Abstract
Background
Individuals on dialysis have a high risk of infection, but risk of infection in earlier stages of chronic kidney disease (CKD) has not been comprehensively described.
Study Design
Observational cohort study.
Setting & Participants
9,697 participants (aged 53–75 years) in the Atherosclerosis Risk in Communities (ARIC) Study. The participants were followed up from 1996–1998 through 2011.
Predictors
Estimated glomerular filtration rate (eGFR) and urinary albumin-creatinine ratio (ACR)
Outcomes
Risk of hospitalization with infection, and death during or within 30-days of hospitalization with infection.
Results
During follow-up (median, 13.6 years), there were 2,701 incident hospitalizations with infection (incidence rate, 23.6 per 1,000 person-years) and 523 infection-related deaths. In multivariable analysis, the HRs of incident hospitalization with infection as compared to eGFR ≥90 ml/min/1.73 m2 were 2.55 (95% CI, 1.43–4.55), 1.48 (95% CI, 1.28–1.71), and 1.07 (95% CI, 0.98–1.16) for eGFR 15–29, 30–59, and 60–89 ml/min/1.73 m2, respectively. Corresponding HRs were 3.76 (95% CI, 1.48–9.58), 1.62 (95% CI, 1.20–2.19), and 0.99 (95% CI, 0.80–1.21) for infection-related death. Compared to ACR <10 mg/g, the HRs of incident hospitalization with infection were 2.30 (95% CI, 1.81–2.91), 1.56 (95% CI, 1.36–1.78), and 1.34 (95% CI, 1.20–1.50) for ACR ≥300, 30–299, and 10–29 mg/g, respectively. Corresponding HRs were 3.44 (95% CI, 2.28–5.19), 1.57 (95% CI, 1.18–2.09), and 1.39 (95% CI, 1.09–1.78) for infection-related death. Results were consistent when separately assessing risk for pneumonia, kidney and urinary tract infections, blood stream infections, and cellulitis, and when taking into account recurrent episodes of infection.
Limitations
Outcome ascertainment relied on diagnostic codes at time of discharge.
Conclusions
Increasing provider awareness of CKD as a risk factor for infection is needed to reduce infection-related morbidity and mortality.
Keywords: Chronic kidney disease (CKD), Chronic renal Insufficiency, Chronic kidney failure, Glomerular filtration rate (GFR), Albuminuria, Proteinuria, Infection, Infectious disease, Pneumonia, Respiratory Tract Infections, Urinary Tract Infections, Bacteremia, Cellulitis, hospitalization, kidney function
Chronic kidney disease (CKD) is a rapidly growing public health problem. More than 20 million adults in the United States are estimated to have some level of CKD defined as either reduced estimated glomerular filtration rate (eGFR) or elevated urinary albumin-creatinine ratio (ACR).1 Numerous studies have shown that together reduced eGFR and albuminuria increase risk for cardiovascular disease,2 but they are also known to increase risk for non-cardiovascular outcomes such as acute kidney injury3 and fracture4 independent of one another.
Infection may be another important complication of CKD. Individuals on dialysis have remarkably high risk of infection,5 particularly for bloodstream infections, foot infections, and pneumonia.6, 7 Increased risk of hospitalization with infection has also been observed among individuals with less severely decreased kidney function that does not require dialysis8–10 although results vary across types of infection. Of importance, data for the other key element of defining and staging CKD, e.g. albuminuria, are sparse in this context. Two studies demonstrated that albuminuria is associated with risk for overall infection-related and pneumonia-related hospitalizations, but these studies exclusively investigated diabetic patients.11, 12 In the general population, one study simultaneously explored the infection risk of both eGFR and albuminuria, but that study investigated only mortality related to infection.13
Understanding the contributions of eGFR and albuminuria to infection risk will provide insights about identifying CKD patients who are at particularly high risk for infectious disease and may benefit from preventive approaches (e.g., vaccination). Thus, we evaluated the association of eGFR and albuminuria with risk of hospitalization with infection and subsequent mortality in a bi-ethnic community-based cohort, the Atherosclerosis Risk in Communities (ARIC) study. We also evaluated specific types of infections, including pneumonia, kidney and urinary tract infection, bloodstream infections, and cellulitis, separately, as well as the rate of recurrent hospitalizations.
Methods
Study Population
The ARIC Study is a community-based prospective cohort study with 15,792 participants who were aged 45–64 years and enrolled in 1987–1989 (visit 1) from four US communities (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD). Short-term follow-up visits were conducted in 1990–1992 (visit 2), 1993–1995 (visit 3), and 1996–1998 (visit 4). Further details of the ARIC study were described elsewhere.14 We set visit 4 as baseline because eGFR and albuminuria were simultaneously assessed for the first time in the ARIC study at this visit. Of 11,656 individuals who attended visit 4, we excluded individuals with a prior hospitalization with infection (as defined later) (n=1,023), missing either serum creatinine or cystatin C (n=323), missing albuminuria (n=117), who declined informed consent for non-cardiovascular research (n=39), with ethnicities other than white or African-American (n=31), with end stage renal disease (ESRD) or eGFR <15 ml/min/1.73 m2 (n=20), and missing covariates (n=406), leaving 9,697 participants for analysis (Figure S1, available as online supplementary material). Written informed consent was obtained from all participants, and the institutional review board at each study site approved the study (#H.34.99.07.02.A1 at Johns Hopkins University).
Exposures of Interest
Primary exposures of interest were eGFR and ACR. The eGFR was based on the CKD-EPI (CKD Epidemiology Collaboration) serum creatinine and cystatin C equation.15 Serum creatinine was measured using a modified kinetic Jaffé method, calibrated to the Cleveland Clinic laboratory measurements,16 and then standardized to an isotope-dilution mass spectrometry–traceable method.17 Serum cystatin C was measured using an enhanced immunonephelometric assay (Siemens Healthcare Diagnostics). Urine albumin and creatinine were measured by nephelometry and the Jaffé method, respectively. The detection threshold was <2 mg/dL for urine albumin and <1 mg/dL for urine creatinine. Participants with urine albumin <2 mg/dL were assumed to have 1 mg/dL for the purpose of calculating ACR (4,364 participants). There were no participants with urine creatinine <1 mg/dL.
Outcomes of Interest
The primary outcome of interest was first incident hospitalization with infection. Hospital discharge records with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes have been captured through active surveillance for all the ARIC study participants. The definitions of infection-related ICD-9 codes were based on the analytical methods used in US Renal Data System (USRDS),18 including any pathogen-, organ-, or symptom-based diagnoses (Table S1). In addition, the a priori determined four most common types of infection19 were separately analyzed, including pneumonia (ICD-9, 480–486), kidney and urinary tract infections (590, 590.0–4, 597, 598, 599.0, 601, 604, 607, and 608), bloodstream infections (038 and 790.7), and cellulitis (681 and 682). Secondary outcomes were all hospitalizations with infection including recurrent cases as well as subsequent death during or within 30 days after discharge from hospitalization with infection, similar to previous studies.20, 21 Participants who did not develop the primary outcome were censored when they died or were lost-to-follow-up or were administratively censored on December 31, 2011.
Other Covariates of Interest
All the covariates were assessed at baseline (visit 4, 1996–1998) except for years of education, which was assessed at visit 1 (1987–1989). Age, sex, race, smoking status, alcohol consumption, and years of education were based on self-reported questionnaires. Information about the use of antineoplastic agents and steroids or immunosuppressive agents was obtained from the medication records at visit 4. Hypertension was defined as taking an antihypertensive drug; systolic blood pressure ≥140 mmHg; or diastolic blood pressure ≥90 mmHg. Diabetes was defined as a self-reported physician diagnosis of diabetes; taking an antidiabetic drug; a fasting glucose ≥126 mg/dL; or a casual glucose ≥200 mg/dL. The ICD-9 codes indicating history of cancer (ICD-9, 140–149,150–159, 160–165, 170–175, 176, 179–189, 190–199, 200–208, 209, 235–238, and 239) and chronic obstructive pulmonary disease (490–491, 492, 494, and 496) were retrieved from hospital discharge records between visit 1 and visit 4. History of cardiovascular disease including heart failure, coronary heart disease, and stroke was determined if there was a self-reported history at visit 1 or clinical event between visit 1 and visit 4. Data on incident ESRD were obtained by the linkage to USRDS.
Statistical Analysis
Baseline characteristics were compared across eGFR and ACR categories using Student’s t-tests, analysis of variance (ANOVA) tests, and chi-square tests. Crude incidence rate and its 95% confidence interval (CI) was estimated using Poisson regression models. Hazard ratios (HRs) were assessed using Cox proportional hazards models across four categories of eGFR (≥90 [reference], 60–89, 30–59, and 15–29 ml/min/1.73 m2) and ACR (<10 [reference], 10–29, 30–299, and ≥300 mg/g). Models were adjusted for age, sex, race, body mass index (BMI), smoking status, alcohol consumption, education level, use of antineoplastic agents and steroids, hypertension, diabetes, history of cancer and chronic obstructive pulmonary disease, prior heart failure, prior coronary disease, and prior stroke, and ACR for the analysis of eGFR, and eGFR for the analysis of ACR. Interaction between eGFR and ACR categories was assessed by contrasting models with and without their production terms using the log-likelihood test.
To provide implications on the current CKD staging system in the KDIGO (Kidney Disease: Improving Global Outcomes) guidelines,22 we subsequently quantified the risk of hospitalization with infection in cross-categories of eGFR and ACR. For this part of the analysis, those with eGFR <30 ml/min/1.73 m2 were grouped together for obtaining a reliable estimate.
Potential effect modifications of kidney function and albuminuria with the subgroups by baseline age (< versus ≥ 65 years), sex (male versus female), race (whites versus African Americans), diabetes (yes versus no), and prior cardiovascular disease (yes versus no) were assessed by comparing two models with and without the interaction term using likelihood ratio tests. To obtain reliable estimates in each subgroup, we dichotomized CKD measures, eGFR 15–59 versus ≥60 ml/min/1.73 m2 and ACR <30 vs. ≥30 mg/g.
Several sensitivity analyses were performed. First, we assessed the risk if we censored at incident ESRD. Second, we restricted the outcome to hospitalizations with infectious diseases as the primary diagnosis (i.e., the first position). Third, we excluded individuals with ACR ≥2,000 mg/g, potentially indicating nephrotic syndrome.23 Fourth, we analyzed the data when glomerular filtration rate (GFR) was estimated using only creatinine by the CKD-EPI creatinine equation.24
Finally, we explored the analysis incorporating recurrent hospitalization with infections. For this, we ran negative binomial regression models to account for overdispersion based on the Poisson distribution. A two-sided p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 13 (StataCorp LP, Collage Station, TX).
Results
Baseline Characteristics
At baseline, the mean age of the study population was 63±6 (standard deviation) years; 5,478 (56%) of the study cohort were female, and 2,145 (22%) were African-Americans. Baseline characteristics stratified by eGFR categories are shown in Table 1. Lower eGFR was associated with older age, black race, higher BMI, fewer years of education, and higher prevalence of hypertension, diabetes, prior heart failure, coronary heart disease, and stroke. Similar patterns were observed when stratifying by ACR categories (Table S2).
Table 1.
Baseline characteristics of the study cohort
| Characteristics |
eGFR category (ml/min/1.73 m2) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (N=9697) |
15–29 (n=19) |
30–45 (n=151) |
45–59 (n=441) |
60–74 (n=1535) |
75–89 (n=3387) |
≥90 (n=4164) |
Pfor trend | |
| Age, y | 63 ±6 | 66 ±5 | 66 ±5 | 66 ±5 | 65 ±5 | 63 ±5 | 61 ±5 | <0.001 |
|
| ||||||||
| Female sex | 5478 (56) | 10 (53) | 87 (58) | 240 (54) | 892 (58) | 1866 (55) | 2383 (57) | 0.3 |
|
| ||||||||
| Black race | 2145 (22) | 6 (32) | 33 (22) | 79 (18) | 234 (15) | 547 (16) | 1246 (30) | <0.001 |
|
| ||||||||
| BMI,(kg/m2) | 29 ±6 | 30 ±6 | 30 ±7 | 30±6 | 29 ±5 | 29 ±5 | 28 ±6 | <0.001 |
|
| ||||||||
| Smoking statusˆ | 0.2 | |||||||
|
| ||||||||
| Current smoker | 1437 (15) | 2 (11) | 30 (20) | 70 (16) | 197 (13) | 524 (15) | 614 (15) | |
|
| ||||||||
| Former smoker | 4182 (43) | 9 (47) | 59 (39) | 199 (45) | 658 (43) | 1446 (43) | 1811 (43) | |
|
| ||||||||
| Never smoker | 4078 (42) | 8 (42) | 62 (41) | 172 (39) | 680 (44) | 1417 (42) | 1739 (42) | |
|
| ||||||||
| Current/Former alcohol consumer | 7696 (79) | 14 (74) | 103 (68) | 330 (75) | 1182 (77) | 2731 (81) | 3336 (80) | <0.001 |
|
| ||||||||
| ≥12 y of education | 7896 (81) | 13 (68) | 110 (73) | 325 (74) | 1206 (79) | 2795 (83) | 3447 (83) | <0.001 |
|
| ||||||||
| ACRˆ | <0.001 | |||||||
|
| ||||||||
| <10 mg/g | 7821 (81) | 6 (32) | 76 (50) | 289 (66) | 1216 (79) | 2804 (83) | 3430 (82) | |
|
| ||||||||
| 10–29 mg/g | 1131 (12) | 1 (5) | 22 (15) | 64 (15) | 174 (11) | 382 (11) | 488 (12) | |
|
| ||||||||
| 30–299 mg/g | 609 (6) | 3 (16) | 34 (23) | 64 (15) | 121 (8) | 174 (5) | 213 (5) | |
|
| ||||||||
| ≥300 mg/g | 136 (1) | 9 (47) | 19 (13) | 24 (5) | 24 (2) | 27 (1) | 33 (1) | |
|
| ||||||||
| Medication use | ||||||||
|
| ||||||||
| Antineoplastic agents | 103 (1) | 0 (0) | 3 (2) | 9 (2) | 24 (2) | 35 (1) | 32 (1) | 0.03 |
|
| ||||||||
| Steroids or immunosuppressive agents | 167 (2) | 0 (0) | 8 (5) | 19 (4) | 33 (2) | 59 (2) | 48 (1) | <0.001 |
|
| ||||||||
| Medical history | ||||||||
|
| ||||||||
| Hypertension | 3599 (37) | 16 (84) | 100 (66) | 277 (63) | 677 (44) | 1177 (35) | 1352 (32) | <0.001 |
|
| ||||||||
| Diabetes | 1537 (16) | 8 (42) | 52 (34) | 92 (21) | 231 (15) | 465 (14) | 689 (17) | <0.001 |
|
| ||||||||
| Cancer | 466 (5) | 1 (5) | 11 (7) | 34 (8) | 98 (6) | 179 (5) | 143 (3) | <0.001 |
|
| ||||||||
| COPD | 151 (2) | 0 (0) | 9 (6) | 13 (3) | 40 (3) | 48 (1) | 41 (1) | <0.001 |
|
| ||||||||
| Prior heart failure | 426 (4) | 5 (26) | 18 (12) | 40 (9) | 73 (5) | 154 (5) | 136 (3) | <0.001 |
|
| ||||||||
| Prior coronary heart disease | 699 (7) | 7 (37) | 30 (20) | 49 (11) | 139 (9) | 251 (7) | 223 (5) | <0.001 |
|
| ||||||||
| Prior stroke | 186 (2) | 2 (11) | 10 (7) | 17 (4) | 42 (3) | 59 (2) | 56 (1) | <0.001 |
Note: Unless otherwise indicated, values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation.
Abbreviations:BMI, body mass index; COPD, Chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio
Number (column percentage).
Incident All-Type Hospitalization With Infection
During a median follow-up of 13.6 years, 2,701 (28%) had incident hospitalization with infection, with a crude incidence rate of 23.6 (95% confidence interval [CI], 22.8–24.6) per 1,000 person-years. In crude analysis, lower eGFR and higher ACR were associated with high risk for incident hospitalization with infection. Crude incidence rates (per 1,000 person-years) were 19.3 (95% CI, 18.1–20.5) for eGFR ≥90 ml/min/1.73 m2 and 20.9 (95% CI, 20.0–21.8) for ACR <10 mg/g but were 92.7 (95% CI, 52.7–163.3) for eGFR 15–29 ml/min/1.73 m2 and 82.5 (95% CI, 66.4–102.6) for ACR ≥300 mg/g (Table 2). These associations were stronger than those for hospitalizations without infection (Table S3).
Table 2.
Crude incident rate and adjusted relative hazard for hospitalization with infection
| Category | Person-year | No. of events | Crude IR per 1000 person-y (95%CI) | Adjusted HR (95% CI)* |
|---|---|---|---|---|
| eGFR | ||||
| ≥90 ml/min/1.73 m2 (n=4164) | 51206 | 987 | 19.28 (18.11–20.52) | 1.00 (reference) |
| 60–89 ml/min/1.73 m2 (n=4922) | 57231 | 1428 | 24.95 (23.69–26.28) | 1.07 (0.98–1.16) |
| 30–59 ml/min/1.73 m2 (n=592) | 5671 | 274 | 48.32 (42.92–54.39) | 1.48 (1.28–1.71) |
| 15–29 ml/min/1.73 m2 (n=19) | 129 | 12 | 92.74 (52.67–163.30) | 2.55 (1.43–4.55) |
|
| ||||
| ACR | ||||
| <10 mg/g (n=7821) | 94764 | 1977 | 20.86 (19.96–21.80) | 1.00 (reference) |
| 10–29 mg/g (n=1131) | 12483 | 383 | 30.68 (27.76–33.91) | 1.34 (1.20–1.50) |
| 30–299 mg/g (n=609) | 6008 | 260 | 43.27 (38.32–48.87) | 1.56 (1.36–1.78) |
| ≥300 mg/g (n=136) | 982 | 81 | 82.52 (66.37–102.59) | 2.30 (1.81–2.91) |
Abbreviations: eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; CI, confidence interval; IR, incidence rate; HR, hazard ratio.
The model was adjusted for age, race, sex, body mass index, smoking status, alcohol consumption, education level, use of antineoplastic agents and steroids, hypertension, diabetes, history of cancer, chronic obstructive pulmonary disease, prior heart failure, prior coronary disease, prior stroke, and the ACR categories for the analysis of eGFR and the eGFR categories for the analysis of ACR.
Multivariable Cox proportional hazards analyses revealed that reduced eGFR and elevated ACR were both associated with high incidence of hospitalization with infection in a graded fashion, independently of each other and potential confounders (Table 2). As compared to eGFR ≥90 ml/min/1.73 m2, adjusted HR of infection was 2.5-fold greater in severely reduced eGFR of 15–29 ml/min/1.73 m2 (HR, 2.55; 95% CI, 1.43–4.55) and 50% higher in moderately reduced eGFR of 30–59 ml/min/1.73 m2 (HR, 1.48; 95% CI, 1.28–1.71; Table 2). As compared to normal albuminuria (ACR <10 mg/g), ACR ≥300 mg/g and ACR 30–299 mg/g demonstrated 2.3-fold and 1.6-fold higher risk for incident hospitalization with infection (HRs of 2.30 [95% CI, 1.81–2.91] and 1.56 [95% CI, 1.36–1.78], respectively). Mild albuminuria (ACR, 10–29 mg/g) also demonstrated a significant association (HR, 1.34; 95% CI, 1.20–1.50). There was no significant interaction between eGFR and ACR categories (P for interaction = 0.3).
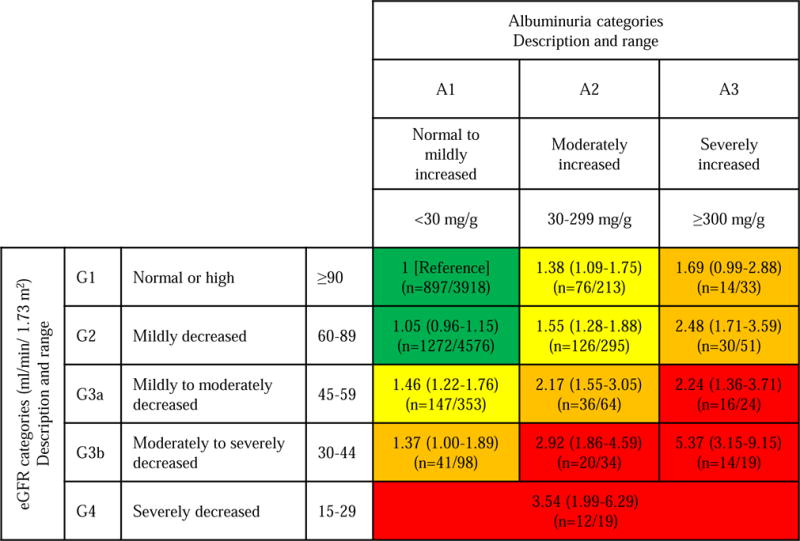
When the infection risk was assessed in the context of the KDIGO risk classification system based on cross-categories of GFR and ACR,22 lower eGFR and higher ACR demonstrated independent and multiplicative contributions (Figure 1). Participants with eGFR 15–29 ml/min/1.73 m2 showed ~3.5 fold higher risk of infection comparing to those with eGFR ≥90 ml/min/1.73 m2 plus ACR <30 mg/g. Of note, some categories with less severely decreased kidney function demonstrated similar or even greater risk when albuminuria is elevated (HRs of 5.37 [95% CI, 3.15–9.15] in eGFR 30–44 ml/min/1.73 m2 when ACR ≥300 mg/g and 2.92 [95% CI, 1.86–4.59] when ACR 30–299 mg/g). Of note, some cross-categories had 2-to 2.5-fold increased risk of hospitalizations with infection (i.e., eGFR 45–59 ml/min/1.73 m2 plus ACR ≥30 mg/g and eGFR 60–89 ml/min/1.73 m2 plus ACR ≥300 mg/g).
Figure 1. Adjusted hazard ratio of hospitalization with infection by eGFR and ACR categories.
Abbreviations: GFR, glomerular filtration rate; ACR, albumin-creatinine ratio. Green: low risk; yellow: moderately increased risk; orange: high risk; red, very high risk. For each category, hazard ratio and its 95% confidence interval were presented in the first row, and n= denotes number of events and number of individuals in the second row. The model was adjusted for age, race, sex, body mass index, smoking status, alcohol consumption, education level, use of antineoplastic agents and steroids, hypertension, diabetes, history of cancer, chronic obstructive pulmonary disease, prior heart failure, prior coronary disease, and prior stroke.
In sensitivity analysis, no significant interaction was observed by age (<65 versus ≥65 years), gender (male versus female), the status of diabetes, smoking (never versus current/former smoker), and prior CVD for both eGFR and ACR. The association of reduced eGFR with risk for infection was stronger in African-Americans than in whites (HR for eGFR <60 versus ≥60 ml/min/1.73 m2 of 1.75 [95% CI, 1.32–2.31] versus 1.50 [95% CI, 1.30–1.73], respectively; p-for-interaction, 0.04), but no significant interaction by race was observed for ACR (Figure S2). The association did not change when censoring incident ESRD cases (table a of Item S1). The associations were much the same when restricting to the cases with primary diagnosis of infection (table b of Item S1). Excluding individuals with ACR ≥2,000 mg/g did not change the associations (table c of Item S1). Finally, when GFR was estimated using a creatinine based equation,24 the associations were much the same (table d of Item S1).
Type-Specific Incident Hospitalization With Infections
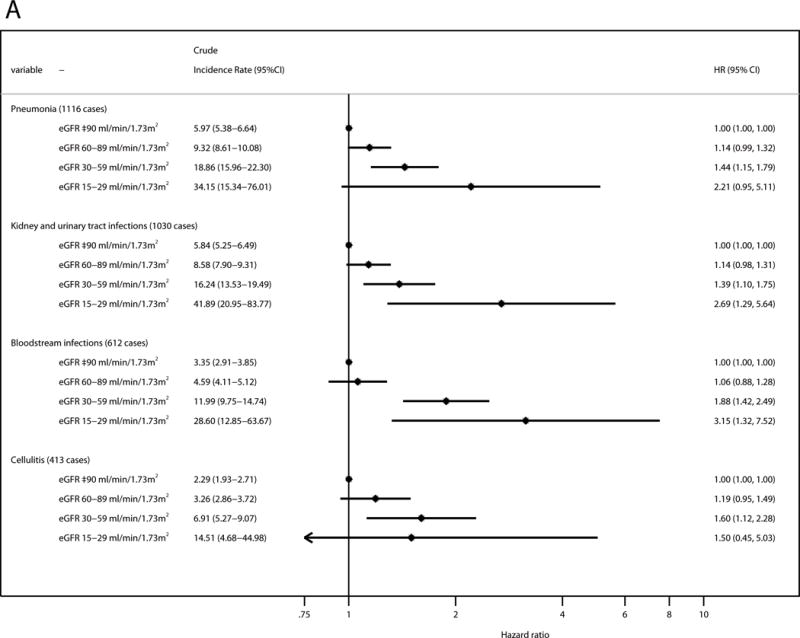
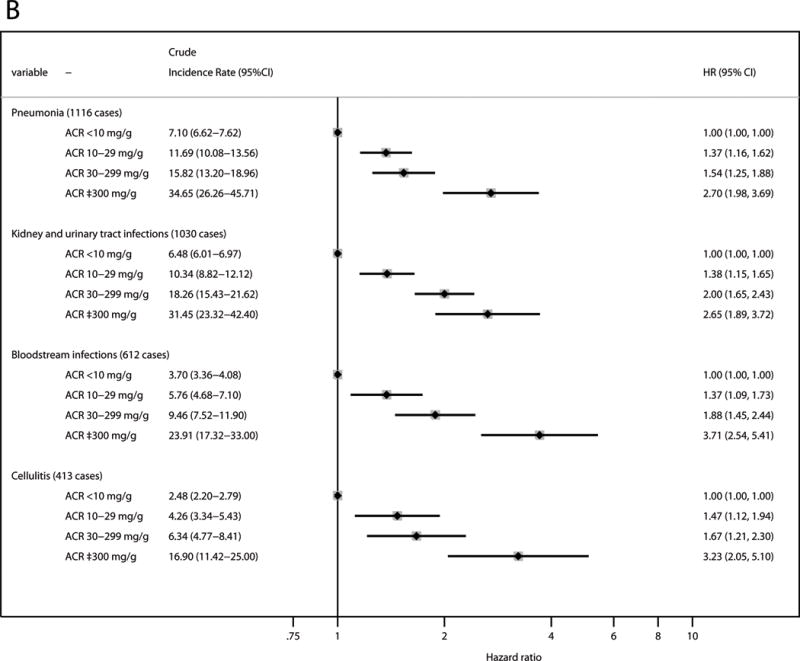
Assessing incidence of four types of infection known a priori to be common, pneumonia was the most frequent type of incident hospitalization with infection (1,116 cases), followed by kidney and urinary tract infections (1,030 cases), bloodstream infections (612 cases), and cellulitis (413 cases). Reduced eGFR and elevated ACR were independently associated with increased risk for all of these types of infection (Figure 2). Bloodstream infections demonstrated the highest HR for both eGFR 15–29 ml/min/1.73 m2 (HR, 3.15; 95% CI, 1.32–7.52) and ACR ≥300 mg/g (HR, 3.71; 95% CI, 2.54–5.41). Of note, all three categories of mild, moderate, and severe albuminuria showed significant associations in any type of infection.
Figure 2. Adjusted relative hazards for hospitalization with infection stratified by the types of infection.
(A) eGFR; and (B) ACR. The model was adjusted for age, race, sex, body mass index, smoking status, alcohol consumption, education level, use of antineoplastic agents and steroids, history of hypertension, diabetes, cancer, chronic obstructive pulmonary disease, prior heart failure, prior coronary disease, and prior stroke, and the ACR categories for the analysis of eGFR and the eGFR categories for the analysis of ACR. The squares represent the point estimate of relative hazard, and the horizontal lines indicate corresponding 95% confidence intervals. Abbreviations: eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; HR, hazard ratio; CI, confidence interval.
Overall All-Type Hospitalization With Infection Including Recurrent Cases
Of 2,701 individuals with incident hospitalization with infection, 1,146 individuals had more than one (range, 2–16) hospitalization with infection (event rate, 44.5 [95% CI, 41.5–45.6] per 1,000 person-years). Recurrent infections were more common among participants with lower eGFR (8.9%, 11.5%, 22.5%, and 42.1% in those with eGFR ≥90, 60–89, 30–59, and 15–29 ml/min/1.73 m2, respectively; Table 3). Similar trends were observed for higher ACR (9.5%, 15.1%, 19.7%, and 36.1% in those with ACR <10, 10–29, 30–299, and ≥300 mg/g, respectively). These patterns remained consistent after adjusting for potential confounders (event rate ratios of 1.10 [95% CI, 1.00–1.22], 1.57 [95% CI 1.31–1.89], and 1.61 [95% CI, 0.70–3.70] for eGFR 60–89, 30–59, and 15–29 ml/min/1.73 m2, respectively, as compared to eGFR ≥90 ml/min/1.73 m2; and of 1.40 [95% CI, 1.23–1.60], 1.67 [95% CI, 1.41–1.97], and 3.12 [95% CI, 2.26–4.31] for ACR 10–29 mg/g, 30–299, and ≥300 mg/g, respectively, as compared to ACR <10 mg/g; Table 3).
Table 3.
Multiple hospitalizations related to infectious disease and adjusted event rate ratio
| Category | No. of participants (row percentage), categorized by no. of infection-related hospitalizations | Total No. of Events | Adjusted event rate ratio (95% CI)c | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 or more | |||
| Overall | 6996 (72.1) | 1619 (16.7) | 617 (6.4) | 215 (2.2) | 98 (1.0) | 152 (1.6) | 4861 | |
|
| ||||||||
| eGFR | ||||||||
| ≥90 ml/min/1.73 m2 (n=4164) | 3177 (76.3) | 615 (14.8) | 232 (5.6) | 73 (1.8) | 30 (0.7) | 37 (0.9) | 1637 | 1.00 (reference) |
| 60–89 ml/min/1.73 m2 (n=4922) | 3494 (71) | 859 (17.5) | 301 (6.1) | 121 (2.5) | 58 (1.2) | 89 (1.8) | 2624 | 1.10 (1.00–1.22) |
| 30–59 ml/min/1.73 m2 (n=592) | 318 (53.7) | 141 (23.8) | 79 (13.3) | 21 (3.5) | 9 (1.5) | 24 (4.1) | 572 | 1.57 (1.31–1.89) |
| 15–29 ml/min/1.73 m2 (n=19) | 7 (36.8) | 4 (21.1) | 5 (26.3) | 1 (5.3) | 2 (10.5) | 0 (0) | 28 | 1.61 (0.70–3.70) |
|
| ||||||||
| ACR | ||||||||
| <10 mg/g (n=7821) | 5844 (74.7) | 1234 (15.8) | 436 (5.6) | 152 (1.9) | 65 (0.8) | 90 (1.2) | 3379 | 1.00 (reference) |
| 10–29 mg/g (n=1131) | 748 (66.1) | 213 (18.8) | 92 (8.1) | 36 (3.2) | 18 (1.6) | 24 (2.1) | 729 | 1.40 (1.23–1.60) |
| 30–299 mg/g (n=609) | 349 (57.3) | 140 (23.0) | 66 (10.8) | 21 (3.4) | 8 (1.3) | 25 (4.1) | 540 | 1.67 (1.41–1.97) |
| ≥300 mg/g (n=136) | 55 (40.4) | 32 (23.5) | 23 (16.9) | 6 (4.4) | 7 (5.1) | 13 (9.6) | 213 | 3.12 (2.26–4.31) |
Abbreviations: eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; CI, confidence interval.
The model was adjusted for age, race, sex, body mass index, smoking status, alcohol consumption, education level, use of antineoplastic agents and steroids, hypertension, diabetes, history of cancer, chronic obstructive pulmonary disease, prior heart failure, prior coronary disease, prior stroke, and the ACR categories for the analysis of eGFR and the eGFR categories for the analysis of ACR.
Mortality Risk Related to Hospitalization With Infection
There were 523 deaths during or within 30 days of hospitalization with infection, including 81 deaths during hospitalization with infection and 442 deaths within 30 days after discharge. Reduced eGFR and elevated ACR were independently associated with higher risk for infection-related death (HRs of 0.99 [95% CI, 0.80–1.21], 1.62 [95% CI, 1.20–2.19], 3.76 [95% CI, 1.48–9.58] for eGFR 60–89, 30–59, and 15–29 ml/min/1.73 m2, respectively, as compared to eGFR ≥90 ml/min/1.73 m2; and of 1.39 [95% CI, 1.09–1.78], 1.57 [95% CI, 1.18–2.09], 3.44 [95% CI, 2.28–5.19] for ACR 10–29, 30–299, and ≥300 mg/g, respectively, as compared to ACR <10 mg/g; Table 4).
Table 4.
Risk for infection-related death according to eGFR and ACR categories
| Category | Person-y | No. of events | Mortality rate per 1000 person-y (95% CI) | Adjusted HR (95% CI)* |
|---|---|---|---|---|
| eGFR | ||||
| ≥90 ml/min/1.73 m2 (n=4164) | 55866 | 170 | 3.04 (2.62–3.54) | 1.00 (reference) |
| 60–89 ml/min/1.73 m2 (n=4922) | 64053 | 274 | 4.28 (3.80–4.82) | 0.99 (0.80–1.21) |
| 30–59 ml/min/1.73 m2 (n=592) | 6659 | 74 | 11.11 (8.85–13.96) | 1.62 (1.20–2.19) |
| 15–29 ml/min/1.73 m2 (n=19) | 167 | 5 | 29.93 (12.46–71.91) | 3.76 (1.48–9.58) |
|
| ||||
| ACR | ||||
| <10 mg/g (n=7821) | 104215 | 353 | 3.39 (3.05–3.76) | 1.00 (reference) |
| 10–29 mg/g (n=1131) | 14121 | 80 | 5.67 (4.55–7.05) | 1.39 (1.09–1.78) |
| 30–299 mg/g (n=609) | 7106 | 60 | 8.44 (6.56–10.88) | 1.57 (1.18–2.09) |
| ≥300 mg/g (n=136) | 1303 | 30 | 23.03 (16.10–32.94) | 3.44 (2.28–5.19) |
Abbreviations: eGFR, estimated glomerular filtration rate; HR, hazard ratio; ACR, albumin-creatinine ratio; CI, confidence interval.
The model was adjusted for age, race, sex, body mass index, smoking status, alcohol consumption, education level, use of antineoplastic agents and steroids, hypertension, diabetes, history of cancer, chronic obstructive pulmonary disease, prior heart failure, prior coronary disease, prior stroke, and the ACR categories for the analysis of eGFR and the eGFR categories for the analysis of ACR.
Discussion
In this community-based bi-ethnic cohort of nearly 10,000 middle-aged and older individuals with follow-up for up to 15 years, reduced eGFR and elevated ACR were associated with higher risk for hospitalization with infection and subsequent mortality. The associations were independent of each other and of confounders, and were present even at mild to moderate stages of reduced eGFR and elevated ACR. An independent association of reduced eGFR and elevated ACR was consistently observed in risk for pneumonia, kidney and urinary tract infections, bloodstream infections, and cellulitis. The present study highlighted the high burden of hospitalization with infection and subsequent outcome in broad spectrum of CKD.
The results in the present study were consistent with the previous findings in terms of overall relationship between reduced kidney function and infection,8–10, 12, 13 but had several unique aspects. First, the use of the creatinine and cystatin C equation, which is considered the best estimate for GFR, allowed us to better quantify the risk of infection across clinical categories of eGFR. Second, despite somewhat conflicting results in the previous studies,8, 10 we observed consistent associations between reduced eGFR and the risk for all major infection types including pneumonia, kidney and urinary tract infections, bloodstream infections, and cellulitis, suggesting broad implications of reduced eGFR in the pathophysiology of infectious diseases. Finally, we confirmed the consistent association of reduced eGFR across incident and recurrent hospitalizations as well as subsequent mortality related to infection in a single study population.
Our study is to our knowledge the first to demonstrate a clear graded association between albuminuria and infection-related outcomes. The association was significant even at mild albuminuria (ACR, 10–29 mg/g), a category currently not considered as clinically abnormal, and was consistent for four major types of infection. A previous study using data from the Third National Health and Nutrition Examination Survey (NHANES III) explored the association of albuminuria with infection in the general population. However, this study had only data on mortality due to infections, thus limiting power.13 Indeed, with ACR <30 mg/g as a reference, moderate ACR 30–299 mg/g did not show a significant relationship to infection mortality.13 In addition, two studies of patients with diabetes mellitus reported the association of albuminuria with hospitalization with infection.11, 12 However, by design, they could not distinguish whether albuminuria itself was a risk factor or whether it was a surrogate for severity of diabetes.
There are several potential mechanisms linking between CKD and infection. In patients with advanced CKD, uremic toxins impair the function of T-lymphocyte and antigen-presenting cells,25 which play important roles in both cellular and humoral immunity. Similarly, oxidative stress in CKD disrupts the function of inflammatory cytokines, and may impair response during initial development of an infection.26 The mechanism by which albuminuria increases risk of infection is less clear. Endothelial dysfunction, which is believed to underlie albuminuria,27 may play an important role. For example, degraded endothelial glycocalyx leads to the disruption of neutrophil adhesion.28 Further investigations are warranted to explore whether and how pathophysiological conditions leading to albuminuria interfere with the immune system.
The present study has several clinical and research implications. Infection prevention programs (e.g., vaccination and preventive procedures for healthcare-acquired infections) usually feature patients on dialysis and individuals with severely decreased kidney function (eGFR <30 ml/min/1.73 m2).29–32 Currently, albuminuria is not taken into account in this context. Thus, our results suggest that these programs could be expanded to include persons with less severely decreased kidney function, including those with elevated albuminuria. Future studies are required to evaluate whether such an approach indeed improves the outcomes and is cost-effective. Also, given the robustness of the albuminuria-infection relationship, it would be interesting to assess whether interventions reducing albuminuria (e.g., renin-angiotensin system inhibitors) can modify the risk of infection.
The present study has certain limitations. First, we did not capture mild cases of infection that were treated solely in an outpatient setting. However, hospitalizations with infection are likely the most costly and morbid.19 Second, our outcome ascertainments based on ICD-9 codes could overestimate the incidence of infection as compared to the estimate made by adjudication with chart review.33 Nonetheless, the associations were consistent when we restricted to cases with the primary diagnosis of infectious diseases. Third, individuals with CKD might be monitored more closely than those without CKD. This could result in an ascertainment bias whereby patients with CKD could be more likely to be hospitalized with an infection. However, infections often present as an acute constellation of symptoms and signs, and thus are probably less prone to ascertainment bias relative to some other chronic conditions such as depression and cognitive dysfunction. Also, we note that the associations with CKD measures were stronger for hospitalizations with infection than those without, further supporting a unique link between CKD and infection. Fourth, our study population was restricted to participants aged 53–75 years, and thus extrapolation of our results to populations outside of this age range must be done carefully. Finally, as with all observational studies, we could not exclude the possibility of residual confounding. For example, we did not have information on cause of kidney disease (e.g., glomerulonephritis).
In conclusion, among community-dwelling middle-aged and older adults in the United States, reduced eGFR and elevated ACR were independently associated with increased risk for hospitalization with infection and subsequent mortality, even at their mild to moderate stages. The associations were consistent in pneumonia, kidney and urinary tract infections, bloodstream infections, and cellulitis. Although the risk and prevention of infection are often discussed for those with severely reduced eGFR, our results suggest their relevance in less severe stages of reduced eGFR as well as among people with CKD manifested solely by elevated ACR.
Supplementary Material
N SECTION.
Because the Editor-in-Chief recused himself from consideration of this article, the Deputy Editor (Daniel E. Weiner, MD, MS) served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Information for Authors & Journal Policies.
Acknowledgments
We thank the staff and participants of the ARIC Study for their important contributions. Dr Ishigami thanks Prof Shinichi Uchida (Department of Nephrology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan) for his mentorship.
Support: The ARIC Study is performed as a collaborative study supported by National Heart, Lung and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The funders of this study did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: JI, KM; data acquisition: JI, KM; data analysis/interpretation: JI, MEG, ARC, JJC, JC, KM; statistical analysis: JI, KM; supervision or mentorship: MEG, JC, KM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. JI and KM take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by two external peer reviewers, a Statistical Editor, a Co-Editor, and an Acting Editor-in-Chief.
References
- 1.Kidney Disease Statistics for the United States. Available: http://www.niddk.nih.gov/health-information/health-statistics/Pages/kidney-disease-statistics-united-states.aspx. Accessed 3 Feb 2016.
- 2.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. Journal of the American Society of Nephrology: JASN. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daya NR, Voskertchian A, Schneider AL, et al. Kidney Function and Fracture Risk: The Atherosclerosis Risk in Communities (ARIC) Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2016;67:218–226. doi: 10.1053/j.ajkd.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman SJ, Johnson EW, Nakatsu C, Alkan M, Chen R, LeDuc J. Burden of infection in patients with end-stage renal disease requiring long-term dialysis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;39:1747–1753. doi: 10.1086/424516. [DOI] [PubMed] [Google Scholar]
- 6.Allon M, Radeva M, Bailey J, et al. The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association -European Renal Association. 2005;20:1180–1186. doi: 10.1093/ndt/gfh729. [DOI] [PubMed] [Google Scholar]
- 7.Slinin Y, Foley RN, Collins AJ. Clinical epidemiology of pneumonia in hemodialysis patients: the USRDS waves 1, 3, and 4 study. Kidney international. 2006;70:1135–1141. doi: 10.1038/sj.ki.5001714. [DOI] [PubMed] [Google Scholar]
- 8.James MT, Laupland KB, Tonelli M, et al. Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Archives of internal medicine. 2008;168:2333–2339. doi: 10.1001/archinte.168.21.2333. [DOI] [PubMed] [Google Scholar]
- 9.James MT, Quan H, Tonelli M, et al. CKD and risk of hospitalization and death with pneumonia. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2009;54:24–32. doi: 10.1053/j.ajkd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Dalrymple LS, Katz R, Kestenbaum B, et al. The risk of infection-related hospitalization with decreased kidney function. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;59:356–363. doi: 10.1053/j.ajkd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton EJ, Martin N, Makepeace A, Sillars BA, Davis WA, Davis TM. Incidence and predictors of hospitalization for bacterial infection in community-based patients with type 2 diabetes: the fremantle diabetes study. PloS one. 2013;8:e60502. doi: 10.1371/journal.pone.0060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald HI, Thomas SL, Millett ER, Nitsch D. CKD and the risk of acute, community-acquired infections among older people with diabetes mellitus: a retrospective cohort study using electronic health records. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;66:60–68. doi: 10.1053/j.ajkd.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HE, Gamboa C, Warnock DG, Muntner P. Chronic kidney disease and risk of death from infection. American journal of nephrology. 2011;34:330–336. doi: 10.1159/000330673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 annual data report. Am J Kidney Dis. 2014;63(1 suppl 1):e1–e420. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Christensen KL, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB. Infectious disease hospitalizations in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;49:1025–1035. doi: 10.1086/605562. [DOI] [PubMed] [Google Scholar]
- 20.30-day risk-standardized readmission measures in the Centers for Medicare & Medicaid Services (CMS) and Hospital Quality Alliance (HQA) Available: https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/hospitalqualityinits/outcomemeasures.html. Accessed 14 Feb 2016.
- 21.Metersky ML, Tate JP, Fine MJ, Petrillo MK, Meehan TP. Temporal trends in outcomes of older patients with pneumonia. Archives of internal medicine. 2000;160:3385–3391. doi: 10.1001/archinte.160.22.3385. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter, Suppl. 2013;3:1–150. [Google Scholar]
- 23.Crowe E, Halpin D, Stevens P, Guideline Development G Early identification and management of chronic kidney disease: summary of NICE guidance. BMJ. 2008;337:a1530. doi: 10.1136/bmj.a1530. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Seminars in dialysis. 2007;20:440–451. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 26.Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Seminars in dialysis. 2009;22:636–643. doi: 10.1111/j.1525-139X.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 27.Salmon AH, Ferguson JK, Burford JL, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. Journal of the American Society of Nephrology: JASN. 2012;23:1339–1350. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nature medicine. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2001;50:1–43. [PubMed] [Google Scholar]
- 30.Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports/Centers for Disease Control. 1997;46:1–24. [PubMed] [Google Scholar]
- 31.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 32.Ford DW, Goodwin AJ, Simpson AN, Johnson E, Nadig N, Simpson KN. A Severe Sepsis Mortality Prediction Model and Score for Use With Administrative Data. Critical care medicine. 2016;44:319–327. doi: 10.1097/CCM.0000000000001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain S, Self WH, Wunderink RG, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. The New England journal of medicine. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


