Abstract
The humane euthanasia of animals in research is of paramount importance. Neonatal mice frequently respond differently to euthanasia agents when compared with adults. The AVMA's Guidelines for the Euthanasia of Animals includes intraperitoneal injection of ethanol as “acceptable with conditions,” and recent work confirmed that this method is appropriate for euthanizing adult mice, but neonatal mice have not been tested. To explore this method in neonatal mice, mouse pups (C57BL/6 and CD1, 162 total) were injected with 100% ethanol, a pentobarbital–phenytoin combination, or saline at 7, 14, 21, 28, or 35 d of age. Electrocardiograms, respiratory rates, and times to loss of righting reflex and death were recorded. Time to death (TTD) differed significantly between ethanol and pentobarbital–phenytoin at 7, 14, and 21 d and between ethanol groups at 7, 14, and 21 d compared with 35 d. The average TTD (± 1 SD) for ethanol-injected mice were: 7 d, 70.3 ± 39.8 min; 14 d, 51.7 ± 30.5 min; 21 d, 32.3 ± 20.8 min, 28 d, 14.0 ± 15.2; and 35 d, 4.9 ± 1.4. Mean TTD in pentobarbital–phenytoin-injected mice were: 7 d, 2.8 ± 0.4 min; 14 d, 2.9 ± 0.5 min; 21 d, 3.9 ± 1.2 min; 28 d, 3.9 ± 0.7 min; and 35 d, 4.4 ± 0.5. Although TTD did not differ between ethanol and pentobarbital–phenytoin at 28 d of age, the TTD in 3 of 12 mice was longer than 15 min after ethanol administration at this age. Therefore, ethanol should not be used as a method of euthanasia for mice younger than 35 d, because the criteria for humane euthanasia were met only in mice 35 d or older.
Abbreviations: GABA, γ-aminobutyric acid; LORR, loss of the righting reflex; TTD, time to death
The humane euthanasia of animals in research is of paramount importance. However, euthanizing young mammals, particularly neonatal and preweanling mice (Mus musculus), can be challenging in the research setting, because the common and easily accessible euthanasia methods, such as carbon dioxide and isoflurane overdose, require long exposure times.24,27 The AVMA Guidelines for the Euthanasia of Animals state, “Altricial neonatal and preweanling mammals are relatively resistant to euthanasia methods that rely on hypoxia as their mode of action. It is also difficult . . . to gain venous access. Therefore intraperitoneal injection of pentobarbital is the recommended method of euthanasia in preweanling . . . small mammals.”1 The aforementioned resistance to hypoxia is the result of neonatal adaptive mechanisms, including a decreased metabolism and slower progression to anaerobic metabolism, in the face of hypoxemia.12,29 As a result, methods that are both efficacious and have been sanctioned by the AVMA Guidelines for neonatal animals, such as pentobarbital overdose, decapitation, and hypothermia, require either a controlled substance or are unpleasant to execute. Therefore, other methods of euthanasia for neonatal mice that are rapid and minimally distressful and that do not require the use of a controlled substance need to be investigated.
Unlike CO2 euthanasia and pentobarbital injection, intraperitoneal administration of ethanol for euthanasia does not require specialized equipment or licensure from the Drug Enforcement Agency and is easily transportable; in addition, ethanol can be stored at room temperature and has a long shelf life.3 To be classified as an acceptable means of euthanasia, the AVMA Guidelines state that the chosen method must result in a rapid loss of consciousness, with minimal pain or distress, ultimately resulting in a rapid and irreversible death.1 Intraperitoneal administration of ethanol for the purpose of euthanasia is described in the AVMA Guidelines as “acceptable with conditions,”1 in light of previous studies.19,20 The working group of the European Commission recommends that the use of ethanol as a means of euthanasia is acceptable only when a mouse is anesthetized or sedated, due to concern for irritation to the peritoneal cavity at concentrations exceeding 10%.13 In an effort to elaborate on these recommendations, one group conducted a follow-up study comparing ethanol injection with the more widely accepted method of pentobarbital administration in adult mice and rats.3 The study reported that 70% ethanol administered intraperitoneally in mice led to loss of the righting reflex (LORR) in 45 s and cardiac arrest in 6 min. The average time to death (TTD) when using ethanol was not statistically different from that for pentobarbital. In addition, ethanol was not more distressful or painful than either pentobarbital or saline, according to objective scoring methods. Ethanol was ineffective for euthanasia in rats, and its use in neonatal and immature mice was not tested.3 The current study aimed to test the efficacy of ethanol euthanasia in young mice and, if ineffective, to define the acceptable age range for euthanasia by this method.
Because of their small size, neonatal and preweanling mice present challenges regarding oral and intravenous delivery of drugs and compounds. By default, a common method of delivery is intraperitoneal injection.10,26,33 Intraperitoneal injection in adult mice has been associated with error rates of 13% to 24%,21 12%4 and 14%,30 but no studies to date have assessed the accuracy of intraperitoneal injections in neonatal and preweanling mice. This deficiency is particularly relevant because one study3 reported a delay in the loss of consciousness when injections were not truly intraperitoneal, thus increasing the time period that mice would be susceptible to pain.
The purpose of the current study was to evaluate whether 100% ethanol administered intraperitoneally resulted in rapid, minimally distressful euthanasia in awake neonatal and preweanling mice, compared with the approved method of intraperitoneal pentobarbital. In addition, we wanted to evaluate the accuracy of intraperitoneal injection in neonatal and preweanling mice.
Materials and Methods
Mice.
Inbred (C57BL/6NCrl) and outbred (Crl:CD1[ICR]) mice (Mus musculus) were included in the study. These mice differed significantly in regard to the euthanasia of neonatal mice using CO2.24 In addition to breeding performed inhouse, pregnant dams and sires were purchased from Charles River Laboratories International (Wilmington, MA). All pups were born at the University of Pennsylvania so that the actual date of birth could be recorded; the day of litter discovery was recorded as day 1. Descriptive data of the mice used are presented in Table 1. All procedures involving mice were approved by the IACUC of the University of Pennsylvania.
Table 1.
Body weight (g) and baseline heart rate (beats per minute) of C57BL/6 and CDI mice prior to injection
| C57BL/6 |
CD1 |
|||
| Age (d) | Weight | Heart rate | Weight | Heart rate |
| 7 | 3.8 ± 0.3a | 406.7 ± 36.9a | 5.3 ± 0.1a | 491.7 ± 64.3a |
| 3.4–4.3 (n = 6) | 377–395 (n = 3) | 3.7–7.8 (n = 36) | 432–567 (n = 6) | |
| 14 | 6.3 ± 1.2a,b | 607.9 ± 134.4b | 7.4 ± 1.8b | 707.9 ± 63.8b,* |
| 4.8–7.8 (n = 11) | 410–761 (n = 8) | 5.0–11.8 (n = 39) | 513–766 (n = 17) | |
| 21 | 7.7 ± 2.5b | 718.2 ± 59.4c | 10.9 ± 0.2c,* | 808.8 ± 82.7c,* |
| 4.2–12.0 (n = 11) | 613–758 (n = 9) | 8.7–12.5 (n = 12) | 222–859 (n = 11) | |
| 28 | 11.2 ± 3.7c | 753.2 ± 33.4c | 18.4 ± 0.5d,* | 821.4 ± 30.4c,* |
| 7.6–17.5 (n = 9) | 715–768 (n = 9) | 14.3–25.0 (n = 14) | 778–840 (n = 8) | |
| 35 | 16.0 ± 2.3d | 752.9 ± 48c | 24.7 ± 3.5e,* | 766.8 ± 55.2 b,c |
| 11.0–21.0 (n = 17) | 648–824 (n = 14) | 20–35 (n = 17) | 678–830 (n = 13) | |
Data are given as mean ± 1 SD followed by range (no. evaluated). For each parameter, different lowercase letters indicate significant (P < 0.05) differences within strain, whereas an asterisk (*) indicates a significant (P < 0.05) difference between strains at the same age.
Mice were held in a conventional facility in accordance with the Guide for the Care and Use of Laboratory Animals14 and were housed in polycarbonate static isolation cages (Max 75, Alternative Design, Siloam Springs, AR) containing corncob bedding (0.12-in. Bed-O-Cobs, The Andersons, Maumee, OH). Mice had unlimited access to standard rodent chow (LabDiet 5001, St. Louis, MO) and water bottles filled with water from a municipal source. Cotton squares (Ancare) were placed in all cages as a form of enrichment and for nest building. Room lights were on a timed 12:12-h light:dark cycle.
Sentinel mice exposed to dirty bedding from mice housed on the same rack were tested each quarter for fur mites and pinworms (Syphacia spp. and Aspiculuris spp.) in addition to antibodies to mouse hepatitis virus, mouse parvovirus, minute virus of mice, rotavirus, and Theiler murine encephalomyelitis virus by inhouse serology. Sentinels were shipped to Charles River Laboratories International once each quarter to be tested for all pathogens outlined in the comprehensive panel (HM Plus panel, Charles River Laboratories). All sentinels tested negative for ectoparasites and murine viruses throughout the course of the study.
Intraperitoneal injection of mice.
Mouse pups were weighed by using a gram scale (model 21762, US Balance, Vincennes, IN) and then were placed on the device platform for baseline recording of heart rate (ECGenie, Mouse Specifics, Quincy, MA; Table 1). All intraperitoneal injections were performed by the same person (CDD). Ethanol (100%, Decon Labs, King of Prussia, PA), a pentobarbital–phenytoin solution (Euthasol, 390 mg pentobarbital sodium and 50 mg phenytoin sodium per mL, Virbac Animal Health, Fort Worth, TX), or saline (0.9%) was drawn into a U40 insulin syringe carrying a 27-gauge, 0.5-in. needle. The volume of administration for all mice was 0.1 mL per 5 g of body weight, which resulted in an ethanol dose of 15.8 g/kg and a pentobarbital dose of 7.8 g/kg. We used 100% ethanol instead of 70% ethanol because a small pilot study suggested a prolonged time to death in young mice even at the higher concentration of ethanol. Ultimately, 88 mice received ethanol, 47 received pentobarbital–phenytoin, and 27 received saline. The outside of the needle was wiped with nonsterile gauze to avoid irritation at the site of skin penetration. The person who administered the injections was blinded to the contents of the syringe for ethanol or saline injections so that assessments of reactions to the injections would be unbiased. However, the pink color of the pentobarbital–phenytoin solution prevented blinding when administering this drug.
The operator restrained each conscious mouse pup by gently gathering the skin over the neck and back, by using the thumb and forefinger of the nondominant hand. The pup's head was tilted downward to displace abdominal organs cranially, and the needle was slowly advanced no more than 0.25 in. into the peritoneal cavity through the skin and body wall in the lower right quadrant of the abdomen. The plunger was withdrawn, and after confirmation of negative pressure and the lack of ingesta, urine, or blood in the hub of the needle, the mouse was injected with either ethanol or saline over 2 to 3 s; given the viscosity of the solution, injections of pentobarbital–phenytoin took as long as 5 s. After several intraperitoneal injections had been performed, the authors noted that ethanol frequently leaked at the site of the injection if the needle was removed from the peritoneal cavity immediately. Thereafter, the needle was held in place for at least 10 s in all mice, in an effort to reduce this leaking. Once the needle was removed from the abdomen, the mouse was replaced on the ECGenie platform for additional recordings. Mice that received saline did not become sedated and were returned to their home cages.
Assessment of pain and distress.
Pain and distress were measured as reaction to the injection, such as squirming and kicking at the needle. In a previous study,3 the Mouse Grimace Scoring system17 and other behavioral indications of discomfort, such as abdominal press, were applied during the time period after injection but before the loss of consciousness. However, given the immature facial features of mice 14 d and younger, this scoring system could not be consistently applied to many of the mice in the current study, making it an inadequate measure of pain or distress across all groups. As a result, pain and distress were assessed only at the time of injection.
Assessment of LORR and TTD.
To determine loss of consciousness, mice were turned on their backs every 15 to 30 seconds until they were no longer able to right themselves; this event was defined as the LORR and was used as an estimate for the loss of consciousness.
TTD was defined as 2 min beyond the cessation of respiration. Using cessation of respiration was the most reliable way to measure TTD because, in several cases, ECG recordings were lost due to weak signals prior to cardiac arrest in very small mice. After death, mice were either set aside to observe for recovery or, in a few mice, the thorax was opened immediately to examine myocardial activity. Then an abdominal incision was made in all mice, to note gross changes to the abdominal viscera and evidence of misinjection. An injection was classified as a misinjection when evidence of ethanol or pentobarbital–phenytoin was present within an abdominal organ or when the majority of the solution injected was visible as a swelling within the subcutaneous tissue. An accurate injection was classified as one that did not appear to involve any abdominal organs or subcutaneous tissues.
ECG recording.
The ECGenie recording device was used to obtain an ECG from all mice. Pups were placed on the platform after weighing and were allowed to acclimate to the space. When the pup was still and at least 2 paws were in contact with the electrodes, short clips of interpretable ECG were recorded. Because young mice (7 or 14 d old) were at risk of hypothermia if left for too long on the recording platform prior to injection, they were allowed to acclimate to the platform for a maximum of 2 min before injection. In addition, a small amount of ultrasound gel (Aquasonic 100 Ultrasound Transmission Gel, Parker Laboratories, Hannover, Germany) was applied to the bottom of all 4 paws of 7- or 14-d-old mice to facilitate detection of the cardiac electrical signal by the electrode footplates. Once a baseline recording was achieved, the mouse was removed from the platform, given its intraperitoneal injection, and then returned to the ECG platform. Heart rate was calculated from the ECG by using ECG analysis software (eMouse 12.3, Mouse Specifics, Quincy, MA) at 5-min intervals. Occasionally the ECG signal was weak or lost in the young mice within minutes of respiratory arrest; in other mice, the electrical activity of the heart continued for as long as 10 min beyond respiratory arrest. In mice with prolonged ECG activity, thoracotomy (after no response to a firm toe pinch) confirmed that the myocardium lacked organized, productive contractions.
Preparation and examination of histologic slides.
After confirmation of euthanasia, a gross postmortem examination was performed to confirm the site of injection and observe any effects of ethanol or pentobarbital–phenytoin on the peritoneum and abdominal organs. From a subset of mice (4 each from the pentobarbital and ethanol groups, one mouse at each time point), samples of stomach, intestines, cecum, spleen, liver, and peritoneal lining were collected and fixed in 10% neutral buffered formalin (Thermo Fisher Scientific, Waltham, MA), processed, and stained with hematoxylin and eosin (Histology Laboratory, Veterinary Hospital of the University of Pennsylvania, Philadelphia, PA). The slides were examined by a board-certified veterinary pathologist (AKB), who was blinded to the euthanasia method and age of the mouse. Inflammation and congestion were examined and scored as no change, mild, moderate, or severe.
Effect of supplemental heat on TTD.
In an additional experiment, 14-d-old CD1 mice (n = 9) were placed in sternal recumbancy on a recirculating warm-water blanket (Gaymar Industries, Orchard Park, NY) set to 42 °C and covered by a c-fold paper towel, immediately after loss of the righting reflex. Of the 9 heated pups, 4 underwent continuous digital rectal thermometry (19-mm probe [model RET3], connected to a model TW2-193, MicroTherma thermometer, Thermoworks, Lindon, UT) for serial temperature measurements. The other 5 heated pups did not have continuous digital rectal thermometry. In addition, 3 pups with the same signalment but without heat support underwent continuous rectal thermometry as described. Because the mice that were warmed could not be returned to the ECG platform, ECG paddles (ECGenie, Mouse Specifics) were secured with conduction putty to the forepaws of both the heated and nonheated mice to obtain ECG recordings. All recordings and postmortem processing were conducted as described previously.
Accuracy of Intraperitoneal Injection in 7 and 14 Day-Old Mice.
A sterile, synthetic dye used for human endoscopic tattooing (GI Spot, GI Supply, Camp Hill, PA) was injected intraperitoneally at the same volume as ethanol, pentobarbital–phenytoin, or saline (that is, 0.1 mL per 5 g body weight) into 7- (n = 30) or 14- (n = 15) d-old pups. After the pup was weighed, the appropriate volume of endoscopic dye was drawn into an insulin syringe, the needle was wiped with nonsterile gauze to remove excess dye, and the dye was injected intraperitoneally as described earlier. After 5 min, each pup was anesthetized either with CO2 in a slow-flow chamber with a total exposure time of 10 min and a displacement rate of 10% to 30% of the chamber volume per minute, or with 2% to 4% isoflurane (by vaporizer). After a negative toe-pinch response was confirmed, the pup was euthanized via decapitation, a ventral midline incision was made, and the abdominal cavity was rinsed to remove any dye successfully injected into the peritoneal cavity. The presence of dye within any abdominal organ, muscle, or subcutaneous tissue or along the path of the needle was recorded; misinjection was defined as described earlier.
Statistical analysis.
We used 3-way ANOVA to compare the average time to LORR and average TTD, with main effects of age, strain, and method of euthanasia; a posthoc Tukey test was run on all significant results to determine significant differences between groups. A t test was used to compare average TTD of 14-d-old CD1 pups that received supplemental heat with the age-matched, nonheated cohort. A χ2 test was used to analyze the proportion of mice that exhibited an adverse response to the injection. Two-way ANOVA was used to compare body weight and baseline heart rates of the mice, with age and background strain as main effects. Results were considered statistically significant when P values were less than or equal to 0.05. All statistical analysis was performed using SigmaPlot 12.3 (Systat Software, San Jose, CA.)
Results
LORR, TTD, and response to injection.
The average time to loss of consciousness, measured as the time to LORR, was calculated for a total of 98 mice across all age groups, methods of euthanasia, and strains (Table 2). Because none of the mice that received saline lost consciousness or progressed to death, they were not included in the statistical analysis. No significant differences were detected between inbred and outbred mice in any of the analyses. The time to LORR was significantly greater in those mice that received ethanol compared with those that received pentobarbital–phenytoin at 7 (P = 0.023), 14 (P = 0.009), or 21 (P = 0.015) days of age, but the time to LORR did not differ between any ages within each method of euthanasia. Among the mice that were misinjected with ethanol (n = 9), 88% retained a righting reflex for 2 min or longer.
Table 2.
Time to LORR (s; mean ± 1 SD [range]) in mice (C57BL/6 and CD1 mice are pooled) injected intraperitoneally with ethanol (15.8 g/kg) or pentobarbital (7.8 g/kg) at 7, 14, 21, 28, and 35 d of age.
| Age (d) | Ethanol | Pentobarbital–phenytoin |
| 7 | 100 ± 34.6 | 33.8 ± 14.4a |
| 6–120 (n = 3) | 15–45 (n = 4) | |
| 14 | 71.6 ± 35.3 | 29.0 ± 10.8a |
| 30–180 (n = 19) | 15–45 (n = 5) | |
| 21 | 80.0 ± 63.2 | 39.5 ± 18.8a |
| 10–240 (n = 11) | 30–90 (n = 10) | |
| 28 | 66.3 ± 53.4 | 42.9 ± 13.5 |
| 30–240 (n = 12) | 30–60 (n = 7) | |
| 35 | 55.6 ± 22.8 | 57.0 ± 19.7 |
| 30–90 (n = 17) | 30–90 (n = 10) |
The table includes mice that were provided with an external heat source.
Time to LORR differed significantly (P < 0.05) between the methods of euthanasia.
TTD differed significantly between ethanol and pentobarbital–phenytoin at 7, 14, and 21 d of age (P < 0.001), as well as between mice euthanized with ethanol at 7, 14, or 21 d of age compared with 35 d (P < 0.001). Although the mean TTD did not significantly differ between accurately injected ethanol and pentobarbital–phenytoin mice at the 28-d time point, the TTD was greater than 15 min in 3 of 12 mice and longer than 30 min in 2 of these 3 mice. TTD did not differ between pentobarbital–phenytoin and ethanol in mice that were accurately injected at 35 d of age.
Mice that were identified as receiving a misinjection (n = 11, 9 ethanol and 2 pentobarbital–phenytoin; all 21 d or older) were removed from the primary data set for analysis. Misinjections were difficult to identify in 7- and 14-d-old mice due to their small size and prolonged time to death. Among the 11 mice that were identified as misinjected, 7 received intracecal injections, 2 mice received subcutaneous injections, 1 mouse received an intrauterine injection, and 1 mouse received a small intestinal injection. In the subset of mice misinjected with ethanol and that were tested for LORR (n = 8), the average time to LORR was 206.7 ± 162.6 s at 21 d (n = 3), 150.0 ± 42.4 s at 28 d (n = 2), and 220.0 ± 69.2 s at 35 d (n = 3). In addition, in 6 of the 8 mice, the time to LORR was 2 SD or greater from the mean for the accurately injected mice in each age group.
The TTD in mice misinjected with ethanol (n = 9) was 83.3 ± 39.8 min at 21 d (n = 3), 52.5 ± 19.1 min at 28 d (n = 2), and 57.0 ± 51.1 min at 35 d (n = 4). In 6 of the 9 mice that were misinjected with ethanol, TTD was 2 SD or more from the mean of the accurately injected mice in each age group. The LORR in those mice that were misinjected with pentobarbital–phenytoin was 52.5 ± 10.6 s (n = 2), and the TTD was 4.0 ± 1.4 min (n = 2) at 28 d of age; these times are similar to the time to LORR and TTD in the mice that were accurately injected with pentobarbital–phenytoin. Overall, misinjections were associated with prolonged times to LORR and death only in the ethanol-injected mice.
The responses to injection did not differ between ethanol, saline, or pentobarbital–phenytoin.
ECG.
The average baseline heart rate for each age group prior to injection can be found in Table 1. In several instances, notable changes in ECG recordings occurred just before or after respiratory arrest in mouse pups. These changes included ventricular arrhythmias (n = 19), and atrioventricular block of various grades (n = 12; Figure 1). In C57BL/6 mice 21 d of age or younger, the heart rate rapidly declined within the first few minutes after the injection and then remained steady, at 200 to 300 beats per minute, prior to respiratory arrest (Figure 2). Shortly before respiratory arrest, the heart rate rapidly declined again, ultimately resulting in cardiac arrest. In CD1 mice, this pattern occurred in the 7-d-old mice only. Weak signals combined with prolonged pulseless electrical activity made determining the exact time to functional cardiac arrest challenging in very young mice.
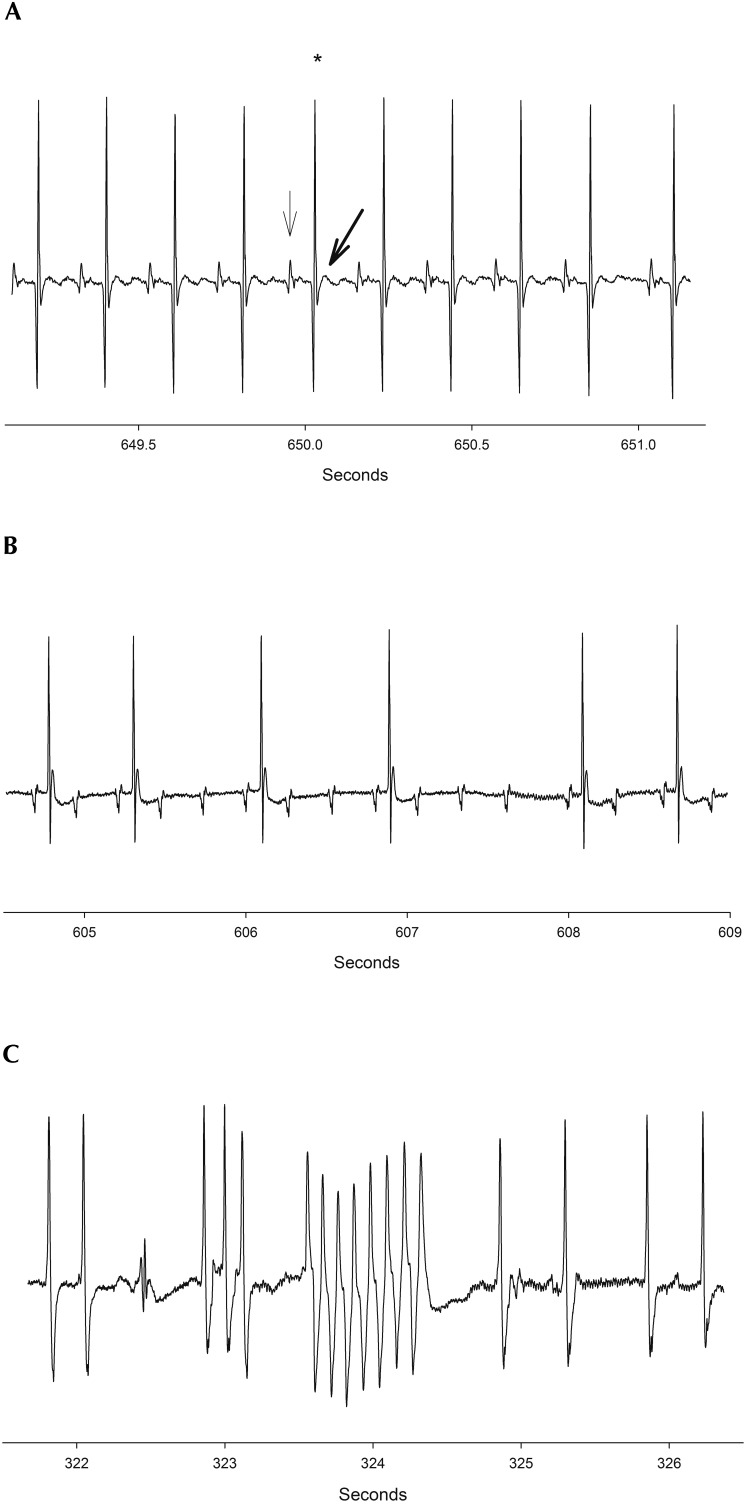
Figure 1.
(A) A normal ECG recording in a mouse. Note that every P wave (solid arrow) is followed by a QRS complex (star) and T wave (bolded arrow). (B) AV block in a mouse just prior to respiratory arrest. 5,6,16 (C) Ventricular arrhythmia (ventricular tachycardia) in a mouse just prior to respiratory arrest. Arrhythmias were common in mice at all ages just before or after respiratory arrest.
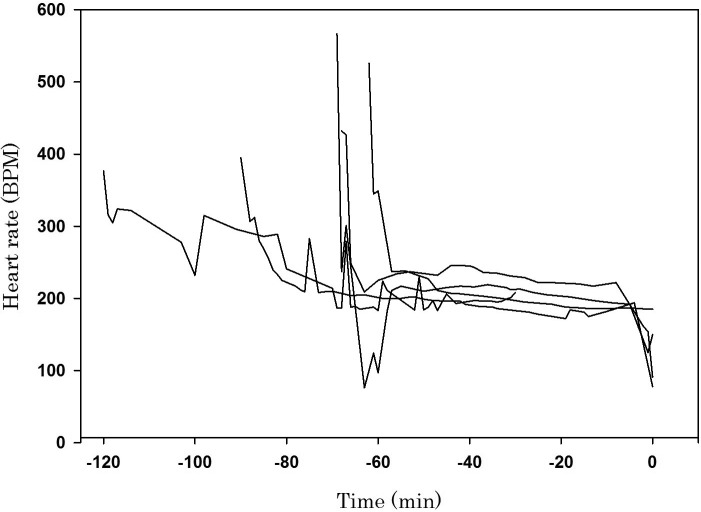
Figure 2.
Change in heart rate (beats per minute) compared with time (min) prior to death in 7-d-old pups. Each line represents 1 of 5 mice; minute 0 is the time of death. The heart rate declined rapidly during the first few minutes of anesthesia and then remained steady, between 200 and 300 beats per minute, prior to respiratory arrest. Around the time of respiratory arrest, the heart rate rapidly declined again, ultimately resulting in cardiac arrest.
Gross pathology and histology.
Grossly, in mice that received an accurate intraperitoneal injection with ethanol, the visceral and parietal peritoneum and serosal surface of the gastrointestinal tract were noticeably hyperemic (Figure 3) at all ages, particularly in mice 21 d of age or younger, in which TTD was prolonged. Whether the gross appearance of the peritoneal cavity correlated with any sensation of pain prior to unconsciousness is unknown, but evidence of inflammation was present histologically. In addition, the gross hyperemia might have been secondary to the vasodilatory properties of ethanol, given the histologic evidence of congestion. Mice that were misinjected had hyperemia of the punctured organ and erythema at the needle-puncture site.
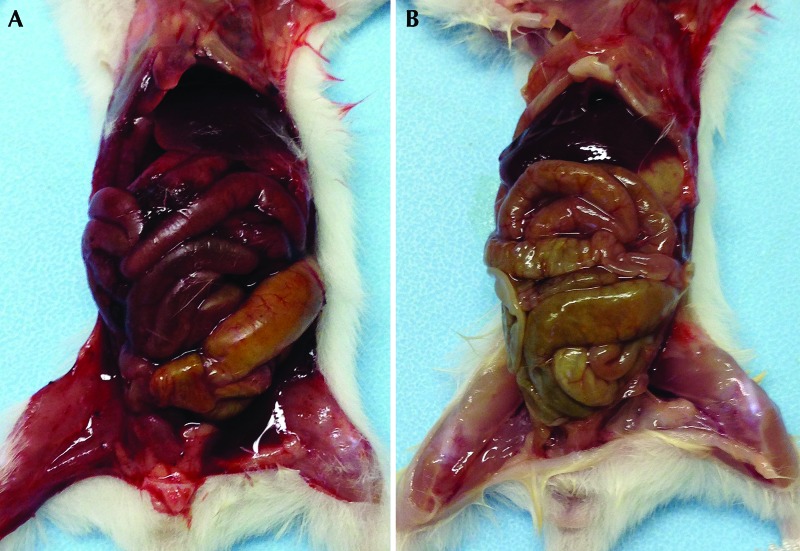
Figure 3.
(A) Representative mouse pup that was euthanized with intraperitoneal 100% ethanol at 21 d of age. Marked erythema of the visceral and parietal peritoneum and the serosal surface of the abdominal organs and serosanguinous peritoneal effusion are present. The red discoloration of the musculature of the hind legs is secondary to this effusion. (B) Representative mouse pup that was euthanized with intraperitoneal pentobarbital–phenytoin at 21 d of age. There is only mild, diffuse erythema of the serosal surface of the gastrointestinal tract, with no evidence of peritoneal effusion.
Tissues from a representative mouse that received either ethanol or pentobarbital–phenytoin at each of 4 time points (7, 14, 21, and 28 d) were processed for microscopic examination. Histologically, mice that received ethanol (n = 4, 1 mouse each at 7, 14, 21, and 28 d of age) exhibited moderate to marked submucosal and serosal lymphohistiocytic inflammation and submucosal and serosal congestion, compared with mice that received pentobarbital–phenytoin. Due to the small sample sizes, histologic results were not compared statistically between euthanasia methods.
Role of supplemental heat in TTD after ethanol injection.
After observing a prolonged TTD in the 7- and 14 d-old pups that received ethanol, we wanted to determine the role of hypothermia in resistance to ethanol toxicity,9 because the pups on the recording platform were cool to the touch after injection. TTD was 29.9 ± 17.7 min in 14-d-old pups that were maintained on a heating pad, significantly (P = 0.015) faster than those mice that remained on the bench top (63.3 ± 32 min ). Mice that were placed on the heating pad had an initial decrease in body temperature immediately after ethanol injection, but the body temperature eventually increased and stabilized at an average of 32.3 ± 1.0 °C until death. Mice that were not maintained on an external heat source exhibited a similar, gradual decline in temperature, which eventually stabilized; the average core temperature was 21.6°± 0.25 °C at the time of death. No differences were noted in the heart rates of mice that received supplemental heat compared with those placed on the bench top.
Table 3.
TTD (min; mean ± 1 SD [range]) in mice (C57BL/6 and CD1 mice are pooled) injected intraperitoneally with ethanol (15.8 g/kg) or pentobarbital–phenytoin (7.8 g/kg).
| Age (d) | Ethanol | Pentobarbital–phenytoin |
| 7 | 70.3 ± 39.8a | 2.8 ± 0.4* |
| 2–120 (n = 6) | 2–3 (n = 6) | |
| 14 | 51.7 ± 30.5a,b | 2.9 ± 0.5* |
| 15–108 (n = 15) | 2–4 (n = 11) | |
| 21 | 32.3 ± 20.8b | 3.9 ± 1.2* |
| 7–80 (n = 11) | 3–6 (n = 11) | |
| 28 | 14.0 ± 15.2c | 3.9 ± 0.7 |
| 4–55 (n = 12) | 3–5 (n = 7) | |
| 35 | 4.9 ± 1.4c | 4.4 ± 0.5 |
| 3–8 (n = 20) | 4–5 (n = 10) |
The table includes only accurately injected mice and 14-d-old pups that were not provided a form of heat support. Significant (P < 0.05) differences in TTD within a method of euthanasia are denoted by different lowercase letters; an asterisk (*) indicates a significant difference in TTD between methods of euthanasia.
Accuracy of intraperitoneal injection in 7- and 14-d-old mice.
After intraperitoneal injections had been performed in several young mice, we noted that ethanol often leaked from the site of injection onto the mouse's ventrum. In addition, determining the accuracy of injection in the 7- and 14-d-old mice that received ethanol or pentobarbital–phenytoin was challenging due to the small injection volumes and, in the case of ethanol, prolonged time to death. Therefore, the purpose of this experiment was to determine the accuracy of intraperitoneal injection in young mice. Most of the injected solution entered the peritoneal cavity in 41 of 45 (91.1%) 7- and 14 d-old mice injected with the dye product, with no evidence of puncture of any abdominal organs (Figure 4 A). All mice had evidence of leakage of a small amount of dye (at least 10% of the volume) from the injection site onto the ventral abdomen, and almost half of the mice (18 of 45 [40%]) had evidence of staining in the subcutaneous space along the needle path (Figure 4 B). However, despite the subcutaneous staining, the dye was primarily found within the peritoneal cavity. In mice that were misinjected, more than 60% of the dye was located in the subcutaneous tissue on the ventral abdomen.
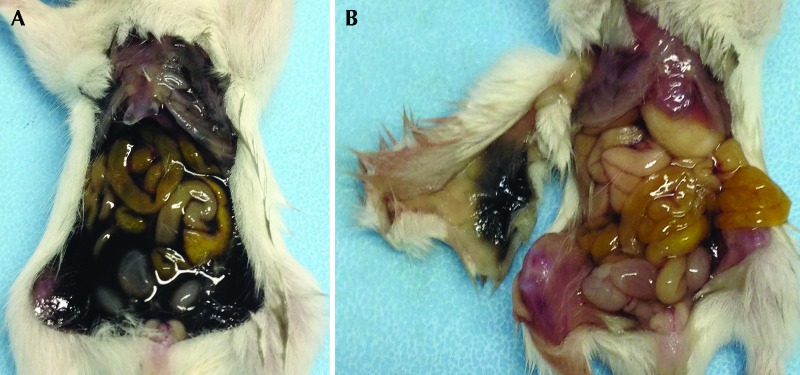
Figure 4.
(A) A 14-d-old CD1 mouse that received a dye product to assess the accuracy of intraperitoneal injection. The endoscopic dye fills the abdomen, indicating successful intraperitoneal injection. (B) A 14-d-old CD1 mouse that received dye product. This mouse was injected precisely (endoscopic dye was present within the peritoneal cavity on necropsy), but after the peritoneal cavity was rinsed, it became apparent that the dye had stained the subcutaneous tissue along the path of the needle, as seen on the underside of the reflected skin.
Discussion
The purpose of this study was to determine the age at which 100% ethanol, administered intraperitoneally, becomes a rapid, reliable, method of euthanasia in mice and to evaluate whether susceptibility to ethanol euthanasia differed between inbred and outbred mice. In addition, we were interested in examining the accuracy of intraperitoneal injection in neonatal and preweanling mice. TTD was significantly prolonged in 7-, 14-, and 21-d-old mice after intraperitoneal administration of 100% ethanol compared with pentobarbital–phenytoin injection at those ages. In addition, TTD at 7, 14, or 21 d of age was significantly longer than at 35 d of age in mice that received ethanol. Furthermore, the time to LORR was prolonged in pups that received 100% ethanol at 7, 14 and 21 d compared with pentobarbital–phenytoin at the same ages. Although the TTD and LORR in the ethanol group at 28 d was not significantly longer than those of any of the pentobarbital–phenytoin groups or of the 35-d ethanol group, the TTD for 2 of 12 mice in the 28-d group exceeded 30 min. Our results demonstrate that, due to a prolonged TTD, 100% ethanol administered intraperitoneally is not an acceptable or practical means of euthanasia for mice younger than 35 d. Finally, the percentage of mice that were misinjected was similar to previously reported percentages in adult mice; therefore intraperitoneal injection was determined to be an adequate means of compound or drug delivery in neonatal and preweanling mice. However, given the frequency of leakage at the injection site at the current volumes, we recommend using a different route for compounds or drugs that might cause skin irritation.
There are several possible physiologic explanations for the prolonged TTD after ethanol administration in neonates compared with adults. First and foremost, previous studies have shown that the mechanism of action, as well as the location and type of receptors that are affected by ethanol, differ between adult and neonatal mammals.18,22,23,25 Ethanol exerts its affects primarily through γ-aminobutyric acid (GABA) and N-methyl-d-aspartate receptors;2,6,32 and whereas the GABAA receptor is typically inhibitory in adult neural circuits, it is excitatory in young animals with developing synapses.7,18,22 In the perinatal brain, Na+–K+–Cl– cotransporters favor the accumulation of intracellular chloride, permitting depolarization of the cell after binding of GABA agonists to receptors and ultimately resulting in an excitatory rather than an inhibitory cellular response.18,23 Although its action at various receptors has been classified, the exact mechanism by which ethanol causes death has yet to be determined.31 The prolonged TTD in neonates compared with adults might be a result of opposite (excitatory compared with inhibitory) responses to ethanol at the GABAA receptor.
Once neonatal mice succumb to CNS depression and respiratory suppression ensues, fetal adaptive mechanisms to reduce the metabolic rate offer protection from the consequences of hypoxia. These adaptations include reducing efforts to regulate body temperature, decrease in heart rate, and upregulation of anaerobic metabolism.11,28,29 These mechanisms are a residual adaption from the relative hypoxia experienced in utero. Generally hypoxia is associated with compensatory tachycardia in adult mammals. However, fetal adaptive mechanisms work to preserve cardiac function by inducing bradycardia during periods of hypoxia, subsequently lowering the metabolic requirements of the myocardium.15 In addition, bradycardia reduces the blood supply to peripheral organs, and this response is ultimately protective of core functions. In the current study, we noted a reduction in the heart rate to a range of 200 to 300 beats per minute for a prolonged period of time in 7-d-old mouse pups, and this rate remained steady until death—an excellent illustration of the fetal adaptive mechanism of bradycardia.
Notably, when perfusion to peripheral tissues decreases and efforts to maintain body temperature are reduced, hypothermia sets in, perhaps conferring resistance to euthanasia by intraperitoneal ethanol.29 Previous studies have shown that hypothermia is protective against ethanol toxicity.8,9 The exact mechanism of this protective affect is not fully understood but is suspected to involve changes in the relative fluidity of the cell membrane.9 The lethal dose of ethanol was decreased in adult mice when an external heat source was provided and the body temperature was raised above physiologic normal.9 Interested in exploring this effect ourselves, we compared TTD in 14-d-old mice that were warmed with mice maintained on the bench top at room temperature after injection. We were able to demonstrate similar results as those from the previous study,9 because TTD was significantly prolonged in the mice that were placed on the bench top compared with those provided external heat support. Therefore, taking into account neonatal resistance to CNS depression at the GABA receptor, one of the main sites of action for ethanol, resistance to hypoxia (perinatal mechanisms of survival), as well as the protective effects of hypothermia, the prolonged TTD in very young mice after administration of ethanol is not surprising.
Other authors24 noted a significant difference in susceptibility to CO2 euthanasia when comparing inbred and outbred neonatal mice, with inbred mice being more resistant to the toxic effects of CO2 than outbred mice at the same age. We did not find this pattern to be true in regard to ethanol toxicity, given the lack of differences between the C57BL/6 and CD1 mice.
As previously mentioned, determining the accuracy of injection when ethanol or pentobarbital–phenytoin was administered intraperitoneally to 7- and 14-d-old mice was challenging, due to the long TTD and small injection volumes. Therefore, we determined the accuracy of injection by using dye injection in mice at these ages. At 7 and 14 d of age, 4 of 45 mice (8.9%) showed no evidence of the dye within the peritoneal cavity and were classified as misinjected. At 21, 28, and 35 d of age, 11 of 82 (13.4%) mice were misinjected. These error rates were within the range of previously reported data regarding the accuracy of intraperitoneal injection (12% to 24%) in adult mice.4,21,30 Noticeable leakage occurred at the site of injection when either ethanol or the dye product was injected, but leaking did not occur with saline or pentobarbital–phenytoin. We speculate that the physical properties of the injected substance, such as density and viscosity, may affect the propensity of the substance to leak, because the dye product had a similar viscosity to the ethanol. The likelihood of leakage should be considered when performing intraperitoneal injections in very young mice, because this effect might result in underdosage of the drug or cause skin irritation on contact.
At necropsy, the visceral and parietal peritoneum and serosal surfaces of the gastrointestinal tract were grossly hyperemic in all mice accurately injected with ethanol compared with mice that received the pentobarbital–phenytoin solution. We were concerned that intraperitoneal injection of ethanol might result in pain, given the erythematous appearance of the peritoneal cavity in mice of all ages, yet when ethanol was compared with saline and pentobarbital–phenytoin, the immediate reaction to injection did not differ significantly. In addition, older mice that received an accurate injection were rendered unconscious rapidly, as indicated by the average time to LORR, and any discomfort that might have resulted when ethanol contacted the peritoneum was likely brief. However we remain concerned that younger mice and misinjected mice, all of whom had a lengthy time to LORR, might have experienced discomfort due to prolonged consciousness after the ethanol injection.
In conclusion, intraperitoneal administration of ethanol is not an acceptable means of euthanasia in mice younger than 35 d, because of the prolonged time to unconsciousness and death and the potential for discomfort after injection. Furthermore, unlike pentobarbital–phenytoin, misinjection of ethanol can further prolong the time to unconsciousness and death in neonatal mice. Although an effective means of euthanasia in adult mice, intraperitoneal ethanol does not consistently result in rapid euthanasia of mice younger than 35 d and therefore is not recommended for neonatal and preweanling mice.
Acknowledgments
Funding for this project was provided by the Office of the Vice Provost for Research, University of Pennsylvania. We would like to thank Rebecca Erickson and Krystal Allen-Worthington for their technical support in the study and Marianne Saunders, Perelman School of Medicine, for the generous donation of the GI SPOT Endoscopic Dye used in the study.
References
- 1.American Veterinary Medical Association 2013. AVMA guidelines for the euthanasia of animals: 2013 ed. Schaumburg (IL): American Veterinary Medical Association. [Google Scholar]
- 2.Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. 2002. GABA-A receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem 2:869–885. [DOI] [PubMed] [Google Scholar]
- 3.Allen-Worthington KH, Brice AK, Marx JO, Hankenson FC. 2015. Intraperitoneal injection of ethanol for the euthanasia of laboratory mice (Mus musculus) and rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 54:769–778. [PMC free article] [PubMed] [Google Scholar]
- 4.Arioli V, Rossi E. 1970. Errors related to different techniques of intraperitoneal injection in mice. Appl Microbiol 19:704–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltazar RF. 2009. Basic and beside electrocardiography. Philadelphia (PA): Lippincott Williams and Wilkins. [Google Scholar]
- 6.Bisaga A, Popik P. 2000. In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-d-aspartate (NMDA) antagonists. Drug Alcohol Depend 59:1–15. [DOI] [PubMed] [Google Scholar]
- 7.Briggs SW, Galanopoulou AS. 2011. Altered GABA signaling in early-life epilepsies. Neural Plast 2011:527605. 10.1155/2011/527605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. 1989. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev 41:489–537. [PubMed] [Google Scholar]
- 9.Dinh TKH, Gailis L. 1979. Effect of body temperature on acute ethanol toxicity. Life Sci 25:547–551. [DOI] [PubMed] [Google Scholar]
- 10.Foust KD, Poirier A, Pacak CA, Mandel RJ, Flotte TR. 2008. Neonatal intraperitoneal or intravenous injections of recombinant adeno-associated virus type 8 transduce dorsal root ganglia and lower motor neurons. Hum Gene Ther 19:61–70. [DOI] [PubMed] [Google Scholar]
- 11.Gautier H. 1998. Invited editorial on “Oxygen transport in conscious newborn dogs during hypoxic hypometabolism.” J Appl Physiol (1985) 84:761–762. [DOI] [PubMed] [Google Scholar]
- 12.Glass HG, Snyder FF, Webster E. 1944. The rate of decline in resistance to anoxia of rabbits, dogs, and guinea pigs from the onset of viability to adult life. Am J Physiol 140:609–615. [Google Scholar]
- 13.Harger RN, Hulpieu HR.1956The pharmacology of alcohol. p 103–232. In: Thompson GN. Alcoholism. Springfield (IL): Charles C Thomas. [Google Scholar]
- 14.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 15.Jensen A, Berger R. 1991. Fetal circulatory responses to oxygen lack. J Dev Physiol 16:181–207. [PubMed] [Google Scholar]
- 16.Kowalak JL, Turkington C. 2007. ECG interpretation. Philadelphia (PA): Lippincott Williams and Wilkins. [Google Scholar]
- 17.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. [DOI] [PubMed] [Google Scholar]
- 18.Leinekugel X, Khalilov I, McLean H, Caillard O, Gaiarsa JL, Ben-Ari Y, Khazipov R. 1999. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol 79:189–201. [PubMed] [Google Scholar]
- 19.Lord R. 1989. Use of ethanol for euthanasia of mice. Aust Vet J 66:268–268. [DOI] [PubMed] [Google Scholar]
- 20.Lord R, Jones GL, Spencer L. 1991. Ethanol euthanasia and its effect on the binding of antibody generated against an immunogenic peptide construct. Res Vet Sci 51:164–168. [DOI] [PubMed] [Google Scholar]
- 21.Miner NA, Koehler J, Greenaway L. 1969. Intraperitoneal injection of mice. Appl Microbiol 17:250–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens DF, Boyce LH, Davis MB, Kriegstein AR. 1996. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci 16:6414–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotkin MD, Snyder EY, Hebert SC, Delpire E. 1997. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol 33:781–795. [DOI] [PubMed] [Google Scholar]
- 24.Pritchett-Corning K, Corrow D, Stockwell J, Smith A. 2005. Euthanasia of neonatal mice with carbon dioxide. Comp Med 55:275–281. [PubMed] [Google Scholar]
- 25.Santhakumar V, Wallner M, Otis TS. 2007. Ethanol acts directly on extrasynaptic subtypes of GABA(A) receptors to increase tonic inhibition. Alcohol 41:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setiady YY, Samy ET, Tung KS. 2003. Maternal autoantibody triggers de novo T cell-mediated neonatal autoimmune disease. J Immunol 170:4656–4664. [DOI] [PubMed] [Google Scholar]
- 27.Seymour TL, Nagamine CM. 2016. Evaluation of isoflurane overdose for euthanasia of neonatal mice. J Am Assoc Lab Anim Sci 55:321–323. [PMC free article] [PubMed] [Google Scholar]
- 28.Singer D. 1998. Thermometry and calorimetry in the neonate: recent advances in monitoring and research. Thermochim Acta 309:39–47. [Google Scholar]
- 29.Singer D. 1999. Neonatal tolerance to hypoxia: a comparative–physiologic approach. Comp Biochem Physiol A Mol Integr Physiol 123:221–234. [DOI] [PubMed] [Google Scholar]
- 30.Steward JP, Ornellas EP, Beernink KD, Northway WH. 1968. Errors in the technique of intraperitoneal injection of mice. Appl Microbiol 16:1418–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsibulsky VL, Amit Z. 1993. Tolerance to effects of high doses of ethanol. 1. Lethal effects in mice. Pharmacol Biochem Behav 45:465–472. [DOI] [PubMed] [Google Scholar]
- 32.Wiberg GS, Trenholm HL, Coldwell BB. 1970. Increased ethanol toxicity in old rats: changes in LD50, in vivo and in vitro metabolism, and liver alcohol dehydrogenase activity. Toxicol Appl Pharmacol 16:718–727. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. 2008. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci USA 105:7528–7533. [DOI] [PMC free article] [PubMed] [Google Scholar]