Abstract
Valproic Acid (Valproate) has been used for over 30 years as a first line treatment for epilepsy. In recent years, prenatal exposure to valproate has been associated with teratogenic effects, limiting its use in women that are pregnant or of childbearing age. However, despite its potential detrimental effects on development, valproate continues to be prescribed at high rates in pediatric populations in some countries. Animal models allow us to test hypotheses regarding the potential effects of postnatal valproate exposure on neurobehavioral development, as well as identify potential mechanisms mediating observed effects. Here, we test the effect of early postnatal (P4-P11) valproate exposure (100mg/kg and 200mg/kg) on motor and affective development in two strains of mice, SVE129 and C57Bl/6N. We also assessed the effect of early valproate exposure on regional BDNF protein levels, a potential target of valproate, and mediator of neurodevelopmental outcomes. We found that early life valproate led to significant motor impairments in both SVE129 and C57Bl/6N mice. Both lines of mice showed significant delays in weight gain, as well as impairments in the righting reflex (P7–8), wire hang (P17), open field (P12 and P21), and rotarod (P25 and P45) tasks. Interestingly, some of the early locomotor effects were strain and dose dependent. We observed no effects of valproate on early markers of anxiety-like behavior. Importantly, early life valproate had significant effects on regional BDNF expression, leading to a near 50% decrease in BDNF levels in the cerebellum of both strains of mice, while not impacting hippocampus BDNF protein levels. These observations indicate that postnatal exposure to valproate may have significant, and region specific effects, on neural and behavioral development, with specific consequences for cerebellar development and motor function.
Keywords: BDNF, Valproate, Early life, Behavior, Development
1. INTRODUCTION
Epilepsy is the most common neurological condition in pediatric populations, affecting 10.5 million or 1% of children under the age of 15, constituting ~25% of individuals with epilepsy [1, 2]. While a number of treatments have been developed for pediatric seizures, valproate is still heavily utilized in many pediatric populations around the world, with prescribing rates as high as 50% in Jordan [3], 40% in Singapore [4], 25% in India [5] and 22% in the US [6]. While valproate is known to have teratogenic effects on fetal development, the impact of pediatric exposure on neurobehavioral development has remained less clear [7]. In humans, few effects of drug exposure (between the ages of 6 and 15 years old) have been identified on measures of cognitive functioning [8, 9], and some studies have actually identified possible improvements in cognitive functioning and IQ scores in individuals with epilepsy on valproate [10]. However, these studies contradict previous work that failed to identify beneficial effects of valproate on cognitive outcomes and identified potential negative effects on motor performance and visuospatial functioning [11] as well as high rates of co-morbid pathology [6].
In animal models, the majority of work related to valproate exposure has focused on the teratogenic effects of prenatal exposure and its possible contribution to the development of ASD-like phenotypes [12, 13]. The study of the effects of postnatal exposure to valproate have been limited, with a great deal of variation in the dosing regimen as well as the timing and duration of drug exposure. For example, work in mice (BALB/c) have found that postnatal exposure (P14) to a single does of valproate (200 or 400mg/kg) led to transient impairments in midair righting reflex and negative geotaxis, effects that rapidly resolved, and found no effect of drug treatment on general locomotor activity [14, 15]. Interestingly, using a near identical paradigm of drug exposure in C57BL/6 mice, other groups have found more limited effects of valproate on these same measures [16]. Long Evans rats exposed during the early postnatal period (P6-P12) to valproate (150mg/kg) were found to have sensory-motor gating abnormalities and impairments in fine motor performance [17], while Sprague Dawley rats exposed exposed from P6-P18 to 150mg/kg of valproate showed deficits in the development of social play [18]. Relevant to neural development, P4-P18 rats exposed to 75–200mg/kg of valproate were found to have a significant decrease in brain weight [19] and rats exposed to three days of 50 to 400mg/kg of valproate between the ages of P3 and P30 were found to have increased apoptotic cell death throughout the brain, suggesting wide spread loss of cells [20–22]. Together, these results provide compelling support for more in depth studies of the effects of valproate on postnatal brain development.
The mechanisms underlying the potential adverse effects of postnatal exposure to valproate remain unknown. Recent evidence suggests that valproate may impact expression of key genes that support neural development, such as brain derived neurotrophic factor (BDNF) [21, 23, 24], potentially through epigenetic effects on the timing or level of expression [25]. BDNF is a critical signaling molecule that is highly expressed in the brain and supports such basic processes as cell proliferation, migration, survival, and growth [26–28]. In addition, BDNF expression is regionally selective, and changes dynamically over development [29]. Studies of valproate-induced changes in BDNF expression during the prenatal and postnatal period in rodents have also been limited. Rats receiving high doses of valproate (600mg/kg) during the prenatal period (E8) were shown to have elevated expression of bdnf in the neural tube [23]. Similarly, in vitro cortical neuronal cultures from postnatal rat showed elevations in bdnf mRNA levels following valproate treatment [24]. Rats receiving valproate during the postnatal period have been found to show reduced bdnf gene expression within 24 hours of treatment [21, 30], however, these studies all report only transcript levels, and are based upon whole brain lysates. Whether these observations translate into changes in BDNF protein, or if the effects of valproate on BDNF levels are regionally restricted at these points in development remain open questions.
Here, we sought to test the behavioral consequences of early postnatal exposure to valproate on motor and affective outcomes, and to compare and contrast results from two independent strains of mice. In addition, we tested the effect of early postnatal valproate treatment on BDNF protein levels by ELISA, across strains of mice and doses, and in two separate brain regions, the cerebellum and the hippocampus. Based upon these results, we hope to better understand the effects of postnatal exposure to valproate on brain and behavioral development, identify possible mechanism driving observed outcomes, and test for possible regional differences in susceptibility to drug exposure.
2. METHODS
2.1 Animals
Male and female C57Bl/6N as well as SVE129 founder mice were obtained from Charles River labs and maintained as separate breeding lines. All mice in the current study were bred in house from approximately 10 breeding pairs (5 for each line), weaned and sex segregated at P21, and were housed under a 12-hour light/dark cycle with food and water ad libitum. Testing occurred prior to sexual maturation, and we did not observe any apparent sex differences on behavioral outcomes. Thus, data from both males and females were merged. All handling, injections, and behavioral tests were done between the hours of 9AM and 12PM, during the lights on phase of the diurnal cycle. All testing was approved by the Brown internal animal care and use committee (IACUC) and consistent with the guide for the care and use of laboratory animals.
2.2 Drug Delivery
To eliminate potential effects of differences in maternal care on behavioral outcomes, mice from all treatment groups were included in each litter (e.g. each litter included saline, Valp100, and/or Valp200 treated mice). Specifically, pups within litter were color coded by daily marking of the tail with lab marker (red, black, or blue). Within each litter, one group of pups received saline injection and the remaining animals received treatment with valproate. Color associated with saline treatment was counterbalanced between litters. Valproate was prepared by dissolving valproic acid (10mg or 20mg; Sigma Aldrich) in sterile saline (1mL). Drug delivery began on postnatal day 4, with daily injections occurring through postnatal day 11. Mice received one of two doses, 100mg/kg (Valp100) or 200mg/kg (Valp200) valproate. Injections were administered subcutaneously, under the nape of the neck, once daily from postnatal day 4 (P4) to P11 between the hours of 9AM and 12PM.
2.3 Righting Reflex
To measure basic motor coordination during early postnatal development, we tested mice on the righting reflex on postnatal days 7 and 8. Testing occurred in the AM prior to the administration of saline or valproate for that day, to insure that effects were not the result of acute effects of drug exposure or stress associated with injection. Briefly, mice were placed onto their backs on a flat dry surface and the latency to right themselves (as defined as all four paws on the ground) was measured over six consecutive trials. The surface was thoroughly cleaned with 70% ethanol between animals. Behavior of the mice was video recorded and latency to right was assessed from video recordings by an observer blind to the treatment condition. If a mouse failed to right, it was given a time of 90 seconds (maximum trial duration) indicating the failure to right. The average latency to right for each animal was calculated and mean latency to right was compared across groups within each mouse strain. For SVE129 mice, we tested n = 8 saline and n = 4 Valp200. For C57Bl/6N mice, we tested n = 16 saline, n = 3 Valp100, and n = 7 Valp200.
2.4 Small Open Field
To measure effect of early life valproate treatment on early locomotor behavior, mice were tested on the small open field test at postnatal day 12. Briefly, mice were placed into a small open field (12 × 6 × 5 in.) under low light (~5–10 lux) and allowed to freely explore for a period of 5 minutes. Mice were digitally recorded and locomotor activity was assessed with the aid of digital tracking software (Noldus Ethovision XT 8.5). Total distance traveled was measured for each mouse. The boxes were thoroughly cleaned with 70% ethanol between subjects. For SVE129 mice, we tested n = 18 saline and n = 6 Valp200. For C57Bl/6N mice, we tested n = 42 saline, n = 14, Valp100, and n = 16 Valp200.
2.5 Large Open Field
To test the effect of early life valproate on locomotor activity and anxiety-like behavior at the time of weaning (P21), mice were tested in the large open field. Briefly, mice were placed into a large empty box (24×20×12 in.) under low light (~5–10 lux) and allowed to freely explore for 10 minutes. Behavior of the mice was digitally recorded and tracked with the aid of digital tracking software (Noldus Ethovision XT 8.5). Boxes were thoroughly cleaned with 70% ethanol between animals. To test for drug effects on locomotion, we tracked the total distance traveled during the trial. To assess anxiety-like behavior, the arena was digitally divided into 12 equal zones and the two center zones defined as the center area. The percent entries as well as percent time spent in the center zones was used as an index of anxiety-like behavior. For SVE129 mice, we tested n = 13 saline and n = 6 Valp200. For C57Bl/6N mice, we tested n = 48 saline, n = 14 Valp100, and n = 15 Valp200.
2.6 Wire Hang
To measure both strength and stamina of mice receiving early valproate treatment, we employed the wire hang test at postnatal day 17 (P17). Using a standard cage top (Allentown Cages), mice were allowed to grip the metal grid cage top and then the grid was slowly inverted and suspended 10 inches above a padded surface. Trials were digitally recorded and the duration until the mouse fell from the grid was collected from video, with the observe blind to treatment. Mice were tested on 6 consecutive trials with a 1-minute interval between trials. We collected the average and max duration that mice were able to hang for group comparisons. The wire hang apparatus was thoroughly cleaned with 70% ethanol between animals. For SVE129 mice, we tested n = 15 saline and n = 9 Valp200. For C57Bl/6N mice, we tested n = 23 saline, n = 3 Valp100, and n = 8 Valp200.
2.7 Rotarod
To assess the effects of early life valproate treatment on motor coordination and motor learning mice were tested at P25 and P45 on the accelerating rotarod test. Briefly, prior to the first trial, mice were acclimated to the rotarod at 4 RPM (revolutions per min). Once mice could sustain walking at 4 RMP for 1-minute testing was begun. For testing, mice were placed on the rotarod at 4RPM and the rotarod began accelerating at a constant rate to 40RPM over 5 minutes. The latency to fall off and maximum speed attained was recorded digitally when the mouse fell from the rotarod and broke an infrared beam. If mice did not fall off, the trial was ended by the experimenter when the rotatod reached maximum speed (40RPM) at 300 seconds (5minutes). Animals were given a 1-minute rest period and tested on four additional trials for a total of five trials per day. The average latency to fall for each trial was calculated for each group and plotted. For SVE129 mice, we tested n = 11 saline and n = 8 Valp200. For C57Bl/6N mice, we tested n = 26 saline, n = 9 Valp100, and n = 8 Valp200.
2.8 BDNF ELISA
To measure the effects of valproate treatment on regional brain BDNF protein levels, we used a BDNF enzyme-linked immunosorbent assay (ELISA- BDNF Emax Immunoassay System, Promega, Madison, WI) with recombinant BDNF as a standard. This assay demonstrates low cross-reactivity (<3%) with other neurotrophic factors and is capable of detecting a minimum of 15.6 pg/ml of BDNF. Briefly, control and valproate treated mice were sacrificed by rapid decapitation at P12. Brains were collected on ice and total bilateral hippocampi and cerebellum were dissected and then lysed in 700 μl TNE lysis buffer (0.1 M Tris HCl, 0.15 M NaCl, 0.001 M EDTA, 1% NP-40). Lysates were centrifuged for 10 min at 4°C and the clarified supernatant was collected. Total levels of protein were quantified for each region using the Bradford method, and equal amounts of protein were loaded in each well. Tissue and assay were prepared and run in accordance with the manufacturers suggested protocol. For SVE129 mice, we tested n = 3 saline and n = 4 Valp200. For C57Bl/6N mice, we tested n = 5 saline, n = 5 Valp100, and n = 5 Valp200.
2.9 Data Analysis
For the righting reflex, wire hang, small and large open field, and BDNF levels, group comparisons were made through ANOVA (C57Bl/6N, with 3 groups) or through t-test (SVE129-2 groups). For rotarod, we used a GLM repeated measures ANOVA with trial as the within subject factor and drug treatment as the between subjects factor. For all tests, alpha was set at 0.05, and eta2 is reported as an estimate of effect size.
3. RESULTS
3.1 Developmental and Motor Effects
3.1.1 Weight Gain
To test the effects of valproate exposure on somatic development, we tracked the weight gain of mice across the injection period as well as prior to each behavioral manipulation. For SVE129 mice, we found a highly significant overall effect of treatment (F(1,683) = 414.342, p < 0.000; eta2 = .378) where mice exposed to valproate (Valp200– 200mg/kg valproate) weighed significantly less than saline treated controls. This effect was present as early as 1 day following the beginning of drug treatment (P5) and continued to be present throughout testing (Figure 1A). In C57 mice, we also observed a significant main effect of treatment on weight (F(2,1082) = 478.186, p < .000; eta2 = .469) and treatment × age interaction (F(22,1028) = 14.764, p < .000; eta2 = .231). Valproate exposure (Valp100 and Valp200) was associated with a significantly lower weight beginning at P5 and remained lower throughout the end of testing (Figure 1B).
Figure 1.
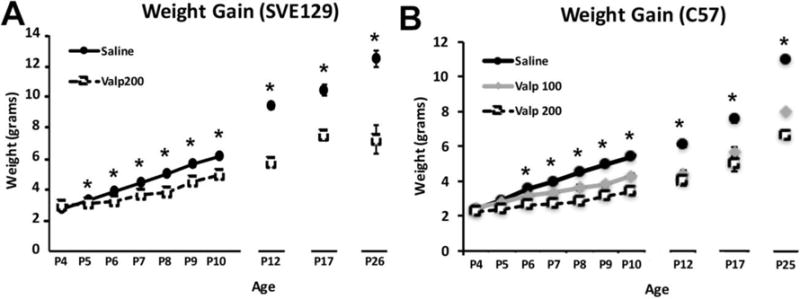
Weight measurements for the two strains of mice (SVE129 and C57) treated with valproate (100mg/kg- Valp100) or 200mg/kg- Valp200) during early postnatal development (P4-P11). (*p < .05, error bars depict the standard error of the mean).
3.1.2 Righting Reflex
To test early motor coordination, we focused on the ability of mice to right themselves from a prone position on postnatal days (P7) and (P8). For SVE129 mice, we found that Valp200 mice showed significantly longer latencies to right themselves at P7 (t(10) = 2.193, p < .053) and P8 (t(7) = 6.502, p < .001; Figure 2A). Similarly, in C57 mice we observed a significant main effect of treatment on the latency to right at P7 (F(2,19) = 35.871, p < .001; eta2 = .791). Mice receiving the higher dose of valproate (Valp200) showed significantly longer latencies to right themselves (p < .001; Figure 2A). In C57 mice we also observed a significant main effect of treatment on the latency to right at P8 (F(2,27) = 9.944, p < .001; eta2 = .424). Mice receiving either dose of valproate (Valp100; p < .003) and (Valp200; p < .001) showed significantly longer latencies to right themselves compared with saline treated controls (Figure 2A).
Figure 2.
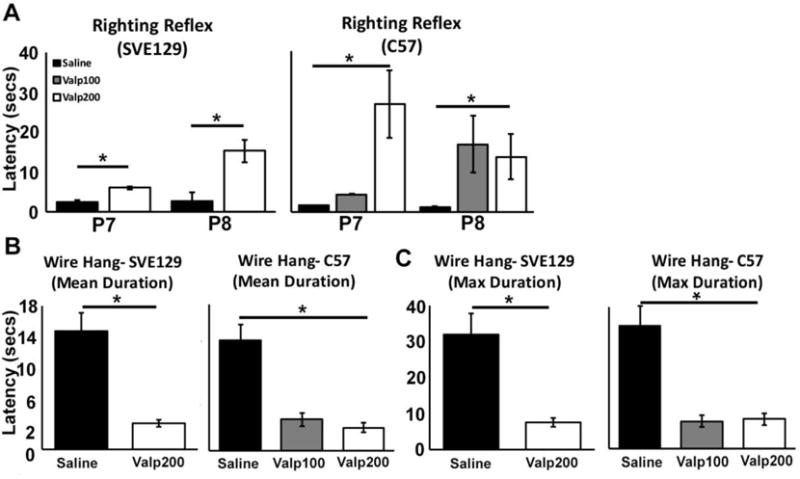
Effect of postnatal valproate on early motor development. (A) Latency to right for SVE129 and C57 mice at P7 and P8 days of age. For the wire hang task, data is plotted for the (B) mean hang durations, and (C) maximum hang durations. (*p < .05, error bars depict the standard error of the mean).
3.1.3 Wire Hang
The Wire Hang test was used to assess the strength and stamina of the mice at P17. Deficits were observed across all drug treatments for both strains. SVE129 mice treated with saline were able to hang for an average of 14.8 seconds, while those treated with valproate showed a significantly shorter latency to fall, averaging a hang duration of only 3.27 seconds (t(22) = 3.704, p < 0.001; Figure 2B). We also assessed max hang duration, to investigate the best possible performance for a given animal over the six trials, and observed similar effects, with valproate treated mice showing a significantly lower max hang duration (t(22) = 3.151, p < .005; Figure 2C). For C57 mice we found a significant main effect of valproate treatment on mean hang duration (F(2,31) = 6.341, p < .005; eta2 = .290; Figure 2B) and max hang duration (F(2,31) = 5.213, p < .011; eta2 = .252; Figure 2C). Again, with valproate treated mice showing lower hang durations than saline treated controls.
3.1.4 Rotarod
SVE129 Mice
The Rotarod test was used to assesses coordination, balance, and motor learning. Here, we first employed this test at P25 as an initial measure of motor coordination and learning and then again at P45 to assess the maintenance of performance, relearning of the task, and overall coordination. In SVE129 mice, at postnatal day 25 (P25) we found that the baseline performance (e.g. Trial 1) was not different between groups (t(15) = .747, p = .466; Figure 3A). We found that both saline and Valp200 SVE129 mice showed a significant improvement in performance over the course of the 5 trials of testing (GLM Repeated Measures- Trial; F(4,12) = 4.199, p < .024; eta2 = .583; Figure 3A). At P25, we found no main effect of treatment on performance in SVE129 mice (F(1,15) = .339, p =.569; eta2 = .022; Figure 3A). At P45, the performance of saline treated mice on Trial 1 (T1) was not different from their final trial (T5) at P25 (t(18) = .757, p = .459; Figure 3A), indicating retention of learned performance. In Valp200 treated SVE129 mice, we found significantly poorer performance on the first trial (T1) at P45 relative to the final trail (T5) at P25 (t(12) = 4.147, p < .001; Figure 3A). At P45, we observed a significant main effect of treatment on performance for SVE129 mice with Valp200 mice performing significantly worse than saline treated controls (GLM Repeated Measures- Treatment; F(1,15) = 17.014, p < .001; eta2 = .531; Figure 3A). At P45, we also did not observe a significant effect of trial, with both saline and Valp200 mice failing to show significant improvements in performance over the five trials (GLM Repeated Measures- Trial; F(4,12) = 2.554, p = .093; eta2 = .460; Figure 3A).
Figure 3.
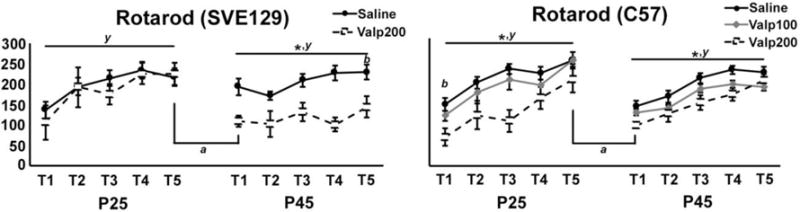
Behavioral performance of mice on the rotarod task in mice tested at P25 and again at P45. (y) denotes significant effect of trial within day. (*) denotes significant main effect of treatment within day. (b) denotes a main effect of treatment for trial 1 (T1). (a) denotes a significant change in performance between trials T5 on P25 and T1 on P45. (For all comparisons, alpha = .05, error bars depict the standard error of the mean).
C57 Mice
For C57 mice, at P25 we found a significant effect of treatment on baseline performance at trial 1 (T1; F(2,40) = 4.214, p < .022; Figure 3B), with Valp200 mice performing significantly worse than saline (p < .006) but not different from Valp100 mice (p < .121). At P25, we observed an overall effect of trial for C57 mice, with all groups of animals showing significant improvements in performance over the course of the five trials (GLM Repeated Measures- Trial; F(4,37) = 25.624, p < .001; eta2 = .735; Figure 3B). We also observed a significant main effect of treatment (GLM Repeated Measures- Treatment; F(2,40) = 8.200, p < .001; eta2 =.291; Figure 3B) with Valp200 mice performing worse than both other groups of mice. To investigate motor memory, we tested for changes in performance between the final trial on P25 and the first trial on P45. We found significant decrements in performance in saline treated mice (t(51) = 7.154, p < .000), in valp100 mice (t(15) = 4.862, p < .000) and Valp200 mice (t(17) = 4.018, p < .001). We also tested for re-learning effects at P45, and found that all groups of mice show significant improvements in performance over the course of the 5 trails (GLM Repeated Measures- Trial; F(4,40) = 17.529, p < .001; eta2 = .637; Figure 3B). Furthermore, we continued to observe a significant main effect of treatment (GLM Repeated Measures- Treatment; F(2,43) = 4.826, p < .013; eta2 = .183; Figure 3B), with saline treated mice performing better than valproate treated animals at this age.
3.2 Activity and Anxiety-like Behavior
3.2.1 Small Open Field
To test the effects of valproate treatment on activity in young animals, we compared the distance traveled of the various groups of mice at P12. Interestingly, at this time point we found that valproate treatment had opposite effects in the two strains. In SVE129 mice, valproate treatment led to significantly lower levels of activity and an overall decrease in distance traveled compared with saline treated mice (t(22) = 2.967, p < 0.007; Figure 4A). SVE129 mice treated with valproate (Valp200) traveled an average of 136.11 inches while saline treated mice traveled an average of 311.15 inches. Conversely, in the C57 strain of mice, greater dosages of valproate were associated with higher levels of activity (F(2,69) = 17.246, p < .001; eta2 = .333; Figure 4B). Compared with saline treated mice, we found significantly greater distance traveled by Valp100 (p < .01) and Valp200 (p < .000) mice. In addition, Valp200 mice traveled on average a significantly greater distance than Valp100 mice (p < .018).
Figure 4.
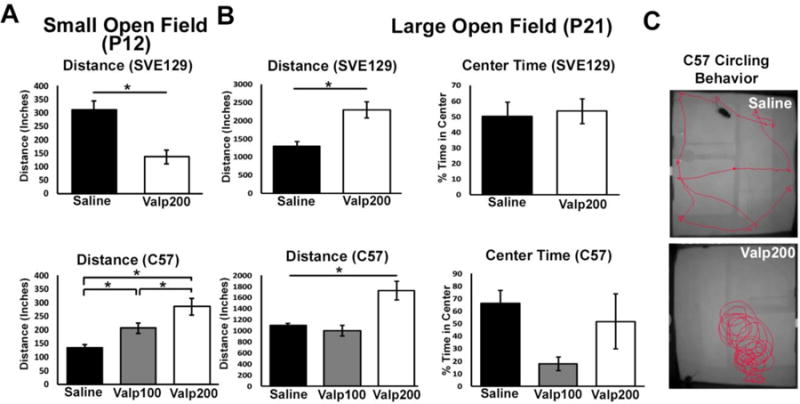
(A) Distance traveled in the small open field at P12. (B) Distance traveled in the large open field at P21, as well as percent time spent in the center of the open field. (C) Activity traces of mouse behavior for two mice over a period of 2 minutes in large open field task. A subset (n = 4) of Valp200 mice showed significant circling behavior, an effect that was not observed in saline controls or any other group of mice. (* p < .05, error bars depict the standard error of the mean)
3.1.2. Large Open Field
Activity
To test the effects of valproate treatment on locomotor function in animals at the time of weaning, we used the large open field test at P21. At this age, we found that valproate treatment led to consistent effects between strains, with both strains of animals showing an increase in locomotion following early life exposure to valproate. Specifically, we found that SVE129 mice treated with valproate showed increased activity when compared with saline treated control mice (t(17) = 4.031, p < .001; Figure 4B). SVE129 mice treated with saline traveled an average of 1291.8 inches, while those treated with valproate traveled an average of 2292.2 inches. In the C57 strain of mice, we also observed a significant main effect of treatment on distance traveled (F(2,74) = 16.856, p < .001; eta2 = .313; Figure 4B), with Valp200 mice traveling significantly greater distance than both saline treated (p < .001) and Valp100 (p < .001; Figure 4B) treated mice. During large open field testing, we noted that a subset of Valp200 treated C57 mice (3 of 6 tested) demonstrated high levels of circling behavior. An example of this behavior is presented in Figure 4C. We did not observe circling behavior in Valp200 treated SVE129 mice, Valp100 treated C57 mice, or saline controls from either strain.
Anxiety-like behavior
At P21, we also tested for potential effect of valproate treatment on anxiety-like behavior in the open field task. To investigate anxiety-like behavior, we measured the relative abundance of time that mice spent in the center of the open field. Typically, when mice are placed into a new environment (i.e. the large open field arena), they initially avoid the center section of the field, and over the course of the trial begin to explore the center section, an effect that many attribute to acclimation to the arena, or decreasing anxiety. A continued failure to enter or spend time in the center of the arena is used as an index of heightened anxiety-like behavior. For SVE129 mice, we did not observe an effect of treatment on center time (t(17) = .227, p = .823), with average center durations of 50.3 and 53.5 seconds, respectively. For C57 mice, while there was a trend toward decreased time in center for the Valp100 treated mice, we did not observe a significant overall effect of treatment on center time (F(2,72) = 2.368, p = .101; eta2 = .062).
3.3 BDNF ELISA
Valproate impacts CNS function through multiple mechanisms of action, including effects on gene expression through modulation of histone deacetylation [23, 25]. One gene target that has been proposed to be impacted by valproate exposure is BDNF [30], a member of the neurotrophin family of signaling molecule, and regulator of multiple aspects of neural development. Here, we tested the effects of early life exposure to valproate on BDNF protein levels at P12 in two brain regions, the cerebellum and the hippocampus of SVE129 and C57Bl/6 mice. In the cerebellum, we found that valproate led to reduced BDNF expression in both strains of mice. In SVE129 mice, BDNF levels were approximately half of that observed in saline treated mice, an effect that approached significance (t(5) = 1.974, p <.105; Figure 5A). In C57 mice, there was a main effect of treatment on BDNF levels (F(2,11) = 9.330, p < .004; eta2= .629; Figure 5B), with significant reductions in BDNF expression relative to controls observed for Valp100 (p < .002) and Valp200 (p < .006) treated mice. In the hippocampus, we found no effect of valproate treatment on BDNF expression for either the SVE129 (t(5) = 1.648, p < .160; Figure 5C) or C57 (F(2,11) = .334, p = .723, eta2 = .057; Figure 5D) strains of mice.
Figure 5.
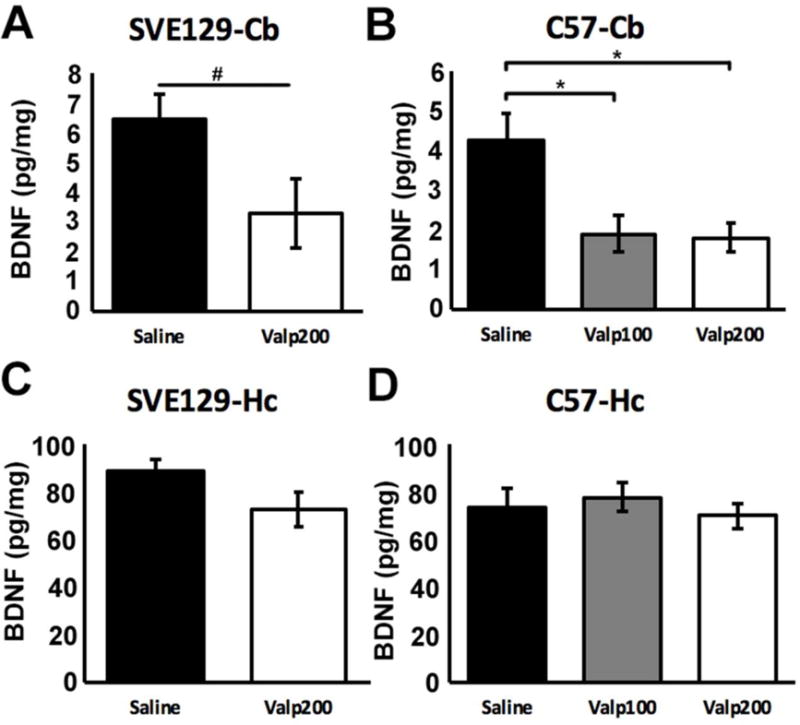
BDNF protein levels in the cerebellum (A & B) and hippocampus (C & D) measured by ELISA at P12. (* p < .05, # p = .06, error bars depict the standard error of the mean).
4. DISCUSSION
Here, we found that early life valproate exposure significantly impacted early motor development in two strains of mice, but did not impact the expression of anxiety-like behavior. These effects were associated with region specific effects on BDNF protein levels, with cerebellar BDNF levels being significantly reduced in valproate treated mice, but no effects on BDNF levels in the hippocampus. The current findings are suggestive of regionally selective effects of postnatal valproate exposure on motor development and the development of key brain centers that control motor output.
In valproate treated mice, we observed significant impairments in the righting reflex, a marker task used to assess early neurodevelopmental milestones, similar to the development of coordinated motor abilities that support the ability of infants to roll over unassisted, crawl, and then walk. Based upon animal work, the development of this reflex depends upon intact cerebellar function [31]. In both strains of mice, treatment with valproate increased the amount of time it took for the mice to complete this task, suggesting significant impairments in the ability to efficiently coordinate the motor movements necessary to right themselves from a prone position. In addition, valproate treated mice demonstrated diminished strength and stamina in the wire hang task, suggesting possible significant effects on motor or muscular development.
Based upon our behavioral results, it is not clear whether the effects of valproate on righting reflex and wire hang were due to effects on motor development at the level of the muscle and/or through central effects, such as the development of motor centers in the brain. Evidence from studies of prenatal valproate exposure suggest that valproate can decrease the number of motor neurons in the earliest-forming cranial nerve motor nuclei [32] with no impact on later developing cranial nerves such as the facial nucleus and the dorsal motor nucleus of the vagus nerve [32]. In addition, prenatal valproate exposure has been associated with the selective loss of neurons derived from the basal (or motor) plate of the hindbrain and a reduction in the number of Purkinje neurons in the posterior cerebellum [32]. More recently, short-term (24–72 hour) postnatal valproate exposure was associated with wide spread cell death in the brain of mice, including effects observed in the cerebellum and hippocampus [15, 20, 21], suggesting potential wide spread effects on neural development. Interestingly, valproate treatment in adults with spinal muscular atrophy have shown that valproate treatment increases muscle strength and function by increasing survival motor neuron protein [33], as well as preserving motor neurons following injury in in vitro models [34]. Thus, valproate may have complex effects on neural centers involved in motor coordination, with the selectivity and direction of these effects depending upon the timing of drug exposures. Despite these observations at the level of the CNS, we are not aware of any work assessing the direct effects of postnatal or prenatal valproate exposure on muscle or neuromuscular junction development. Based upon the currently available data, we interpret our results to mean that valproate is likely affecting the development of key brain centers supporting motor function. However, more work will be required to investigate the potential consequences of postnatal drug exposure on peripheral motor development.
Here, we found that valproate exposure also significantly impacted motor performance on the rotarod, which assesses motor coordination, balance, and motor learning. We found several effects of valproate treatment on the rotarod task, indicative of both impairments in basic motor coordination and balance, as well as effects that could be interpreted as impairments in motor learning. Specifically, in the C57 strain of mice, we observed a significant main effect of drug exposure on rotarod performance, with valproate treated mice continually underperforming saline treated control animals, suggestive of significant effects of valproate on basic motor abilities. Importantly, all of the C57 mice, including valproate treated animals, showed significant improvements over the course of multiple trials of testing on each day, indicating that while basal motor performance may be compromised, they still demonstrated plasticity on this task. In the case of the SVE129 mice, we observed a more complex phenotype. SVE129 mice tested early in development (P25) showed no impairments in motor performance compared with saline treated control mice, and showed normal learning on the rotarod task at this developmental stage. However, by the time these mice reached 45 days of age, we observed a significant drop in performance of valproate treated mice, and no indication of intact motor learning at this age. Based upon these observations, one possible interpretation is that in the SVE129 mice, motor coordination may continue to degrade well after the cessation of valproate treatment, leading to a progressive worsen of the motor phenotype. However, more work would be required to confirm such a prediction, as well as histological assessment of cumulative effect of valproate exposure on neural structures supporting this behavior.
We also observed significant effects of valproate exposure on basal locomotor activity, effects that were dependent upon the strain of mouse and age of testing. At P12, in the small open field, we found that drug treatment led to significant but opposing effects on locomotor activity in the two strains. SVE129 mice exposed to the drug were hypoactive compared with saline treated controls, while valproate treated C57 mice were hyperactive compared to saline treated controls of the same strain, an effect that increased with increasing dose. There are several reasons that may have contributed to the observed effects, including strain differences in basal activity rates, with SVE129 mice being more labile in general than the C57 strains. However, by the time the mice reached 21 days of age, both SVE129 and C57 mice showed effects in a uniform direction, with both demonstrating a hyperactive phenotype associated with prior valproate exposure. The neurobiological underpinnings of these effects are largely unknown, but may be the consequence of more widespread disturbance. However, as we did not investigate morphological or biochemical effects of valproate exposure on dopaminergic development, this remains speculation, and more work will be required to empirically test the effect of valproate on development of these systems.
Previous work investigating co-morbid pathology associated with pediatric exposure to valproate in patient populations have demonstrated high rates of anxiety and mood disturbance [6, 11, 35]. Here, we tested for possible effects of early life valproate exposure on development of anxiety-like behavior in mice. In the SVE129 strain, we found no indication of changes in anxiety-like behavior in valproate treated animals compared with saline treated controls. However, in C57 mice, treatment with 100 mg/kg of valproate resulted in what appeared to be a trend toward elevated anxiety-like behavior, but this effect was not present in C57 mice receiving high dose (200mg/kg) of valproate. The failure to observe effects in the Valp200 mice, could be interpreted as being consistent with the null effect observed in the SVE129 strain. Alternatively, the null effect in Valp200 treated mice may merely be the consequence of disturbance in general locomotor function, including the enhanced circling behavior observed in a significant proportion of those mice. The repetitive circling may have impacted the animals ability to navigate and avoid the center of the arena, and a failure to demonstrate a change in center time relative to saline treated mice may have merely been due to these motor disturbances. More work investigating alternative tasks that tap into emotional processing and rely less upon locomotor activity could provide additional insights into the effects of valproate exposure on emotional development.
The mechanisms underlying the observed behavioral effects of valproate are still unknown. Previous work has demonstrated that valproate exposure can significantly impact the expression of neurotrophins, specifically BDNF. Disturbance in BDNF expression has been proposed to contribute to altered cellular and circuit development and increases in cell death observed in valproate treated animals [20, 21]. Here, we assessed the effect of valproate exposure on BDNF protein levels in multiple brain regions and found a sizable decrease in BDNF expression in both strains of mice in the cerebellum, a primary center controlling motor coordination. The observation of decreased BDNF expression is consistent with prior work showing decreased bdnf gene expression in the whole brain of rodents treated for 72 hours with valproate [20, 21]. However, despite this provocative correlation, more work will be required in which genetic or pharmacological methods can be employed to restore BDNF levels in valproate treated animals to test if the drop in BDNF is causally linked to the observed behavioral deficits. For example, BDNF heterozygous mice, in which a single copy of the BDNF gene has been deleted do not show significant disruptions in motor development, similar to those observed here. However, the failure to observe such effects in BDNF heterozygous mice could either be due to accommodation from lacking BDNF throughout embryonic development, or secondary and tertiary effects of valproate on circuit activity and function over development. However, the observation of regionally selective effects of valproate on cerebellum and not hippocampus BDNF expression deserved additional attention. Such effects could be important for understanding the particularly profound effect of drug exposure on motor development.
In the attempt to exclude potential third variables that may have contributed to the observed behavioral and biochemical phenotypes observed here, we included multiple controls. For example, it is possible that acute effects of valproate can impact motor functioning. To control for this, for all testing that occurred during the valproate treatment phase (P4-P11), tasks were carried out prior to that days administration of drug. For tasks such as the wire hang, open field, and rotarod testing occurred several days, and in some cases weeks, after the injection period had ended. Therefore, several of the motor phenotypes cannot be attributed to acute effects of valproate on motor function or sedation. In recent work, we have shown that early life stress induced by alterations in maternal care can profoundly impact behavioral development of mice, with significant effects on somatic development [36]. In order to account for potential differences in maternal care, each litter contained mice from all treatment groups including saline controls (e.g. for a given litter, a subset of pups were treated with drug, while the other pups received saline). Thus, possible alterations in maternal behavior that may have been driven by distress or malaise of the pups are not likely to account for the behavioral effects observed here.
5. CONCLUSIONS
Valproate is an effective therapeutic for those suffering from a broad range of seizure types. While teratogenic effects have been observed following prenatal exposure, postnatal exposure may also have significant, albeit more subtle effects on brain and behavioral development. In the case of any treatment, the benefits must we weighed against the potential adverse effects. In the current study, we provide additional evidence from a model organism that postnatal exposure may carry with it potential adverse effects on motor development, and identify possible mechanisms underlying those effects. These data provide support for more intensive investigation of possible deleterious effects of valproate on postnatal brain and behavioral development in patient populations, and possibly diminished use of this drug in developing populations.
Highlights.
Early life valproate leads to motor impairments in two strains of mice.
Early life valproate does not contribute to development of anxiety-like behaviors.
Early life valproate leads to regional effects on BDNF expression in brain.
Acknowledgments
This work was funded by a grant from the Epilepsy Foundation (KGB), the Robert and Nancy Carney Fund for Scientific Innovation (KGB), as well as in part by an Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES CITED
- 1.Forsgren L, Wallace SJ, Farrell K. In: Epilepsy in children. 2nd. Incidence and Prevalence, editor. Arnold; London: 2004. Arnold. [Google Scholar]
- 2.Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129(2):256–64. doi: 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- 3.Albsoul-Younes A, et al. Patterns of antiepileptic drugs use in epileptic pediatric patients in Jordan. Neurosciences (Riyadh) 2016;21(3):264–7. doi: 10.17712/nsj.2016.3.20150766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan WW, et al. A retrospective study on the usage of antiepileptic drugs in Asian children from 2000 to 2009 in the largest pediatric hospital in Singapore. Pharmacoepidemiol Drug Saf. 2012;21(10):1074–80. doi: 10.1002/pds.3293. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt KM, et al. Drug utilization in pediatric neurology outpatient department: A prospective study at a tertiary care teaching hospital. J Basic Clin Pharm. 2014;5(3):68–73. doi: 10.4103/0976-0105.139729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glauser TA. Effects of antiepileptic medications on psychiatric and behavioral comorbidities in children and adolescents with epilepsy. Epilepsy Behav. 2004;5(Suppl 3):S25–32. doi: 10.1016/j.yebeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Bath KG, Scharfman HE. Impact of early life exposure to antiepileptic drugs on neurobehavioral outcomes based on laboratory animal and clinical research. Epilepsy Behav. 2013;26(3):427–39. doi: 10.1016/j.yebeh.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trimble MR, Thompson PJ. Sodium valproate and cognitive function. Epilepsia. 1984;25(Suppl 1):S60–4. doi: 10.1111/j.1528-1157.1984.tb05640.x. [DOI] [PubMed] [Google Scholar]
- 9.Vining EP, et al. Psychologic and behavioral effects of antiepileptic drugs in children: a double-blind comparison between phenobarbital and valproic acid. Pediatrics. 1987;80(2):165–74. [PubMed] [Google Scholar]
- 10.Vining EP. Cognitive dysfunction associated with antiepileptic drug therapy. Epilepsia. 1987;28(Suppl 2):S18–22. doi: 10.1111/j.1528-1157.1987.tb05767.x. [DOI] [PubMed] [Google Scholar]
- 11.Sommerbeck KW, et al. Valproate sodium: evaluation of so-called psychotropic effect. A controlled study. Epilepsia. 1977;18(2):159–67. doi: 10.1111/j.1528-1157.1977.tb04464.x. [DOI] [PubMed] [Google Scholar]
- 12.Chomiak T, Turner N, Hu B. What We Have Learned about Autism Spectrum Disorder from Valproic Acid. Patholog Res Int. 2013;2013:712758. doi: 10.1155/2013/712758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabunga DF, et al. Exploring the Validity of Valproic Acid Animal Model of Autism. Exp Neurobiol. 2015;24(4):285–300. doi: 10.5607/en.2015.24.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner GC, et al. A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord. 2006;36(6):779–93. doi: 10.1007/s10803-006-0117-y. [DOI] [PubMed] [Google Scholar]
- 15.Yochum CL, et al. VPA-induced apoptosis and behavioral deficits in neonatal mice. Brain Res. 2008;1203:126–32. doi: 10.1016/j.brainres.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 16.Furnari MA, et al. Altered behavioral development in Nrf2 knockout mice following early postnatal exposure to valproic acid. Brain Res Bull. 2014;109:132–42. doi: 10.1016/j.brainresbull.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds S, Millette A, Devine DP. Sensory and Motor Characterization in the Postnatal Valproate Rat Model of Autism. Dev Neurosci. 2012 doi: 10.1159/000336646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomiak T, et al. Altering the trajectory of early postnatal cortical development can lead to structural and behavioural features of autism. BMC Neurosci. 2010;11:102. doi: 10.1186/1471-2202-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz J, Shields WD. Effects of dipropylacetate on brain development. Ann Neurol. 1981;10(5):465–8. doi: 10.1002/ana.410100510. [DOI] [PubMed] [Google Scholar]
- 20.Bittigau P, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089–94. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;993:103–14. doi: 10.1111/j.1749-6632.2003.tb07517.x. discussion 123–4. [DOI] [PubMed] [Google Scholar]
- 22.Kim JS, et al. Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia. 2007;48(Suppl 5):19–26. doi: 10.1111/j.1528-1167.2007.01285.x. [DOI] [PubMed] [Google Scholar]
- 23.Bennett GD, et al. Valproic acid-induced alterations in growth and neurotrophic factor gene expression in murine embryos [corrected] Reprod Toxicol. 2000;14(1):1–11. doi: 10.1016/s0890-6238(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 24.Fukuchi M, et al. Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res. 2009;65(1):35–43. doi: 10.1016/j.neures.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Phiel CJ, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276(39):36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 26.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 27.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bath KG, et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28(10):2383–93. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24(17):4250–8. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi XY, et al. Effects of antiepileptic drugs on mRNA levels of BDNF and NT-3 and cell neogenesis in the developing rat brain. Brain Dev. 2010;32(3):229–35. doi: 10.1016/j.braindev.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Petrosini L, Molinari M, Gremoli T. Hemicerebellectomy and motor behaviour in rats. I. Development of motor function after neonatal lesion. Exp Brain Res. 1990;82(3):472–82. doi: 10.1007/BF00228789. [DOI] [PubMed] [Google Scholar]
- 32.Rodier PM, et al. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370(2):247–61. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Weihl CC, Connolly AM, Pestronk A. Valproate may improve strength and function in patients with type III/IV spinal muscle atrophy. Neurology. 2006;67(3):500–1. doi: 10.1212/01.wnl.0000231139.26253.d0. [DOI] [PubMed] [Google Scholar]
- 34.Pandamooz S, et al. Valproic acid preserves motoneurons following contusion in organotypic spinal cord slice culture. J Spinal Cord Med. 2016:1–7. doi: 10.1080/10790268.2016.1213518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glauser TA. Behavioral and psychiatric adverse events associated with antiepileptic drugs commonly used in pediatric patients. J Child Neurol. 2004;19(Suppl 1):S25–38. doi: 10.1177/088307380401900104. [DOI] [PubMed] [Google Scholar]
- 36.Bath KG, Manzano-Nieves G, Goodwill H. Early life stress accelerates neuroal and behavioral maturation of the hippocampus in male mice. Horm Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


