Abstract
Tumor consistency is a critical factor that influences operative strategy and patient counseling. Magnetic resonance imaging (MRI) describes the concentration of water within living tissues and as such, is hypothesized to predict aspects of their biomechanical behavior. In meningiomas, MRI signal intensity has been used to predict the consistency of the tumor and its histopathological subtype, though its predictive capacity is debated in the literature.
We performed a systematic review of the PubMed database since 1990 concerning MRI appearance and tumor consistency to assess whether or not MRI can be used reliably to predict tumor firmness. The inclusion criteria were case series and clinical studies that described attempts to correlate preoperative MRI findings with tumor consistency. The relationship between the pre-operative imaging characteristics, intraoperative findings, and World Health Organization (WHO) histopathological subtype is described.
While T2 signal intensity and MR elastography provide a useful predictive measure of tumor consistency, other techniques have not been validated. T1-weighted imaging was not found to offer any diagnostic or predictive value. A quantitative assessment of T2 signal intensity more reliably predicts consistency than inherently variable qualitative analyses.
Preoperative knowledge of tumor firmness affords the neurosurgeon substantial benefit when planning surgical techniques. Based upon our review of the literature, we currently recommend the use of T2-weighted MRI for predicting consistency, which has been shown to correlate well with analysis of tumor histological subtype. Development of standard measures of tumor consistency, standard MRI quantification metrics, and further exploration of MRI technique may improve the predictive ability of neuroimaging for meningiomas.
Keywords: meningioma, tumor consistency, magnetic resonance imaging, pathology
Introduction
Meningiomas are the most common intracranial brain tumor [1, 2]. Magnetic Resonance Imaging (MRI) is vital to the diagnosis and characterization of these tumors, particularly when selecting a treatment plan. Surgical resection is the current treatment of choice for large meningiomas refractory to radiation treatment or those with symptomatic mass effect, while radiosurgery, chemotherapy, and arterial embolization play a more supplementary role.
Consistency is a critical factor that influences ease of meningioma resection and risk of operative morbidity [3–5]. Meningiomas range in texture from “soft and/or suckable” to “firm and/or fibrous” [1]. Soft meningiomas tend to be easily resectable and are associated with lower surgical morbidity, shorter operative time, and lower rates of recurrence [6, 7]. Meningioma consistency is thought to be a function of water and collagen content, though there is not yet a consensus on the correlation between consistency and histopathological subtype [1, 3–5, 12–16].
The growing prevalence of minimally invasive (e.g. endoscopic) intracranial tumor resection necessitates a reliable, non-invasive technique to predict consistency. Softer tumors would purportedly be easier to remove endoscopically, while firmer tumors may require a traditional open approach, especially around the skull base [20, 25, 32]. A validated preoperative neuroimaging strategy using would obviously be of great value to both surgeon and patient in informing surgical planning, operative strategy, and patient counseling. It may also be useful in determining the need for adjuvant treatment.
Several authors have studied the utility of MRI in predicting tumor consistency. However, previous studies vary widely in scope and quality, and the accuracy of current neuroimaging techniques remains controversial. A recent review by Shiroishi et al provides a good summary of the existing literature but does not address study quality or correlation with tumor pathology [35]. The purpose of this study is to provide an updated review of the use of MRI to predict meningioma consistency. The secondary aim is to summarize studies correlating MRI findings and consistency with histopathological analysis, and to review the application of advanced MRI techniques.
Methods
Literature Search Strategy
We conducted a literature search of the PubMed and Cochrane databases for relevant clinical studies. We limited our search strategy to articles published in the last fifteen years in order to provide the most contemporary review. We queried both databases using a combination of keywords “consistency”"texture”, or “firmness”, and the Medical Subject Heading (MeSH) terms “meningioma” and “magnetic resonance imaging”. The inclusion criteria were case series and clinical studies describing attempts to correlate preoperative MRI findings with tumor consistency and histopathology. Titles and abstracts were reviewed for the following exclusion criteria: 1) does not use MRI; 2) does not study meningiomas; 3) non-human studies; 4) studies not written in English; 5) studies that only correlate MRI findings with histopathology without addressing consistency; and 6) review articles that offer no new information. After redundant articles were removed, full text review was performed for publications that met these criteria. Reference lists of relevant articles were searched to identify additional studies. The date of the last search was October 12 2015.
Assessment of Study Quality
We used a modified scale of methodological quality for clinical studies of radiologic examinations created by Arrivé et al to assess study quality [36]. The original scale includes fifteen standards related to study design, patient population, and imaging analysis, with each standard receiving two, one, or no points for “yes,” “partially,” or “no” in regards to how well the article fulfilled the criteria. We omitted the standard of management of indeterminate examination results, and included one additional standard to the scale: “Was tumor consistency graded on an ordinal scale?”, for a maximum score of 30. Publications scoring between 20–30 were graded as “good,” 10–19 as “fair,” and 0–9 as “poor.”
Data Collection
All studies that described correlation between preoperative MRI findings and meningioma consistency were reviewed. The studies were abstracted for study design, sample size, field strength, imaging modalities, intraoperative assessment of consistency, sensitivity and specificity, and World Health Organization (WHO) histopathological subtype if included. In studies involving different types of brain tumors, only meningioma cases were included in the review. Studies that described new MRI advancements (e.g. elastography, fractional anisotropy) were also included to provide the most comprehensive and contemporary information.
Results
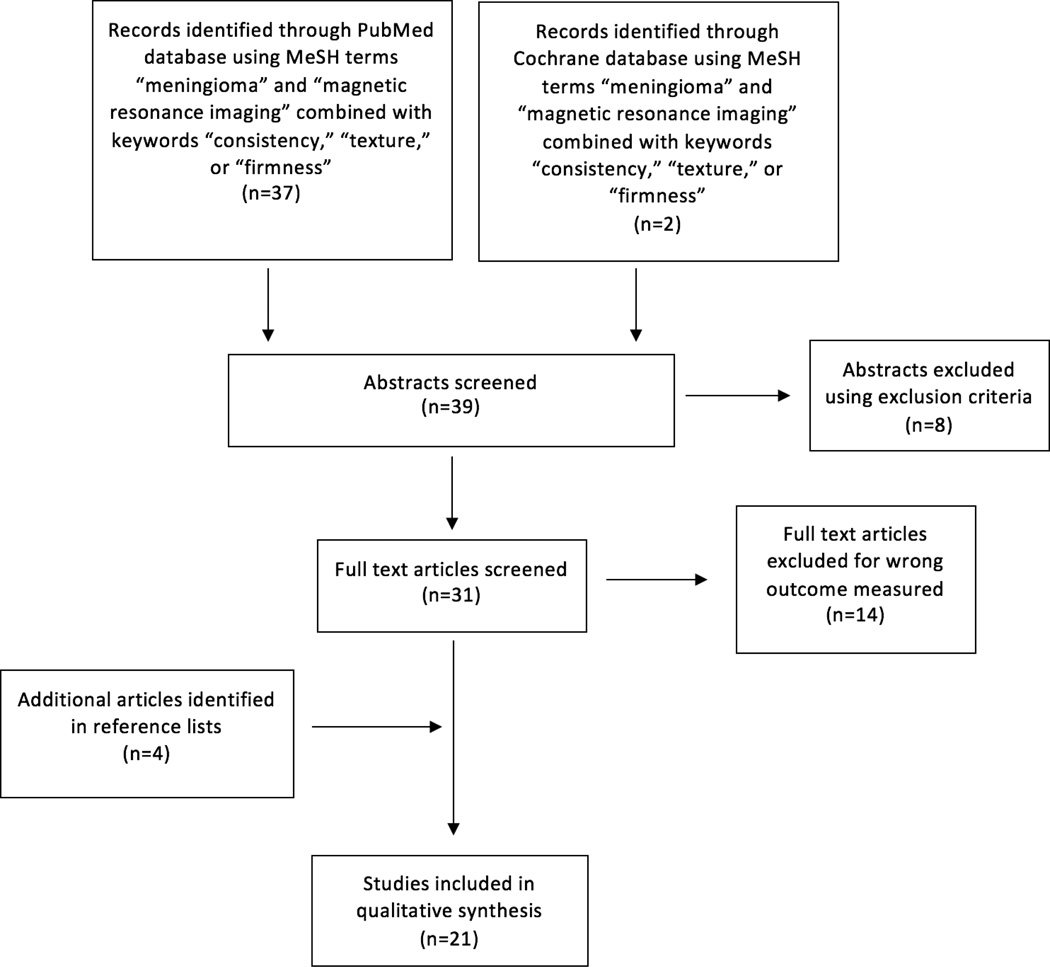
The PubMed search strategy retrieved 37 abstracts. The search of the Cochrane database yielded two additional abstracts. The review of reference lists yielded four additional abstracts. A total of 43 abstracts were reviewed. Figure 1 demonstrates a flow chart outlining the selection process for relevant studies.
Figure 1.
Flow chart outlining the selection process of relevant studies.
Twenty-one studies were eligible to be included in this review. Table 1 provides a summary of findings.
Table 1.
Summary of 21 articles reviewed.
| Paper | Year | N | Field | T1WI | T2WI | QA? | Histology | Angiography | MRE | FA | ADC | FLAIR | FIESTA | PDWI | Score1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hughes | 2015 | 15 | 3T | ns | ns | n/a | ns | ns | + | ns | ns | ns | ns | ns | 20 |
| Watanabe | 2015 | 43 | 3T | − | + | Yes | ns | ns | ns | ns | − | + | + | ns | 20 |
| Ortega-Porcayo* | 2015 | 16 | 3T | + | + | No | − | ns | ns | − | ns | ns | ns | ns | 14 |
| Smith | 2015 | 20 | ? | − | + | Yes | ns | ns | ns | ns | ns | − | ns | − | 15 |
| Murphy | 2014 | 13 | 3T | − | − | No | ns | ns | + | ns | ns | ns | ns | ns | 18 |
| Romani | 2014 | 110 | 3T | − | − | No | ns | ns | ns | + | − | − | ns | ns | 18 |
| Yogi | 2014 | 27 | 1.5T | ns | ns | n/a | ns | ns | ns | ns | + | ns | ns | ns | 18 |
| Sitthinamsuwan | 2012 | 243 | ? | − | + | No | ns | ns | ns | ns | ns | + | ns | ns | 14 |
| Hoover* | 2011 | 38 | ? | + | + | No | ns | − | ns | ns | − | ns | ns | ns | 16 |
| Kashimura | 2007 | 29 | 3T | ns | − | No | + | ns | ns | + | ns | ns | ns | ns | 11 |
| Tropine | 2007 | 30 | 1.5T | ns | ns | n/a | + | ns | ns | + | ns | ns | ns | ns | 11 |
| Xu | 2007 | 6 | 3T | ns | ns | n/a | ns | ns | + | ns | ns | ns | ns | ns | 13 |
| Yrjana | 2006 | 21 | 0.23T | − | + | Yes | + | − | ns | ns | ns | + | ns | ns | 17 |
| Yoneoka | 2002 | 20 | 3T | ns | + | No | + | ns | ns | ns | ns | ns | ns | ns | 11 |
| Maiuri | 1999 | 35 | ? | − | + | No | + | + | ns | ns | ns | ns | ns | ns | 13 |
| Ildan | 1999 | 126 | 1.5T | − | + | No | + | ns | ns | ns | ns | ns | ns | ns | 17 |
| Yamaguchi | 1997 | 50 | 1.5T | − | + | No | − | ns | ns | ns | ns | ns | ns | ns | 9 |
| Soyama | 1995 | 40 | 0.5/1.5T | ns | + | No | + | ns | ns | ns | ns | ns | ns | ns | 16 |
| Suzuki | 1994 | 73 | 0.5/1.5T | − | + | No | + | ns | ns | ns | ns | ns | ns | − | 11 |
| Carpeggiani | 1993 | 43 | 1.5T | − | − | No | − | ns | ns | ns | ns | ns | ns | − | 12 |
| Chen | 1992 | 54 | 1.5T | − | + | No | + | + | ns | ns | ns | ns | ns | ns | 12 |
These studies used a T1–T2 combined assessment designed by Hoover et al
Key: −(not significant at α=0.05); + (significant at α=0.05); NS (not studied)
Maximum score was 30. Studies between 20–30 were graded as “good,” between 10–19 as “fair,” and 9 or less as “poor.”
Abbreviations: T1WI (T1-weighted imaging); T2WI (T2-weighted imaging); QA (quantitative assessment), MRE (Magnetic resonance elastography); FA (Fractional anisotropy); ADC (Apparent diffusion coefficient); FLAIR (Fluid-attenuated inversion recovery); PDWI (Proton density weighted imaging)
Of the 21 publications reviewed, 16 studied the utility of T1-weighted imaging (T1WI) and/or T2-weighted imaging (T2WI) to predict tumor consistency. All concluded that T1WI, when analyzed alone, offered no significant predictive value [4–6, 8, 12–14, 16, 17, 19, 24, 26, 27]. The majority of authors endorsed the predictive ability of T2WI, noting that tumors hyperintense on T2WI relative to cerebral cortex tended to be softer. [1, 4–5, 8, 13, 14, 16–19, 24, 26, 28, 29]. Yamaguchi et al in their review of 50 patients additionally stated that the probability of a soft tumor is up to 100% higher if hyperintensity on T2WI is associated with hypointensity on T1WI [4].
However, four authors did not find that T2WI predicted consistency [2, 6, 12, 21]. All but one of these studies observed a trend of hyperintensity and hypointensity on T2WI paralleling tumor softness and firmness, respectively, though their results did not reach statistical significance. Table 2 presents a summary of the studies that reviewed T2-weighted imaging.
Table 2.
Summary of studies that reviewed the clinical use of T2-weighted imaging alone in predicting consistency.
| Paper | Year | N | Field | T2WI vs. consistency |
Hyperintensity vs. consistency |
Hypointensity vs. consistency |
T2WI vs. histology |
Hyperintensity vs. histology | Hypointensity vs. histology |
|---|---|---|---|---|---|---|---|---|---|
| Watanabe | 2015 | 43 | 3T | + | Soft | Firm | ns | ||
| Smith | 2015 | 20 | ? | + | Soft | Firm | ns | ||
| Murphy | 2014 | 13 | 3T | − | Soft* | Firm* | ns | ||
| Sitthinamsuwan | 2012 | 243 | ? | + | Soft | Firm | ns | ||
| Kashimura | 2007 | 29 | 3T | − | Soft* | Firm* | + | Angiomatous, microcystic | Fibroblastic |
| Yrjana | 2006 | 21 | 0.23T | + | Soft | Firm | + | “High vascularity” | “High collagen” |
| Yoneoka | 2002 | 20 | 3T | + | Soft | Firm | + | Meningothelial, angiomatous, atypical |
Fibroblastic |
| Maiuri | 1999 | 35 | ? | + | Soft | Firm | + | Angioblastic, syncytial | Fibroblastic |
| Ildan | 1999 | 126 | 1.5T | + | Soft | Firm | + | Meningothelial, angioblastic, atypical |
Fibroblastic, transitional |
| Yamaguchi | 1997 | 50 | 1.5T | + | Soft | Firm | − | ||
| Soyama | 1995 | 40 | 0.5/1.5T | + | Soft | Firm | + | Angiomatous, psammomatous, anaplastic |
Fibrous |
| Suzuki | 1994 | 73 | 0.5/1.5T | + | Soft | Firm | + | Angioblastic | Fibroblastic |
| Carpeggiani | 1993 | 43 | 1.5T | − | Soft* | Firm* | − | ||
| Chen | 1992 | 54 | 1.5T | + | Soft | Firm | + | Angioblastic, atypical, melanocytic |
Fibroblastic, transitional |
These findings, while not statistically significant at α=0.05, were notable enough to warrant an observable trend
Key: − (not significant at α=0.05); + (significant at α=0.05); NS (not studied)
Of note, all studies who quantitatively assessed signal intensity, rather than qualitatively comparing tumor to local gray matter, found a significant correlation between T2WI and consistency [13, 19, 24]. Quantitative assessment consisted of calculating signal intensity (SI) ratios of tumor to cerebral cortex or cerebellar peduncle, instead of coding tumors to a binary of hypo- or hyperintensity relative to cortex.
Twelve studies analyzed correlation between MRI findings, tumor consistency, and histopathological subtype. Again, T1WI was not of significant predictive value. Tumors hyperintense on T2WI were more likely to be angioblastic, meningothelial, or syncytial, and also tended to be softer tumors [8, 14, 16, 17]. Chen et al additionally found that tumors that appeared hyperintense relative to gray matter on T2WI were more likely to demonstrate cellular atypia, invasion, angioblastic, or melanocytic components [16]. Tumors hypointense on T2WI were mostly of the fibroblastic subtype, characterized by a dense collagenous matrix, and tended to be firmer [8, 16, 17, 30]. These findings concur with previous studies, which observe a correlation between greater collagen content and hypointensity on T2WI, and between greater water content and vascularity and hyperintensity on T2WI [4, 28].
However, a few authors observed no correlation between MRI findings and histological subtype, citing that signal intensity provides insufficient detail for differentiating between subtypes that are normally analyzed microscopically [12, 27, 31].
Study quality was generally rated as fair. Two studies were graded “good” and one received a grade of “poor.” Virtually no studies addressed intra- and interobserver reliability, though the employment of calculated SI ratios may countermand this potential bias. Less than a quarter of the studies assessed consistency on an ordinal scale. Nearly half the studies did not address blinding at all in their methodology. Only three studies reported sensitivity and specificity values in their statistical analysis. Watanabe et al calculated sensitivity and specificity values of T2WI to predict consistency of 89% and 79%, respectively [19]. Hoover et al devised a combined T1/T2 assessment and reported a sensitivity of 56% for predicting firm meningiomas [1]. Using the same assessment, Ortega-Porcayo et al found a sensitivity and specificity of 25% and 100% respectively for predicting firm tumors [18].
The majority of studies reviewed here assessed a wide range of potential predictive factors for meningioma consistency, including angiography, magnetic resonance elastography (MRE), fractional anisotropy (FA), apparent diffusion coefficient (ADC), fluid-attenuated inversion recovery (FLAIR), Fast Imaging Employing Steady-state Acquisition (FIESTA), and proton density-weighted imaging (PDWI). Of these parameters, few were to found to demonstrate significant predictive capability of tumor consistency. FLAIR was studied in five reports and found to be predictive of consistency in three [5, 19, 24]. Hypointensity on FLAIR correlated with firm tumors. FIESTA was reviewed by Watanabe et al and also found to be predictive [19].
Discussion
Tumor consistency has been frequently and independently linked to certain intraoperative and postoperative surgical risks, necessitating the development of reliable neuroimaging techniques for preoperative study. Meningiomas are increasingly resected via minimally invasive techniques, and therefore both surgeons and patients stand to benefit substantially from reliable preoperative neuroimaging that accurately predicts consistency. However, the existing literature suffers from inconsistent reference standards and poor reproducibility, rendering studies difficult to compare.
Correlation between MRI and tumor consistency
Overall, T2WI was found to be a much more reliable predictor of consistency than T1WI. Unfortunately, the current binary classification of either “firm” or “soft” fails to account for the diverse spectrum of meningioma consistency, particularly in tumors of intermediate consistency or heterogeneous composition. The majority of studies assessed consistency retrospectively by reviewing operative reports or intraoperative video recordings, [17, 19] while others defined consistency as a function of the surgical tools required to remove the tumor [5] Studies involving different surgeons reporting consistency within the same series may have suffered from interobserver bias, which went largely unaddressed by nearly all studies. Zada et al recently proposed a model for evaluating intraoperative consistency, in which tumors are graded from 1–5 based on the difficulty of debulking [15]. This model was prospectively validated, and offers a standardized alternative to the highly subjective interpretation of “firm” versus “soft”.
Another detriment to study quality involved the initial assessment of each MRI. The majority of the studies qualitatively assessed tumor signal intensity relative to cerebral cortex, but little to no detail was given on how these measurements were standardized. Signal intensity of normal brain tissue varies substantially in MRI, particularly at the periphery of the scan, introducing a potential confounding variable that may have hindered statistical analysis. The general lack of methodological specificity and attention to blinding may have introduced additional confounders. Three studies quantified signal intensity relative to local gray matter using regions of interest (ROIs) and calculating signal intensity (SI) ratios. All of these studies subsequently found a significant correlation between SI ratio and meningioma consistency.
Correlation between MRI, tumor consistency, and histopathological analysis
Tumor cellularity, water content, and fibrous content are thought to be the main determinants of different signal intensities of the various meningioma subtypes [17]. Preoperative knowledge of tumor histology may influence the surgical plan. Fibrous meningiomas in particular have been associated with increased risk of postoperative cranial nerve deficits [25]. Most studies found histologic subtype to correlate well with intraoperative consistency. Meningiomas hyperintense on T2WI tended to be angioblastic or atypical, possibly attributable to the increased vascularity. Hypointensity on T2WI correlated well with the fibroblastic subtype. Studies that did not find a significant correlation employed only qualitative assessments of consistency. Moreover, these studies still reported trends that came close to reaching statistical significance and concurred with other publications. For example, Spagnoli et al found no correlation between MRI and tumor subtype, although they noted that psammomatous meningiomas tended to be hypointense relative to cortex on T2WI [31].
The relationship between MRI and histologic subtype is controversial in the literature. Elster et al found that signal intensity and features on T2WI strongly correlated with histopathologic findings in over 75% of their reviewed cases [8]. Demaeral et al used the same visual scoring system proposed by Elster et al and argue that while histologic subtypes may have a different appearance on MRI, the difference is insufficient to reach a histologic diagnosis. MRI is by no means a suitable replacement for pathological analysis, but our review suggests that quantitative assessment of signal intensity may offer some predictive value. We recommend that all future studies include pathology reports in their investigation in order to provide the most thorough analysis.
Other Imaging Techniques
The recent development of MR elastography (MRE) offers an adjunct to traditional MRI. MRE is a dynamic technique that measures shear wave movement through tissue to determine consistency [20]. All three studies that reviewed MRE supported its use to predict consistency [6, 20, 23]. Murphy et al studied found that MRE results had a stronger correlation with the intraoperative assessment of consistency, outperforming traditional T1WI and T2WI especially when measuring heterogeneous meningiomas [6]. Hughes et al also studied MRE and reported similar results, though cautioning that MRE was not as powerful in predicting soft consistency or ruling out hardness. While MRE is a promising development, it is not wholly non-invasive due to to the delivery of shear forces to the patient’s brain.
Two studies found a correlation between fractional anisotropy (FA) and histological subtype [21, 22], and three found a correlation between FA and tumor consistency [2, 21, 22]. Kashimura et al found that of 29 meningiomas, FA values of firm tumors were significantly higher than those of soft tumors. Kashimura et al and Tropine et al both noted that FA values of fibroblastic tumors were significantly higher than those of the meningothelial subtype [21, 22]. Romani et al reported similar results and additional proposed that FA value and FA maps should be considered in the preoperative MRI examination in all patients with intracranial meningiomas. However, Ortega-Porcayo et al found that FA was not an independent predictor of consistency [18].
Apparent diffusion coefficient (ADC) values were tested for their predictive abilities, based upon the hypothesis that increased collagen content seen in firmer tumors would block diffusion and lower the ADC. Three authors analyzed ADC, though only one found a significant correlation between ADC and tumor consistency [11, 19, 33]. Yogi et al found the minimum ADC value of hard tumors was significantly lower than that of soft tumors [34].
The influence of magnetic field strength on predictive ability may warrant further study. The reviewers in the study performed by Elster et al unanimously reported the superior spatial resolution and contrast of higher field imaging (1.5T versus 0.5T) to identify specific tumor features [8]. However, they also noted that signal intensity could be readily assessed regardless of low or high field strength. Yrjana et al reported similar results [24]. However, there are not yet studies comparing 1.5T to 3T or higher field strengths, and the emergence of ultra-high field MRI imaging offer better predictive value for meningiomas of intermediate or indeterminate consistency.
Clinical Practice and Further Study
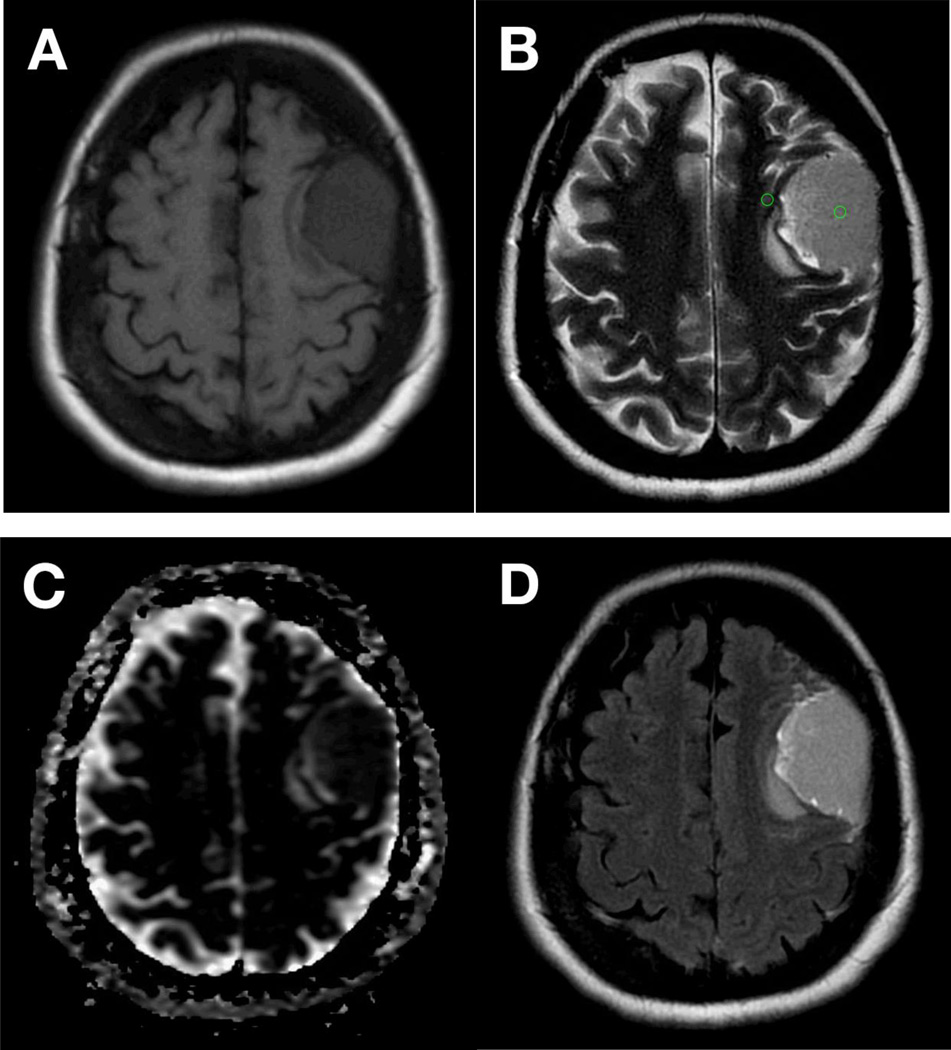
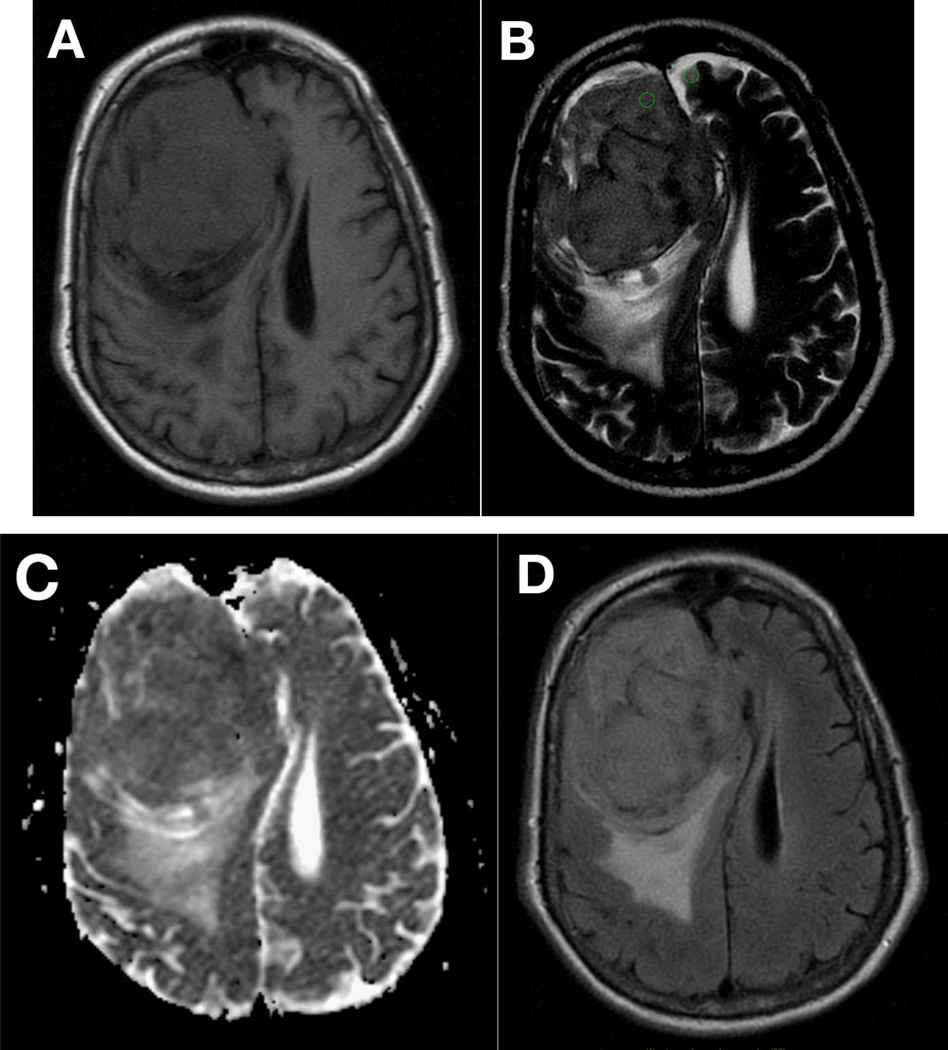
From our analysis, we believe that clinical practice may benefit during the surgical planning stage when tumor signal intensity of T2WI is quantitatively compared to adjacent cortex tissue using SI ratios. Intraoperative consistency should be assessed using a standardized model, such as the one proposed by Zada et al, and should be correlated to postoperative pathological analysis. Figure 2 demonstrates corresponding images of a large meningioma scanned at 1.5T, qualitatively assessed isointense relative to adjacent gray matter on T1WI (Fig. 2A), hyperintense on T2WI (Fig. 2B), isointense on ADC (Fig. 2C) and hyperintense on T2 FLAIR (Fig. 2D), found to be relatively soft intraoperatively. Hyperintensity on T2WI was quantitatively confirmed using ROIs calculated as SI of the lesion divided by SI of the adjacent gray matter. Figure 3 shows corresponding images of a meningioma found to be firm and fibrous intraoperatively, scanned at 1.5T and qualitatively assessed as isointense on T1WI (Fig. 3A), iso- to hypointense on T2WI (Fig. 3B), isointense on ADC (Fig. 3C), and isointense on T2 FLAIR (Fig. 3D). The SI ratio was calculated and found to be consistent with a hypointense mass relative to adjacent gray matter.
Figure 2.
Corresponding axial MR imaging of a large L anterior fronto-parietal meningioma appears iso- to hypo-intense on T1WI (A) and iso- to hyper-intense on T2WI (B) relative to adjacent gray matter. Lesion appears isointense to gray matter on apparent difficusion coefficient imaging (ADC) (C) and hyperintense on T2 FLAIR (D). Imaging performed at 1.5T. This meningioma was found to be soft intraoperatively. ROI analysis, calculated as signal intensity (SI) of lesion/SI of adjacent gray matter (GM) determined the lesion to be hyper-intense to surrounding GM (B, green circles).
Figure 3.
Corresponding axial MR imaging of a large R anterior frontal meningioma appears isointense on T1WI (A) and iso- to hypo-intense on T2WI (B) relative to adjacent gray matter. Lesion appears isointense to gray matter on apparent difficusion coefficient imaging (ADC) (C) and iso- to hyper-intense on T2 FLAIR (D). Imaging performed at 1.5T. Subjective determination of intensity relative to gray matter on T2WI is difficult in this case and warrants quantitative analysis. This meningioma was found to be firm and fibrous intraoperatively. ROI analysis, calculated as signal intensity (SI) of lesion/SI of adjacent gray matter (GM) determined the lesion to be hypo-intense to surrounding GM (B, green circles).
The ubiquitous nature of conventional MRI inclines researchers to devise a reliable predictive scheme for preoperatively determining consistency [1]. Several models have been proposed, though most are only effective in predicting consistency in either very firm or very soft tumors, and limited sensitivity when used in most meningiomas, which are of intermediate firmness [1, 18].
A prospective study including all of these parameters is warranted for a deeper understanding of how neuroimaging contributes to the surgical plan, especially for tumors amenable to minimally invasive resection. T1WI and T2WI are standard sequences that can be used in all fields of MRI, and a robust study would ideally generate a simple algorithm that could be then validated and directly applied to clinical practice.
Conclusion
Tumor consistency is a critical factor that influences operative strategy and patient counseling. Preoperative knowledge of tumor firmness affords the neurosurgeon substantial benefit when planning surgical techniques.
T1WI does not have sufficient value for predicting consistency; however, preoperative T2WI correlates well with intraoperative tumor consistency and postoperative histopathological analysis. Tumors hyperintense relative to gray matter on T2WI tend to be softer and of the angioblastic, meningothelial, and syncytial subtypes, whereas hypointense images tended to be firm of the fibroblastic subtype. Quantitative assessment of signal intensity offers the most robust and reliable predictive capacity of consistency. Intraoperative consistency should be graded using a standardized model to allow for reproducibility and minimize bias.
Advanced MRI techniques such as MRE and FA may be of diagnostic use, especially when used in conjunction with T2WI MRI. These techniques require further study, particularly when evaluating meningiomas of intermediate or indeterminate consistency. Additional exploration of T2WI and other parameters, such as higher field strength and pulse sequences, is necessary to fully ascertain the predictive value of MRI in determining meningioma consistency.
Highlights.
Tumor consistency and histopathological subtype can be anticipated based on pre-operative MRI
T2 weighted images have predictive value for tumor consistency and histopathology, while T1 weighted images do not
Images hyperintense on T2WI relative to gray matter generally correlate with softer tumors, while hypointense images correlate with firmer tumors
Quantitative assessment of tumor signal intensity using calculations of signal intensity ratios reliably predicts tumor consistency
Magnetic resonance elastography and fractional anisotropy are advanced MRI techniques that show potential for preoperative assessment of meningioma consistency
Abbreviations list
- MRI
magnetic resonance imaging
- CT
Computed tomography
- MD
mean diffusivity
- PDWI
proton density weight imaging
- FLAIR
fluid attenuated inversion recovery
- WHO
World Health Organization
- FIESTA
fast imaging employing steady-state acquisition
- MRE
magnetic resonance elastography
- FA
fractional anisography
- ADC
apparent diffusion coefficient
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study formal consent is not required.
References
- 1.Hoover JM, Morris JM, Meyer FB. Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surg Neurol Int. 2011;2:142. doi: 10.4103/2152-7806.85983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romani R, et al. Diffusion tensor magnetic resonance imaging for predicting the consistency of intracranial meningiomas. Acta Neurochir (Wien) 2014;156(10):1837–1845. doi: 10.1007/s00701-014-2149-y. [DOI] [PubMed] [Google Scholar]
- 3.Kendall B, Pullicino P. Comparison of consistency of meningiomas and CT appearances. Neuroradiology. 1979;18(4):173–176. doi: 10.1007/BF00345721. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi N, et al. Prediction of consistency of meningiomas with preoperative magnetic resonance imaging. Surg Neurol. 1997;48(6):579–583. doi: 10.1016/s0090-3019(96)00439-9. [DOI] [PubMed] [Google Scholar]
- 5.Sitthinamsuwan B, et al. Predictors of meningioma consistency: A study in 243 consecutive cases. Acta Neurochir (Wien) 2012;154(8):1383–1389. doi: 10.1007/s00701-012-1427-9. [DOI] [PubMed] [Google Scholar]
- 6.Murphy MC, et al. Preoperative assessment of meningioma stiffness using magnetic resonance elastography. J Neurosurg. 2013;118(3):643–648. doi: 10.3171/2012.9.JNS12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26(5):461–469. doi: 10.1016/0090-3019(86)90259-4. [DOI] [PubMed] [Google Scholar]
- 8.Elster AD, et al. Meningiomas: MR and histopathologic features. Radiology. 1989;170(3 Pt 1):857–862. doi: 10.1148/radiology.170.3.2916043. [DOI] [PubMed] [Google Scholar]
- 9.Watts J, et al. Magnetic resonance imaging of meningiomas: a pictorial review. Insights Imaging. 2014;5(1):113–122. doi: 10.1007/s13244-013-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanverdi SE, et al. Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol. 2012;81(9):2389–2395. doi: 10.1016/j.ejrad.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Santelli L, et al. Diffusion-weighted imaging does not predict histological grading in meningiomas. Acta Neurochir (Wien) 2010;152(8):1315–1319. doi: 10.1007/s00701-010-0657-y. discussion 1319. [DOI] [PubMed] [Google Scholar]
- 12.Carpeggiani P, Crisi G, Trevisan C. MRI of intracranial meningiomas: correlations with histology and physical consistency. Neuroradiology. 1993;35(7):532–536. doi: 10.1007/BF00588715. [DOI] [PubMed] [Google Scholar]
- 13.Smith KA, Leever JD, Chamoun RB. Predicting Consistency of Meningioma by Magnetic Resonance Imaging. J Neurol Surg B Skull Base. 2015;76(3):225–229. doi: 10.1055/s-0034-1543965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki Y, et al. Meningiomas: correlation between MRI characteristics and operative findings including consistency. Acta Neurochir (Wien) 1994;129(1–2):39–46. doi: 10.1007/BF01400871. [DOI] [PubMed] [Google Scholar]
- 15.Zada G, et al. A proposed grading system for standardizing tumor consistency of intracranial meningiomas. Neurosurg Focus. 2013;35(6):E1. doi: 10.3171/2013.8.FOCUS13274. [DOI] [PubMed] [Google Scholar]
- 16.Chen TC, et al. Magnetic resonance imaging and pathological correlates of meningiomas. Neurosurgery. 1992;31(6):1015–1021. doi: 10.1227/00006123-199212000-00005. discussion 1021-2. [DOI] [PubMed] [Google Scholar]
- 17.Maiuri F, et al. Intracranial meningiomas: correlations between MR imaging and histology. Eur J Radiol. 1999;31(1):69–75. doi: 10.1016/s0720-048x(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 18.Ortega-Porcayo LA, et al. Prediction of mechanical properties and subjective consistency of meningiomas using T1-T2 assessment vs Fractional Anisotropy. World Neurosurg. 2015 doi: 10.1016/j.wneu.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K, et al. Prediction of hard meningiomas: quantitative evaluation based on the magnetic resonance signal intensity. Acta Radiol. 2015 doi: 10.1177/0284185115578323. [DOI] [PubMed] [Google Scholar]
- 20.Hughes JD, et al. Higher-Resolution Magnetic Resonance Elastography in Meningiomas to Determine Intratumoral Consistency. Neurosurgery. 2015;77(4):653–659. doi: 10.1227/NEU.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashimura H, et al. Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. J Neurosurg. 2007;107(4):784–787. doi: 10.3171/JNS-07/10/0784. [DOI] [PubMed] [Google Scholar]
- 22.Tropine A, et al. Differentiation of fibroblastic meningiomas from other benign subtypes using diffusion tensor imaging. J Magn Reson Imaging. 2007;25(4):703–708. doi: 10.1002/jmri.20887. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, et al. Magnetic resonance elastography of brain tumors: preliminary results. Acta Radiol. 2007;48(3):327–330. doi: 10.1080/02841850701199967. [DOI] [PubMed] [Google Scholar]
- 24.Yrjana SK, et al. Low-field MR imaging of meningiomas including dynamic contrast enhancement study: evaluation of surgical and histopathologic characteristics. AJNR Am J Neuroradiol. 2006;27(10):2128–2134. [PMC free article] [PubMed] [Google Scholar]
- 25.Little KM, et al. Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery. 2005;56(3):546–559. doi: 10.1227/01.neu.0000153906.12640.62. discussion 546-59. [DOI] [PubMed] [Google Scholar]
- 26.Ildan F, et al. Correlation of the relationships of brain-tumor interfaces, magnetic resonance imaging, and angiographic findings to predict cleavage of meningiomas. J Neurosurg. 1999;91(3):384–390. doi: 10.3171/jns.1999.91.3.0384. [DOI] [PubMed] [Google Scholar]
- 27.Demaerel P, et al. Intracranial meningiomas: correlation between MR imaging and histology in fifty patients. J Comput Assist Tomogr. 1991;15(1):45–51. [PubMed] [Google Scholar]
- 28.Yoneoka Y, et al. Pre-operative histopathological evaluation of meningiomas by 3 0T T2R MRI. Acta Neurochir (Wien) 2002;144(10):953–957. doi: 10.1007/s00701-002-1005-7. discussion 957. [DOI] [PubMed] [Google Scholar]
- 29.Soyama N, Kuratsu J, Ushio Y. Correlation between magnetic resonance images and histology in meningiomas: T2-weighted images indicate collagen contents in tissues. Neurol Med Chir (Tokyo) 1995;35(7):438–441. doi: 10.2176/nmc.35.438. [DOI] [PubMed] [Google Scholar]
- 30.Zee CS, et al. Magnetic resonance imaging of meningiomas. Semin Ultrasound CT MR. 1992;13(3):154–169. [PubMed] [Google Scholar]
- 31.Spagnoli MV, et al. Intracranial meningiomas: high-field MR imaging. Radiology. 1986;161(2):369–375. doi: 10.1148/radiology.161.2.3763903. [DOI] [PubMed] [Google Scholar]
- 32.Pierallini A, et al. Pituitary macroadenomas: preoperative evaluation of consistency with diffusion-weighted MR imaging--initial experience. Radiology. 2006;239(1):223–231. doi: 10.1148/radiol.2383042204. [DOI] [PubMed] [Google Scholar]
- 33.Hakyemez B, et al. The contribution of diffusion-weighted MR imaging to distinguishing typical from atypical meningiomas. Neuroradiology. 2006;48(8):513–520. doi: 10.1007/s00234-006-0094-z. [DOI] [PubMed] [Google Scholar]
- 34.Yogi A, et al. Usefulness of the apparent diffusion coefficient (ADC) for predicting the consistency of intracranial meningiomas. Clin Imaging. 2014;38(6):802–807. doi: 10.1016/j.clinimag.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Shiroishi MS, Cen SY, Tamrazi B, et al. Predicting Meningioma Consistency on Preoperative Neuroimaging Studies. Neurosurgery clinics of North America. 2016;27(2):145–154. doi: 10.1016/j.nec.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arrive L, Renard R, Carrat F, et al. A scale of methodological quality for clinical studies of radiologic examinations. Radiology. 2000;217(1):69–74. doi: 10.1148/radiology.217.1.r00oc0669. [DOI] [PubMed] [Google Scholar]