Abstract
Objective
Sepsis is the number one cause of lung injury in adults. Ex vivo lung perfusion (EVLP) is gaining clinical acceptance for donor lung evaluation and rehabilitation, and may expand the use of marginal organs for transplantation. We hypothesized that four hours of normothermic EVLP would improve compliance and oxygenation in a porcine model of sepsis-induced lung injury.
Methods
We utilized a porcine lung injury model using intravenous lipopolysaccharide (LPS) to induce a systemic inflammatory response. Two groups (n=4 animals/group) received a 2-hour infusion of LPS via the external jugular vein. Serial blood gases were performed every 30 min until the PO2/FiO2 ratio dropped below 150 on two consecutive readings. Lungs were then randomized to treatment with 4 hours of normothermic EVLP with Steen solution or 4 additional hours of in vivo perfusion (Control). Airway pressures and blood gases were recorded for calculation of dynamic lung compliance and PO2/FiO2 ratios. EVLP was performed according to the NOVEL trial protocol with hourly recruitment maneuvers and oxygen challenge.
Results
All animals reached a PO2/FiO2 ratio < 150 mmHg within 3 hours after start of LPS infusion. Animals in the Control group had continued decline of oxygenation and compliance during the 4-hour in vivo perfusion period with three of the four animals dying within 4 hours due to severe hypoxia. The EVLP group demonstrated significant improvements in oxygenation and dynamic compliance from hour 1 to hour 4 (365.8±53.0 vs 584.4±21.0 mmHg, p=0.02; 9.0±2.8 vs 15.0±3.6, p=0.02 mL/cmH2O).
Conclusions
EVLP can successfully rehabilitate LPS-induced lung injury in this preclinical porcine model. Thus EVLP may provide a means to rehabilitate many types of acute lung injury.
Introduction
Acute respiratory distress syndrome (ARDS) is a rapidly progressive state of pulmonary failure affecting over 2 million patients worldwide and 500,000 in the US alone(1). An estimated 75% of these cases are moderate and severe requiring prolonged mechanical and ventilator support with almost no therapeutic options(2–4). Despite numerous clinical trials dedicated to regulating the pro-inflammatory response accompanying ARDS, protective ventilation remains the only proven therapy to consistently reduce mortality(5). The pathophysiologic progression of ARDS through exudative, proliferative, and terminal fibrotic stages results in a life-threatening ventilation-perfusion mismatch (PO2/FiO2 ≤ 300 mmHg) and loss of compliance within affected lungs(6, 7). The multi-factorial pathogenesis and immunologic mechanisms underlying this rapid and potentially irreversible decline in lung function remain poorly understood. Circulatory shock secondary to sepsis is the leading causes of ARDS worldwide(1).
Ex vivo lung perfusion (EVLP) has demonstrated significant potential to assess and rehabilitate injured lungs for lung transplantation(8, 9). This therapy provides ventilation and perfusion of the lungs after excision from a donor for transplant(10). The circuit design is modified from clinical cardiopulmonary bypass and is utilized by our group and others for the ex vivo perfusion of lungs. The circuit membrane deoxygenater enables the evaluation of the isolated lung oxygenation capacity(8, 11). Steen solution, a physiologic solution containing albumin, dextran, and electrolytes, supplemented with steroids, antibiotics, and heparin, is used clinically for EVLP. It is an oncotic solution that may ameliorate the effects of lung edema, provide free radical scavenging to reduce further injury in the lung tissue and reduce harmful inflammation and endothelial damage(10).
Given the growing body of literature supporting the use of EVLP for evaluation and rehabilitation of ischemia-reperfusion injury in marginal donor lungs for transplantation, we sought to evaluate EVLP in other etiologies of lung injury. The current study examines the use of EVLP to rehabilitate sepsis induced lung injury in a porcine model of ARDS. We hypothesized this innovative use of EVLP will reduce pulmonary edema, clear inflammatory mediators, and attenuate lung injury in sepsis-induced ARDS.
Materials and Methods
Animals and Study Groups
The study protocol was approved by the University of Virginia Animal Care and Use Committee (ACUC) and complies with the 1996 Guide for the Care and Use of Laboratory Animals as recommended by the US National Institutes of Health (NIH). The investigators ensured all animals received humane care during the duration of the study. Farm-raised swine of both sexes (30–40 kg) underwent induction of general anesthesia followed by placement of right carotid artery catheter for blood pressure monitoring and left jugular vein catheter placement for vascular access and placement of Swan-Ganz catheter. The animals were all maintained on conventional ventilation (tidal volume 8 mL/kg, respiratory rate 12–16 breaths/min, positive end-expiratory pressure 5 mmHg, FiO2 0.40) and pentobarbital infusion.
Midline sternotomy was performed and baseline measurements of lung compliance and oxygenation were taken. After intravenous heparin (100 U/kg, Hospira Inc., Lake Forest, IL) administration, animals underwent 2 hours of intravenous infusion of lipopolysaccharide (LPS) until PO2/FiO2 ratio fell below 150 mmHg. Animals were then randomized to two groups. The experimental group (n=4) underwent excision of bilateral lungs followed by 4 hours of left lung EVLP (EVLP group). The right lung was taken for fresh tissue samples and histology as a baseline measurement (ex vivo Control). The Control group (n=4) received 4 hours of continued in vivo perfusion and ventilator support. Airway pressure measurements and pulmonary vein blood gases were performed every hour during perfusion for left lung-specific PO2/FiO2 ratio. Hemodynamic goals were pH 7.35–7.45, base excess > −5, and mean arterial pressure > 55 mmHg, which were maintained with use of normal saline, epinephrine, and sodium bicarbonate as necessary. The left lung was explanted after 4 hours for tissue samples and histology (in vivo Control). The animal was then euthanized.
Ex vivo Lung Perfusion
Warm back-table preparation included division of lungs and ligation of the right pulmonary veins at the left atrium. The left lung then underwent cannulation with an 18F arterial cannula tied in the left pulmonary artery, a green cannula (XVIVO Perfusion Inc., Englewood, CO) sewn to the LA cuff, and a 7-0 endotracheal tube was tied into the left main stem bronchus. Prior to initiation of 4 hours of single left lung EVLP, 500 mL of normothermic 5% Albumin was flushed retrograde through the LA cannula. EVLP was initiated on a perfusion circuit as previously described(11). The circuit was primed in the standard fashion with 500 mL Steen Solution supplemented with cefazolin (500 mg, APP Pharmaceuticals, Schaumburg, IL), methylprednisolone (500 mg, Pfizer Inc., New York, NY), and heparin (10,000 IU).
The circuit was perfused similar to a protocol initially described by the Toronto group with flow initiated at 0.2 mL/min and LA pressures maintained between 0–5 mmHg(8). However, contrary to their method of cold perfusion, our perfusate was pre-warmed to 37° and lung ventilation was begun immediately at a tidal volume of 3 mL/kg (single lung), respiratory rate of 8 breaths/minute, positive end-expiratory pressure of 5.0 cm H2O, and FiO2 of 0.21. Flow was titrated up to 8% of estimated cardiac output (100 mL/kg donor body weight) over the first half hour. A standard tri-gas mixture (86% nitrogen, 8% carbon dioxide, 6% oxygen) through an Affinity membrane (Medtronic, Eden Prairie, MN) was used to deoxygenate the perfusate. Samples from the left pulmonary artery inflow and LA outflow were collected every hour following 15-minute challenge period with 1.0 FiO2 and lung recruitment to PEEP of 10 for the measurement of partial pressure of oxygen (PO2). Airway pressures on conventional ventilation were measured hourly to calculate dynamic lung compliance.
Pulmonary Edema Assessment
Three samples of fresh tissue (one upper lobe, two lower lobe) were obtained and weighed from each lung in each of our groups (EVLP, in vivo Control and ex vivo Control). A vacuum oven was used to desiccate the tissues. Wet-to-dry weight ratios were calculated and averaged per lung to assess overall pulmonary edema accumulation.
Measurements of Expression of Cytokines and VCAM-1
Two fresh tissue samples were obtained (upper lobe and lower lobe) from lungs in each of the groups. The right lung (ev vivo Control) was used to establish a baseline for inflammatory markers prior to EVLP and the in vivo Control was used as reference for inflammation after 4 more hours of perfusion. The fresh tissue was flash frozen in liquid nitrogen, and stored at −80°C. After homogenization using a FastPrep®-24 instrument with lysing matrix D tubes (MP Biomedicals, Santa Ana, CA), the total protein concentration in the supernatant was determined with a bicinchoninic acid protein assay (Pierce, Rockford, IL). Multiplex enzyme-linked immunosorbent assay (EMD Millipore, Billerica, MA) was used to measure cytokine levels in homogenized tissue supernatant (normalized to total protein).
Expression of VCAM-1 in lung tissue was assessed by Western blot analysis. In detail, 15 ug of whole cell protein extract was electrophoresed in a 12% Bis-Tris polyacrylamide gel followed by transfer to a nitrocellulose membrane. The membranes were blocked for 1 hour in Tris-buffered saline and Tween 20 with 5% nonfat milk for 1 hour. After blocking, membranes were incubated with anti-VCAM-1 monoclonal antibody (1:1000; Abcam) overnight, followed by 3 washes. Membranes were then incubated in horseradish peroxidase-conjugated secondary antibody, followed by another series of washes. Protein was visualized by autoradiography and then analyzed by Image Analysis software (Bio-Rad, Hercules, CA, USA).
Histology
Lungs were fixed overnight via intratracheal instillation of 10% buffered formalin at 20cm H2O and four representative lung samples were taken from each lung and stored in 70% ethanol until paraffin embedding. After sectioning the samples one section from each lung sample was stained with hematoxylin-eosin (H&E) for review by a masked pathologist to provide lung injury scores. Each section was scored on a standard scale based on polymorphonuclear cells per 40× high-powered field, alveolar edema, and interstitial inflammation as previously described (Supplemental Table 1)(12).
Statistical Analysis
Student’s t-test, Fishers Exact Tests, and ANOVA were used to determine statistical significance. Prism 7 (GraphPad Software Inc., La Jolla, CA) was used to perform statistical calculations and all data were reported as mean ± standard error, and p<0.05 was used for statistical significance.
Results
Injury Model Validation
Preliminary experiments were performed to validate a commonly used porcine model of sepsis-induced lung injury. A two-hour intravenous infusion of LPS demonstrated a dose dependent decrease in PO2/FiO2 ratios in addition to a reduction in dynamic lung compliance over a 4-hour period (Supplemental Figure 1A and 1B).
Lung Function and Edema
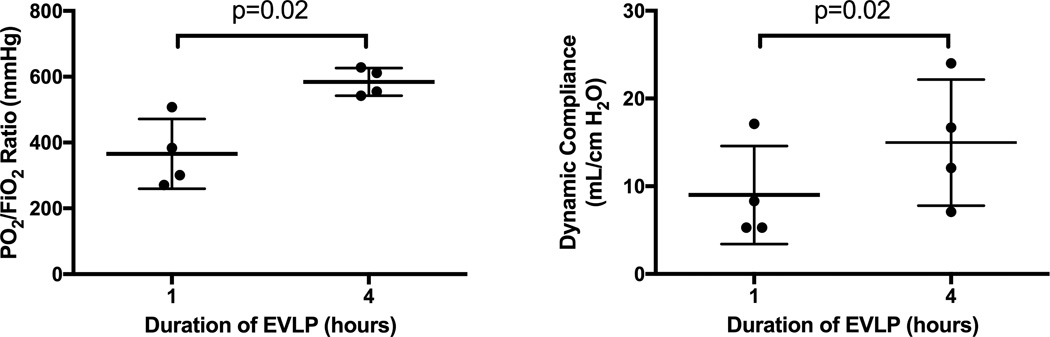
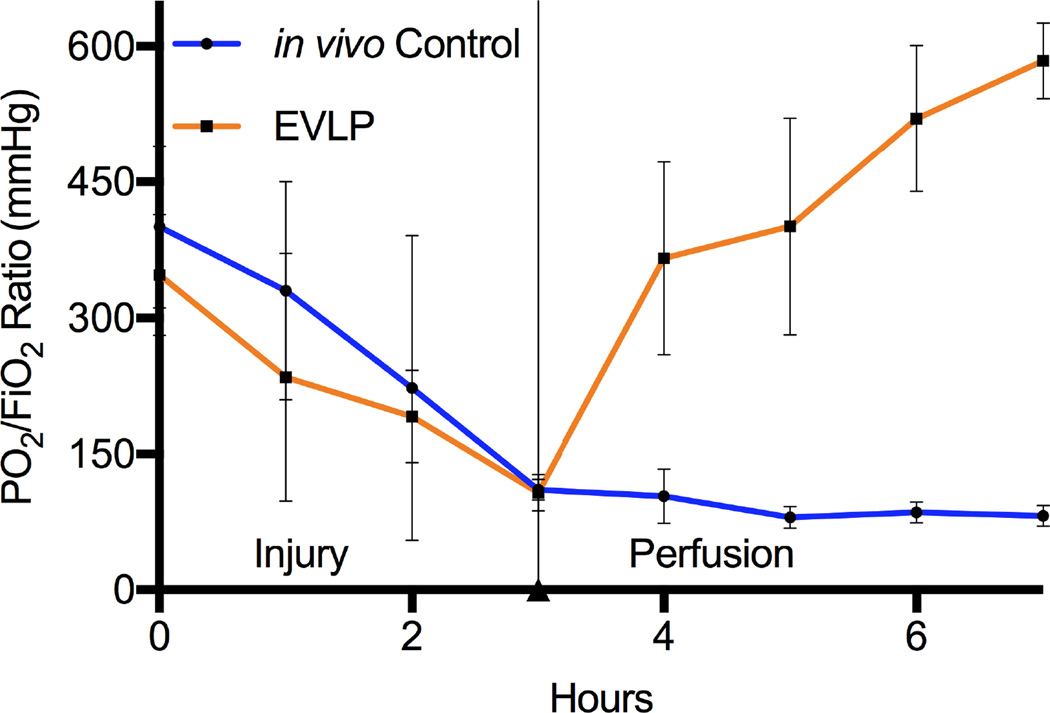
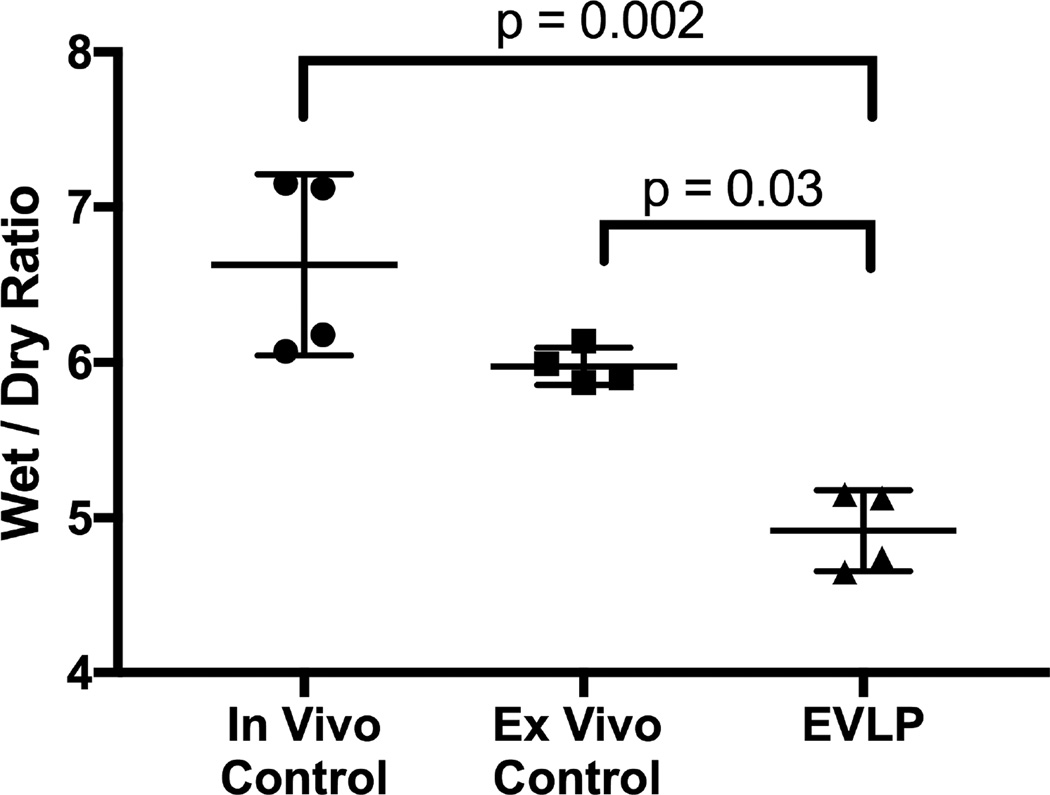
The median procedure time from warm lung harvest to initiation of ventilation and perfusion on the EVLP circuit was 14 minutes with an interquartile range of 12–18. Oxygenation (PO2/FiO2 ratios) and compliance of lungs undergoing LPS-induced lung injury significantly improved over the 4-hour EVLP period compared to initial performance (Figure 1). Compared to in vivo Controls, lungs rehabilitated with EVLP demonstrated major improvement in oxygenation (Figure 2). Three (75%) of the Control group animals did not survive a full 4 hours after lung injury due to severe hypoxia and circulatory collapse (median survival 3.6 hours). Lung wet-to-dry weight ratios for each group reveal a significant reduction of edema in the lungs rehabilitated with EVLP (4.9 ± 0.1) compared to the in vivo Control (6.6 ± 0.3, p=0.002) as well as the ex vivo Controls (6.0 ± 0.1, p=0.03) (Figure 3).
Figure 1.
Significant improvement in both oxygenation and compliance from hour 1 to hour 4 on EVLP in porcine left lung with LPS induced lung injury.
Figure 2.
Major improvement in oxygenation of left lungs placed on EVLP compared to left lungs remaining in vivo after LPS-induced lung injury.
Figure 3.
Significant reduction in edema in the EVLP lungs compared to in vivo Controls (4.9±0.1 vs 6.6 ± 0.3, p=0.002) and ex vivo Controls (4.9±0.1 vs 6.0 ± 0.1, p=0.03).
Inflammatory Markers
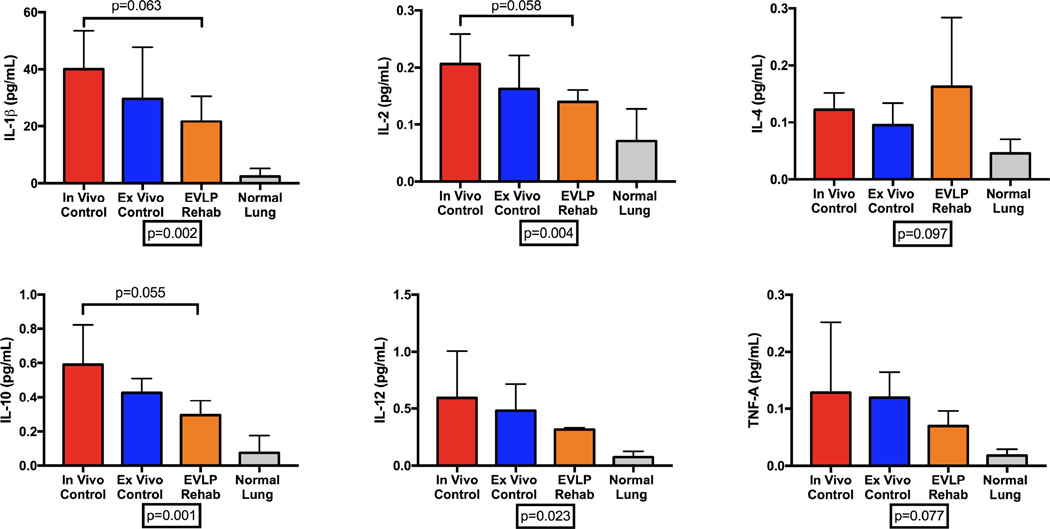
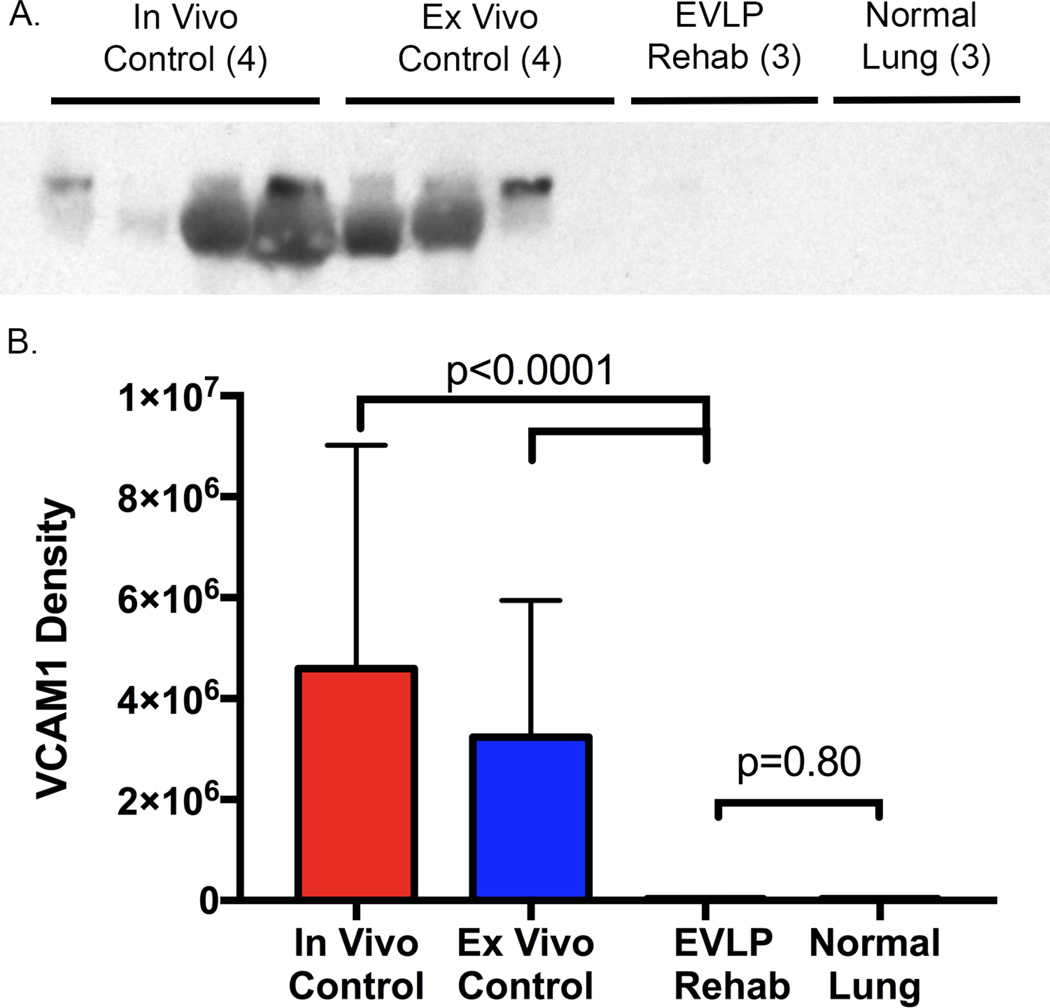
We demonstrated a major reduction in the expression of several biologically relevant pro-inflammatory cytokines in lungs after EVLP compared to Cgroups (Figure 4). There is a higher expression of IL-1β, IL-2, IL-10, IL-12, and TNF-α in lungs from both Control groups compared to both the post-EVLP lung tissue and normal healthy porcine lungs. However, IL-4 expression trended toward higher expression in the EVLP rehabilitated lung tissue. Similarly, expression of VCAM-1, a leukocyte-endothelial cell adhesion molecule upregulated during inflammation, in lung tissue was significantly higher in both of the Control groups (in vivo 4,596,152 ± 2,212,658D, ex vivo 3,239,387 ± 1,352,423 D, both p<0.0001) compared to the EVLP group (3,8701 ± 12,438 D) and normal lung tissue (33,582 ± 5,046 D) by image density (Figure 5).
Figure 4.
Expression of cytokines in lung tissue homogenates
Figure 5.
Western blot for VCAM-1 (top) and densitometry measurements by group (bottom) demonstrate significantly higher VCAM-1 expression in both of the Control groups (in vivo 4,596,152 ± 2,212,658 D, ex vivo 3,239,387 ± 1,352,423 D, both p<0.0001) compared to the EVLP group (38,701 ± 12,438D) and normal lung tissue (33,582 ± 5,046D).
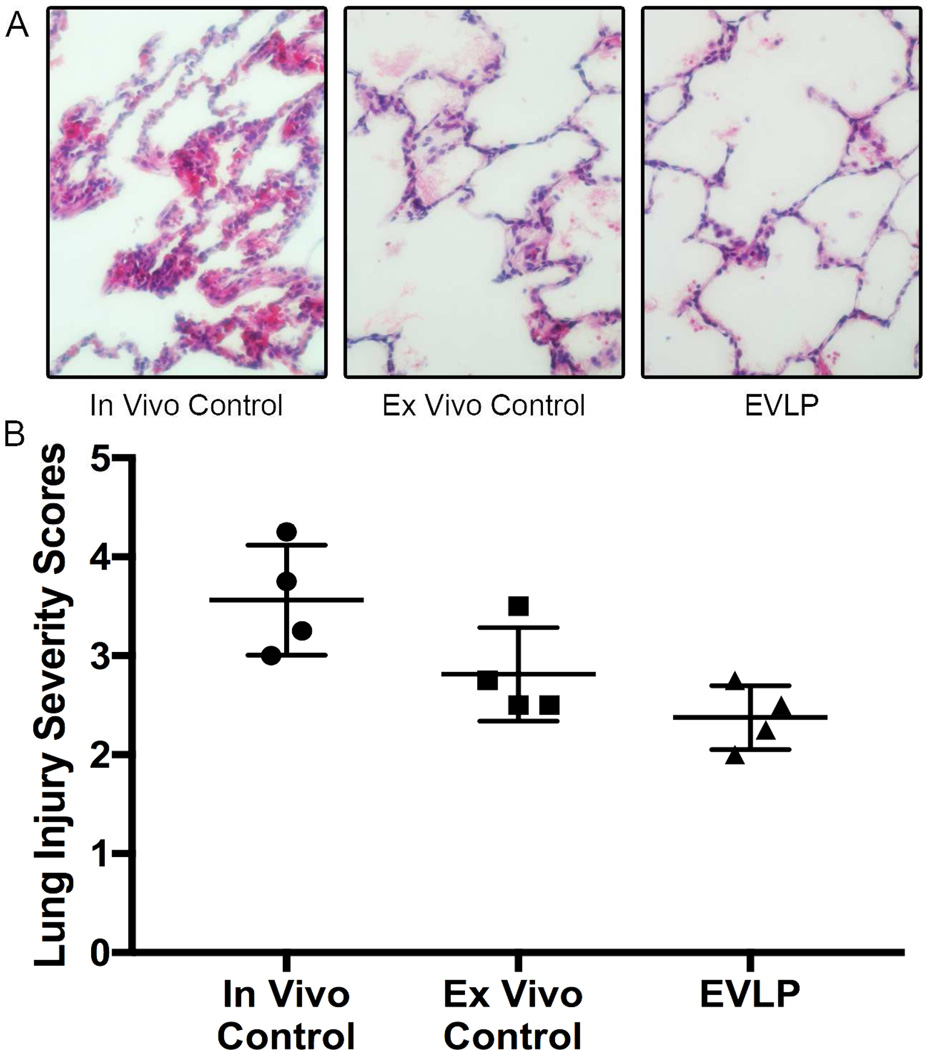
Lung Injury Severity Score
Histologic lung injury severity scores were lower in the EVLP rehabilitated group compared to the in vivo group (2.4 ± 0.2 vs 3.6 ± 0.3, p=0.01). Although the EVLP group was also lower than the ex vivo Controls (2.8 ± 0.2, p=0.18), this difference did not reach significance (Figure 6).
Figure 6.
A. Representative lung histology (H & E staining) for each group. B. EVLP lungs had lower lung injury severity scores compared to in vivo Controls (2.4 ± 0.2 vs 3.6 ± 0.3, p=0.01)
Comment
The present study demonstrates the rehabilitative potential of EVLP in a porcine model of ARDS induced by endotoxin-mediated lung injury. This previously validated model of sepsis-induced ARDS using systemic LPS produces a dose dependent reduction in oxygenation and lung compliance. These data present evidence of improved oxygenation, compliance, lung edema and histologic injury score with a major reduction in cytokine activity and VCAM-1 expression in EVLP-treated lungs. The findings in this study could represent a major paradigm shift in lung perfusion therapy going from an assessment tool for lung transplant to a rehabilitative therapy to ameliorate sepsis-induced ARDS.
Perfusion with Steen solution provides significant reduction in pulmonary edema compared to continued support in the injured animal. This is a key mechanism by which this technique improves lung function. Edema is a critical step in the formation, severity and clinical course of ARDS. Decreased edema will improve the DLCO2 by reducing the distance oxygen must diffuse with compensatory improvement in compliance. Additionally, the statistical difference between the EVLP group and the ex vivo Control right lung from the same animal also represents a clinical difference that suggests successful rehabilitation and reduction of edema in these severely injured lungs.
The inflammatory profile reveals a major reduction in cytokine expression in lungs from the EVLP group compared to both in vivo Control and ex vivo Control, suggesting a ‘resetting’ of the inflammatory milieu(13, 14). The clinical implications of these changes in inflammatory signaling are not completely clear. It seems perfusion with Steen solution including steroids, antibiotics and a scavenging molecule like dextran can clear the inflammatory pathways and return cytokine levels closer to those seen in healthy lung tissue(15, 16). Additionally, the leukocyte-reducing filter in the EVLP circuit, which lowers the burden of circulating inflammatory cells, likely plays a role in clearing leukocytes implicated in many of these signaling cascades(8, 17, 18).
In addition to cytokines, VCAM1 expression in EVLP-rehabilitated lungs was significantly reduced compared to in vivo Control animals, indicative of attenuated vascular inflammation. Several studies have implicated endothelial cell proteins, like VCAM1, to play a major role in ARDS and associated pulmonary edema(19–21). Reducing endothelial permeability results in decreased pulmonary edema, leading to improved oxygenation and compliance. The mechanism(s) for EVLP-mediated down-regulation of inflammation is not clear from this study and will need further evaluation to elucidate the cellular pathways involved.
Importantly, histologic analysis supports the findings of lung injury attenuation by EVLP in this porcine model of LPS induced ARDS. The lung injury scores reflect three critical markers of lung injury and inflammation: alveolar edema, interstitial inflammation and leukocyte infiltration. While there was no statistical difference between the EVLP group and the ex vivo group, it is important to note that lung injury scores were lower in the EVLP group. This is an important distinction because both of these lungs came from the same animal after injury but perfusion on the circuit resulted in histologic improvement.
The limitations of this study include inherent variability between farm-raised large animals. Additionally, there is variability in response to LPS between animals. Each animal demonstrated a significant reduction in oxygenation and lung compliance; however, the timing of drop below our threshold of PO2/FiO2 = 150 mmHg ranged from 60 minutes to 150 minutes. Importantly, 75% of in vivo Control animals did not survive the full 4-hour perfusion period due to severe respiratory failure, hypoxia, and circulatory collapse. The inherent differences between in vivo perfusion and ex vivo perfusion limit the ability to directly compare these groups. This is especially true for measuring inflammatory mediators in lungs perfused with an acellular solution compared to those perfused in vivo with blood. This difference was mitigated by the use of tissue homogenate rather than lavage fluid as well as the measurement of VCAM-1 expression(22). Finally, it is important to note that EVLP lungs were harvested warm with no Perfadex flush and quickly placed on warm circuit with immediate ventilation, which differs vastly from standard donor procurement for EVLP. This was done to simulate an in vivo perfusion scenario where the lung could be isolated and perfused in the animal. While concerns about warm ischemia exist, it is important to note the median time from cross clamp to EVLP perfusion was 14 minutes. Despite these limitations, the present study represents a critical step toward in vivo rehabilitation of ARDS through lung isolation and perfusion techniques.
In conclusion, EVLP with Steen solution successfully rehabilitates LPS induced lung injury in this preclinical porcine model. Several mechanisms for lung injury attenuation have been identified including reduced edema, cessation of inflammatory cascades and down regulation of the vascular cell adhesion molecule VCAM1. These data may represent a critical paradigm shift in lung perfusion technology, however, further elucidation of underlying cellular mechanisms are required. Additionally, EVLP may provide an ideal platform for targeted drug, cellular, or gene therapy for further amelioration of lung injury as future areas of research in ARDS therapy.
Supplementary Material
Acknowledgments
We thank the University of Virginia Research Histology Core for efficient preparation and staining of histology slides. Special thanks to Tony Herring, Cindy Dodson, and Sheila Hammond for their technical support and continued commitment to the completion of this project.
The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007849 (I.L.K.) and R01HL119218 (I.L.K. and V.E.L.) supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: STSA Annual Meeting, November 11th, 2016 in Naples, FL
Disclosure
There are no financial disclosures or conflicts of interest from any of the authors.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.O'Gara B, Fan E, Talmor DS. Controversies in the Management of Severe ARDS: Optimal Ventilator Management and Use of Rescue Therapies. Semin Respir Crit Care Med. 2015;36(6):823–834. doi: 10.1055/s-0035-1564889. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli AR, Zein J, Adams J, Ioannidis JP. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med. 2014;40(6):769–787. doi: 10.1007/s00134-014-3272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England journal of medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz-Diaz E, Festic E, Gajic O, Levitt JE. Emerging pharmacological therapies for prevention and early treatment of acute lung injury. Semin Respir Crit Care Med. 2013;34(4):448–458. doi: 10.1055/s-0033-1351118. [DOI] [PubMed] [Google Scholar]
- 7.Modig J. Adult respiratory distress syndrome. Pathogenesis and treatment. Acta Chir Scand. 1986;152:241–249. [PubMed] [Google Scholar]
- 8.Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Cypel M, Rubacha M, Yeung J, Hirayama S, Torbicki K, Madonik M, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9(10):2262–2269. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 10.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjoberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76(1):244–252. doi: 10.1016/s0003-4975(03)00191-7. discussion 52. [DOI] [PubMed] [Google Scholar]
- 11.Mulloy DP, Stone ML, Crosby IK, Lapar DJ, Sharma AK, Webb DV, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J Thorac Cardiovasc Surg. 2012;144(5):1208–1215. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner CE, Pope NH, Charles EJ, Huerter ME, Sharma AK, Salmon MD, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg. 2015 doi: 10.1016/j.jtcvs.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016;21(3):246–252. doi: 10.1097/MOT.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnevale R, Biondi-Zoccai G, Peruzzi M, De Falco E, Chimenti I, Venuta F, et al. New insights into the steen solution properties: breakthrough in antioxidant effects via NOX2 downregulation. Oxid Med Cell Longev. 2014;2014:242180. doi: 10.1155/2014/242180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Kron IL. Pulmonary macrophages are involved in reperfusion injury after lung transplantation. Ann Thorac Surg. 2001;71(4):1134–1138. doi: 10.1016/s0003-4975(01)02407-9. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 16.George TJ, Arnaoutakis GJ, Beaty CA, Jandu SK, Santhanam L, Berkowitz DE, et al. A physiologic and biochemical profile of clinically rejected lungs on a normothermic ex vivo lung perfusion platform. J Surg Res. 2013;183(1):75–83. doi: 10.1016/j.jss.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charles EJ, Huerter ME, Wagner CE, Sharma AK, Zhao Y, Stoler MH, et al. Donation After Circulatory Death Lungs Transplantable Up to Six Hours After Ex Vivo Lung Perfusion. Ann Thorac Surg. 2016 doi: 10.1016/j.athoracsur.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaPar DJ, Laubach VE, Emaminia A, Crosby IK, Hajzus VA, Sharma AK, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142(4):887–894. doi: 10.1016/j.jtcvs.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meliton AY, Meng F, Tian Y, Sarich N, Mutlu GM, Birukova AA, et al. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol. 2015;308(6):L550–L562. doi: 10.1152/ajplung.00248.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng F, Mambetsariev I, Tian Y, Beckham Y, Meliton A, Leff A, et al. Attenuation of lipopolysaccharide-induced lung vascular stiffening by lipoxin reduces lung inflammation. Am J Respir Cell Mol Biol. 2015;52(2):152–161. doi: 10.1165/rcmb.2013-0468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian Y, Mambetsariev I, Sarich N, Meng F, Birukova AA. Role of microtubules in attenuation of PepG-induced vascular endothelial dysfunction by atrial natriuretic peptide. Biochim Biophys Acta. 2015;1852(1):104–119. doi: 10.1016/j.bbadis.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medeiros IL, Pego-Fernandes PM, Mariani AW, Fernandes FG, do Vale Unterpertinger F, Canzian M, et al. Histologic and functional evaluation of lungs reconditioned by ex vivo lung perfusion. J Heart Lung Transplant. 2012;31(3):305–309. doi: 10.1016/j.healun.2011.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.