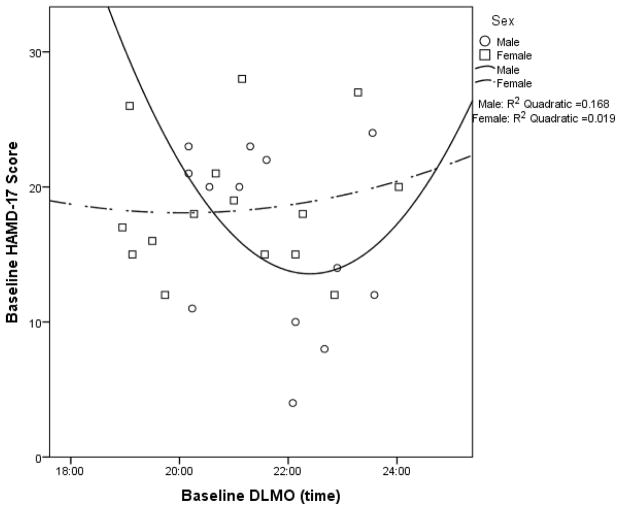
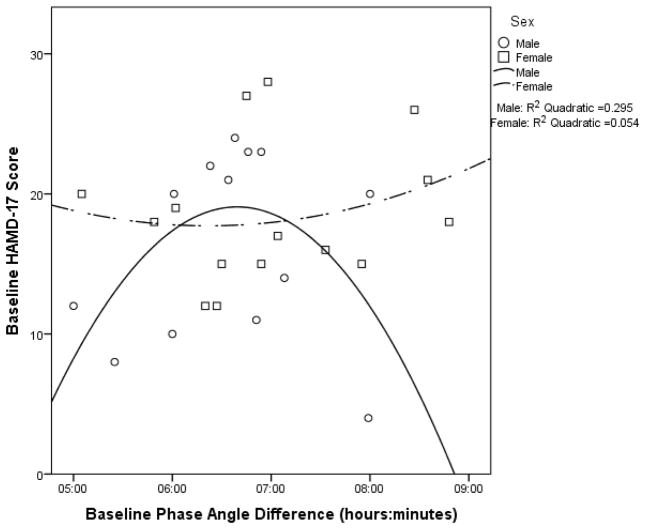
When examining the entire sample, there were no significant associations between Baseline HAMD-17 score and Baseline DLMO or Baseline PAD (see Supplemental Table 2a). Associations were also non-significant when analyzed separately for males and females (see Figures 1 and 2 for the quadratic models and Supplemental Table 2a).
Figure 1.
Baseline HAMD-17 score as a function of baseline DLMO time with quadratic curves fitted for males (p = .398) and females (p = .893).
Figure 2.
Baseline HAMD-17 score as a function of baseline phase angle difference with quadratic curves fitted for males (p = .174) and females (p = .715).
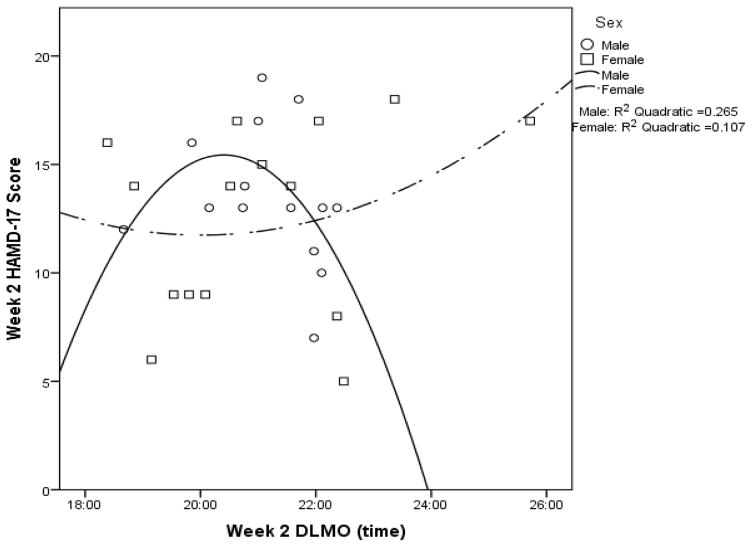
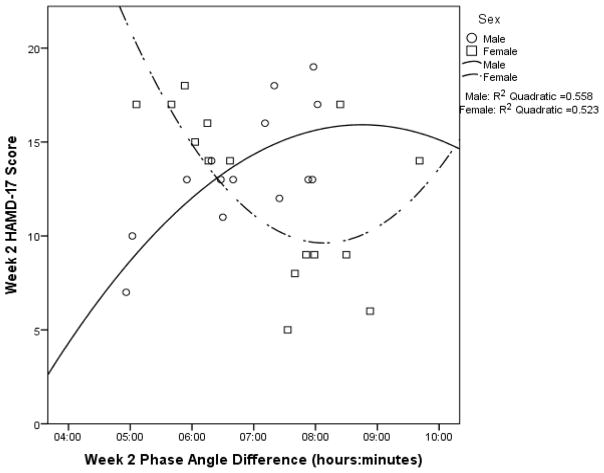
Across the sample, Week 2 HAMD-17 score was not significantly associated with Week 2 DLMO or Week 2 PAD (see Supplemental Table 2b). When analyzed by sex, Week 2 HAMD-17 score was not significantly associated with Week 2 DLMO for males or females (see Figure 3 for the quadratic models and Supplemental Table 2b). However, Week 2 HAMD-17 score was significantly associated with Week 2 PAD for both males and females, albeit in opposite directions. Males showed a positive correlation between Week 2 HAMD-17 score and Week 2 PAD, whereas females had a negative correlation (see Supplemental Table 2b). A similar relationship was observed for the quadratic model (see Figure 4).
Figure 3.
Week 2 HAMD-17 score as a function of week 2 DLMO time with quadratic curves fitted for males (p = .184) and females (p = .508).
Figure 4.
Week 2 HAMD-17 score as a function of week 2 phase angle difference with quadratic curves fitted for males (p = .011) and females (p = .012).
Change in DLMO between Baseline and Week 2 was not associated with Week 2 HAMD-17 score across the sample, nor was change in PAD (see Supplemental Table 2b). When analyzed by sex, change in DLMO was not significantly associated with Week 2 HAMD-17 score for males or females (see Supplemental Table 2b). Change in PAD from Baseline to Week 2 was correlated with Week 2 HAMD-17 scores for males but not females (see Supplemental Table 2b).
When fitted using the entire sample, linear mixed models showed a main effect for time and type of circadian shift, but no interaction for these variables. Thus, only main effects were retained in the final model (model results are shown in Table 3 for the entire sample and by sex). The results from the final model using the entire sample indicate that HAMD-17 scores declined over time for all participants, and those participants who experienced a phase delay had higher HAMD-17 scores across the 8 weeks of study relative to participants who did not experience a phase shift. At the final week of study, the mean HAMD-17 score for participants who did not experience a phase shift was 6.93 (SD = 3.58) versus 12 (SD = 5.10) for those who experienced a phase delay. A similar pattern of results emerged from the model when fit for females. However, males showed only a main effect for time (i.e., their HAMD-17 scores did not differ by circadian phase shift type).
Table 3.
Estimates of fixed effects from linear mixed models predicting depression severity (HAMD-17 score)
| Depression severity (HAMD-17 score)
|
||||||
|---|---|---|---|---|---|---|
| Entire Samplea
|
Malesb
|
Femalesc
|
||||
| B | SE | B | SE | B | SE | |
| Intercept | 15.13** | 5.11 | 17.83 | 15.85 | 13.76** | 4.73 |
| Participant (Level 2) | ||||||
| Baseline | 0.07 | 0.80 | 0.21 | 0.22 | ||
| HAMD-17 | 0.17 | 0.25 | ||||
| Shift Typed | ||||||
| Delay | 3.75* | 1.63 | 1.94 | 3.23 | 5.04** | 1.73 |
| Advance | 1.13 | 1.38 | −0.66 | 2.55 | 2.96 | 1.55 |
| Week of study (Level 1) | ||||||
| Week (linear) | −2.53*** | 0.41 | −2.46*** | 0.56 | −2.59*** | 0.58 |
| Week (quadratic) | 0.14*** | 0.04 | 0.11* | 0.05 | 0.15** | 0.22 |
Note. B = unstandardized coefficient; SE = standard error.
Level 2 N = 28 people; Level 1 N = 241 person-weeks.
Level 2 N = 13 males; Level 1 N = 111 person-weeks.
Level 2 N = 15 females; Level 2 N = 130 person-weeks.
No shift was the reference group for all models.
p < 0.05,
p < 0.01
p ≤.001.
4. Discussion
In the present study, we explored change in circadian timing after 2-weeks of a TIB manipulation administered adjunctively to fluoxetine, and examined relationships between depression symptoms and circadian timing in a randomized controlled trial of time in bed restriction to augment fluoxetine. We also explored whether these relationships were different for males and females.
We did not find any significant relationships between depression symptom severity and DLMO or PAD when we included males and females in the same analyses. However, when analyzed separately by sex, after 2 weeks of fluoxetine and the experimental TIB schedule, Week 2 PAD was negatively correlated with depression symptom severity for females, whereas Week 2 PAD was positively correlated with depression symptom severity for males. Thus, for females, a shorter Week 2 PAD (associated with a shorter duration between the onset of melatonin and mid-sleep, suggestive of a delay of circadian timing relative to the timing of sleep) was linked to more severe depression. In contrast, for males, a longer Week 2 PAD (associated with a longer duration between the onset of melatonin and mid-sleep, suggestive of an advance of circadian timing relative to the timing of sleep) was linked to more severe depression. Further, change in PAD from Baseline to Week 2 was positively correlated with depression symptom severity for males (but not females), such that a lengthening of the PAD at Week 2 (i.e., advance of circadian timing relative to sleep) from Baseline was associated with more severe depression at Week 2.
When we examined the role of a circadian phase shift between baseline and the end of the experimental phase of the study, we found that a phase delay after 2 weeks of fluoxetine was associated with a poorer treatment response across 8 weeks, consistent with our hypothesis. In addition to being statistically significant, this difference clinically significant; the mean depression symptom severity score at the end of the study for participants who did not experience a phase shift is consistent with remission from depression (i.e., ≤ 7; (Frank et al., 1991)), whereas the mean score for participants who experienced a phase delay remained in the symptomatic range. However, when we analyzed the data separately for males and females, we found this effect for females only. Males showed the same response to fluoxetine regardless of whether they experienced a phase shift. This study is the first to our knowledge to use objective methods to determine whether shift in DLMO is associated with antidepressant response.
The lack of a significant relationship between depression severity and DLMO (and PAD when analyzed for males and females together) is consistent with results from Hasler and colleagues (2010), who did not find significant differences between adults with depression and healthy controls on DLMO or the PAD between DLMO and mid-sleep in a sample of both males and females. However, Hasler et al. did show that a delay of core body temperature minimum relative to sleep and a longer PAD between DLMO and core body temperature minimum were associated with greater depression severity. It may be that temperature rhythms are a more sensitive measure of desynchrony than the measure we utilized (midpoint of sleep). Our results confirm and extend the findings of Emens and colleagues (2009), as both studies have shown a linear relationship between depression severity and phase-delayed circadian misalignment as measured by the DLMO-midsleep PAD in females with MDD.
With respect to changes in circadian measures from baseline to post-experimental TIB schedule, on average, across the sample, participants in all three groups experienced minimal changes in DLMO and PAD. Average DLMO at Baseline (21:20) and Week 2 (21:05) were roughly consistent with the only other published report of DLMO in adults with non-seasonal, unipolar depression ((DLMO 21:13; (Emens et al., 2009)). However, the average PAD for our sample at both Baseline and Week 2 of approximately 7 hours is longer than the optimal PAD of 6 hours as suggested by Lewy and colleagues (1998), and longer than the average PAD (6:10) found by Emens and colleagues (2009) in their study of females with depression who were taking an antidepressant. The relatively large standard deviation (approximately 1 hour) in our sample’s PAD could account for some of this difference. Another possible explanation for this discrepancy is the method used to calculate DLMO in the present study (3k threshold), which typically results in DLMOs that are approximately 20 minutes earlier (Molina and Burgess, 2011) than the threshold used by Emens and colleagues (2009) who utilized a 10 pg/ml threshold in their plasma analysis, which corresponds to a 3 pg/ml threshold in saliva (Benloucif et al., 2008). Nevertheless, replication of the PAD in adults with MDD relative to healthy controls will be important to confirm whether the PAD found in the present study is a feature of depression. Although at least one prior study found that depression symptoms were associated with a shorter PAD (indicating a delay; (Emens et al., 2009)) Lewy posits that a PAD longer or shorter than 6 hours indicates misalignment common in seasonal affective disorder (2006), and our results showing a curvilinear relationship between PAD and depression symptoms suggest this may also be true in individuals with MDD.
The stability of the circadian measures between Baseline and Week 2, despite the TIB manipulation administered to two-thirds of the sample, is likely due to an element of the study design. To isolate the effects of TIB restriction from circadian change as an augmentation for fluoxetine, participants were asked to remain in dim light during the period when they would usually be sleeping. Two findings support the efficacy of this instruction in minimizing circadian changes. First, we did not find significant differences in the type of circadian shift from Baseline to Week 2 between the Early Rise time and Late Bedtime groups. Second, although there were no group differences in PAD at baseline, at Week 2, the Late Bedtime group had a significantly longer PAD relative to the Early Rise time and 8h TIB groups. A longer PAD indicates an advanced DLMO relative to the timing of sleep. Thus, the circadian pacemaker did not adjust the time of melatonin onset to correspond with a later midsleep time, as would be expected if participants in the Late Bedtime group were exposed to bioactive light in the hours before their bedtime. Indeed, light intensity data from wrist actigraphy showed excellent compliance for the dim light periods during the 2-week experimental phase of the study, with an average light intensity of approximately 13 lux during the 2h sleep deprivation period across the 2 weeks, well below the threshold known to suppress melatonin. However, there are other possible explanations for the stability of the circadian measures between Baseline and Week 2: first, it remains possible that individuals with MDD are less sensitive to light or other zeitgeibers than healthy controls, although others have not found this to be the case with light exposure (Nathan et al., 1999); second, 2 hours of TIB restriction nightly for 2 weeks may not sufficient to produce significant change in the circadian system; and third, previous work has shown that partial sleep deprivation reduces light-induced circadian phase shifts (Burgess, 2010; Burgess and Eastman, 2006).
Taken together, the results from this preliminary study suggest that phase delay (as evidenced by a shorter PAD and a shift to a later DLMO) after 2 weeks of fluoxetine therapy and an experimental TIB manipulation is associated with more severe depression symptoms and a poorer response to fluoxetine across 8 weeks for females. In contrast, for males, a longer PAD (suggestive of a phase advance) after 2 weeks of fluoxetine therapy and experimental TIB manipulation was associated with more severe depression symptoms; however, phase shift per se (as measured by change in DLMO) was not a predictor of response to fluoxetine across the 8-week study for males. This inverse relationship for females and males may be best understood in the context of sex differences in the timing of sleep relative to the circadian clock. In healthy women without depression, an earlier melatonin onset relative to sleep (i.e., longer PAD) is observed compared to men (Cain et al., 2010). Further, women are more likely to have an earlier chronotype than men (Adan and Natale, 2002). Thus, for females, a phase delay may be more destabilizing to mood (and may potentially negatively influence response to treatment) than an advance, whereas the opposite may hold true for males, who may have more consequences from a lengthening of the interval between melatonin onset and the timing of sleep. However, the exact mechanisms by which circadian and sleep alignment may influence response to antidepressant therapy remain unknown, and future research is necessary to explicate possible mechanisms.
We note that the present study is the first that we are aware of to examine sex differences in the context of circadian rhythms and depression, and thus the first clue that there may be significantly different relationships between circadian timing and depression for males and females. Thus, it will be important for future studies to further elucidate the role of sex in circadian alignment with the timing of sleep and depression. This is particularly critical, as should inverse relationships exist for males and females, lack of consideration for sex differences may lead to a type II error in studies enrolling both men and women.
The findings from the present study must be interpreted as preliminary, and in the context of several limitations. First, some of our null findings could be due to the small sample size, and to the relatively modest circadian changes observed in the sample. Further, as we randomly selected participants for sample assay without controlling by sex, small and unequal numbers of males and females in the subgroups may have limited our power to detect sex differences by condition. Second, our sample was relatively young and mostly Caucasian, which limits the generalizability of the findings. Third, circadian parameters were not measured after the second week of the study, so we do not know whether circadian changes in the last 6 weeks of the study affected treatment response. Fourth, we only assessed relationships between dim light melatonin onset and depression; previous work has also shown relationships between the offset of dim light melatonin and depression (Parry et al., 2008; Sekula et al., 1997). Finally, the influence of fluoxetine must also be considered when interpreting these findings (Schaufler et al., 2016). Baseline circadian assessments were completed before participants started fluoxetine, and at Week 2 they had been taking fluoxetine daily for approximately 14 days. Selective serotonin reuptake inhibitors are known to influence circadian rhythms in animals, with studies showing normalization of circadian patterns in mice bred for anxiety and depression-like behavior (Schaufler et al., 2016), and advancement of rhythms in acute (Sprouse et al., 2006), but not chronic (Duncan et al., 2010) exposure. Other psychotropic medications, such as lithium, are associated with phase delays in both rats and healthy humans (Kripke et al., 1979; McEachron et al., 1982). However, effects of selective serotonin reuptake inhibitors on circadian rhythms in humans have not been elucidated, which limits the context in which we can interpret the findings. A further complicating factor is the potential of sex differences in response to fluoxetine, as some previous studies have shown that females respond better to selective serotonin reuptake inhibitors than men (Berlanga and Flores-Ramos, 2006; Khan et al., 2005; Kornstein et al., 2000).
Nevertheless, the present study represents an important step in the body of literature on major depressive disorder and circadian rhythms. Strengths of this study include a longer period of adaptation to a consistent TIB (14 days relative to 3–7 days used in previous studies), and all participants used the same antidepressant (fluoxetine). Our results provide a kernel of evidence to suggest that circadian parameters may be linked to depression in different ways in men and women. For men, an advancement of the time of melatonin onset relative to sleep is associated with more severe depression symptoms after 2 weeks of fluoxetine and TIB manipulation, whereas for women, a shorter window of time between melatonin onset and sleep is associated with more severe depression symptoms after 2 weeks of fluoxetine and TIB manipulation. Further, for women only, a phase delay when starting an antidepressant was associated with a worse treatment response across 8 weeks of fluoxetine. Replication of these finding are important, as they have implications for the optimal conditions for starting antidepressant therapy to maximize efficacy.
Supplementary Material
HIGHLIGHTS.
Circadian measures have not been studied in antidepressant treatment response.
We examined circadian markers in a trial of repeated partial sleep deprivation to adjunctive to fluoxetine.
A shorter phase angle difference was associated with more depression symptoms in females.
A longer phase angle difference was associated with more depression symptoms in males.
Phase delay was associated with poorer antidepressant response in females, but not males.
Acknowledgments
Funding: This work was supported by the National Institute of Mental Health (Arnedt; R01 MH077690), the National Heart, Lung, and Blood Institute (Swanson; K23HL122461), and the National Center for Advancing Translational Sciences (University of Michigan; UL1TR00043). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
Roseanne Armitage was a paid consultant for the University of Ottawa Institute of Mental Health, Ottawa, Ontario, Canada.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19(4):709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, Sadeh A, Spira AP, Taylor DJ. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav Sleep Med. 2015;13(Suppl 1):S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Swanson LM, Dopp RR, Bertram HS, Mooney AJ, Huntley ED, Hoffmann RF, Armitage R. Effects of Restricted Time in Bed on Antidepressant Treatment Response: A Randomized Controlled Trial. J Clin Psychiatry. 2016 doi: 10.4088/JCP.15m09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med. 2008;4(1):66–69. [PMC free article] [PubMed] [Google Scholar]
- Berlanga C, Flores-Ramos M. Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J Affect Disord. 2006;95(1–3):119–123. doi: 10.1016/j.jad.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Burgess HJ. Partial sleep deprivation reduces phase advances to light in humans. J Biol Rhythms. 2010;25(6):460–468. doi: 10.1177/0748730410385544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Short nights reduce light-induced circadian phase delays in humans. Sleep. 2006;29(1):25–30. doi: 10.1093/sleep/29.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, Schoen MW, Czeisler CA, Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JW, Lam SP, Li SX, Yu MW, Chan NY, Zhang J, Wing YK. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. 2014;37(5):911–917. doi: 10.5665/sleep.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Hester JM, Hopper JA, Franklin KM. The effects of aging and chronic fluoxetine treatment on circadian rhythms and suprachiasmatic nucleus expression of neuropeptide genes and 5-HT(1B) receptors. The European journal of neuroscience. 2010;31(9):1646–1654. doi: 10.1111/j.1460-9568.2010.07186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168(3):259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Gaspar-Barba E, Calati R, Cruz-Fuentes CS, Ontiveros-Uribe MP, Natale V, De Ronchi D, Serretti A. Depressive symptomatology is influenced by chronotypes. J Affect Disord. 2009;119(1–3):100–106. doi: 10.1016/j.jad.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361–377. [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Buysse DJ, Kupfer DJ, Germain A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: Further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 2010;178:205–207. doi: 10.1016/j.psychres.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1. American Academy of Sleep Medicine; Westchester, Illinois: 2007. [Google Scholar]
- Jones SG, Benca RM. Circadian Disruption in Psychiatric Disorders. Sleep Med Clin. 2015;10(4):481–493. doi: 10.1016/j.jsmc.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol. 2005;25(4):318–324. doi: 10.1097/01.jcp.0000168879.03169.ce. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157(9):1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Judd LL, Hubbard B, Janowsky DS, Huey LY. The effect of lithium carbonate on the circadian rhythm of sleep in normal human subjects. Biol Psychiatry. 1979;14(3):545–548. [PubMed] [Google Scholar]
- Lewy AJ. The dim light melatonin onset, melatonin assays and biological rhythm research in humans. Biological signals and receptors. 1999;8(1–2):79–83. doi: 10.1159/000014573. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Cutler NL, Sack RL, Ahmed S, Thomas KH, Blood ML, Jackson JM. Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55(10):890–896. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103(19):7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachron DL, Kripke DF, Hawkins R, Haus E, Pavlinac D, Deftos L. Lithium delays biochemical circadian rhythms in rats. Neuropsychobiology. 1982;8(1):12–29. doi: 10.1159/000117873. [DOI] [PubMed] [Google Scholar]
- Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int. 2011;28(8):714–718. doi: 10.3109/07420528.2011.597531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PJ, Burrows GD, Norman TR. Melatonin sensitivity to dim white light in affective disorders. Neuropsychopharmacology. 1999;21(3):408–413. doi: 10.1016/S0893-133X(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, Hauger RL, Elliott JA. Increased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. J Clin Endocrinol Metab. 2008;93(1):54–60. doi: 10.1210/jc.2006-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack DA, Duncan W, Rosenthal NE, Mendelson WE, Wehr TA. The timing and duration of sleep in partial sleep deprivation therapy of depression. Acta Psychiatr Scand. 1988;77(2):219–224. doi: 10.1111/j.1600-0447.1988.tb05104.x. [DOI] [PubMed] [Google Scholar]
- Schaufler J, Ronovsky M, Savalli G, Cabatic M, Sartori SB, Singewald N, Pollak DD. Fluoxetine normalizes disrupted light-induced entrainment, fragmented ultradian rhythms and altered hippocampal clock gene expression in an animal model of high trait anxiety- and depression-related behavior. Ann Med. 2016;48(1–2):17–27. doi: 10.3109/07853890.2015.1122216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilgen B, Tölle R. Partial sleep deprivation as therapy for depression. Arch Gen Psychiatry. 1980:267–271. doi: 10.1001/archpsyc.1980.01780160037003. [DOI] [PubMed] [Google Scholar]
- Sekula LK, Lucke JF, Heist EK, Czambel RK, Rubin RT. Neuroendocrine aspects of primary endogenous depression. XV: Mathematical modeling of nocturnal melatonin secretion in major depressives and normal controls. Psychiatry Res. 1997;69(2–3):143–153. doi: 10.1016/s0165-1781(96)02937-x. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Braselton J, Reynolds L. Fluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firing. Biol Psychiatry. 2006;60(8):896–899. doi: 10.1016/j.biopsych.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary Melatonin as a Circadian Phase Marker: Validation and Comparison to Plasma Melatonin. Journal of Biological Rhythms. 1997;12(5):457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.