Abstract
Macrophage migration inhibitory factor (MIF) is a key cytokine/chemokine in the activation and recruitment of inflammatory T lymphocytes known to exacerbate experimental stroke severity. MIF effects are mediated through its primary cellular receptor, CD74, the MHC class II invariant chain present on all class II expressing cells, including monocytes, macrophages and dendritic cells (DC). We demonstrated previously that partial MHC class II/peptide constructs (pMHC) can effectively treat mice with experimental stroke, in part through their ability to competitively inhibit MIF/CD74 interactions and downstream signaling. However, the role of MIF and CD74 in human ischemic stroke is not yet well established. To evaluate the therapeutic potential for pMHC, we assessed MIF and CD74 expression levels and their association with disease outcome in subjects with ischemic stroke. MIF levels were assessed in blood plasma by ELISA and CD74 expression was quantified by flow cytometry and qRT-PCR in peripheral blood mononuclear cells (PBMCs) obtained from subjects with ischemic stroke and age and sex-matched healthy controls (HC). MIF levels were increased in plasma and the number of CD74+ cells and CD74 mRNA expression levels were significantly increased in PBMC of subjects with ischemic stroke versus HC, mainly on CD4+ T cells, monocytes and DC. Greater increases of CD74+ cells were seen in subjects with cortical vs. subcortical infarcts and the number of CD74+ cells in blood correlated strongly with infarct size and neurological outcomes. However, differences in MIF and CD74 expression were not affected by age, gender or lesion laterality. Increased CD74 expression levels may serve as a useful biomarker for worse stroke severity and predicted outcomes in subjects with ischemic stroke and provide a rationale for potential future treatment with pMHC constructs.
Keywords: Stroke, Inflammation, Macrophage Migration Inhibitory Factor, CD74, Partial MHC class II constructs
1. Introduction
Ischemic stroke afflicts nearly 800,000 Americans each year, with lethal or disabling consequence. Due to the failure of a number of stroke trials, there is a pressing unmet need for effective stroke therapies, with only one available FDA approved drug, tissue plasminogen activator (tPA), which has limited efficacy. Assessment of new therapeutic approaches may include rationally-based target molecules that are integral to the pathogenic process. This process has been enhanced by development of experimental stroke models and the use of STAIR criteria (Albers et al., 2000).
The pathogenesis of ischemic stroke injury in brain involves inflammation (Campanella et al., 2002; Chamorro et al., 2012; Elkind et al., 2004; Offner et al., 2006; Pennypacker and Offner, 2015; Urra et al., 2009), including early activation of the peripheral immune system and rapid migration of inflammatory cells, including activated T cells, into the infarcted area (Akopov et al., 1996; Gelderblom et al., 2009; Jin et al., 2010; Offner et al., 2006; Petrovic-Djergovic et al., 2016; Urra et al., 2009; Zhou et al., 2013). These T cells perpetuate inflammation and increase neuronal damage (Gronberg et al., 2013; Shichita et al., 2009). In experimental stroke models, the lesion volume is reduced by ~50% in mice genetically deficient in T cells (Hurn et al., 2007; Yilmaz et al., 2006), thus implicating these cells in the pathogenic mechanism and as potential targets of therapy. Our laboratory invented a novel therapeutic approach using partial MHC class II molecules (pMHC) covalently linked to antigenic peptides. One pMHC, called RTL1000, inhibits autoreactive T cell damage by promoting secretion of anti-inflammatory and neuroprotective factors and reducing the ability of inflammatory cells to enter the damaged area of the brain. RTL1000 can reduce clinical deficits and infarct size by ~50% in mice with experimental stroke (Akiyoshi et al., 2011; Subramanian et al., 2009; Zhu et al., 2015a) and is highly effective for treatment of young as well as older mice (Dotson et al., 2014) of both sexes (Pan et al., 2014; Zhu et al., 2015b). This biological agent can be administered up to 6 h after stroke and is compatible with concomitant tPA administration (Zhu et al., 2014). These findings meet essentially all of the STAIR recommendations for clinical testing of new drugs in stroke (Fisher et al., 2009).
RTL1000, comprised of an HLA-DR2 moiety linked to the human MOG-35-55 peptide, can reverse paralysis and demyelination in mice with experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (MS) (Burrows et al., 1999; Offner et al., 2011; Sinha et al., 2007, 2010; Vandenbark et al., 2003; Wang et al., 2006). It was shown to be safe and well tolerated in a Phase 1 trial in MS (Yadav et al., 2012) and will soon be tested in a Phase 1b trial in progressive MS. One key inhibitory activity of the pMHC involves binding to the invariant chain of MHC class II (CD74) and downregulation of its expression on the monocyte cell surface (Vandenbark et al., 2013). This binding to CD74 not only modulates cell surface expression, but also blocks the binding of macrophage migration inhibitory factor (MIF), for which CD74 serves as the major receptor (Benedek et al., 2013).
CD74 is a type II transmembrane protein with a dormant transcription factor in the intracytoplasmic domain, a transmembrane region and an extracellular domain thought to be the receptor for MIF binding (Leng et al., 2003). CD74, in combination with CD44 and CXCR2/4/7, has been reported to transduce signaling by MIF, one of the first described cytokine mediators (Leng et al., 2003; Naujokas et al., 1993; Shi et al., 2006). We demonstrated that CD74 is upregulated on activated CD11b+ macrophages/microglial cells in spinal cord during the acute phase of EAE in mice (Benedek et al., 2013) and in CD11b+CD45int and CD11b+CD45hi cells in the ischemic hemisphere in mice after middle cerebral artery occlusion (MCAO) (unpublished).
The role of MIF in ischemic stroke remains controversial. MIF expression was reported to be elevated in the periphery and around the infarct core in the brain after induction of experimental stroke in rodents (Inacio et al., 2011; Wang et al., 2009). In addition, MIF plasma levels were found to be elevated in patients within a few days after stroke and these levels were correlated with neurological deficits (Wang et al., 2009). Although the upregulation of MIF has been shown in human ischemic stroke, the cellular targets of MIF, including CD74, and their involvement in human brain injury have not been investigated.
In this study, we thus sought to determine in human ischemic stroke the levels of MIF in plasma and the expression of CD74 on peripheral blood mononuclear cells (PBMCs).
2. Methods
2.1. Study population
From May 2016 to August 2016, 33 consecutive subjects with ischemic stroke were recruited into this study and hospitalized in the Department of Neurology of Tianjin Medical University General Hospital. The diagnosis of ischemic stroke was based on clinical symptoms, neurological examination and results of computed tomography (CT) or magnetic resonance imaging (MRI) of the brain as defined according to the World Health Organization criteria. Of these 33 stroke subjects, 13 were excluded because of their history of autoimmune diseases (autoimmune thyroiditis: 3, lupus: 2, asthma: 2, rheumatoid arthritis: 1, autoimmune hepatitis: 1, Idiopathic thrombocytopenic purpura: 1, other autoimmune diseases: 3) and use of immunosuppressant drugs including steroids. The 20 included subjects had a duration of symptom onset to admission within 72 h. These subjects did not exhibit complications of infections or tumors at admission. Fourteen age-matched healthy subjects were recruited into this study as controls. The experimental protocol and supporting documentation were approved by the Tianjin Medical University General Hospital institutional review boards. All participants signed consent forms after being informed of risks and benefits of participating in the study.
2.2. Clinical assessments
Clinical assessments were performed upon subjects admission (<72 h after onset) and at day 14 after onset. The extent of neurologic deficits was evaluated by the National Institutes of Health Stroke Scale (NIHSS) that assesses the levels of consciousness, language, neglect, visual-field loss, extraocular movement, motor strength, ataxia, dysarthria, and sensory loss. Clinical assessments were performed in an evaluator-blinded fashion as previously described (Fu et al., 2014a,b; Zhu et al., 2015c). A trained investigator rated the subjects’ ability to answer questions and perform activities. Ratings for each item were scored with 3–5 grades with 0 as normal, with an allowance for untestable items. Results were reported as a sum of each scored item.
2.3. FACS assessments
Peripheral blood samples were collected from healthy control subjects and ischemic stroke subjects at the time of admission (<72 h after onset). Blood samples were stored in ice-cold tubes containing EDTA prior to flow cytometry analysis. Mononuclear cells were isolated from the whole-blood specimens and stained with fluorescent-labeled antibodies, including CD3-PEcy7, CD4-APC, CD8-FITC, CD19-APC, CD56-PerCP, CD11b-PerCP, CD11c-FITC, CD14-APC, CD16-FITC, and CD74-PE (Becton Dickinson, San Jose, CA). Data were acquired using FACS Caliber (Becton Dickinson, San Jose, CA) and analyzed with Flow Jo 7.6 software.
2.4. Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from human PBMCs using Trizol reagent (Invitrogen, USA). Reverse transcription reactions were performed using PrimeScript™ RT kit (Takara Biotech, China) according to manufacturer’s instructions. All qPCR reactions were performed in duplicate and SYBR Green was used to monitor the amplification in real time. Real-time analysis was performed on a CFX Connect™ Real-Time System (Bio-Rad, USA) using the following conditions: initial denaturation for 10 min at 95 °C, 39 cycles of 15 s denaturation at 95 °C, 1 min for annealing and extension at 60 °C. The mean value was calculated by plotting Ct, and then used for further calculation. Analysis was performed according to the ΔΔCt method using GAPDH as the housekeeping gene. The primer sequences were: 5′-GACGAGAACGGCAACTATCTG-3′ (forward) and 5′-GTTGGGGAAGACACACCAGC-3′ (reverse) for CD74; and 5′-GCAGAACCGCTCCTACAG-3′ (forward) and 5′-CTTAGGCGAAGGTGGAGTTG-3′ (reverse) for MIF.
2.5. Enzyme-linked immunosorbant assay
Plasma levels of MIF were measured using a human MIF ELISA kit (Anoric Biotechnology, China) according to the manufacturer’s instructions. Reactions were analyzed at a wavelength of 450 nm using a 96-well microplate reader (Model 680; Bio-Rad Laboratories, Hercules, CA).
2.6. Neuroimaging
MRI scans were performed within 72 h after stroke onset at admission using a 3.0-T superconducting MRI system (GE, USA), including diffusion-weighted imaging (DWI). Lesion volume was manually outlined on the DWI and measured using Image J 1.38 (National Institutes of Health, Bethesda, MD, USA).
2.7. Statistical analysis
The sample size was determined by power analysis using a significance level of α = 0.05 with 80% power to detect statistical differences. Power analysis and sample size calculations were performed using SAS 9.1 software (SAS Institute Inc. Cary, NC, USA). All data are shown as mean ± s.e.m. Statistical differences among groups were evaluated by two-tailed unpaired Student’s t-test for two groups. Linear regression analyses were performed using GraphPad Prism 5 software. A P value ≤ 0.05 was considered statistically significant.
3. Results
3.1. Upregulation of CD74 in PBMCs after ischemic stroke
Blood samples were collected from 20 subjects with ischemic stroke (<72 h after onset at admission) and 14 healthy individuals (Table 1). The number of CD74-expressing PBMCs was significantly higher in subjects with ischemic stroke versus HC individuals (Fig. 1A–B). CD74 mRNA levels were also significantly increased in patients with ischemic stroke versus HC (Fig. 1C). CD74-expressing cells were assessed In PBMC subsets, including FACS gated CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), B cells (CD3− CD19+), NK cells (CD3− CD56+), CD11b+ cells, CD11c+ cells and monocytes (CD14+CD16−). A significant increase in the number of CD74-expressing cells was found in CD4+ T cells, CD11b+ cells, CD11c+ cells and monocytes in subjects with ischemic stroke versus HC (Fig. 2A and B). Despite no statistical significance, a trend towards increased expression was noted in CD8+ T cells, B cells and NK cells (Fig. 2A and B).
Table 1.
Patient characteristics.
| Control (n = 14) |
Ischemic stroke (n = 20) |
P value | |
|---|---|---|---|
| Age (year, mean ± s.e.m.) | 63.71 ± 0.95 | 66.60 ± 1.44 | P = 0.14 |
| Gender (male vs. female) | 8 vs. 6 | 13 vs. 7 | P = 0.66 |
| NIHSS Score (mean ± s.e.m.) | N/A | 7.15 ± 0.99 | N/A |
| Infarct volume (ml, mean ± s.e.m.) | N/A | 6.03 ± 2.03 | N/A |
| Infarct side (left vs. right) | N/A | 12 vs. 8 | N/A |
| Infarct location (subcortex vs. cortex) | N/A | 11 vs. 9 | N/A |
Fig. 1. Upregulation of CD74 in PBMC of subjects with ischemic stroke.
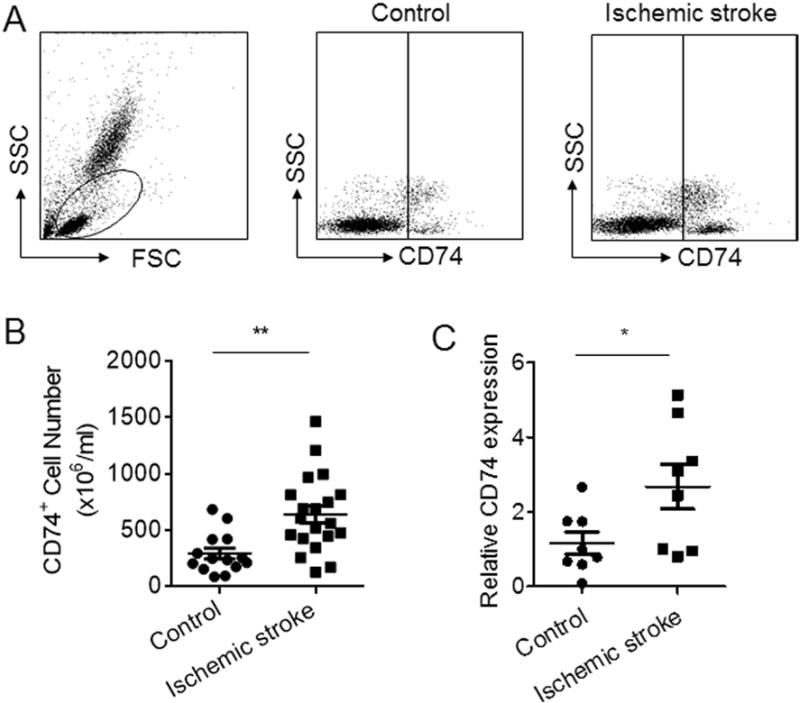
PBMCs were obtained from subjects with ischemic stroke (<72 h after onset) and HC individuals. A. Representative flow cytometry dot plots show gating strategy to assess CD74 expression in PBMC from a subject with ischemic stroke versus a healthy control. B. Absolute numbers of CD74-expressing cells in PBMC of subjects with ischemic stroke versus HC (n = 20 in ischemic stroke group; n = 14 in HC group). C. Relative expression of CD74 mRNA in PBMC of subjects with ischemic stroke versus HC (n = 8 in each group). Data in each group were normalized to internal GAPDH mRNA levels. Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01.
Fig. 2. Expression profile of CD74 in PBMC subsets after brain ischemia.
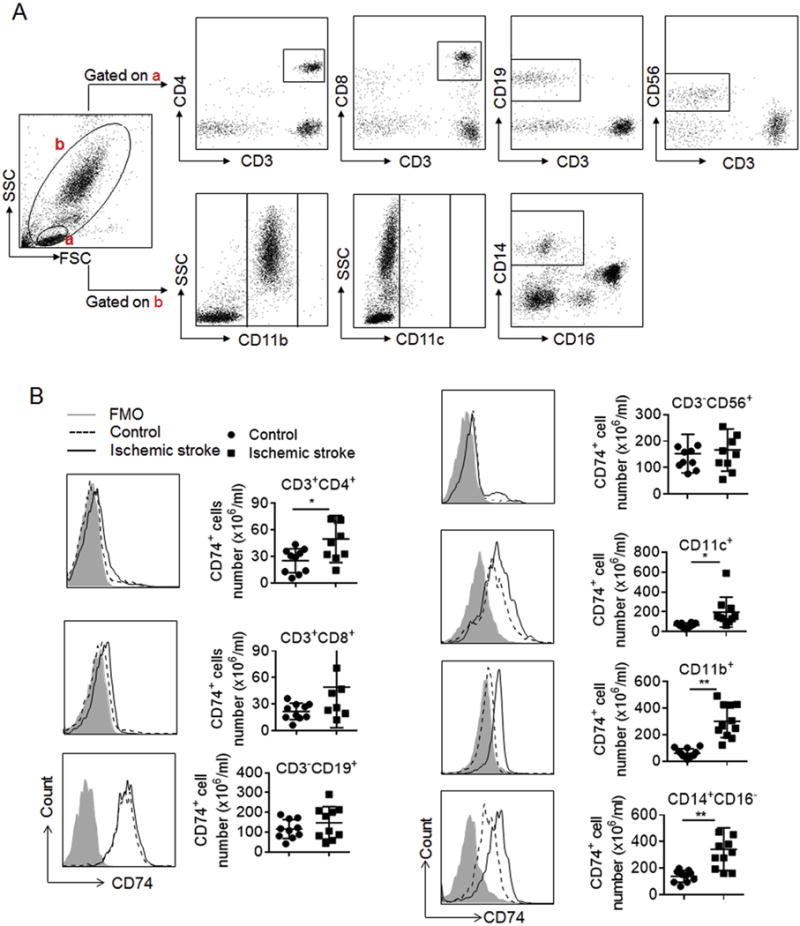
PBMC were obtained from subjects with ischemic stroke (<72 h after onset) and HC. The numbers of CD74-expressing cells were measured by flow cytometry. A. Representative flow cytometry plots show gating strategy of CD74-expressing cell subsets in PBMC, including CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), B cells (CD3−CD19+), NK cells (CD3−CD56+), CD11b+ cells, CD11c+ cells and monocytes (CD14+CD16−). B. Flow cytometry histogram and summarized results show significant increase of CD74 expression in CD4+ T, CD11b+ & CD11c+ cells and monocytes. CD4+ T cells (HC: n = 10; Stroke: n = 9); CD8+ T cells (HC: n = 10; Stroke: n = 8); B cells (HC: n = 10; Stroke: n = 10); NK cells (HC: n = 10; Stroke: n = 10); CD11c+ cells (HC: n = 10; Stroke: n = 10); CD11b+ cells (HC: n = 12; Stroke: n = 11); and monocytes (HC: n = 10; Stroke: n = 11). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01.
3.2. Influence of infarct location and laterality, and subject age and gender on CD74 expression in subjects with ischemic stroke
We further determined whether infarct location, laterality, subject age at stroke onset or subject gender influenced CD74 expression in PBMC after ischemic stroke. Significantly higher numbers of CD74-expressing cells in PBMC were seen in subjects with cortical versus subcortical infarcts (Fig. 3A). In contrast, similar numbers of CD74-expressing cells in PBMC were seen in subjects with the infarct located in the left or right hemispheres (Fig. 3B), suggesting that laterality of infarct may not effect CD74 expression in PBMC. Correlation analyses showed no significant relationship between age and the number of CD74-expressing cells in PBMC of subjects with ischemic stroke (Fig. 3C). Moreover, the numbers of CD74-expressing cells were similar in PBMC of male versus female subjects with ischemic stroke (Fig. 3D).
Fig. 3. Effect of infarct location, laterality, age and sex on CD74 expression.
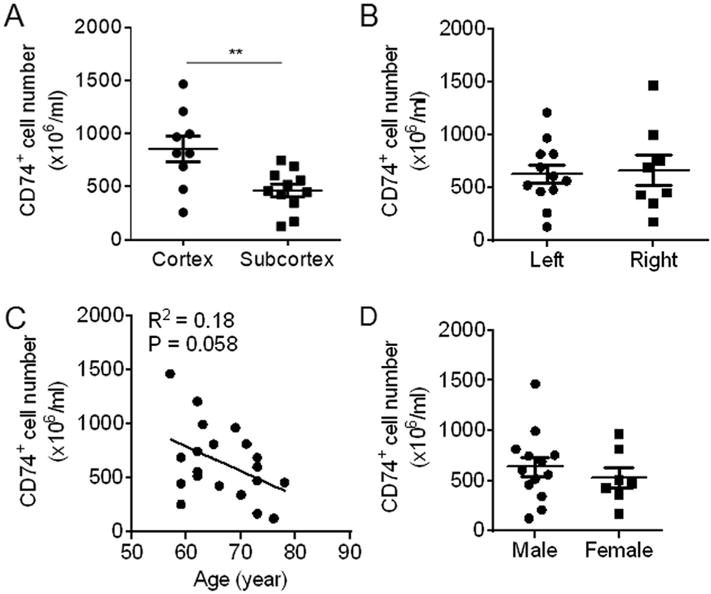
PBMC were obtained from subjects with ischemic stroke (<72 h after onset). The numbers of CD74-expressing cells were measured by flow cytometry. A. Number of CD74-expressing PBMC in ischemic stroke subjects with infarcts in cortex versus subcortex brain regions. (Cortex: n = 9; Subcortex: n = 11). B. Numbers of CD74-expressing PBMC in ischemic stroke subjects with infarct in the left versus the right hemisphere (Left: n = 12; Right: n = 8). C. Correlation between the numbers of CD74-expressing PBMC with the age of ischemic stroke subjects (n = 20). D. Number of CD74-expressing PBMC in male versus female subjects with ischemic stroke (Male: n = 13; Female: n = 7). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01.
3.3. CD74 expression correlated with stroke severity
To understand the potential involvement of CD74 during the progression of ischemic stroke, we evaluated the correlation between the number of CD74-expressing cells in PBMC and stroke severity. This analysis demonstrated a significant correlation between the number of CD74-expressing cells and infarct size measured within 72 h after onset (Fig. 4A). Moreover, there was a highly significant correlation between the number of CD74-expressing cells observed and neurological outcome at admission (<72 h) and on day 14 after stroke onset (Fig. 4B and C).
Fig. 4. Numbers of CD74-expressing cells in PBMC are associated with stroke severity.
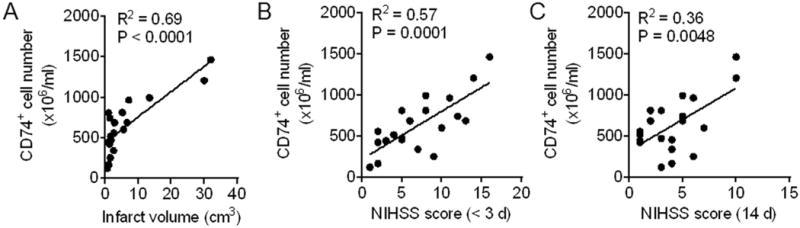
PBMC were obtained from subjects with ischemic stroke (<72 h after onset). Numbers of CD74-expressing cells were measured by flow cytometry. A. Numbers of CD74+ cells in PBMC correlated with infarct volumes measured at admission (<72 h after onset) in subjects with ischemic stroke (n = 20). B. Numbers of CD74+ cells in PBMC correlated with NIHSS Neurodeficit scores at admission (<72 h after onset) (n = 20). C. Numbers of CD74+ cells in PBMCs correlated with NIHSS Neurodeficit scores during the later stage of ischemic stroke (day 14 after stroke onset) (n = 20).
3.4. Upregulation of MIF after ischemic stroke
Because MIF is a ligand of CD74, we also measured the plasma levels of MIF and found a significant increase in MIF in subjects with ischemic stroke at admission (<72 h after onset) compared to HC (Fig. 5A). Moreover, MIF mRNA expression was also significantly higher in stroke subjects (Fig. 5B). Correlation analysis showed no significant relationship between plasma MIF levels and infarct volume at admission or neurological outcomes at admission (<72 h) or at day 14 after stroke onset (Fig. 5C–E).
Fig. 5. Upregulation of MIF expression in PBMC does not correlate with stroke severity.
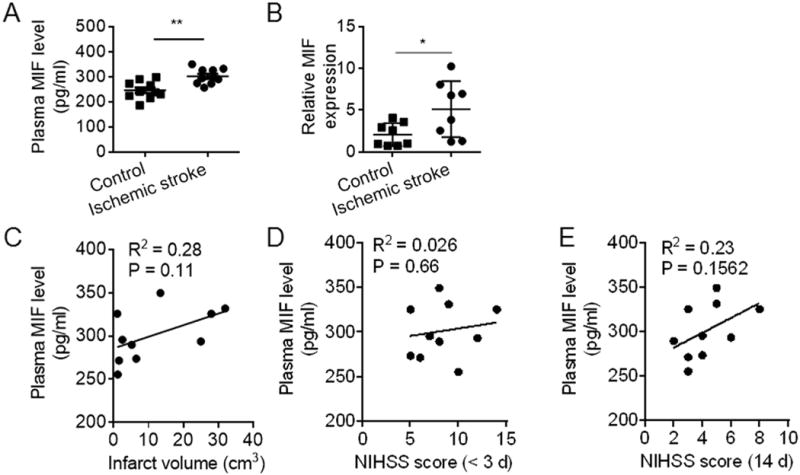
Blood samples were obtained from subjects with ischemic stroke (<72 h after onset) and HC individuals. Plasma levels of MIF were measured by ELISA and mRNA expression of MIF in PBMC was determined by qRT-PCR. A. Plasma levels of MIF in subjects with ischemic stroke versus HC controls (n = 10 per group). B. Relative expression of MIF mRNA in PBMC of subjects with ischemic stroke versus HC (n = 8 in each group). Data are presented as mean ± s.e.m. *P < 0.05, **P < 0.01. C. Correlation between plasma MIF levels versus infarct volumes; D. MIF levels versus NIHSS Neurodeficit scores at admission (<72 h after onset); and E. MIF levels versus NIHSS Neurodeficit scores at day 14 after onset in subjects with ischemic stroke (n = 10).
4. Discussion
The results of this study clearly demonstrate significant activation of the MIF/CD74 axis in blood obtained from acute stroke subjects evaluated upon admission to the hospital within 72 h of infarction. These changes included significant increases in expression of both MIF in blood plasma and of CD74, the primary cellular receptor for MIF, present predominantly on CD11b+CD14+CD16− monocytes and to a lesser extent on CD4+ T cells and DC. Increases in expression of CD74 but not MIF showed highly significant correlations with infarct size and with increasing NIHSS disability scores both in the early (<72 h) and later (14 days) stages of stroke, thus indicating the potential importance of assessing CD74 levels as a biomarker for stroke severity and for predicting short term outcomes. Moreover, the highest CD74 expression levels were observed in subjects with cortical lesions, with lesser changes in those with subcortical infarcts. No differences in MIF or CD74 levels were observed as a function of age, sex or affected hemisphere, although a downward trend was noted with increasing age.
MIF is the first described cytokine/chemokine and the MIF/CD74 axis has been implicated as a potentially crucial contributor to a growing number of inflammatory diseases and conditions, including systemic inflammatory disease, atherosclerosis, tuberculosis, multiple sclerosis (MS) and traumatic brain injury (TBI), among others (Bucala, 2013; O’Reilly et al., 2016). The MIF activation pathway has been well described, with an initial high affinity binding to the CD74/CD44 receptor complex that results in sustained activation of ERK1/2 MAPK (Leng et al., 2003; Shi et al., 2006) and ultimately gene transcription and synthesis of proinflammatory factors, increased cell survival and proliferation and trafficking to the sites of acute and chronic inflammation (Gore et al., 2008; Mitchell et al., 2002). The potential involvement of MIF and CD74 in stroke is consistent with the recent demonstration that adaptive immunity, including T cell responses to brain antigens such as myelin oligodendrocyte glycoprotein (MOG)-35-55 peptide (Ren et al., 2012), contributes to increased infarct volumes and worsened stroke outcomes. Indeed, this notion is supported by our findings of increased CD74-expressing cells and mRNA expression in stroke subjects versus healthy individuals. The extent of increase correlated with cortical versus subcortical infarcts, infarct size and neurological deficits.
Involvement of immune inflammation in experimental stroke has facilitated the evaluation of new therapeutic approaches such as pMHC constructs that can competitively block signaling through the MIF/CD74 axis. The RTL1000 pMHC class II construct containing the DR2 α1β1 domains was initially developed as a therapy for multiple sclerosis (MS), in which HLA-DR2 is the most prevalent allele. However, treatment of ischemic stroke with RTL1000 would currently require HLA-DR2 screening and would be limited to those subjects who are DR2 positive. We recently reported that the HLA-DRα1 domain, like RTL1000, binds to and downregulates cell surface expression CD74 and competitively inhibits binding and downstream signaling of MIF, with profound anti-inflammatory and neuroprotective effects on the CNS (Benedek et al., 2015; Meza-Romero et al., 2014). Moreover, because the DRα1 domain is present in all humans and thus would not be recognized as foreign, treatment using DRα1 constructs would not require HLA screening of potential recipients including those with ischemic stroke.
We demonstrated that DRα1-MOG-35-55 reduces infarct size and reverses splenic atrophy in experimental stroke when administered at the clinically relevant time-point of 4 h after infarction (Benedek et al., 2014, 2016). The neuroprotective effects of DRα1-MOG-35-55 were mediated in part by reduced expression of CD74 and inhibition of migration of CD11b+ monocytes into the ischemic brain. The results of our current study highlighting enhanced CD74 expression in human stroke subjects provide a new incentive for the use of DRα1-MOG-35-55 as therapy for human stroke due to its ability to rapidly downregulate CD74 expression, competitively block MIF/CD74 binding and selectively inhibit inflammation induced by MOG- and other myelin-specific T cells.
Biomarkers are currently used in stroke patients for risk assessment and aid in the diagnosis (Jickling and Sharp, 2015). Most commonly used or currently studied biomarkers include but are not limited to HgA1c, C-reactive protein, glial fibrillary acidic protein (GFAP), S100b, myelin basic protein (MBP), interleukin-6, matrix metalloproteinase (MMP)-9 and others (Jickling and Sharp, 2011; Saver et al., 2012). In comparison with these available stroke biomarkers, CD74 has its own advantages. First, given the heterogeneity of stroke as manifested by variability in infarct size, location, and cause, a biomarker like CD74 may reflect at least some of these alterations. Second, the blood brain barrier is thought to restrict the release of brain-injury markers to the circulation such as MBP, GFAP and S100b. Different than this, CD74 expression in the circulating PBMCs can be easily measured. Third, in terms of clinical use, a biomarker for stroke should be rapidly measured across a diverse range of clinical settings with reproducibility and accuracy. Measurement of circulating CD74 certainly meets this criterion. Although as a single biomarker, CD74 may not be sufficient to reflect the entire complexity and heterogeneity in stroke patients, but it may at least serve as a marker to better reflect stroke onset and severity.
5. Conclusion
Increased CD74 expression levels may serve as a useful biomarker for worse stroke severity and strengthen the rationale for treatment of subjects with ischemic stroke with pMHC constructs. These initial findings in a relatively small cohort of stroke patients warrant further analyses in larger patient cohorts.
Acknowledgments
This work was supported by the National Institutes of Health grants R01NS075887 and R01NS076013 (to HO) and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (AAV and HO). The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The authors wish to thank Gail Kent for assistance with manuscript submission.
Footnotes
Authors’ contributions
H. O. and J. H. formulated the concept and designed the study. Y. L. and H. R. acquired the data. Y. K. collected the patient samples. Y. L., M. L., E. S and W.-N. J. analyzed the data. Y. L., M. L., J. H. and H. O. interpreted the results and drafted the manuscript. A.A.V. reviewed and edited the manuscript.
Conflict of interest
No conflict of interest to report.
References
- Akiyoshi K, Dziennis S, Palmateer J, Ren X, Vandenbark AA, Offner H, Herson PS, Hurn PD. Recombinant T cell receptor ligands improve outcome after experimental cerebral ischemia. Transl Stroke Res. 2011;2:404–410. doi: 10.1007/s12975-011-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283:1145–1150. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- Benedek G, Meza-Romero R, Andrew S, Leng L, Burrows GG, Bourdette D, Offner H, Bucala R, Vandenbark AA. Partial MHC class II constructs inhibit MIF/CD74 binding and downstream effects. Eur J Immunol. 2013;43:1309–1321. doi: 10.1002/eji.201243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Meza-Romero R, Jordan K, Keenlyside L, Offner H, Vandenbark AA. HLA-DRalpha1-mMOG-35-55 treatment of experimental autoimmune encephalomyelitis reduces CNS inflammation, enhances M2 macrophage frequency, and promotes neuroprotection. J Neuroinflamm. 2015;12:123. doi: 10.1186/s12974-015-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Vandenbark AA, Alkayed NJ, Offner H. Partial MHC class II constructs as novel immunomodulatory therapy for stroke. Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Zhu W, Libal N, Casper A, Yu X, Meza-Romero R, Vandenbark AA, Alkayed NJ, Offner H. A novel HLA-DRalpha1-MOG-35-55 construct treats experimental stroke. Metab Brain Dis. 2014;29:37–45. doi: 10.1007/s11011-013-9440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R. MIF, MIF alleles, and prospects for therapeutic intervention in autoimmunity. J Clin Immunol. 2013;33(Suppl 1):S72–S78. doi: 10.1007/s10875-012-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- Dotson AL, Zhu W, Libal N, Alkayed NJ, Offner H. Different immunological mechanisms govern protection from experimental stroke in young and older mice with recombinant TCR ligand therapy. Front Cell Neurosci. 2014;8:284. doi: 10.3389/fncel.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind MS, Cheng J, Rundek T, Boden-Albala B, Sacco RL. Leukocyte count predicts outcome after ischemic stroke: the Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis. 2004;13:220–227. doi: 10.1016/j.jstrokecerebrovasdis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, Group, S Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, Yan Y, Huang D, Yu C, Shi FD. Fingolimod for the treatment of intracerebral hemorrhage: a 2-Arm proof-of-concept study. JAMA Neurol. 2014a;71:1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li Y, Han W, Xue R, Liu Q, Hao J, Yu C, Shi FD. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A. 2014b;111(51):18315–18320. doi: 10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Gore Y, Starlets D, Maharshak N, Becker-Herman S, Kaneyuki U, Leng L, Bucala R, Shachar I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- Gronberg NV, Johansen FF, Kristiansen U, Hasseldam H. Leukocyte infiltration in experimental stroke. J Neuroinflamm. 2013;10:115. doi: 10.1186/1742-2094-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio AR, Ruscher K, Wieloch T. Enriched environment downregulates macrophage migration inhibitory factor and increases parvalbumin in the brain following experimental stroke. Neurobiol Dis. 2011;41:270–278. doi: 10.1016/j.nbd.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurotherapeutics. 2011;8:349–360. doi: 10.1007/s13311-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke. 2015;46:915–920. doi: 10.1161/STROKEAHA.114.005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-Romero R, Benedek G, Yu X, Mooney JL, Dahan R, Duvshani N, Bucala R, Offner H, Reiter Y, Burrows GG, Vandenbark AA. HLA-DRalpha1 constructs block CD74 expression and MIF effects in experimental autoimmune encephalomyelitis. J Immunol. 2014;192:4164–4173. doi: 10.4049/jimmunol.1303118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A. 2002;99:345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Morin M, Anderson MS, Peterson M, Miller J. The chondroitin sulfate form of invariant chain can enhance stimulation of T cell responses through interaction with CD44. Cell. 1993;74:257–268. doi: 10.1016/0092-8674(93)90417-o. [DOI] [PubMed] [Google Scholar]
- O’Reilly C, Doroudian M, Mawhinney L, Donnelly SC. Targeting MIF in cancer: therapeutic strategies, current developments, and future opportunities. Med Res Rev. 2016;36:440–460. doi: 10.1002/med.21385. [DOI] [PubMed] [Google Scholar]
- Offner H, Sinha S, Burrows GG, Ferro AJ, Vandenbark AA. RTL therapy for multiple sclerosis: a Phase I clinical study. J Neuroimmunol. 2011;231:7–14. doi: 10.1016/j.jneuroim.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Pan J, Palmateer J, Schallert T, Hart M, Pandya A, Vandenbark AA, Offner H, Hurn PD. Novel humanized recombinant T cell receptor ligands protect the female brain after experimental stroke. Transl Stroke Res. 2014;5:577–585. doi: 10.1007/s12975-014-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennypacker KR, Offner H. The role of the spleen in ischemic stroke. J Cereb Blood Flow Metab. 2015;35:186–187. doi: 10.1038/jcbfm.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. 2016;119:142–158. doi: 10.1161/CIRCRESAHA.116.308022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Akiyoshi K, Grafe MR, Vandenbark AA, Hurn PD, Herson PS, Offner H. Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metab Brain Dis. 2012;27:7–15. doi: 10.1007/s11011-011-9267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL, Warach S, Janis S, Odenkirchen J, Becker K, Benavente O, Broderick J, Dromerick AW, Duncan P, Elkind MS, Johnston K, Kidwell CS, Meschia JF, Schwamm L, National Institute of Neurological, D., Stroke Stroke Common Data Element Working, G. Standardizing the structure of stroke clinical and epidemiologic research data: the National Institute of Neurological Disorders and Stroke (NINDS) Stroke Common Data Element (CDE) project. Stroke. 2012;43:967–973. doi: 10.1161/STROKEAHA.111.634352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Sinha S, Miller L, Subramanian S, McCarty OJ, Proctor T, Meza-Romero R, Huan J, Burrows GG, Vandenbark AA, Offner H. Binding of recombinant T cell receptor ligands (RTL) to antigen presenting cells prevents upregulation of CD11b and inhibits T cell activation and transfer of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;225:52–61. doi: 10.1016/j.jneuroim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Subramanian S, Proctor TM, Kaler LJ, Grafe M, Dahan R, Huan J, Vandenbark AA, Burrows GG, Offner H. A promising therapeutic approach for multiple sclerosis: recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J Neurosci. 2007;27:12531–12539. doi: 10.1523/JNEUROSCI.3599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Zhang B, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, Hurn PD, Offner H. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40:2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–1268. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- Vandenbark AA, Meza-Romero R, Benedek G, Andrew S, Huan J, Chou YK, Buenafe AC, Dahan R, Reiter Y, Mooney JL, Offner H, Burrows GG. A novel regulatory pathway for autoimmune disease: binding of partial MHC class II constructs to monocytes reduces CD74 expression and induces both specific and bystander T-cell tolerance. J Autoimmun. 2013;40:96–110. doi: 10.1016/j.jaut.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J Immunol. 2003;171:127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- Wang C, Gold BG, Kaler LJ, Yu X, Afentoulis ME, Burrows GG, Vandenbark AA, Bourdette DN, Offner H. Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem. 2006;98:1817–1827. doi: 10.1111/j.1471-4159.2006.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zis O, Ma G, Shan Z, Zhang X, Wang S, Dai C, Zhao J, Lin Q, Lin S, Song W. Upregulation of macrophage migration inhibitory factor gene expression in stroke. Stroke. 2009;40:973–976. doi: 10.1161/STROKEAHA.108.530535. [DOI] [PubMed] [Google Scholar]
- Yadav V, Bourdette DN, Bowen JD, Lynch SG, Mattson D, Preiningerova J, Bever CT, Jr, Simon J, Goldstein A, Burrows GG, Offner H, Ferro AJ, Vandenbark AA. Recombinant T-cell receptor ligand (RTL) for treatment of multiple sclerosis: a double-blind, placebo-controlled, phase 1, dose-escalation study. Autoimmune Dis. 2012;2012:954739. doi: 10.1155/2012/954739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liesz A, Bauer H, Sommer C, Lahrmann B, Valous N, Grabe N, Veltkamp R. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. 2013;23:34–44. doi: 10.1111/j.1750-3639.2012.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Casper A, Libal NL, Murphy SJ, Bodhankar S, Offner H, Alkayed NJ. Preclinical evaluation of recombinant T cell receptor ligand RTL1000 as a therapeutic agent in ischemic stroke. Transl Stroke Res. 2015a;6:60–68. doi: 10.1007/s12975-014-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Dotson AL, Libal NL, Lapato AS, Bodhankar S, Offner H, Alkayed NJ. Recombinant T-cell receptor ligand RTL1000 limits inflammation and decreases infarct size after experimental ischemic stroke in middle-aged mice. Neuroscience. 2015b;288:112–119. doi: 10.1016/j.neuroscience.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Libal NL, Casper A, Bodhankar S, Offner H, Alkayed NJ. Recombinant T cell receptor ligand treatment improves neurological outcome in the presence of tissue plasminogen activator in experimental ischemic stroke. Transl Stroke Res. 2014;5:612–617. doi: 10.1007/s12975-014-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, Shi FD. Combination of an immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015c doi: 10.1161/CIRCULATIONAHA.115.016371. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


