Abstract
Objective
Cartilage fatigue, due to mechanical work, may account for the early development of degenerative joint disease in the temporomandibular joint (TMJ), and why women are 3-times more likely to be afflicted. This study tested for gender differences in mechanical energy densities in women and men with healthy TMJs.
Design
Eighteen women and eighteen men gave informed consent. Research diagnostic criteria including imaging were used to ensure that subjects’ TMJs were normal, without disc displacement or signs of degenerative joint disease. Numerical modeling determined TMJ loads (Fnormal). Jaw tracking and three-dimensional dynamic stereometry characterized individual-specific data of stress-field dynamic mechanics during 10 symmetrical jaw closing cycles. These data were used to estimate tractional forces (Ftraction). Energy densities were then calculated, where: Energy Density=W/Q (W=work done or mechanical energy input=Ftraction* distance of stress-field translation, Q=volume of cartilage). Two-way analysis of variance and follow-up two-group comparisons tested mean energy densities for ipsilateral and contralateral TMJs in women versus men.
Results
Mean energy densities ± standard deviations in ipsilateral and contralateral TMJs in women were 9.0 ± 9.7 and 8.4 ± 5.5 mJ/mm3, respectively, and were significantly larger (P=0.004 and 0.001, respectively) compared to ipsilateral and contralateral TMJs in men, which were 5.6 ± 4.2 and 6.3 ± 4.2 mJ/mm3, respectively.
Conclusions
Energy densities were significantly larger in healthy TMJs of women than men. Larger TMJ energy densities during normal jaw functions could predispose earlier mechanical fatigue of the TMJ disc.
Keywords: energy density, gender, human, mechanical fatigue, mechanics, temporomandibular joint
Introduction
The osseous changes associated with degenerative joint disease (DJD) of the temporomandibular joint (TMJ) are late-stage events1 seen in 20–30 year-olds. This is over a decade earlier than DJD in other human joints2. TMJ disc displacement appears to precede development of DJD in animal models3 and in some humans4. Why TMJ tissues fail so early in life, and why women are 2–3 times more likely to develop osteoarthritis compared to men5, is not understood.
Early mechanical fatigue is thought to be a factor6 associated with DJD of the TMJ and may be playing a role in disease progression. As in the human knee7, mechanical work imposed on the articular surfaces of the TMJ is a consequence of tractional (sum of plowing and frictional) forces (Ftraction) resulting from stress-field translation,8 which is the movement of the loaded contact area that occurs during jaw function. The magnitude of work done per volume of cartilage, also known as energy density9 (mJ/mm3), may influence the rate of fatigue of the collagen fibers of the TMJ fibrocartilaginous disc. This is especially likely along the mediolateral axis of the disc which is more prone to tissue fatigue due to the anisotropic nature of the TMJ disc10 and because high stress-field translation velocities, up to 110 mm/s, have been recorded along this axis11.
Given the predilection of TMJ DJD in women, the objective of the current project was to test for gender differences in mechanical energy densities in men and women with healthy TMJs.
Methods
The protocols used in this project were approved by both the University at Buffalo and University of Missouri-Kansas City Institutional Review Boards. Procedures followed were in accordance with the ethical standards of the two university institutional review boards and with the Helsinki declaration of 1975, as revised in 2000. All subjects gave informed consent to participate in this study. Inclusion and classification of subjects were based on Diagnostic Criteria for Temporomandibular Disorders12 and magnetic resonance (MR) and cone-beam computed tomography (CBCT) images of the TMJs. Individuals were excluded from participating if they were pregnant; had a diagnosed systemic musculoskeletal or rheumatological disease such as fibromyalgia or muscular atrophy; had TMJ disc displacement or degenerative disease based on MR and CBCT images; or if they had multiple missing teeth, large dental restorations, or fixed orthodontic appliances.
As previously described9, dynamic stereometry was used to estimate the magnitudes of the variables (Table 1) used in energy density calculations of each subject’s right and left TMJs. Briefly, dynamic stereometry processes involved the three-dimensional reconstruction of subject-specific anatomical structures captured from MR images, and the animation of these structures using motion data captured from jaw tracking recordings. Anatomy and motion data sets were linked via a common head reference system attached to a custom occlusal registration appliance that each subject bit into and held stable during both types of data collection. The MR images of each reference system and TMJ were made at serial oblique sagittal slices perpendicular to the main condylar axis using 1.5 T MR equipment with TMJ surface coils of 12 cm radius.9 Jaw tracking recordings were made in another clinical setting via an optoelectronic system with 3 linear cameras mounted at fixed geometry and resolution >5 µm at a sampling frequency of 200 Hz. These cameras captured the relative positions of triangular target frames, carrying 3 non-collinear light emitting diodes (LEDs) each, attached to the head reference system and the upper and lower jaws via the teeth. During the jaw tracking sessions, static positions of the jaws and reference system were recorded while each subject bit into the occlusal registration appliance. In addition, time-varying upper and lower jaw LED positions were recorded during 10 symmetrical jaw opening-closing movements.
Table 1.
Symbols and units for variables of interest
| Symbol | Variable | Units |
|---|---|---|
| Ftraction | Surface tractional forces | N |
| h | Thickness of TMJ disc | mm |
| D | Mediolateral position of the centroid of the stress-field | mm |
| a | Radius of stress-field | mm |
| Q | TMJ disc volume under the translating stress-field | mm3 |
| y | Velocity of stress-field translation | mm/s |
| μtraction | Tractional coefficient = Ftraction/Fnormal | (none) |
| a | Constant=0.23* | (none) |
| x | Aspect ratio•compressive strain3 = a/h•(Δh/h)3 | (none) |
| a/h | Aspect ratio | (none) |
| Δh/h | Compressive strain ≈ 0.1 =TMJ disc thickness change due to loading/original thickness |
(none) |
| x0 | Constant=0.82* | (none) |
| b | Constant=0.49* | (none) |
| y0 | Constant=260* | mm/s |
| c | Constant=141* | mm/s |
| Fnormal | TMJ load | N |
| ΔD | Distance of stress-field translation | mm |
| Ftraction•ΔD | Work done | mJ |
| ψ | Energy density | mJ/mm3 |
Constant derived from previous laboratory experiments8.
Reconstruction and animation of each TMJ to within errors of ≤0.9% were performed on a graphics workstation as previously described9. Briefly, the reconstruction processes entailed first tracing on each MR slice anatomical and reference structure contours defined by driving points of spline functions, then triangulating the contour sets to depict the three-dimensional surface relationships. Visualization and resolution were improved by use of shading and smoothing algorithms, respectively. The reconstructed TMJ structures were animated via mathematical transformations based on the spatial positions of the vertices of polygons that represented the recorded surfaces as these changed during the jaw opening-closing movements.
For each TMJ, the condylar long axis was defined as the mediolateral axis and magnitudes of the variables of interest (Table 1) were determined from the reconstructed and animated MR images over 5 ms time intervals of jaw closing as previously described9. That is, to quantify the geometry of the stress-field for each time interval, first the area of minimum distance between TMJ fossa and eminence was identified. Next, the 30 smallest adjacent fossa-eminence distances, measured between polygon vertices, were identified and:
the distances averaged to determine the minimum disc thickness, h;
the centroid of the area defined by these distances was calculated to determine the mediolateral position of the stress-field, D;
the standard deviation about the centroid was quantified to determine the radius of the stress-field, a.
Once D was determined, the local volume of cartilage (Q, mm3) under the translating stress-field was calculated by integrating under the area of the stress-field for each time interval. Then, the coordinates of D were smoothed over 30 ms and velocity of the stress-field translation (y, mm/s) quantified. The magnitudes of these variables were used in an empirical model8 that describes the relationship between stress-field geometry and dynamics and a tractional coefficient (μtraction):
where a, b, c, x0 and y0 are constants derived from laboratory experiments,8 x is aspect ratio•compressive strain3 (a/h•(Δh/h)3, where Δh/h≈0.1) and Fnormal is the perpendicular TMJ loading force. Fnormal for right and left TMJs in each subject for an ordinary bite-force (20 N) applied on the mandibular canine at a range of directions representing normal function were determined using established numerical modeling techniques9, 13. The computer-assisted numerical model employed each subject’s three-dimensional craniomandibular geometry extracted from CBCT images and right and left TMJ eminence morphologies derived from dynamic stereometry plus the objective function of minimization of muscle effort (sum of muscle forces squared) to predict right and left Fnormal for a given biting condition. Average Fnormal for the range of biting angles represented were then calculated for TMJs ipsilateral and contralateral to the applied bite-force and used in the rearranged μtraction equation to determine subject- and TMJ-specific Ftraction. Ultimately, these results then applied to determine mechanical work done (Ftraction*ΔD) and energy densities (ψ = (Ftraction*ΔD)/Q, mJ/mm3) in the ipsilateral and contralateral TMJs of each subject during 5 ms intervals of jaw closing.
The series of instantaneous energy densities calculated at 5 ms intervals during 10 consecutive symmetrical closing cycles of the mandible were then averaged for ipsilateral and contralateral TMJ in each subject. Descriptive statistics were reported as mean and standard deviation. Two-way analysis of variance (ANOVA) investigated whether or not significant effects existed in gender, side, and the interaction of gender and side. Follow-up two-group comparisons tested mean energy densities in the ipsilateral and contralateral TMJs of women versus men. Partial eta2 for effect size was estimated in the two-way ANOVA. Statistical significance was indicated by P<0.05. All analyses were performed using commercial software (SPSS version 23, IBM SPSS Inc., Chicago, IL).
Results
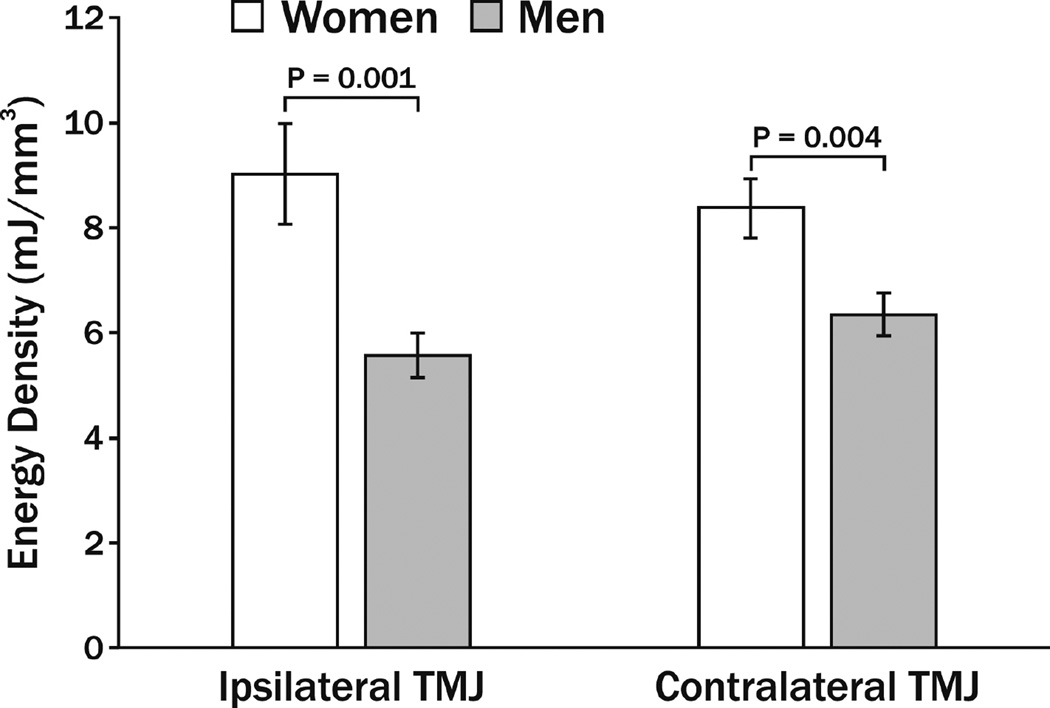
Eighteen women and eighteen men with average (±standard deviation) ages of 31.1 (±9.4) and 30.9 (±10.7) years, respectively, participated. Contralateral TMJs showed average Fnormal that were generally higher than for ipsilateral TMJs and were not markedly different between women (16.3±4.2 N) and men (15.7±2.6 N). Instantaneous energy densities per closing cycle for all subjects ranged from 0.1 to 146.1 mJ/mm3. Mean (±standard deviation) energy densities per closing cycle for ipsilateral and contralateral TMJs in women were 9.0 (±9.6) and 8.4 (±5.5) mJ/mm3, respectively, and in men were 5.6 (±4.2) and 6.3 (±4.2) mJ/mm3, respectively (Fig. 1). That is, mean energy densities for TMJs in women were significantly higher than men by 38% on the ipsilateral side (P=0.001; 95% confidence interval of mean difference 1.38–5.54) and 25% on the contralateral side (P=0.004; 95% confidence interval of mean difference 0.67–3.42). The effect of gender on TMJ energy densities showed partial eta2 of 0.046 in two-way ANOVA at the overall level.
Fig. 1.
Mean energy densities (mJ/mm3) in the ipsilateral and contralateral TMJs of women and men during jaw closing movements, where vertical bars indicate ±standard errors. P-values show results of follow-up comparisons after significance was found at the overall level in the two-way ANOVA. Significance was defined by P<0.05.
Discussion
The current work demonstrates how individual- and joint-specific contact mechanics can be quantified in living humans. The results show significantly higher mean energy densities during jaw closing in women compared to men with healthy TMJs. TMJ loads (Fnormal) do not appear to account for these gender differences. However, velocity of stress-field translation (y, mm/s), which contributes to the magnitude of surface Ftraction, and ultimately energy densities imposed on articular cartilages, may be an important variable that has been shown previously to vary between joints and functions11, 14 and should be investigated further in future research. Reports show remarkable similarities for y measured in human TMJs, where average peak values were 47 mm/s in normal joints during jaw closing,14 and in human knees, where average values at heal strike were 29.1 and 43.9 mm/s in healthy and arthritic joints, respectively7. Due to the lack of vascularity of both the TMJ disc and knee meniscus, solute movement within the cartilage matrix is determined by osmotic pressure for fluid absorption and mechanical loading for exudation. Higher cell densities and nutrient consumption rates in the TMJ disc compared to hyaline cartilages and the intervertebral disc, increase the vulnerability of TMJ disc fibroblasts to oxidative stress.15 Stress-field translation related plowing forces8 can move fluids through the TMJ disc to ensure adequate nutrition to fibroblasts. However, energy densities of sufficient magnitude potentiate the fatigue of the collagen fibers of the disc. The significantly higher energy densities in the TMJs of women compared to men could escalate the rate of mechanical fatigue of articulating surfaces, and may explain, in part, the gender bias of degenerative joint disease in the TMJ.
Acknowledgments
The authors thank the subjects for their participation. At the University of Zurich, Stefan Erni, Alessandro Gallo, Vera Colombo, and Eveline Studer contributed to computer programing and data analysis. At the University at Buffalo, Dr. Khawaja Nasir helped in subject diagnosis and Dr. Heidi Crow provided general support.
Role of the funding source
This project was supported by the National Institute for Dental and Craniofacial Research (NIDCR; R01 DE016417, JN-PI). NIDCR had no involvement in the design, collection, analysis and interpretation of data, nor in the writing or submission of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
All authors made substantial contributions to design of the study, interpretation of data, revisions of the manuscript, and provided final approval for submission of the manuscript. Specifically, Y.M. Gonzalez oversaw subject recruitment, diagnoses and management plus image acquisition. J.C. Nickel, L.R. Iwasaki, and H. Liu were responsible for data collection and numerical modeling. L.M. Gallo and M. Markova were responsible for all aspects of dynamic stereometric analyses. Y. Liu was responsible for statistical analyses. J.C. Nickel (nickeljc@umkc.edu) and L.R. Iwasaki (iwasakil@umkc.edu) take responsibility for the integrity of the work as a whole.
Competing interest statement
None of the authors have any financial or personal relationships that could potentially influence the outcome of this work.
Contributor Information
L.R. Iwasaki, University of Missouri-Kansas City, School of Dentistry, Departments of Orthodontics & Dentofacial Orthopedics and Oral & Craniofacial Sciences, iwasakil@umkc.edu
Y.M. Gonzalez, University at Buffalo, School of Dental Medicine, Department of Oral Diagnostic Sciences, ymg@buffalo.edu
Y. Liu, East Tennessee State University, Department of Biostatistics & Epidemiology, LIUY09@mail.etsu.edu
H. Liu, University of Missouri-Kansas City, School of Dentistry, Department of Oral & Craniofacial Sciences, liuhong@umkc.edu
M. Markova, University of Zurich, Center for Dental & Oral Medicine & Oral Maxillofacial Surgery, Clinic for Masticatory Disorders, michala.markova@zzm.uzh.ch
L.M. Gallo, University of Zurich, Center for Dental & Oral Medicine & Oral Maxillofacial Surgery, Clinic for Masticatory Disorders, Luigi.Gallo@zzm.uzh.ch
J.C. Nickel, University of Missouri-Kansas City, School of Dentistry, Departments of Orthodontics & Dentofacial Orthopedics and Oral & Craniofacial Sciences
References
- 1.Wiese M, Wenzel A, Hintze H, Petersson A, Knutsson K, Bakke M, et al. Osseous changes and condyle position in TMJ tomograms: impact of RDC/TMD clinical diagnoses on agreement between expected and actual findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:e52–e63. doi: 10.1016/j.tripleo.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki LR, Crosby MJ, Marx DB, Gonzalez Y, McCall WD, Jr, Ohrbach R, et al. Human temporomandibular joint eminence shape and load minimization. J Dent Res. 2010;89:722–727. doi: 10.1177/0022034510364492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharawy M, Ali AM, Choi WS. Experimental induction of anterior disk displacement of the rabbit craniomandibular joint: an immuno-electron microscopic study of collagen and proteoglycan occurrence in the condylar cartilage. J Oral Pathol Med. 2003;32:176–184. doi: 10.1034/j.1600-0714.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 4.Roh HS, Kim W, Kim YK, Lee JY. Relationships between disk displacement, joint effusion, and degenerative changes of the TMJ in TMD patients based on MRI findings. J Craniomaxillofac Surg. 2012;40:283–286. doi: 10.1016/j.jcms.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim K, Wojczynska A, Lee JY. The incidence of osteoarthritic change on computed tomography of Korean temporomandibular disorder patients diagnosed by RDC/TMD; a retrospective study. Acta Odontol Scand. 2016;74:337–342. doi: 10.3109/00016357.2015.1136678. [DOI] [PubMed] [Google Scholar]
- 6.Juran CM, Dolwick MF, McFetridge PS. Shear mechanics of the TMJ disc: relationship to common clinical observations. J Dent Res. 2013;92:193–198. doi: 10.1177/0022034512468749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrokhi S, Voycheck CA, Gustafson JA, Fitzgerald GK, Tashman S. Knee joint contact mechanics during downhill gait and its relationship with varus/valgus motion and muscle strength in patients with knee osteoarthritis. Knee. 2016;23:49–56. doi: 10.1016/j.knee.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickel JC, Iwasaki LR, Beatty MW, Marx DB. Tractional forces on porcine temporomandibular joint discs. J Dent Res. 2009;88:736–740. doi: 10.1177/0022034509340161. [DOI] [PubMed] [Google Scholar]
- 9.Gallo LM, Iwasaki LR, Gonzalez YM, Liu H, Marx DB, Nickel JC. Diagnostic group differences in temporomandibular joint energy densities. Orthod Craniofac Res. 2015;18(Suppl 1):164–169. doi: 10.1111/ocr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beatty MW, Nickel JC, Iwasaki LR, Leiker M. Mechanical response of the porcine temporomandibular joint disc to an impact event and repeated tensile loading. J Orofac Pain. 2003;17:160–166. [PubMed] [Google Scholar]
- 11.Gallo LM, Chiaravalloti G, Iwasaki LR, Nickel JC, Palla S. Mechanical work during stress-field translation in the human TMJ. J Dent Res. 2006;85:1006–1010. doi: 10.1177/154405910608501106. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki LR, Crosby MJ, Gonzalez Y, McCall WD, Marx DB, Ohrbach R, et al. Temporomandibular joint loads in subjects with and without disc displacement. Orthop Rev (Pavia) 2009;1:90–93. doi: 10.4081/or.2009.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo LM, Nickel JC, Iwasaki LR, Palla S. Stress-field translation in the healthy human temporomandibular joint. J Dent Res. 2000;79:1740–1746. doi: 10.1177/00220345000790100201. [DOI] [PubMed] [Google Scholar]
- 15.Kuo J, Shi C, Cisewski S, Zhang L, Kern MJ, Yao H. Regional cell density distribution and oxygen consumption rates in porcine TMJ discs: an explant study. Osteoarthritis Cartilage. 2011;19:911–918. doi: 10.1016/j.joca.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]