Abstract
Hyaluronan and versican are extracellular matrix (ECM) components that are enriched in the provisional matrices that form during the early stages of development and disease. These two molecules interact to create pericellular “coats” and “open space” that facilitate cell sorting, proliferation, migration, and survival. Such complexes also impact the recruitment of leukocytes during development and in the early stages of disease. Once thought to be inert components of the ECM that help hold cells together, it is now quite clear that they play important roles in controlling cell phenotype, shaping tissue response to injury and maintaining tissue homeostasis. Conversion of hyaluronan-/versican-enriched provisional matrix to collagen-rich matrix is a “hallmark” of tissue fibrosis .Targeting the hyaluronan and versican content of provisional matrices in a variety of diseases including, cardiovascular disease and cancer, is becoming an attractive strategy for intervention.
Keywords: Versican, Hyaluronan, Glycocaylx, Extracellular matrix, Leukocytes, Development, Remodeling
Introduction
Throughout life, the extracellular matrix (ECM) constantly changes! ECM composition is controlled by the coordinate and differential regulation of synthesis and turnover of each of its individual components. These changes are driven by cytokines and growth factors and can involve the formation of matrices that are open and loose or compact and rigid. Disease often recapitulates development. Early in development, the ECM remodels to form a loose open network of molecules that facilitate cell division, cell movement, and cell sorting. While, in disease, the first stages of repair are the creation of an ECM that is open and loose, allowing cells to enter and repair tissue damage created by disease insult. Such an open and loosely organized ECM is, in both cases, referred to as a Provisional Matrix.
Components of the ECM interact by entanglement, cross-linking, and charge-dependent interactions to form bioactive polymers which, in part, regulate the biomechanical properties of tissues and interact with cells to affect cell phenotype [1]. Usually, matrices that are soft and compliant are enriched in proteoglycans and hyaluronan, while matrices that are stiff and rigid are enriched in collagens and other fibrous proteins. The relative contributions of different ECM molecules vary with tissue type and result in mechanical and chemical properties appropriate to each tissue environment [2]. However, during development and in disease, the ECM undergoes changes to accommodate cellular events, such as proliferation and migration, and to maintain tissue homeostasis. Disturbances in the balance of these components creates altered tissue architecture that impacts tissue function. In fact, such ECM disturbances may determine or dictate the course of disease pathogenesis. Targeting the ECM is becoming an effective therapeutic strategy to treat human disease [3–5]. To understand the dynamic nature of the ECM and the functional consequences of ECM changes as tissues develop and remodel, classic wound healing is often given as an example of the transitions that occur [6–8]. Wound healing shares certain common features of ECM remodeling and with early events during embryonic development, such as in the development of the chick cornea or neural crest, the complete regeneration of a limb in amphibians [9–11], and in epithelial-to-mesenchymal transitions (EMT) [12]. With wounding, an ECM forms in a cell-free space, followed by the migration of mesenchymal cells into this ECM. These early changes involve the wound filling with a wave of cytokines and growth factors originating from broken or leaky blood vessels and cells entering and originating from within the damaged tissue. The early “provisional ECM” is formed by plasma proteins, such as fibrin, fibrinogen, and fibronectin, seeping into the wound site along with hyaluronan, either from the plasma or released from migrating mesenchymal cells. These components interact to form a crossed-linked gel which acts as a temporary scaffold for cellular events needed to repair the wound [6, 8, 11, 13, 14]. Thus, the provisional matrix serves as a scaffold and substrate for other cells entering into the wound bed, such as fibroblasts and smooth muscle cells. Such a substrate impacts the phenotype of the cells in contact with this matrix. For example, we showed that culturing arterial smooth muscle cells (ASMCs) on fibrin gels dramatically enriches these gels with decorin and biglycan, thus changing the mechanical properties of the fibrin gel. Such changes lead to alterations in the phenotype of the ASMCs [15].
In addition, during the early provisional phase of wound healing, inflammatory cells are drawn into the wound bed together with additional fibroblasts which further modify the composition of the provisional matrix. Such modifications include the synthesis and accumulation of hyaluronan and versican [16–18]. This is referred to by some as a “second order” of provisional ECM [19, 20]. This review will consider the importance of hyaluronan and versican as specific ECM components of the provisional matrix and their role in regulating the phenotype of cells embedded in this matrix.
Hyaluronan and Versican
Hyaluronan is a glycosaminoglycan (GAG) consisting of a linear polymer of repeating disaccharides of glucuronic acid and N-acetylglucosamine [β(1,4)-GlcUAβ(1,3)-GlcNAc-]n, and is synthesized by three different, but related, hyaluronan synthases, HAS1, HAS2, and HAS3 [21–24]. These are enzymes with multiple transmembrane domains and are situated on the inner surface of the plasma membrane of cells. During synthesis, the growing polymer is extruded through the membrane into the pericellular space. This is in contrast to the mode of synthesis of other GAGs, which are made and covalently linked to core proteins in the Golgi apparatus of the cell to form a proteoglycan, and then secreted by normal exocytotic mechanisms [25, 26]. Hyaluronan chains can be anchored to the cell surface via the synthase enzyme or through binding to a cell surface receptor such as CD44 or RHAMM (receptor for hyaluronan-mediated motility). Hyaluronan is cleaved by one of several hyaluronidases. There are six hyaluronidase genes in humans, encoding enzymes with different properties and different cell locations [27, 28]. Under normal physiological conditions, hyaluronan ranges in relative molecular mass from 104–107 Da (see reviews [29, 30]). Hyaluronan can also self-associate to form fibers (cables), networks, and stacks. When retained at the cell surface, hyaluronan forms a voluminous pericellular matrix or “coat,” which has also been termed “glycocalyx.” The hyaluronan-dependent coat has multiple important roles, from serving structural and mechanochemical functions, to the regulation of cell division and motility [31]. High molecular weight hyaluronan (> 500 kDa) has anti-inflammatory properties while low molecular weight (fragments < 500 kDa) promote inflammation [29, 30]. Hyaluronan interacts with a number of cells including leukocytes, playing a critical role in immunity and inflammation [29, 32, 33]. Such interactions are prominent in diseases such as cancer and affect events that promote tumor formation such as progression and metastasis [34–39]. Hyaluronan is an integral component of the provisional matrix.
Versican is a large chondroitin sulfate (CS)-containing proteoglycan that interacts with hyaluronan through specific domains in its core protein [40]. Versican is synthesized by a variety of cells and in humans it is encoded from a single gene locus on chromosome 5q14.3 [41]. It is 86% identical between mouse and human [42], indicating the importance and highly conserved nature of this proteoglycan. Versican is encoded by 15 exons that are arrayed over 90 kb of continuous genomic DNA [43]. The central, GAG-bearing domain of the versican core protein is coded by two large exons, α-GAG and β-GAG, which can be alternately spliced with exon 7, which codes for the α-GAG region, and exon 8, which codes for the β-GAG region. When both the entire exons 7 and 8 are present and no splicing occurs, versican V0 is formed. When exon 7 is spliced out, versican V1 is formed. When exon 8 is spliced out, versican V2 is formed. When both exons 7 and 8 are spliced out, versican V3 is formed. This form of alternate splicing gives rise to versican variants that differ in the number of CS chains attached to the consensus sequence attachment sites in the core proteins (see reviews [43–45]). Since V3 does not contain any CS chains, it cannot be considered a proteoglycan, but it is frequently grouped with proteoglycans and characterized as such [46]. It is of interest that while V0, V1, and V3 are found in most tissues, V2 is mostly restricted to the central nervous system [47]. The CS GAG chains attached to the different isoforms of versican may differ in size and composition, depending upon the species, the tissue of origin, or the culture conditions that promote versican synthesis. For example, CS chains isolated from versican synthesized by ASMCs have a 6-sulfate:4-sulfate ratio of 2 which increases to approximately 4 upon platelet-derived growth factor (PDGF) (mitogenic) stimulation of the cells [48, 49]. Such stimulation also increases the length of the CS chains attached to versican from 45 to 70 kDa leading to an overall increase in the hydrodynamic size of the proteoglycan and its capacity to trap water. Versican is cleaved by a number of proteases [50] including members of the a disintegrin and metalloproteinase with a thrombospondin type-1 motif (ADAMTS) family [51]. Versican fragments (versikines) are capable of eliciting biological activities, such as promoting cell death (apoptosis) [52] in some systems. The modifications in the structure of versican contribute to different degrees of matrix expansion and the altered mechanical properties of provisional matrices. Both stromal cells [44] and leukocytes (macrophages) [53, 54] are capable of synthesizing and secreting versican.
Hyaluronan Binding Proteins–the Hyaladherins
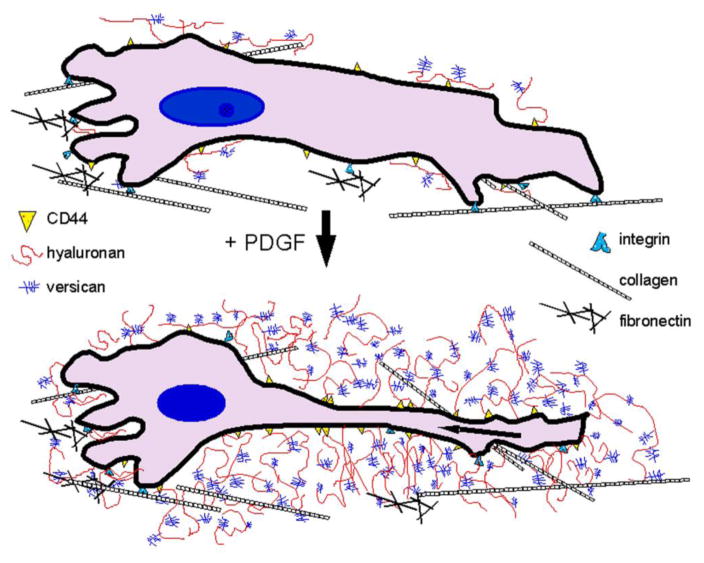
Hyaluronan is a “sticky” molecule and several proteins involved in provisional matrix formation bind hyaluronan, including fibrin, fibrinogen, fibronectin, and the family of hyaluronan binding proteoglycans, the hyaladherins of which versican is a member [47, 55]. Versican binds hyaluronan via a 100-amino acid domain within the G1 domain of the proteoglycan termed a Link Module [40, 56–60]. Examination of the vertebrate genomes reveals at least 13 hyaluronan binding proteins, with one or two link module domains, plus an unknown number of proteins that contain the hyaluronan binding motifs B(X7)B. This motif defines B as arginine or lysine and X is any non-acidic amino acid [61]. The interaction of versican with hyaluronan is the basis for formation of the pericellular matrix that surrounds cells and, in part, controls their phenotype, as seen in development and disease (Figure 1). The interaction of these two molecules with one another has a dramatic impact on how they are assembled into complex structures. For example, we recently found that adding the G1 domain of versican to dermal fibroblasts in vitro promoted hyaluronan aggregation on the surface of the fibroblasts and the formation of hyaluronan cables [62] (Figure 2A,B). In cell culture, such structures can be seen to connect adjacent cells and may be important in cell-cell communication. These hyaluronan cables can serve as “landing strips” for leukocytes that come into contact with this matrix during inflammation (Figure 2B). It is also clear that different isoforms of versican can impact the organization of the ECM in such a manner as to create either a pro- or anti-inflammatory form of the ECM [44, 45, 63]. For example, overexpression of the V3 isoform of versican by stromal cells decreases the expression of the V0 and V1 isoforms as well as the synthesis and accumulation of hyaluronan. These changes are accompanied by increases in elastic fiber assembly and decreases in the capacity of this remodeled ECM to bind and activate macrophages [64–69].
Figure 1.
ECM transitions in the pericellular matrix required for cell shape changes in r cell proliferation and migration. Growth factors such as PDGF and/or TGF-β stimulate arterial smooth muscle cells to produce hyaluronan and versican which interact and expand the tissue space by entrapping water. Reprinted from Advanced Drug Delivery Reviews, Volume 59, Evanko SP, Tammi, MI, Tammi, RH, Wight, TN. Hyaluronan-dependent pericellular matrix, pages 1351–65, Copyright 2007, with permission from Elsevier.
Figure 2.
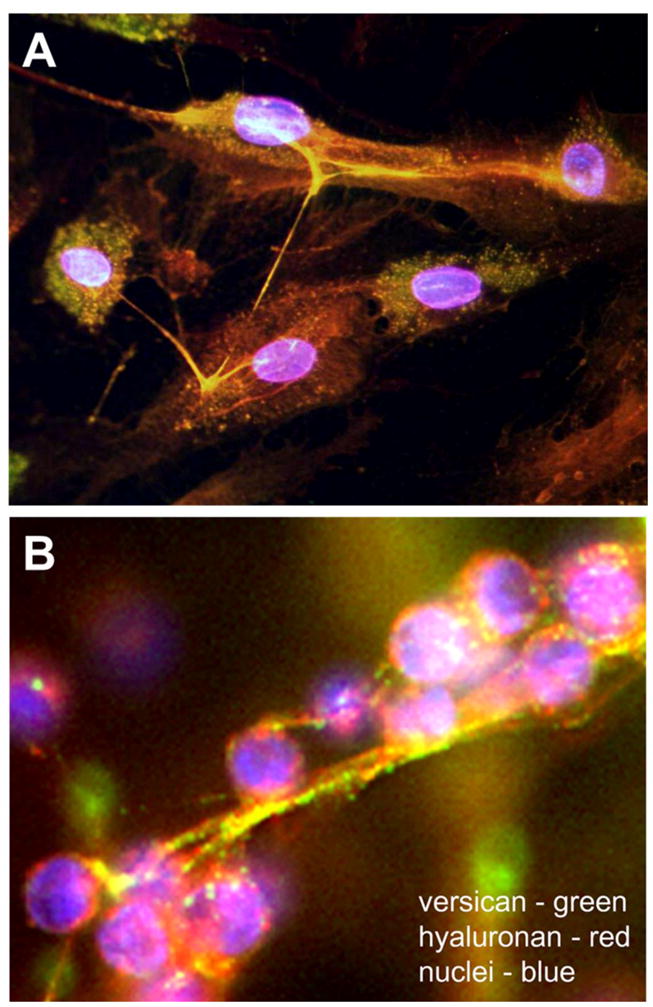
(A) Pericellular hyaluronan cables. Recombinant G1 domain of versican (red) was added to cultured human dermal fibroblasts and the cultures stained for hyaluronan (green). The G1 domain bound to the hyaluronan strands causing them to aggregate (yellow). These cable-like strands were observed connecting adjacent cells in a perinuclear distribution. (B) Pericellular hyaluronan/versican cables bind leukocytes. Adhesion of T lymphocytes (purple) to a hyaluronan (red)/versican (green)-enriched cable in the pericellular matrix of fibroblasts. Panel A is reprinted from Merrilees MJ, Zuo N, Evanko SP, Day AJ, Wight TN. G1 domain of versican regulates hyaluronan organization and the phenotype of cultured human dermal fibroblasts. J Histochem Cytochem. 64:353–363, 2016. Panel B is reprinted from Biochimica et Biophysica Acta (BBA) - General Subjects, Vol. 1840, Issue 8, Wight TN, Kinsella MG, Evanko SP, Potter-Perigo S, Merrilees MJ. Versican and the regulation of cell phenotype in disease, pages 2441–2451, Copyright 2014, with permission from Elsevier.
While versican and hyaluronan are usually found together in complex formation within the pericellular ECM, there can be situations where only versican is present without hyaluronan and vice versa. Such alterations have a dramatic effect on how cells respond to various stimuli [70]. Other proteins such as inter-alpha-inhibitor (IαI) and tumor necrosis factor-stimulated gene-6 (TSG-6), have link domains and interact through covalent cross linking, promoting the formation and organization of hyaluronan strands [29, 58–60, 71–74]. IαI is a serum protein that forms cross links with hyaluronan monomers through the catalytic activity of TSG-6 [58, 59, 71–73]. This type of cross linking stabilizes the hyaluronan-enriched matrices and is prominent during provisional matrix formation. This is the only covalent modification known to occur on hyaluronan that facilitates changes in its organization and assembly critical to specific events in development and disease [29, 75, 76]. These interactions are critical to shaping both the microenvironment and the provisional matrix, and to a large extent may regulate the ability of hyaluronan to impact cell phenotype [29, 58, 59]. A recent study suggests that complexes that contain hyaluronan, versican, TSG-6, and IαI found on the surface (glycocalyx) of umbilical cord mesenchymal stem cells have immunosuppressive properties and protect the stem cells from immunodestruction [77]. Furthermore, this study went on to show that this versican/hyaluronan-enriched glycocalyx impacted both T cell and macrophage inflammatory phenotypes. Such results suggest that this hyaluronan/versican complex may be a valuable resource for immnunosuppressive and anti-inflammatory therapeutic approaches. Interestingly, in tumors, cancer stem cells synthesize and secrete prominent pericellular matrices enriched in hyaluronan and versican [78]. Such a “cell coat” may account, in part, for the resistance to chemotherapy that these cells exhibit, as well as protection from immune surveillance.
Hyaluronan and Versican as Components of the Pericellular Matrix
Cells alter the composition and organization of their microenvironment when they divide and migrate [31, 79]. This remodeling is driven by cytokines and growth factors. For example, PDGF promotes cell proliferation as part of the early growth response that occurs in development and in the early phase of wound repair. PDGF also stimulates cells to produce a pericellular matrix enriched in hyaluronan and versican forming a viscoelastic cell coat. This cell coat allows the cells to change shape and facilitates cell division and migration [31, 49, 79–82]. In fact, interfering with the formation of this ECM coat by treating the cells with short oligosaccharides of hyaluronan blocks their proliferation even in the presence of a mitogen [79]. It is postulated that all cells use this modification to different degrees for division and migration.
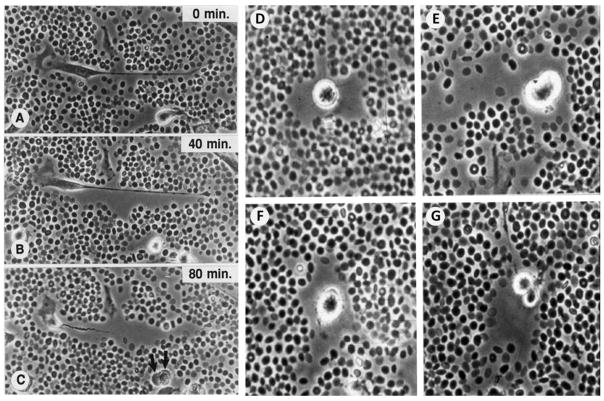
Several studies have investigated the structure and formation of the hyaluronan/versican-rich pericellular matrix that forms the provisional matrix for a number of cells. A technique to view the hyaluronan-dependent pericellular matrix is the particle exclusion assay, which was first utilized nearly forty years ago [83]. In this cell culture assay, a suspension of particles, usually fixed erythrocytes, is allowed to settle onto a cell culture dish containing cells. A clear zone surrounding the cell is made apparent by virtue of the exclusion of the red blood cells (RBCs) by the gel-like hyaluronan coat (Figure 3). When cells proliferate and/or migrate, hyaluronan and versican are secreted and form a coat in the pericellular space [31, 79]. This coat excludes the RBCs used in the particle exclusion assay as it is possible to watch the coat spread and expand when using living cells (Figure 3A, B). Treatment of cells with hyaluronan-specific Streptomyces hyaluronidase removes the pericellular coat, indicating that the coat is hyaluronan-dependent. The size of the pericellular coat is dependent on the presence of an aggregating proteoglycan, such as aggrecan or versican, and is required to exclude erythrocytes in the particle assay [84, 85]. The presence of aggregating proteoglycans in the pericellular matrix confers a high fixed negative charge density due to the numerous CS chains, and can have important effects on the material properties and permeability of the matrix. In addition, this matrix allows cells to change shape and guides their proliferation and migration [44, 86]. Thus, the osmotic swelling pressure of the pericellular matrix is increased when more proteoglycans are present. The importance of the aggregating proteoglycans in the formation of the pericellular coat and determination of cell phenotype is highlighted by a study examining dermal fibroblasts from ADAMTS5−/− mice which lack this versican-degrading protease [87]. The cells from these mice had thickened pericellular coats due to elevated versican content and exhibited a myofibroblast phenotype. However, crossing these mice to versican haplo-insufficient mice generated dermal fibroblasts with normal pericellular coats and normal cell phenotypes. Such findings indicate that the composition of the pericellular matrix can, in part, regulate cell phenotype.
Figure 3.
Video microscopic images of migrating (A, B, C) and dividing (D-G) human vascular smooth muscle cells treated with PDGF. Fixed red blood cells (particles) have been added to the living culture and are being excluded from the pericellular matrix by the viscoelastic versican/hyaluronan matrix. The formation of this matrix facilitates cell shape change and is permissive for cell proliferation and migration. Reprinted from Chemistry and Biology of Hyaluronan, Garg HG, Hales CA, eds. Wight TN, Evanko S, Kolodgie F, Farb A, Virmani R, Chapter 14, Hyaluronan in atherosclerosis and restenosis, pages 307–321, Copyright 2004, with permission from Elsevier.
Hyaluronan and Versican in Provisional Matrices in Development
Provisional matrices are highly malleable, viscoelastic, and compliant. They are important in the development of several organ systems in that hyaluronan and versican create tissue space for cell sorting and shifting during development. For example, expression and accumulation of versican occur in the dermal papillae and associated mesenchyme in the skin in distinct temporal and spatial patterns during hair follicle development, implicating versican in hair follicle maturation [88, 89]. Versican also increases concomitantly with hyaluronan during the pre-ovulatory follicular development period and expansion of the cumulus cell oocyte complex during ovulation [90, 91]. The uterine cervix undergoes changes during pregnancy and labor that transforms it from a closed rigid structure to a dilated distensible structure enriched in versican and hyaluronan to allow birth. This involves significant remodeling of the ECM with increases in hyaluronan and versican [92]. Versican expression is also high in the developing mesenchyme during limb development as the ECM expands prior to mesenchymal condensation [93, 94]. Transient expression of versican also occurs in migratory pathways of melanoblasts [95–98] and in neural crest migratory pathways and provides migratory cues for these developmental events [86, 99, 100]. Expression and processing of versican appear important during embryonic stem cell differentiation since the different splice variants of versican are upregulated and deposited along with ADAMTS-generated versican fragments during embryoid body formation, with localization in the developing mesenchyme consistent with a role in epithelial-to-mesenchymal transition [101–103]. While a distinct mechanistic role for hyaluronan and versican in stem cell differentiation is not known, it is of interest that changes in the expression of these ECM components accompany cardiomyocyte differentiation from undifferentiated human embryonic stem cells [104]. Provisional matrices enriched in hyaluronan and versican are involved in migratory activity of cells in endocardial cushion formation in the developing heart [105–107]. Interestingly, knocking out the gene for either hyaluronan or versican is embryonic lethal due to failure of the heart to form. In addition, the different isoforms of versican and/or the processing of versican together with hyaluronan in these matrices may control different aspects of cardiomyocyte differentiation and heart development as well [108–110].
Hyaluronan and Versican in Provisional Matrices in Blood Vessel Disease
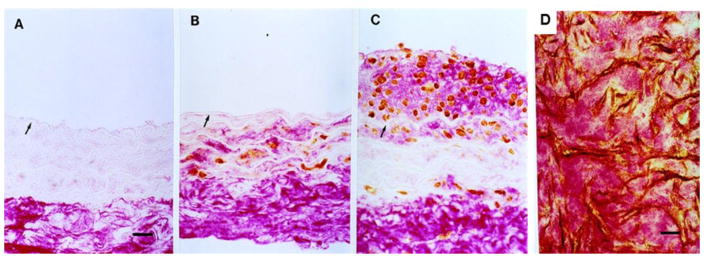
The in vitro generation of hyaluronan/versican pericellular matrix can be seen in vivo as well. In blood vessels, these provisional matrices containing few collagen or elastic fibers [81, 111–114] (Figure 4) participate in early intimal hyperplasia in response to arterial injury, such as occurs in vascular angioplasty, stenting, and bypass grafting [63, 81, 115]. Such matrices are postulated to support ASMC proliferative and migratory intimal expansion characteristic of early vascular disease. Inhibiting the accumulation of hyaluronan following arterial injury by treatment with 4-methylbelliferone (4-MU) in this animal model inhibits intimal hyperplasia [116]. Furthermore, animals deficient in HAS3, one of the enzymes responsible for hyaluronan synthesis, do not develop intimal hyperplasia when subjected to arterial injury [117]. Collectively, these studies indicate a critical role for hyaluronan in matrix expansion and ASMC proliferation in early arterial disease. We also know that these changes are found not only in experimental animals subjected to experimental injury, but also in humans who have undergone balloon angioplasty to debulk vascular lesions [111–114]. The restenotic lesions that occur following balloon angioplasty in patients can occur in less than a year after intervention and are composed primarily of an ECM of hyaluronan and versican creating an open wound bed [111–114] (Figure 4). In fact, this ECM has been likened to a provisional matrix in early wounds [118]. Such matrices are often termed “myxoid” and are devoid of collagens and other fibrous proteins necessary to offset the swelling pressure of the large proteoglycans and hyaluronan. Thus, it may be that the rapid closure of vessels that have undergone balloon angioplasty is due to edema and tissue swelling. On the other hand, loss or breakdown of the hyaluronan/versican complex could lead to the expulsion of water and tissue shrinkage (inward remodeling) and a compromise in arterial circumference [63]. This myxoid matrix is also characteristic of artery/vein anastomoses and failed vein grafts which are enriched in versican and hyaluronan with little collagen or elastic fibers [111–113, 119]. These myxoid matrices promote leukocyte invasion and accumulation, (see reviews: [29, 45, 120]) as described above. Interfering with the generation of this provisional matrix, such as inhibiting the accumulation of versican, completely blocks macrophage influx and lipid accumulation in an animal model of vascular injury with high fat feeding [68]. Other vascular diseases, such as idiopathic pulmonary hypertension, involve intimal hyperplasia, vascular remodeling, and inflammatory cell recruitment and are characterized by increases in hyaluronan [71, 121] and versican [122].
Figure 4.
Hyaluronan accumulates in blood vessels following balloon angioplasty. Rat carotid artery stained for hyaluronan (red) and proliferative cell nuclear antigen (brown). (A) Uninjured. Hyaluronan is confined to the adventitia with little to no staining for PCNA. (B) 3 days after balloon injury, positive PCNA staining appears in the media surrounded by hyaluronan staining (red). (C) By 7 days, a neointima has formed consisting of PCNA-positive cells in a “sea” of hyaluronan (red). Versican is also present with hyaluronan, but little to no collagen or elastic fibers are present. (D) A section of a human coronary restenotic lesion retrieved 3 months after angioplasty and doubled stained for PCNA (brown) and hyaluronan (red). This myxoid ECM is also enriched in versican (not shown) with little to no collagen or elastic fibers present. Originally published in Circulation. Riessen R, Wight TN, Pastore C, Henley C, Isner JM. Distribution of hyaluronan during extracellular remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996; 93:1141–1147. Wolters Kluwer Health Lippincott Williams & Wilkins©.
Hyaluronan and Versican in Provisional Matrices in Cancer
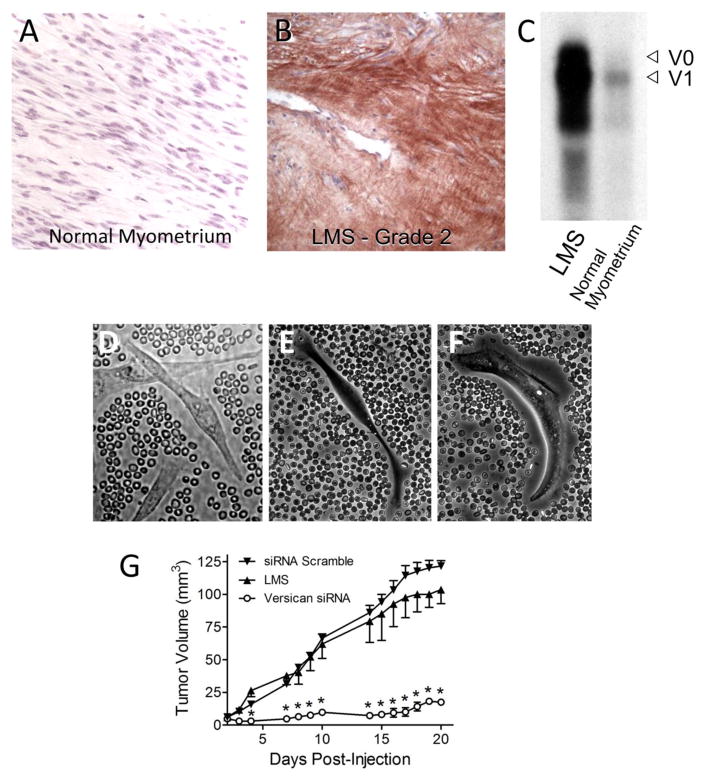
In addition, myxoid provisional matrices are formed in different types and stages in cancer [34–37, 123–127]. These ECMs are found in tumor stroma [124, 125, 128, 129] and have prompted the suggestion that “tumors are wounds that do not heal” [129, 130]. Clearly tumor stroma is enriched in fibrin, hyaluronan, and versican. Such a matrix that fails to transit to a more fibrous, cross-linked mature ECM could provide an environment for continued tumor growth, promoting tumor cell proliferation and migration [129]. In cancer, either the stromal cells surrounding the tumor or the tumor cells themselves produce hyaluronan/versican provisional matrices impacting tumor cell proliferation, migration, and metastasis [78, 123–125, 131]. For example, in a study of human leiomyosarcoma smooth muscle tumors which are enriched in versican, we found that the tumor cells exhibited thick pericellular coats in culture which could be reduced by inhibiting versican synthesis using siRNA treatment of these cells (Figure 5) [123]. Interestingly, blocking versican synthesis and accumulation in the pericellular matrices reduced tumor cell growth in vitro and the ability of these cells to form tumors in vivo in a mouse model of leiomyosarcoma [123] (Figure 5). Such results indicate that controlling provisional matrix formation by tumor or stromal cells may represent a therapeutic approach for treating certain cancers.
Figure 5.
Versican in tumor provisional matrix. (A) Section of normal human myometrium stained for versican showing no staining. (B) Grade 2 leiomyosarcoma stained for versican (brown) showing extensive immunostaining. (C) Northern blot analyses showing enrichment for the versican signal in the tumor compared to control. (D–F) Leiomyosarcoma smooth muscle (LMS) cells in culture treated with fixed red blood cells to image the pericellular matrix. (D) The LMS cells exhibit extensive pericellular coats. E. Pericellular matrix of the LMS cells after treatment with siRNA to versican. (F) LMS pericellular coat 24 hours after adding back versican to cells shown in B. (G) Tumor growth in a mouse model of LMS using LMS cells treated or not treated with siRNA to versican. This figure shows reduced tumor growth in the animals receiving siRNA versican LMS cells. This research was originally published in Journal of Biological Chemistry. Keire PA, Bressler SL, Lemire JM, Edris B, Rubin BP, Rahmani M, McManus BM, van de Rijn M, Wight TN. A role for versican in the development of leiomyosarcoma. J Biol Chem. 2014; 289:34089–103. © by the American Society for Biochemistry and Molecular Biology.
Hyaluronan and Versican in Provisional Matrices in Angiogenesis
Support for the importance of such a provisional matrix in tumor growth is the impact of the ECM on growth of new blood vessels [132]. Angiogenesis is a normal and vital process in development, wound healing, and disease and, in part, takes place in matrices that are open and compliant such as found in the provisional ECM. Versican and hyaluronan also play a central role in angiogenesis [133–144]. We found, for example, that human stromal stem cells can regulate the angiogenic phenotype of endothelial cells by modulating the formation of hyaluronan- and versican-enriched provisional matrices [145]. Stromal stem cells that produce elevated levels of versican formed the most extensive vascular network when co-cultured with vascular endothelial cells. Furthermore, patches containing these pro-angiogenic cells, when transplanted onto uninjured athymic rat hearts, developed 50-fold more vessels than stromal cells with low versican expression [145]. The deposition of versican has been found to be linearly correlated with the number of microvessels in tumor stroma [146, 147]. Versican is actively processed during the early stages of VEGF-A-induced pathological angiogenesis in tumors [136]. These observations, plus the fact that the tumor stroma contains provisional matrix components such as fibrin, fibronectin, and hyaluronan [129, 130, 148], highlight the importance of this specialized ECM in the pathogenesis of cancer. In angiogenic models, increased versican expression is often accompanied by increased expression of hyaluronan [149, 150]. Clearly hyaluronan and fragments of hyaluronan have been shown to play a key role in new blood vessel formation, affecting the behavior of endothelial cells [137–144]. The involvement of immune cells as part of this angiogenic phenotype in disease such as cancer is critical [151].
Hyaluronan and Versican in Provisional Matrices in Immune Regulation and Inflammation
Immune and inflammatory cells penetrate provisional matrices and come into contact with hyaluronan and versican. For example, intact hyaluronan interacts with regulatory T cells through CD44 and promotes their functional suppression of T cell responder proliferation, whereas degraded low molecular weight hyaluronan does not exhibit this activity [152, 153]. In addition, high molecular weight hyaluronan promotes the induction of IL-10 by TR-1 regulatory cells. Such treatment is capable of abrogating an IL-10-dependent colitis in a mouse model, while fragmented hyaluronan is ineffective [154]. Both hyaluronan and versican, when produced by stromal cells under stress, such as endoplasmic reticulum (ER) stress, are strongly adhesive for T cells and this limits their migration [155]. The important role that hyaluronan and the provisional matrix play in disease progression has recently been highlighted by studies showing that interfering with hyaluronan accumulation in pancreatic islets and the brain can block the development of type 1 diabetes (T1D) [156] and the progression of multiple sclerosis [157] in experimental animals.
Leukocytes interact with complexes of hyaluronan and versican that have formed cable-like structures within the ECM [32, 45, 70, 158–162]. It has been proposed that the presentation of hyaluronan in a crosslinked form, together with other hyaluronan binding molecules, leads to receptor clustering on leukocytes (or co-receptor engagement through the presence of accessory molecules on the cables) which promotes adhesion [58, 160]. Furthermore, these versican/hyaluronan-rich ECMs may be degraded by infiltrating leukocytes, generating small versican and hyaluronan fragments, which by themselves impact inflammatory events [163] as described above. ECM degradation is often referred to as a “danger signal” alerting the cells to remodel and repair the ECM so that homeostasis can be restored [164]. Some ECM fragments can promote vascularization as part of the repair process, such as is seen for hyaluronan fragments promoting new blood vessel growth [144]. In addition, hyaluronan fragments can be pro-inflammatory by stimulating inflammatory cytokine release by myeloid cells [165, 166]. The bioactive members of the ADAMTS family of proteases can degrade versican to form versican fragments such as versikine [167] which can be pro-apoptotic in some systems [52] and/or immunoregulatory [168] in other systems. Furthermore, fragments of hyaluronan promote collagen production in some cells [140]. It has been suggested also that this form of “early inflammation” if not resolved may lead to further leukocyte infiltration and chronic inflammation and thus, eventually to fibrosis [169, 170]. Others have suggested that hyaluronan/versican complexes could be anti-inflammatory and control leukocyte adhesion and activation, preventing the leukocytes from getting to their site of action [58, 158, 160]. Such examples highlight the importance of provisional matrix components controlling events such as immunity and inflammation. Other diseases that involve hyaluronan and versican impacting immune and inflammatory cell invasion include lung disease [76, 171–176], inflammatory bowel disease (IBD) [32, 158], kidney disease [162, 177], and autoimmune diseases, such as T1D [178, 179]. In diseases of the lung, both versican and hyaluronan accumulate as part of provisional matrices in subepithelial regions and perivascular compartments of the lung, impacting the recruitment of inflammatory cells, such as neutrophils, eosinophils, and macrophages. Hyaluronan-rich matrices in pancreatic islets are also prominent in autoimmune diseases, such as T1D in areas of insulitis where leukocyte invasion and destruction occur. Such observations raise questions as to the source of these ECM components in the diabetic islet and whether changes in the hyaluronan and versican content of the islet is the cause or the result of islet destruction by leukocytes.
Hyaluronan and versican interact with the surface of immune and inflammatory cells [33, 180–182]. Current wisdom suggests that size matters when it comes to both hyaluronan and versican impacting immune cell phenotype [33, 182, 183]. Receptors, such as CD44 on lymphoid and myeloid cells, bind high molecular weight hyaluronan inducing clustering of CD44 and provoking signaling pathways involving p38, ERK1,2, Akt, MEK ,FAK resulting in the production of anti-inflammatory cytokines such as IL-10 [33, 182]. On the other hand, fragmented hyaluronan binds toll-like receptors (TLRs), such as TLR2 and TLR4, which initiates signaling cascades that involve MYD88, IRAK, TRAFs resulting in upregulation of NFκB. This pathway drives the expression of pro-inflammmatory cytokines by myeloid cells such as IL-6, TNFα and IL1β [180]. While receptors and signaling pathways have been identified for hyaluronan on immune cells [181], less is known about the binding of versican to immune and inflammatory cells. One way versican can affect immune cell phenotype is through its binding to hyaluronan. In fact, we found that incubating versican with hyaluronan before adding to activated T lymphocytes inhibited the binding of hyaluronan to the lymphocytes and blocked the migration of T lymphocytes [155]. In addition, versican and versican fragments, like hyaluronan fragments , can bind TLR2 and 4 and induce inflammatory cytokine release from monocytes/macrophages [168, 184–192] providing yet another example of how ECM components possess bioactivity and regulate critical events during the early inflammatory response.
Dynamic ECM Remodeling of Hyaluronan and Versican: From Provisional Matrix to Fibrotic Matrix
Once the proliferative phase has occurred and leukocytes have migrated into a wound site, the provisional ECM is remodeled [193]. This coincides with the appearance of the myofibroblast, a specialized form of fibroblast whose differentiation is primarily driven by cytokines, such as TGF-β and mechanical tension [194, 195]. A number of other cytokines such as connective tissue growth factor, insulin-like growth factor-1 and PDGF have been identified as pro-fibrotic cytokines. Myofibroblasts are responsible for wound closure and for the formation of a collagen-rich scar [194, 196]. It is also the myofibroblasts that are thought to be responsible for the excessive production of collagen leading to fibrosis and tissue destruction in multiple diseases.
Interestingly, the myofibroblast seems to be dependent upon the synthesis and secretion hyaluronan and versican which are prominent players in the provisional matrix stage of ECM maturation. Hyaluronan secretion has been intimately connected with the maintenance of the myofibroblast phenotype and thus may have a role in fibrosis [169, 197–203]. Association of hyaluronan with CD44 influences the positioning of TGF-β receptors which can have an impact on TGF-β signaling [204, 205]. Furthermore, blocking the synthesis of hyaluronan in fibroblasts inhibits the increase in α-actin expression induced by TGF-β during the fibroblast-to-myofibroblast conversion [201]. Since removal of cell surface hyaluronan is known to destabilize focal adhesions involved in cell attachment [206], these findings point to the possibility that hyaluronan, as a constituent of the cross-linked pericellular matrix, may cooperate with focal adhesions to provide the mechanical tension needed to maintain the myofibroblast phenotype. Molecules, such as hyaluronan, that interact with fibrillar collagens, will modulate the mechanical properties of the collagen and alter the contractile forces that can be generated by the cells [207]. Furthermore, release of mechanical tension in myofibroblasts can result in a wave of apoptosis and cell loss [208, 209], suggesting that pericellular hyaluronan may promote survival of the myofibroblast. In addition, the capacity of a cell to synthesize and secrete hyaluronan in response to TGF-β has been linked to a fibrotic cell phenotype. For example, fibroblasts isolated from human oral mucosa are resistant to TGF-β-driven myofibroblast conversion [198] and this difference has been associated with scar-free healing of the oral mucosa. However, human dermal fibroblasts are readily converted to myofibroblasts by TGF-β and form scars with healing. In dermal fibroblasts, myofibroblast conversion is associated with an induction of the hyaluronan synthases, HAS1 and HAS2, and formation of a pericellular coat. Changes in these enzymes and pericellular coat formation were not observed for the oral fibroblasts in response to TGF-β [198]. While it is not entirely clear how pericellular hyaluronan promotes events leading to fibrosis, one possibility is that the hyaluronan-enriched ECM that forms around cells in response to pro-inflammatory agonists attracts and retains inflammatory cells, thus driving the inflammation associated with fibrosis [70, 158, 198]. Recent studies by Paul Noble’s group have demonstrated that overexpression of HAS2 in murine mesenchymal cells regulates the invasiveness of fibroblasts and promotes pulmonary fibrosis [210]. Furthermore, this same group has shown that HAS2 controls fibroblast senescence [211] and that targeting HAS2 could be an attractive therapeutic approach to resolve tissue fibrosis. These studies however have been further complicated by studies from our own group [212] which showed that removal of hyaluronan from TGF-β-stimulated myofibroblasts promotes the fibrotic phenotype by increasing, rather than inhibiting, the expression and accumulation of collagen I and fibronectin (Figure 6). These results differ from those cited above and raise questions as to how these different ECM components interact and how this interaction creates altered cell phenotypes. It may be that it matters if other hyaluronan binding molecules such as versican participate. It also may be a timing issue in that short treatments to reduce hyaluronan may impact phenotype in one way whereas longer treatment periods may have opposite effects. Versican also may exhibit pro-fibrotic activity. For example, forced expression of versican V1 in cultured fibroblasts leads to the induction of the myofibroblast phenotype and the production of collagens [213]. Thus, while versican and hyaluronan are prominent players in the generation and activity of the provisional matrix, their relative concentration and availability may also be critical for the transition of this matrix to a more fibrotic matrix. Such questions need to be answered if progress in preventing fibrosis is to be achieved.
Figure 6.
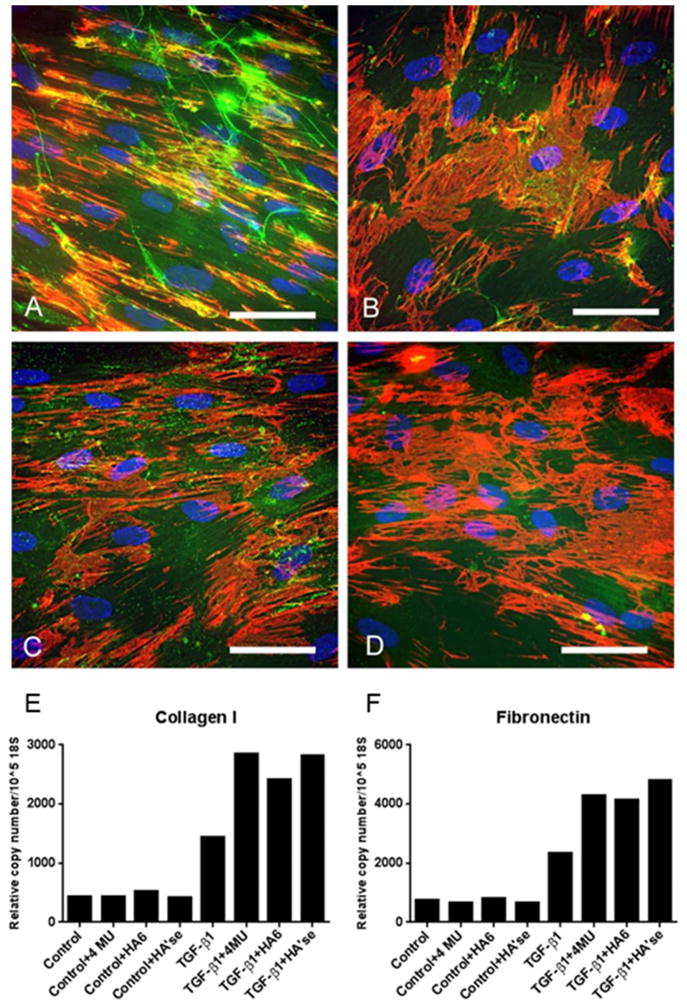
Impact of hyaluronan on synthesis and accumulation of fibronectin and collagen. TGFβ1-treated myofibroblasts were left untreated (A) or treated with 4-MU (B), hyaluronan oligosaccharides (C) or Streptomyces hyaluronidase (D) for 4 days. Cultures were stained for fibronectin (red) and hyaluronan (green). Parallel cultures undergoing the same treatments were evaluated for collagen I (E) and fibronectin (F) gene expression using qRT-PCR. Results indicate that chronic removal of HA from TGF-β1-treated myofibroblasts increases their fibrotic response by promoting collagen and fibronectin synthesis and accumulation. Reprinted from Matrix Biology, Vol. 42, Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN, Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-β1 induction of lung myofibroblasts, Pages 74–92, Copyright (2015), with permission from Elsevier.
Summary and Conclusions
In summary, the provisional matrices that form early in both development and disease pathogenesis are enriched in hyaluronan and versican. These molecules can act singularly, together, or as part of a complex to promote assembly of a specialized ECM that is open, viscoelastic, and compliant. Not only do they contribute to mechanical properties of tissue, but also they influence the phenotype of the cells with which they interact. Thus, hyaluronan and versican are emerging as potential targets for therapeutic intervention across a variety of disease conditions where tissues form provisional matrices as a part of early pathogenesis.
Highlights.
Versican and hyaluronan are enriched in Provisional Matrices
Provisional matrices occur in development and early stages of disease pathogenesis
Versican/hyaluronan complexes facilitate cell proliferation and migration
Versican/hyaluronan interact with immune and inflammatory cells
Inhibition of versican and/or hyaluronan accumulation alters disease progression
Acknowledgments
I would like to thank all the members of my group and my collaborators, past and present, who have worked tirelessly to unravel the many mysteries of hyaluronan and versican. Special thanks to Drs. Stephen P. Evanko, Michael G. Kinsella, Jens Fischer, Paul Bollyky and Susan Potter-Perigo whose early and continuing work on these molecules helped recognize their importance in the provisional matrix and unravel some of the ways that versican and hyaluronan impact cell phenotype. I would also like to thank current members of my group: Drs Inkyung Kang, Ingrid Harten, Marika Bogdani, Steve Reeves, and Paul Keire, as well as collaborators Bob Vernon and John Gebe whose work continues to inspire us moving forward. Special thanks also to Dr. Virginia M. Green for her thorough and careful editing of this manuscript and “holding” all of us together. This review was prepared with support from the National Institutes of Health grants P01 HL098067, U01 AI101984, R01 EB012558, U01 AI101990 Pilot Projects, and from The Helmsley Charitable Trust nPOD (Network for Pancreatic Organ Donors) Award for Team Science.
List of Abbreviations
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transitions
- ASMCs
arterial smooth muscle cells
- GAG
glycosaminoglycan
- RHAMM
receptor for hyaluronan-mediated motility
- CS
chondroitin sulfate
- PDGF
platelet-derived growth factor
- ADAMTS
a disintegrin and metalloproteinase with a thrombospondin type-1 motif
- HAS
hyaluronan synthase
- IαI
inter-alpha-inhibitor
- TSG-6
tumor necrosis factor-stimulated gene-6
- RBCs
red blood cells
- ER
endoplasmic reticulum
- T1D
type 1 diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bissell M, Hall HG, Parry G. How does the extracellular matrix control gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 2.Hay ED. Cell biology of extracellular matrix. Plenum Press; New York: 1991. [Google Scholar]
- 3.Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merrilees MJ, Wight TN. Targeting the matrix: potential benefits for versican therapeutics. 2012 < http://www.elsevierblogs.com/currentcomments/?p=519>.
- 5.Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224. doi: 10.3389/fonc.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark RAF. Cutaneous tissue repair: basic biologic considerations. J Am Acad Derm. 1985;13:701–725. doi: 10.1016/s0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- 7.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 8.Clark RA. Basics of cutaneous wound repair. J Dermatol Surg Oncol. 1993;19:693–706. doi: 10.1111/j.1524-4725.1993.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 9.Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1980;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- 10.Toole BP. Morphogenetic role of glycosaminoglycans (acid mucopolysaccharides) in brain and other tissues. In: Barondes SH, editor. Neuronal Recognition. Plenum Press; New York: 1976. pp. 275–329. [Google Scholar]
- 11.Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrinogen in the early events of inflammatory response and wound healing. J Theoret Biol. 1986;119:219–234. doi: 10.1016/s0022-5193(86)80076-5. [DOI] [PubMed] [Google Scholar]
- 12.Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39:2153–60. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Yamada KM, Clark RAF. Provisional matrix. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. Plenum Press; New York: 1996. pp. 51–93. [Google Scholar]
- 14.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–62. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson PY, Potter-Perigo S, Gooden MD, Vernon RB, Wight TN. Decorin synthesized by arterial smooth muscle cells is retained in fibrin gels and modulates fibrin contraction. J Cell Biochem. 2007;101:281–94. doi: 10.1002/jcb.21182. [DOI] [PubMed] [Google Scholar]
- 16.Oksala O, Salo T, Tammi R, Hakkinen L, Jalkanen M, Inki P, Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995;43:125–35. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- 17.Pierce GF, Berg JV, Rudolph R, Tarpley J, Mustoe TA. Platelet-derived growth factor-BB and transforming growth factor beta1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991;138:629–646. [PMC free article] [PubMed] [Google Scholar]
- 18.Maytin EV. Hyaluronan: More than just a wrinkle filler. Glycobiology. 2016;26:553–9. doi: 10.1093/glycob/cww033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van De Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993;142:793–801. [PMC free article] [PubMed] [Google Scholar]
- 20.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 22.Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 2002;277:4581–4. doi: 10.1074/jbc.R100037200. [DOI] [PubMed] [Google Scholar]
- 23.Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem. 2007;282:36777–81. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 24.Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB Life. 2002;54:195–9. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 25.Hascall VC, Heinegård DK, Wight TN. Proteoglycans: metabolism and pathology. In: Hay ED, editor. Cell Biology of Extracellular Matrix. Plenum Press; New York: 1991. pp. 149–175. [Google Scholar]
- 26.Wight TN, Heinegård DK, Hascall VC. Proteoglycans: structure and function. In: Hay ED, editor. Cell Biology of Extracellular Matrix. Plenum Press; New York: 1991. pp. 45–78. [Google Scholar]
- 27.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–39. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern R, Kogan G, Jedrzejas MJ, Soltes L. The many ways to cleave hyaluronan. Biotechnol Adv. 2007;25:537–57. doi: 10.1016/j.biotechadv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 31.Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59:1351–65. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-α-trypsin inhibitor is crucial to structure and function. Am J Pathol. 2003;163:121–133. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bollyky PL, Bogdani M, Bollyky JB, Hull RL, Wight TN. The role of hyaluronan and the extracellular matrix in islet inflammation and immune regulation. Curr Diab Rep. 2012;12:471–80. doi: 10.1007/s11892-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toole BP. Hyaluronan promotes the malignant phenotype. Glycobiology. 2002;12:37R-42R. doi: 10.1093/glycob/12.3.37r. [DOI] [PubMed] [Google Scholar]
- 35.Toole BP. Hyaluronan-CD44 Interactions in cancer: Paradoxes and possibilities. Clin Cancer Res. 2009;15:7462–7468. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toole BP, Slomiany MG. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resist Updat. 2008;11:110–21. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–6. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 38.Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011;278:1429–43. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAtee CO, Berkebile AR, Elowsky CG, Fangman T, Barycki JJ, Wahl JK, 3rd, Khalimonchuk O, Naslavsky N, Caplan S, Simpson MA. Hyaluronidase Hyal1 increases tumor cell proliferation and motility through accelerated vesicle trafficking. J Biol Chem. 2015;290:13144–56. doi: 10.1074/jbc.M115.647446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267:10003–10010. [PubMed] [Google Scholar]
- 41.Iozzo RV, Naso MF, Cannizzaro LA, Wasmuth JJ, McPherson JD. Mapping of the versican proteoglycan gene (CSPG2) to the long arm of human chromosome 5 (5q12–5q14) Genomics. 1992;14:845–851. doi: 10.1016/s0888-7543(05)80103-x. [DOI] [PubMed] [Google Scholar]
- 42.Naso MF, Zimmermann DR, Iozzo RV. Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J Biol Chem. 1994;269:32999–33008. [PubMed] [Google Scholar]
- 43.Zimmermann D. Versican. In: Iozzo R, editor. Proteoglycans: Structure, Biology and Molecular Interactions. Marcel Dekker, Inc; New York: 2000. pp. 327–341. [Google Scholar]
- 44.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 45.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–61. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zako M, Shinomura T, Ujita M, Ito K, Kimata K. Expression of PG-M (V3), an alternatively spliced form of PG-M without a chondroitin sulfate attachment region in mouse and human tissues. J Biol Chem. 1995;270:3914–3918. doi: 10.1074/jbc.270.8.3914. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–53. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 48.Cardoso LE, Little PJ, Ballinger ML, Chan CK, Braun KR, Potter-Perigo S, Bornfeldt KE, Kinsella MG, Wight TN. Platelet-derived growth factor differentially regulates the expression and post-translational modification of versican by arterial smooth muscle cells through distinct protein kinase C and extracellular signal-regulated kinase pathways. J Biol Chem. 2010;285:6987–95. doi: 10.1074/jbc.M109.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schönherr E, Järveläinen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-β 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- 50.Kenagy RD, Plaas AH, Wight TN. Versican degradation and vascular disease. Trends Cardiovasc Med. 2006;16:209–15. doi: 10.1016/j.tcm.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–7. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17:687–98. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, Wight TN, Frevert CW. A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol. 2014;34:1–12. doi: 10.1016/j.matbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sotoodehnejadnematalahi F, Staples KJ, Chrysanthou E, Pearson H, Ziegler-Heitbrock L, Burke B. Mechanisms of hypoxic up-regulation of versican gene expression in macrophages. PLoS ONE. 2015;10:e0125799. doi: 10.1371/journal.pone.0125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wight T, Toole B, Hascall V. Hyaluronan and the aggregating proteoglycans. In: Mecham R, editor. The Extracellular Matrix: an Overview. Springer; Heidelberg: 2011. pp. 147–195. [Google Scholar]
- 56.Blundell CD, Almond A, Mahoney DJ, DeAngelis PL, Campbell ID, Day AJ. Towards a structure for a TSG-6.hyaluronan complex by modeling and NMR spectroscopy: insights into other members of the link module superfamily. J Biol Chem. 2005;280:18189–201. doi: 10.1074/jbc.M414343200. [DOI] [PubMed] [Google Scholar]
- 57.Blundell CD, Mahoney DJ, Almond A, DeAngelis PL, Kahmann JD, Teriete P, Pickford AR, Campbell ID, Day AJ. The link module from ovulation- and inflammation-associated protein TSG-6 changes conformation on hyaluronan binding. J Biol Chem. 2003;278:49261–70. doi: 10.1074/jbc.M309623200. [DOI] [PubMed] [Google Scholar]
- 58.Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–43. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–8. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- 60.Day AJ, Sheehan JK. Hyaluronan: polysaccharide chaos to protein organisation. Curr Opin Struct Biol. 2001;11:617–22. doi: 10.1016/s0959-440x(00)00256-6. [DOI] [PubMed] [Google Scholar]
- 61.Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merrilees MJ, Zuo N, Evanko SP, Day AJ, Wight TN. G1 domain of versican regulates hyaluronan organization and the phenotype of cultured human dermal fibroblasts. J Histochem Cytochem. 2016;64:353–63. doi: 10.1369/0022155416643913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158–67. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 64.Hinek A, Braun KR, Liu K, Wang Y, Wight TN. Retrovirally mediated overexpression of versican v3 reverses impaired elastogenesis and heightened proliferation exhibited by fibroblasts from Costello syndrome and Hurler disease patients. Am J Pathol. 2004;164:119–31. doi: 10.1016/S0002-9440(10)63103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang JY, Johnson PY, Braun KR, Hinek A, Fischer JW, O'Brien KD, Starcher B, Clowes AW, Merrilees MJ, Wight TN. Retrovirally mediated overexpression of glycosaminoglycan-deficient biglycan in arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointimae after vascular injury. Am J Pathol. 2008;173:1919–28. doi: 10.2353/ajpath.2008.070875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang I, Barth JL, Sproul EP, Yoon DW, Braun KR, Argraves WS, Wight TN. Expression of V3 versican by rat arterial smooth muscle cells promotes differentiated and anti-inflammatory phenotypes. J Biol Chem. 2015;290:21629–41. doi: 10.1074/jbc.M115.657486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang I, Yoon DW, Braun KR, Wight TN. Expression of versican V3 by arterial smooth muscle cells alters TGFβ-, EGF-, and NFκB-dependent signaling pathways, creating a microenvironment that resists monocyte adhesion. J Biol Chem. 2014;289:15393–15404. doi: 10.1074/jbc.M113.544338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merrilees MJ, Beaumont BW, Braun KR, Thomas AC, Kang I, Hinek A, Passi A, Wight TN. Neointima formed by arterial smooth muscle cells expressing versican variant v3 is resistant to lipid and macrophage accumulation. Arterioscler Thromb Vasc Biol. 2011;31:1309–16. doi: 10.1161/ATVBAHA.111.225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, Wight TN. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–7. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- 70.Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion. Am J Respir Cell Mol Biol. 2010;43:109–20. doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lauer ME, Aytekin M, Comhair SA, Loftis J, Tian L, Farver CF, Hascall VC, Dweik RA. Modification of hyaluronan by heavy chains of inter-alpha-inhibitor in idiopathic pulmonary arterial hypertension. J Biol Chem. 2014;289:6791–8. doi: 10.1074/jbc.M113.512491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lauer ME, Cheng G, Swaidani S, Aronica MA, Weigel PH, Hascall VC. Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J Biol Chem. 2013;288:423–31. doi: 10.1074/jbc.M112.389882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauer ME, Glant TT, Mikecz K, DeAngelis PL, Haller FM, Husni ME, Hascall VC, Calabro A. Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. J Biol Chem. 2013;288:205–14. doi: 10.1074/jbc.M112.403998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milner C, Tongsoongnoen W, Rugg M, Day A. The molecular basis of inter-alpha-inhibitor heavy chain transfer on to hyaluronan. Biochem Soc Trans. 2007;35:672–6. doi: 10.1042/BST0350672. [DOI] [PubMed] [Google Scholar]
- 75.Csoka AB, Stern R. Hypotheses on the evolution of hyaluronan: a highly ironic acid. Glycobiology. 2013;23:398–411. doi: 10.1093/glycob/cws218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lauer ME, Dweik RA, Garantziotis S, Aronica MA. The rise and fall of hyaluronan in respiratory diseases. Int J Cell Biol. 2015;2015:712507. doi: 10.1155/2015/712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coulson-Thomas VJ, Gesteira TF, Hascall V, Kao W. Umbilical cord mesenchymal stem cells suppress host rejection: The role of the glycocalyx. J Biol Chem. 2014;289:23465–81. doi: 10.1074/jbc.M114.557447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Avnet S, Cortini M. Role of pericellular matrix in the regulation of cancer stemness. Stem Cell Rev. 2016;12:464–75. doi: 10.1007/s12015-016-9660-x. [DOI] [PubMed] [Google Scholar]
- 79.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 80.Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhia J, Wight TN. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch Biochem Biophys. 2001;394:29–38. doi: 10.1006/abbi.2001.2507. [DOI] [PubMed] [Google Scholar]
- 81.Riessen R, Wight TN, Pastore C, Henley C, Isner JM. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996;93:1141–1147. doi: 10.1161/01.cir.93.6.1141. [DOI] [PubMed] [Google Scholar]
- 82.Schönherr E, Kinsella MG, Wight TN. Genistein selectively inhibits platelet-derived growth factor stimulated versican biosynthesis in monkey arterial smooth muscle cells. Arch Biochem Biophys. 1997;339:353–361. doi: 10.1006/abbi.1996.9854. [DOI] [PubMed] [Google Scholar]
- 83.Clarris BJ, Fraser JR. On the pericellular zone of some mammalian cells in vitro. Exp Cell Res. 1968;49:181–93. doi: 10.1016/0014-4827(68)90530-2. [DOI] [PubMed] [Google Scholar]
- 84.Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120:825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang Y, Askew EB, Knudson CB, Knudson W. CRISPR/Cas9 knockout of HAS2 in rat chondrosarcoma chondrocytes demonstrates the requirement of hyaluronan for aggrecan retention. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szabo A, Melchionda M, Nastasi G, Woods ML, Campo S, Perris R, Mayor R. In vivo confinement promotes collective migration of neural crest cells. J Cell Biol. 2016;213:543–55. doi: 10.1083/jcb.201602083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hattori N, Carrino DA, Lauer ME, Vasanji A, Wylie JD, Nelson CM, Apte SS. Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis. J Biol Chem. 2011;286:34298–310. doi: 10.1074/jbc.M111.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.du Cros DL, LeBaron RG, Couchman JR. Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J Invest Dermatol. 1995;105:426–31. doi: 10.1111/1523-1747.ep12321131. [DOI] [PubMed] [Google Scholar]
- 89.Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc Natl Acad Sci USA. 1999;96:7336–41. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Russell D, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24:217–27. doi: 10.1055/s-2006-948551. [DOI] [PubMed] [Google Scholar]
- 91.Russell DL, Ochsner SA, Hsieh M, Mulders S, Richards JS. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 2003;144:1020–31. doi: 10.1210/en.2002-220434. [DOI] [PubMed] [Google Scholar]
- 92.Ruscheinsky M, De la Motte C, Mahendroo M. Hyaluronan and its binding proteins during cervical ripening and parturition: dynamic changes in size, distribution and temporal sequence. Matrix Biol. 2008;27:487–97. doi: 10.1016/j.matbio.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shinomura T, Nishida Y, Kimata K, Ito K. cDNA cloning of PG-M, a large chondroitin sulfate proteoglycan expressed during chondrogenesis in chick limb buds. Alternative spliced multiforms of PG-M and their relationship to versican. J Biol Chem. 1993;268:14461–14469. [PubMed] [Google Scholar]
- 94.Shinomura T, Jensen KL, Yamagata M, Kimata K, Solursh M. The distribution of mesenchyme proteoglycan (PG-M) during wing bud outgrowth. Anat Embryol (Berl) 1990;181:227–233. doi: 10.1007/BF00174617. [DOI] [PubMed] [Google Scholar]
- 95.Stigson M, Lofberg J, Kjellen L. Reduced epidermal expression of a PG-M/versican-like proteoglycan in embryos of the white mutant axolotl. Exp Cell Res. 1997;236:57–65. doi: 10.1006/excr.1997.3702. [DOI] [PubMed] [Google Scholar]
- 96.Stigson M, Lofberg J, Kjellen L. PG-M/versican-like proteoglycans are components of large disulfide- stabilized complexes in the axolotl embryo. J Biol Chem. 1997;272:3246–53. doi: 10.1074/jbc.272.6.3246. [DOI] [PubMed] [Google Scholar]
- 97.Perris R, Johansson S. Inhibition of neural crest cell migration by aggregating chondroitin sulfate proteoglycans is mediated by their hyaluronan-binding region. Dev Biol. 1990;137:1–12. doi: 10.1016/0012-1606(90)90002-z. [DOI] [PubMed] [Google Scholar]
- 98.Perris R, Lofberg J, Fallstrom C, von Boxberg Y, Olsson L, Newgreen DF. Structural and compositional divergencies in the extracellular matrix encountered by neural crest cells in the white mutant axolotl embryo. Development. 1990;109:533–551. doi: 10.1242/dev.109.3.533. [DOI] [PubMed] [Google Scholar]
- 99.Perris R, Perissinotto D. Role of the extracellular matrix during neural crest cell migration. Mech Dev. 2000;95:3–21. doi: 10.1016/s0925-4773(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 100.Perris R, Perissinotto D, Pettway Z, Bronner-Fraser M, Morgelin M, Kimata K. Inhibitory effects of PG-H/aggrecan and PG-M/versican on avian neural crest cell migration. FASEB J. 1996;10:293–301. doi: 10.1096/fasebj.10.2.8641562. [DOI] [PubMed] [Google Scholar]
- 101.Choudhary M, Zhang X, Stojkovic P, Hyslop L, Anyfantis G, Herbert M, Murdoch AP, Stojkovic M, Lako M. Putative role of hyaluronan and its related genes, HAS2 and RHAMM, in human early preimplantation embryogenesis and embryonic stem cell characterization. Stem Cells. 2007;25:3045–57. doi: 10.1634/stemcells.2007-0296. [DOI] [PubMed] [Google Scholar]
- 102.Nairn AV, Kinoshita-Toyoda A, Toyoda H, Xie J, Harris K, Dalton S, Kulik M, Pierce JM, Toida T, Moremen KW, Linhardt RJ. Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J Proteome Res. 2007;6:4374–87. doi: 10.1021/pr070446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shukla S, Nair R, Rolle MW, Braun KR, Chan CK, Johnson PY, Wight TN, McDevitt TC. Synthesis and organization of hyaluronan and versican by embryonic stem cells undergoing embryoid body differentiation. J Histochem Cytochem. 2010;58:345–58. doi: 10.1369/jhc.2009.954826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chan CK, Rolle MW, Potter-Perigo S, Braun KR, Van Biber BP, Laflamme MA, Murry CE, Wight TN. Differentiation of cardiomyocytes from human embryonic stem cells is accompanied by changes in the extracellular matrix production of versican and hyaluronan. J Cell Biochem. 2010;111:585–96. doi: 10.1002/jcb.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- 106.Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186:58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- 107.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kern CB, Norris RA, Thompson RP, Argraves WS, Fairey SE, Reyes L, Hoffman S, Markwald RR, Mjaatvedt CH. Versican proteolysis mediates myocardial regression during outflow tract development. Dev Dyn. 2007;236:671–83. doi: 10.1002/dvdy.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kern CB, Twal WO, Mjaatvedt CH, Fairey SE, Toole BP, Iruela-Arispe ML, Argraves WS. Proteolytic cleavage of versican during cardiac cushion morphogenesis. Dev Dyn. 2006;235:2238–47. doi: 10.1002/dvdy.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010;29:304–16. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chung IM, Gold HK, Schwartz SM, Ikari Y, Reidy MA, Wight TN. Enhanced extracellular matrix accumulation in restenosis of coronary arteries after stent deployment. J Am Coll Cardiol. 2002;40:2072–2081. doi: 10.1016/s0735-1097(02)02598-6. [DOI] [PubMed] [Google Scholar]
- 112.Farb A, Kolodgie FD, Hwang JY, Burke AP, Tefera K, Weber DK, Wight TN, Virmani R. Extracellular matrix changes in stented human coronary arteries. Circulation. 2004;110:940–7. doi: 10.1161/01.CIR.0000139337.56084.30. [DOI] [PubMed] [Google Scholar]
- 113.Wight TN, Lara S, Reissen R, LeBaron R, Isner J. Selective deposits of versican in the extracellular matrix of restenotic lesions from human peripheral arteries. Am J Pathol. 1997;151:963–973. [PMC free article] [PubMed] [Google Scholar]
- 114.Matsuura R, Isaka N, Imanaka-Yoshida K, Yoshida T, Sakakura T, Nakano T. Deposition of PG-M/versican is a major cause of human coronary restenosis after percutaneous transluminal coronary angioplasty. J Pathol. 1996;180:311–316. doi: 10.1002/(SICI)1096-9896(199611)180:3<311::AID-PATH657>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 115.Wight T. The vascular extracellular matrix. In: Fuster V, Topol E, Nabel E, editors. Atherothrombosis and Coronary Artery Disease. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 421–437. [Google Scholar]
- 116.Kashima Y, Takahashi M, Shiba Y, Itano N, Izawa A, Koyama J, Nakayama J, Taniguchi S, Kimata K, Ikeda U. Crucial role of hyaluronan in neointimal formation after vascular injury. PLoS ONE. 2013;8:e58760. doi: 10.1371/journal.pone.0058760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kiene LS, Homann S, Suvorava T, Rabausch B, Muller J, Kojda G, Kretschmer I, Twarock S, Dai G, Deenen R, Hartwig S, Lehr S, Kohrer K, Savani RC, Grandoch M, Fischer JW. Deletion of hyaluronan synthase 3 inhibits neointimal hyperplasia in mice. Arterioscler Thromb Vasc Biol. 2016;36:e9–e16. doi: 10.1161/ATVBAHA.115.306607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH. Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg. 1998;27:96–106. doi: 10.1016/s0741-5214(98)70296-4. [DOI] [PubMed] [Google Scholar]
- 119.Swedberg SH, Brown BG, Sigley R, Wight TN, Gordon D, Nicholls SC. Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Clinical, immunocytochemical, light and electron microscopic assessment. Circulation. 1989;80:1726–36. doi: 10.1161/01.cir.80.6.1726. [DOI] [PubMed] [Google Scholar]
- 120.de la Motte CA. Hyaluronan in intestinal homeostasis and inflammation: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G945–9. doi: 10.1152/ajpgi.00063.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L789–99. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang YT, Chan CK, Eriksson I, Johnson PY, Cao X, Westoo C, Norvik C, Andersson-Sjoland A, Westergren-Thorsson G, Johansson S, Hedin U, Kjellen L, Wight TN, Tran-Lundmark K. Versican accumulates in vascular lesions in pulmonary arterial hypertension. Pulm Circ. 2016;6:347–59. doi: 10.1086/686994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keire PA, Bressler SL, Lemire JM, Edris B, Rubin BP, Rahmani M, McManus BM, van de Rijn M, Wight TN. A role for versican in the development of leiomyosarcoma. J Biol Chem. 2014;289:34089–103. doi: 10.1074/jbc.M114.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ricciardelli C, Russell DL, Ween MP, Mayne K, Suwiwat S, Byers S, Marshall VR, Tilley WD, Horsfall DJ. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem. 2007;282:10814–25. doi: 10.1074/jbc.M606991200. [DOI] [PubMed] [Google Scholar]
- 125.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–45. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 126.Sato N, Kohi S, Hirata K, Goggins M. Role of hyaluronan in pancreatic cancer biology and therapy: Once again in the spotlight. Cancer Sci. 2016;107:569–75. doi: 10.1111/cas.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwertfeger KL, Cowman MK, Telmer PG, Turley EA, McCarthy JB. Hyaluronan, inflammation, and breast cancer progression. Front Immunol. 2015;6:236. doi: 10.3389/fimmu.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Narunsky L, Oren R, Bochner F, Neeman M. Imaging aspects of the tumor stroma with therapeutic implications. Pharmacol Ther. 2014;141:192–208. doi: 10.1016/j.pharmthera.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 130.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3:1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Keire PA, Bressler SL, Mulvihill ER, Starcher BC, Kang I, Wight TN. Inhibition of versican expression by siRNA facilitates tropoelastin synthesis and elastic fiber formation by human SK-LMS-1 leiomyosarcoma smooth muscle cells in vitro and in vivo. Matrix Biol. 2016;50:67–81. doi: 10.1016/j.matbio.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vernon RB, Sage EH. Between molecules and morphology. Extracellular matrix and creation of vascular form. Am J Pathol. 1995;147:873–83. [PMC free article] [PubMed] [Google Scholar]
- 133.Rivera CG, Bader JS, Popel AS. Angiogenesis-associated crosstalk between collagens, CXC chemokines, and thrombospondin domain-containing proteins. Ann Biomed Eng. 2011;39:2213–22. doi: 10.1007/s10439-011-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y, Wu Y, Kerbel RS, Yang BB. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004;18:754–6. doi: 10.1096/fj.03-0545fje. [DOI] [PubMed] [Google Scholar]
- 135.Du WW, Yang W, Yee AJ. Roles of versican in cancer biology--tumorigenesis, progression and metastasis. Histol Histopathol. 2013;28:701–13. doi: 10.14670/HH-28.701. [DOI] [PubMed] [Google Scholar]
- 136.Fu Y, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, Dvorak HF, Wight TN. Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. J Histochem Cytochem. 2011;59:463–73. doi: 10.1369/0022155411401748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feinberg RN, Beebe DC. Hyaluronate in vasculogenesis. Science. 1983;220:1177–1179. doi: 10.1126/science.6857242. [DOI] [PubMed] [Google Scholar]
- 138.Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest. 1996;75:249–262. [PubMed] [Google Scholar]
- 139.Rooney P, Kumar S, Ponting J, Wang M. The role of hyaluronan in tumor neovascularization (review) Int J Cancer. 1995;60:632–636. doi: 10.1002/ijc.2910600511. [DOI] [PubMed] [Google Scholar]
- 140.Rooney P, Wang M, Kumar P, Kumar S. Angiogenic oligosaccharides of hyaluronan enhance the production of collagens by endothelial cells. J Cell Sci. 1993;105:213–218. doi: 10.1242/jcs.105.1.213. [DOI] [PubMed] [Google Scholar]
- 141.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 142.West DC, Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res. 1989;183:179–196. doi: 10.1016/0014-4827(89)90428-x. [DOI] [PubMed] [Google Scholar]
- 143.Slevin M, Krupinski J, Badimon L. Controlling the angiogenic switch in developing atherosclerotic plaques: possible targets for therapeutic intervention. J Angiogenes Res. 2009;1:4. doi: 10.1186/2040-2384-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 145.Kreutziger KL, Muskheli V, Johnson P, Braun K, Wight TN, Murry CE. Developing vasculature and stroma in engineered human myocardium. Tissue Eng Part A. 2011;17:1219–28. doi: 10.1089/ten.tea.2010.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghosh S, Albitar L, LeBaron R, Welch WR, Samimi G, Birrer MJ, Berkowitz RS, Mok SC. Up-regulation of stromal versican expression in advanced stage serous ovarian cancer. Gynecol Oncol. 2010;119:114–20. doi: 10.1016/j.ygyno.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Labropoulou VT, Theocharis AD, Ravazoula P, Perimenis P, Hjerpe A, Karamanos NK, Kalofonos HP. Versican but not decorin accumulation is related to metastatic potential and neovascularization in testicular germ cell tumours. Histopathology. 2006;49:582–93. doi: 10.1111/j.1365-2559.2006.02558.x. [DOI] [PubMed] [Google Scholar]
- 148.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 149.Koyama H, Hibi T, Isogai Z, Yoneda M, Fujimori M, Amano J, Kawakubo M, Kannagi R, Kimata K, Taniguchi S, Itano N. Hyperproduction of hyaluronan in neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol. 2007;170:1086–99. doi: 10.2353/ajpath.2007.060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nara Y, Kato Y, Torii Y, Tsuji Y, Nakagaki S, Goto S, Isobe H, Nakashima N, Takeuchi J. Immunohistochemical localization of extracellular matrix components in human breast tumours with special reference to PG-M/versican. Histochem J. 1997;29:21–30. doi: 10.1023/a:1026460700592. [DOI] [PubMed] [Google Scholar]
- 151.Spinelli FM, Vitale DL, Demarchi G, Cristina C, Alaniz L. The immunological effect of hyaluronan in tumor angiogenesis. Clin Transl Immunology. 2015;4:e52. doi: 10.1038/cti.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol. 2009;86:567–72. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–7. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 154.Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, Teng B, Holt GE, Standifer NE, Braun KR, Xie CF, Samuels PL, Vernon RB, Gebe JA, Wight TN, Nepom GT. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci USA. 2011;108:7938–43. doi: 10.1073/pnas.1017360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31:90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nagy N, Kaber G, Johnson P, Gebe J, Preisinger A, Falk B, Sunkari V, Gooden M, Vernon R, Bogdani M, Kuipers H, Day A, Campbell D, Wight T, Bollyky P. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J Clin Invest. 2015;185:372–81. doi: 10.1172/JCI79271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kuipers HF, Rieck M, Gurevich I, Nagy N, Butte MJ, Negrin RS, Wight TN, Steinman L, Bollyky PL. Hyaluronan synthesis is necessary for autoreactive T-cell trafficking, activation, and Th1 polarization. Proc Natl Acad Sci USA. 2016;113:1339–44. doi: 10.1073/pnas.1525086113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.de la Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C) J Biol Chem. 1999;274:30747–30755. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- 159.Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J Histochem Cytochem. 2009;57:1041–60. doi: 10.1369/jhc.2009.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 161.Selbi W, de la Motte C, Hascall V, Phillips A. BMP-7 modulates hyaluronan-mediated proximal tubular cell-monocyte interaction. J Am Soc Nephrol. 2004;15:1199–211. doi: 10.1097/01.asn.0000125619.27422.8e. [DOI] [PubMed] [Google Scholar]
- 162.Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 2004;279:10279–85. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- 163.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–10. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Powell JD, Horton MR. Threat matrix: low-molecular-weight hyaluronan (HA) as a danger signal. Immunol Res. 2005;31:207–18. doi: 10.1385/IR:31:3:207. [DOI] [PubMed] [Google Scholar]
- 165.Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc Am Thorac Soc. 2006;3:401–4. doi: 10.1513/pats.200604-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-κB/I- κBalpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183:2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nandadasa S, Foulcer S, Apte SS. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014;35:34–41. doi: 10.1016/j.matbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hope C, Foulcer S, Jagodinsky J, Chen SX, Jensen JL, Patel S, Leith C, Maroulakou I, Callander N, Miyamoto S, Hematti P, Apte SS, Asimakopoulos F. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016 doi: 10.1182/blood-2016-03-705780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Albeiroti S, Soroosh A, de la Motte CA. Hyaluronan's role in fibrosis: A pathogenic factor or a passive player? Biomed Res Int. 2015;2015:790203. doi: 10.1155/2015/790203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem. 2003;278:47223–31. doi: 10.1074/jbc.M304871200. [DOI] [PubMed] [Google Scholar]
- 171.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 2011;30:126–34. doi: 10.1016/j.matbio.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology. 2013;23:43–58. doi: 10.1093/glycob/cws122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reeves SR, Kaber G, Sheih A, Cheng G, Aronica MA, Merrilees MJ, Debley JS, Frevert CW, Ziegler SF, Wight TN. Subepithelial accumulation of versican in a cockroach antigen-induced murine model of allerigic asthma. J Histochem Cytochem. 2016;64:364–80. doi: 10.1369/0022155416642989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol. 2011;301:L137–47. doi: 10.1152/ajplung.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec (Hoboken) 2010;293:968–81. doi: 10.1002/ar.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Andersson-Sjoland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, Rydell-Tormanen K, Bjermer L, Malmstrom A, Karlsson JC, Westergren-Thorsson G. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology. 2015;25:243–51. doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Selbi W, de la Motte CA, Hascall VC, Day AJ, Bowen T, Phillips AO. Characterization of hyaluronan cable structure and function in renal proximal tubular epithelial cells. Kidney Int. 2006;70:1287–95. doi: 10.1038/sj.ki.5001760. [DOI] [PubMed] [Google Scholar]
- 178.Bogdani M, Johnson PY, Potter-Perigo S, Nagy N, Day AJ, Bollyky PL, Wight TN. Hyaluronan and hyaluronan binding proteins accumulate in both human type 1 diabetic islets and lymphoid tissues and associate with inflammatory cells in insulitis. Diabetes. 2014;63:2727–2743. doi: 10.2337/db13-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bogdani M, Korpos E, Simeonovic CJ, Parish CR, Sorokin L, Wight TN. Extracellular matrix components in the pathogenesis of type 1 diabetes. Curr Diab Rep. 2014;14:552. doi: 10.1007/s11892-014-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–64. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P. The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol. 2015;6:150. doi: 10.3389/fimmu.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ruppert SM, Hawn TR, Arrigoni A, Wight TN, Bollyky PL. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol Res. 2014;58:186–92. doi: 10.1007/s12026-014-8495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015;2015:563818. doi: 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bogels M, Braster R, Nijland PG, Gul N, van de Luijtgaarden W, Fijneman RJ, Meijer GA, Jimenez CR, Beelen RH, van Egmond M. Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunology. 2012;1:798–809. doi: 10.4161/onci.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hope C, Ollar SJ, Heninger E, Hebron E, Jensen JL, Kim J, Maroulakou I, Miyamoto S, Leith C, Yang DT, Callander N, Hematti P, Chesi M, Bergsagel PL, Asimakopoulos F. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood. 2014;123:3305–15. doi: 10.1182/blood-2014-02-554071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang F, Wu KY, Wan HY. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS ONE. 2013;8:e56616. doi: 10.1371/journal.pone.0056616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Said N, Sanchez-Carbayo M, Smith SC, Theodorescu D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Invest. 2012;122:1503–18. doi: 10.1172/JCI61392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Said N, Theodorescu D. RhoGDI2 suppresses bladder cancer metastasis via reduction of inflammation in the tumor microenvironment. Oncoimmunology. 2012;1:1175–1177. doi: 10.4161/onci.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating IL-6 and IL-10 receptor signaling. Cell Rep. 2015;13:2851–64. doi: 10.1016/j.celrep.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 191.Wang W, Xu GL, Jia WD, Ma JL, Li JS, Ge YS, Ren WH, Yu JH, Liu WB. Ligation of TLR2 by versican: a link between inflammation and metastasis. Arch Med Res. 2009;40:321–3. doi: 10.1016/j.arcmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Z, Miao L, Wang L. Inflammation amplification by versican: the first mediator. Int J Mol Sci. 2012;13:6873–82. doi: 10.3390/ijms13066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Clark RAF, Henson PM. The molecular and cellular biology of wound repair. Plenum Press; New York: 1988. [Google Scholar]
- 194.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 195.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 197.Jenkins RH, Thomas GJ, Williams JD, Steadman R. Myofibroblastic differentiation leads to hyaluronan accumulation through reduced hyaluronan turnover. J Biol Chem. 2004;279:41453–60. doi: 10.1074/jbc.M401678200. [DOI] [PubMed] [Google Scholar]
- 198.Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, Steadman R. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem. 2007;282:25687–97. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- 199.Meran S, Thomas DW, Stephens P, Enoch S, Martin J, Steadman R, Phillips AO. Hyaluronan facilitates transforming growth factor-beta1-mediated fibroblast proliferation. J Biol Chem. 2008;283:6530–45. doi: 10.1074/jbc.M704819200. [DOI] [PubMed] [Google Scholar]
- 200.Simpson RM, Meran S, Thomas D, Stephens P, Bowen T, Steadman R, Phillips A. Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblast differentiation. Am J Pathol. 2009;175:1915–28. doi: 10.2353/ajpath.2009.090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFβ1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 2009;175:148–60. doi: 10.2353/ajpath.2009.080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-β1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 2009;284:9083–92. doi: 10.1074/jbc.M806989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guo N, Li X, Mann MM, Funderburgh ML, Du Y, Funderburgh JL. Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor beta. J Biol Chem. 2010;285:32012–9. doi: 10.1074/jbc.M110.127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ito T, Williams JD, Fraser D, Phillips AO. Hyaluronan attenuates transforming growth factor- β1-mediated signaling in renal proximal tubular epithelial cells. Am J Pathol. 2004;164:1979–88. doi: 10.1016/s0002-9440(10)63758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ito T, Williams JD, Fraser DJ, Phillips AO. Hyaluronan regulates transforming growth factor-β1 receptor compartmentalization. J Biol Chem. 2004;279:25326–32. doi: 10.1074/jbc.M403135200. [DOI] [PubMed] [Google Scholar]
- 206.Twarock S, Tammi MI, Savani RC, Fischer JW. Hyaluronan stabilizes focal adhesions, filopodia, and the proliferative phenotype in esophageal squamous carcinoma cells. J Biol Chem. 2010;285:23276–84. doi: 10.1074/jbc.M109.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Allison DD, Wight TN, Ripp NJ, Braun KR, Grande-Allen KJ. Endogenous overexpression of hyaluronan synthases within dynamically cultured collagen gels: Implications for vascular and valvular disease. Biomaterials. 2008;29:2969–76. doi: 10.1016/j.biomaterials.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 208.Grinnell F, Zhu M, Carlson MA, Abrams JM. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res. 1999;248:608–19. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- 209.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538–46. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 210.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–71. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li Y, Liang J, Yang T, Monterrosa Mena J, Huan C, Xie T, Kurkciyan A, Liu N, Jiang D, Noble PW. Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis. Matrix Biol. 2016;55:35–48. doi: 10.1016/j.matbio.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-beta1 induction of lung myofibroblasts. Matrix Biol. 2015;42:74–92. doi: 10.1016/j.matbio.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Carthy JM, Meredith AJ, Boroomand S, Abraham T, Luo Z, Knight D, McManus BM. Versican V1 overexpression induces a myofibroblast-like phenotype in cultured fibroblasts. PLoS ONE. 2015;10:e0133056. doi: 10.1371/journal.pone.0133056. [DOI] [PMC free article] [PubMed] [Google Scholar]