Abstract
Tail tip amputation with minimal restraint is not widely used for mouse phlebotomy. In part, this infrequency may reflect policies influenced by tail tip amputation procedures for genotyping, which involve greater handling and tissue removal. To assess tail tip amputation with minimal restraint as a phlebotomy technique, we compared it with 2 more common methods: scruffing with facial vein puncture and lateral tail vein incision with minimal restraint. Blood glucose levels, audible and ultrasonic vocalizations, postphlebotomy activity and grooming behavior, open field and elevated plus maze behaviors, nest-building scores, and histologic changes at the phlebotomy site were evaluated. Mice in the facial vein phlebotomy group produced more audible vocalizations, exhibited lower postphlebotomy activity in the open field, and had more severe histologic changes than did mice in the tail incision and tail tip amputation groups. Facial vein phlebotomy did not affect grooming behavior relative to sham groups, whereas tail vein incision—but not tail tip amputation—increased tail grooming compared with that in control mice. Blood glucose levels, nest-building scores, and elevated plus maze behavior did not differ between groups, and no mice in any group produced ultrasonic vocalizations. Tail tip amputation mice did not perform differently than sham mice in any metric analyzed, indicating that this technique is a potentially superior method of blood collection in mice in terms of animal wellbeing.
Abbreviations: FV, facial vein puncture; SF, sham facial vein puncture; ST, sham tail tip amputation; TA, tail tip amputation; TI, tail incision
Nonterminal blood sample collection from mice is a common procedure in biomedical research. Because multiple phlebotomy sites are accessible in mice, researchers must weigh the benefits and disadvantages of each technique during experimental design. A method that requires minimal restraint, minimal handling, and no anesthesia or analgesia administration is less likely to affect stress-responsive physiologic parameters and impair animal wellbeing, thereby reducing variability in research data.5,15,27 Stress associated with phlebotomy likely varies with the amount of related pain, the type of handling and restraint used, and the procedure length. In addition, common procedures such as moving cages, handling, and immobilization can cause substantial increases in stress responsive physiologic parameters.13,18,32 We sought to evaluate how tail tip amputation for phlebotomy, compared with facial vein puncture and lateral tail vein incision, affected indicators of pain and distress in mice.
In addition, the practical feasibility of a phlebotomy method must be considered, including the ability to obtain an adequate sample volume, the ease of training the phlebotomist, and the skill and dexterity required. The availability of visual landmarks at the site, and its proximity to other important anatomic structures, also are likely to affect success rates. Previous studies have indicated that training and experience influence animal outcomes after retroorbital phlebotomy in rats and mice. However, such experience did not significantly affect the outcome after tail tip amputation, suggesting that this technique is easier to master than retroorbital phlebotomy.2,12,33
Methods used to evaluate the effect of phlebotomy on animal welfare and data quality vary, making comparisons between studies difficult. However, the influence of an experimental procedure on mice can be evaluated by quantifying and assessing relevant physical and behavioral parameters. Physical parameters include blood glucose levels and the presence of stress- and pain-responsive hormones, such as glucocorticoids.20,26 In addition, stress and anxiety in mice can be measured by using well-established behavioral tests.4,9 The open field and elevated plus maze tests are 2 common assessments of unconditioned activity that evaluate aspects of natural rodent behavior, including general physical activity, exploratory behavior, anxiety, and aversion to exposed, open areas.21 Nesting is another natural mouse behavior that increasingly is used to evaluate rodent wellbeing, as well as the results of various genetic mutations.10,14,24,28 By assessing multiple physical and behavioral metrics, the effects of different phlebotomy methods on animal wellbeing can be broadly evaluated.
Recommendations for rodent phlebotomy proposed in individual reports, by working groups, and developed inhouse by institutions vary. Further complicating matters, descriptions of collecting blood by tail tip amputation differ widely, from minimal-restraint approaches2 to those applying restraining devices for as long as 8 min.31 Tail tip amputation has historically not been favored for blood collection,11 and institutional policies often recommend or require anesthesia or analgesia (or both) in adult mice prior to tail tip amputation for genotyping. However, reports suggest that the effects of anesthesia on animal physiology and wellbeing may exceed those of tail biopsy or amputation in both severity and duration.5,15 Other reports emphasize that the handling involved in rodent procedures is a major factor affecting animal physiology, more so than the sampling procedure itself.8 Because many reports that discourage tail tip amputation for phlebotomy differ in methodology from what several of our investigators propose to use for tail tip blood collection, we sought to compare tail tip amputation under minimal restraint with 2 approved methods of blood collection, facial vein puncture and tail vein incision, to guide our own institutional policy regarding this technique in adult mice. We hypothesized that phlebotomy via tail tip amputation would have no greater influence on animal physiology or behavior than the tail vein incision or facial vein puncture method.
Materials and Methods
Animals and housing conditions.
Mice were randomly assigned to one of the following phlebotomy methods: facial vein puncture (FV, n = 12), tail tip amputation (TA, n = 9), tail vein incision (TI, n = 9), sham facial vein puncture (SF, n = 8), and sham tail amputation (ST, n = 8). All animals were cared for in compliance with the Guide for the Care and Use of Laboratory Animals16 and American Association of Laboratory Animal Science Position Statements,3 and all procedures were approved by the Cornell University IACUC. Mice were housed in an AAALAS-accredited facility. Mice were obtained free of Sendai virus, mouse hepatitis virus, mouse parvovirus, minute virus of mice, epizootic diarrhea of infant mice, reovirus type 3, pneumonia virus of mice, ectromelia virus, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, mouse adenovirus, polyoma virus, norovirus, Helicobacter species, Klebsiella, Pasteurella, Mycoplasma pulmonis, cilia-associated respiratory bacillus, murine pinworms (Aspicularis and Syphacia spp.), and mouse ectoparasites. Mice were acclimated to the room and other environmental conditions in which experimental procedures were conducted for at least 1 wk prior to study initiation. Mice were housed and tested in a behavioral testing suite under conventional conditions in static, open-top cages (Mouse Cage PC7115HT, Allentown, Allentown, NJ) containing 1/4-in. autoclaved corncob bedding (7097A, Harlan Teklad, Frederick, MD), a cardboard hut (Refuge XKA-2450-087, Ketchum Manufacturing, Brockville, Ontario), and a sterile nesting pad (Nestlets, Ancare, Bellmore, NY). Mice were housed in randomly assigned groups of 5 until 24 h before phlebotomy, at which time they were moved to single housing. Irradiated rodent diet (7912, Harlan Teklad, Madison, WI) and municipal chlorinated water were provided without restriction. Mice were on a 12:12-h light:dark cycle with transitions at noon (nightfall) and midnight (daybreak). Room temperature and humidity were controlled between 20 and 22 °C and 30% to 70%, respectively. The study population comprised male C57BL/6J mice (age, 10 to 12 wk; The Jackson Laboratory, Bar Harbor, ME).
Timeline.
Experimental procedures were conducted between 0900 and 1200 (at the end of the animals’ light cycle). A singly housed mouse was carried in its home cage to the experimental station in the same room. One of 5 procedures was conducted in each mouse: tail tip amputation, tail vein incision, facial vein puncture, sham tail tip amputation, or sham facial vein puncture (described below). After phlebotomy by means of tail tip amputation or tail vein incision and after the sham tail amputation procedure, the tail was gently ‘milked’ for 2 min to obtain drops of blood. Phlebotomy by facial vein incision lasted approximately 15 to 20 s for mice in which blood was obtained on the first puncture attempt and for as long 60 s for animals for which the first bleeding attempt was unsuccessful. In such cases, a maximum of 3 attempts were made on the same side. The sham facial vein phlebotomy procedure lasted 15 to 20 s. Immediately after phlebotomy or sham procedures, mice were placed into a new, empty, observation cage and scored during free movement for 10 min. Vocalizations were recorded from the time that the mice were removed from their home cages for the procedure through the first 2 min in the postprocedural observation cage.
After the 10-min observation period, mice were moved into an open field apparatus and scored for 5 min. Open field testing was followed by another 5 min in the observation cage, after which mice were transferred to the elevated plus maze for 5 min of testing. After the elevated plus maze, animals were returned to their original home cage. At 6 h after the phlebotomy or sham procedure, each mouse was moved to another clean cage for 4 h to score nest-building behaviors. Mice were removed from the nest-scoring cage after 4 h (that is, at about 10.5 h postprocedure) and returned to their original cage. When all of the mice had completed their 4 h in the nest-building cage, all mice were euthanized, and tissues were fixed for histologic processing. This time point was chosen to best evaluate the acute phase of tissue inflammation to allow comparison of subtle differences in neutrophil infiltration related to tissue injury without potential complication from inflammation due to secondary bacterial invasion.
Phlebotomy and observations during the procedure.
Phlebotomy procedures were designed to standardize the amount of blood collected, rather than the length of the procedure, because appropriate sample volume is a realistic requirement for obtaining study data. For all methods of blood collection, the first drop of blood was used immediately for measurement of unfasted blood glucose (OneTouch UltraMini Glucometer, LifeScan, Chesterbrook, PA). The approximate volumes of blood and plasma obtained for each mouse were recorded at the end of all procedures. The phlebotomist was the same person throughout the study and was trained and practiced in all methods. The phlebotomist did not, however, have extensive experience, reflecting the training level of research personnel who might be performing phlebotomy after required institutional training. Facial vein puncture was performed by scruffing the mouse gently but firmly enough to achieve near immobility of the head, neck, and forelimbs without impairing respiration and puncturing the right lateral facial vein with a 22-gauge needle.
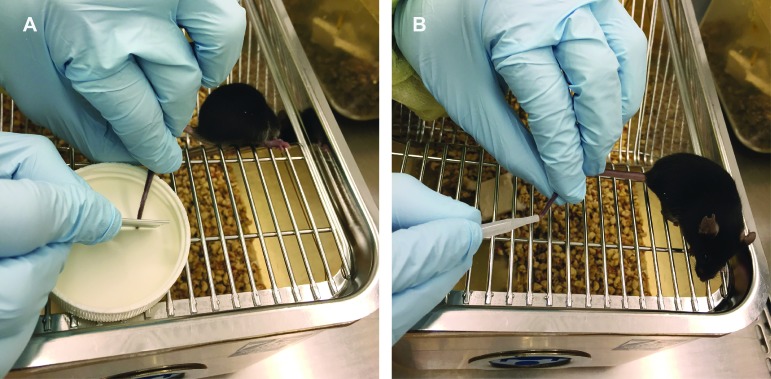
Tail tip amputation was performed similarly to a previously reported technique.2 Mice were gently removed from their home cage by lifting from the tail base and placed immediately on the wire cage lid. They were aligned by manipulation of the tail such that the tail tip was placed over a small flat surface (water bottle cap). A new, flat scalpel was used to amputate the distal 1 to 2 millimeters of tail against the flat surface (Figure 1). The mouse was allowed to move freely atop the wire cage lid as the tail was gently held and milked for 2 min; this duration was determined in a pilot study to be the amount of time required to collect approximately the same volume of blood achieved by a facial vein puncture. Tail vein incision was performed in a similar manner with minimal restraint: a new, flat scalpel blade was used to make a 2- to 3-mm incision over the lateral tail vein at mid-tail, and the mouse was allowed to move freely atop the wire cage lid while the tail was gently held and milked for 2 min.
Figure 1.
Tail tip amputation phlebotomy technique. Mice were removed from their home cage by grasping the base of the tail and immediately placed on top of their wire-bar food hopper. (A) The tail was positioned so that the tip rested over a flat surface (for example, a water bottle cap), and the distal 1 to 2 mm was amputated using a scalpel blade. (B) The mouse was allowed freedom of movement with minimal tail restraint while the desired volume of blood was collected by gently ‘milking’ the tail.
The sham facial vein puncture involved the same scruffing as facial vein puncture, and an unopened needle gently touched the normal site of puncture; no blood was collected. The sham tail amputation involved the same handling as the tail tip amputation, and the nonsharp side of a blade gently touched the tail tip; no amputation or blood collection was performed. At the time of phlebotomy or sham procedure, an observer recorded the following behaviors: audible noise, body flinch, urination, defecation, and aggression. The number of phlebotomy attempts was recorded. Immediately after phlebotomy or sham procedures, mice were placed into a new, empty observation cage.
Postprocedural observation.
After the phlebotomy or sham procedure, the same observer continued to watch the mouse for 10 min in the observation cage, recording all behaviors noted during 10 periods (1 min each). Posture, movement and activity, and grooming behaviors were specifically noted. Inactive episodes were defined as periods of 15 s or more during which a mouse was either largely or completely immobile.
Recording audible and ultrasonic vocalizations.
We used a high-frequency microphone connected to a desktop running Avisoft Recorder (USG 116-200 UltraSoundGate Kit, Avisoft, Berlin, Germany) to record audio during our trials. The microphone was placed approximately 10 cm from where the head of the mouse was located during phlebotomy. Recording began before the cage lid was lifted to remove the mouse and ended after 2 min in the observation cage after the procedure. For a subset of these trials (FV, n = 7; TA, n = 5; TI, n = 5; SF, n = 4; ST, n = 4), we also recorded ultrasonic vocalizations at either a 125 kHz (n = 7) or 250 kHz (n = 18) sampling rate. Discrete procedural events, including cage opening, picking up the mouse, scruffing (if applicable), puncture (if applicable), and returning the mouse to the observation cage, were timestamped to correlate the audible and ultrasonic recordings with particular events in each procedure. The spectrograms were visualized by using Raven Pro 1.4 (FFT, 1024; Hanning window, 50% overlap; Cornell Lab of Ornithology). Two independent researchers (blind to treatment groups) manually searched the spectrograms for the presence of audible and ultrasonic vocalizations; detections were in 100% agreement between the researchers. To supplement these manual searches, an automated detector in Raven was used to identify any acoustic energy above 20 kHz that was distinct from background noise.
Behavioral testing.
Open field.
After 10 min of postprocedural observation, mice were placed into the center of a square open-field arena (47 cm × 47 cm; wall height, 44 cm). A digital videocamera was mounted approximately 120 cm above the arena, and mouse movements were recorded (Any-Maze, Stoelting, Wood Dale, IL) for 5 min. After each mouse, the arena was cleaned with 70% ethanol, followed by water, and dried to remove excreta and odors. Parameters analyzed included movement velocity and time spent in the center compared with the periphery of the arena. Two-factor ANOVA was performed to assess the effects of phlebotomy treatment and region (central or peripheral zones of the open field), and their interaction. Mice were then returned to the observation cage for 5 min before being placed into an elevated plus maze.
Elevated plus maze.
The maze was elevated 60 cm above the floor and consisted of 4 arms (length, 30 cm; width, 5 cm). Two opposite ‘closed’ arms had 15-cm walls, whereas the 2 ‘open’ arms did not have walls. Arms were lined with 1/4-in. thick rubber pads to eliminate the lip surrounding the open arms. The maze was cleaned between mice by using the same method as for the open field arena. Mice were initially released into the maze onto the central platform facing a closed arm and recorded for 5 min using Any-Maze software. Parameters analyzed were average movement velocity and total time spent within the closed arms, open arms, and center. Both the open field and elevated plus maze apparati were constructed in-house.
Nest scoring.
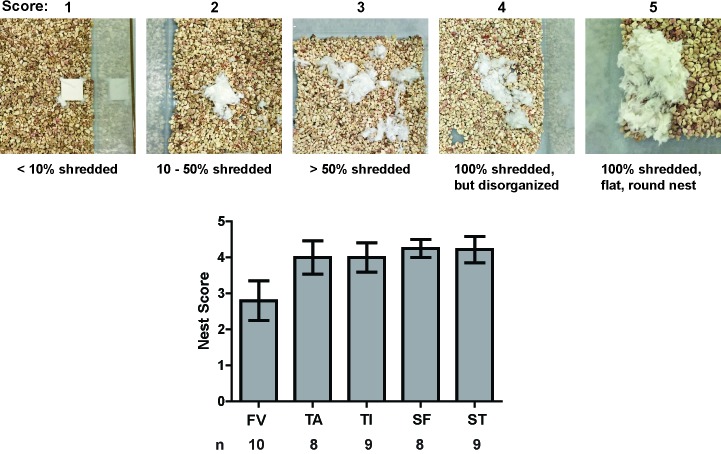
At 6 h postphlebotomy, to assess nest-building behaviors, each mouse was moved to a new cage containing a new, compressed cotton nesting pad (Nestlets, Ancare, Bellmore, NY). After 4 h in this cage, the mouse was removed, and photos were taken from above the cage to assess nest building. The nests were scored by a single scorer blinded to experimental group, using a modification of a published scoring system.10 Ordinal scores from 1 through 5 were assigned as follows: 1, less than 10% shredded; 2, 10% to 50% shredded; 3, more than 50% shredded; 4, 100% shredded but disorganized; and 5, 100% shredded with a flat, round nest. In addition, nests were scored according to the location of the nest: corner (nest within 1 cm of 2 walls) or central (all other locations).
Tissue processing and histologic scoring.
Mice were euthanized via CO2 inhalation at 10.5 to 13.0 h after phlebotomy. Heads and tails were fixed in neutral-buffered 10% formalin for 24 h, followed by decalcification and tissue trimming and embedding. Heads from the FV and SF groups were sectioned just cranial to the angle of the mandible. For TI mice, the location of the tail incision was located visually and that area of tail was sectioned transversely for histologic analysis. The caudal 5 mm of tail from TA group mice was longitudinally sectioned. Sections analogous to those taken for both TI and TA groups were made from ST mice. Sections stained with hematoxylin and eosin were scored by a board-certified veterinary pathologist blinded to the experimental groups to assess hemorrhage, inflammation, and tissue trauma.
Statistical analysis.
Data were analyzed using R 3.2.3 (R Foundation) and JMP Pro 12 software (SAS Institute, Cary, NC). Blood glucose data were analyzed using a one-way ANOVA with Tukey's Honestly Significant Difference post hoc tests. Audible vocalization data were analyzed using a χ2 test for independence followed by 10 Mann–Whitney U tests using a Bonferroni-corrected α of 0.005 for pairwise comparisons. The number of inactive episodes was analyzed using a one-way ANOVA and Tukey post hoc tests. The impact of facial and tail phlebotomies on the grooming of head, body, and tail locations was analyzed using MANOVA; facial and tail phlebotomy data were analyzed separately, and Tukey post hoc tests were used for pairwise comparisons. Elevated plus maze data were analyzed through MANOVA. Open field data were analyzed by ANOVA with Tukey post hoc tests for pairwise comparisons of phlebotomy groups. Modified Deacon nest scores were rank transformed and analyzed with the Kruskal–Wallis test for ordinal data. Summary data are reported as means ± SEM. Experiment-wise P values of less than 0.05 were considered significant.
Results
Tail tip amputation as a phlebotomy technique.
In a pilot study, we found that all blood collection methods evaluated enabled collection of the allowable blood volume based on animal weight, although the time required to do so varied. For the current study, we chose to limit blood collection time for both the TA and TI methods to 2 min to standardize the procedures across mice. There were no significant differences between the blood volumes collected by using FV (50.63 ± 8.986 μL), TI (56.67 ± 10.37 μL), and TA (65 ± 4.167 μL; one-way ANOVA, P = 0.4806). All 9 TA procedures were performed successfully on the first attempt. Of the 9 TI procedures, 6 were successful on the first attempt, whereas 3 mice required a second attempt to deepen the incision sufficiently to obtain blood. FV puncture was the least reliable method in our study: only 4 of 12 mice had successful venipuncture on the first attempt, and 6 mice required 2 or 3 attempts for success. Because we had decided a priori that no mouse would undergo more than 3 attempts, we were unable to collect blood through facial vein puncture in the remaining 2 mice (these mice were included in analyses other than blood glucose).
Blood glucose in phlebotomy groups.
We compared blood glucose levels among the 3 phlebotomy groups and included the values reported on the Mouse Phenome Database19 for 7- to 9-wk-old C57BL/6J male mice, fed standard chow, and fasted 4 h prior to testing (one-way ANOVA, F3,36 = 7.205, P = 0.001). We found no differences in blood glucose levels among the 3 phlebotomy groups (Figure 2; P > 0.05 [Tukey test] for all comparisons). However, the FV and TI groups had significantly lower average blood glucose values than those reported in the Mouse Phenome Database (P < 0.001, P = 0.014, respectively), whereas the TA group exhibited blood glucose levels similar to the values in the Mouse Phenome Database (P > 0.05).
Figure 2.
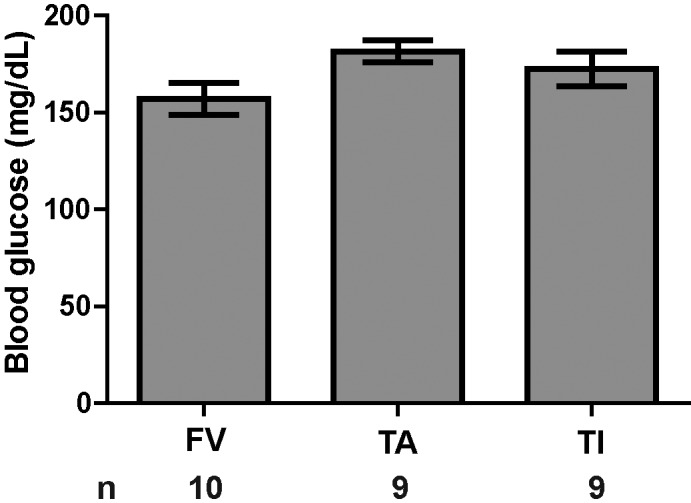
Blood glucose (mean ± SEM) did not differ between phlebotomy methods. The first drop of blood obtained by the indicated methods was used to measure blood glucose. Group sizes are indicated. No differences existed between groups (one-way ANOVA, P = 0.095).
Audible vocalizations.
Among the behaviors recorded at the time of phlebotomy or sham procedure, phlebotomy group significantly influenced the proportion of mice that produced audible vocalizations (χ2 test for independence, χ24,46 = 31.6, P < 0.001). Specifically, the FV and SF groups had significantly more audible vocalizations than all other groups (pairwise Mann–Whitney U tests, Bonferroni-corrected for multiple comparisons; P < 0.005). No mice in the TA (n = 9) or ST (n = 8) groups made an audible noise during the procedure, and only 1 of 9 mice in the TI group vocalized. The single TI mouse that produced audible vocalizations did so during positioning, tail vein incision, and blood collection. In contrast, the majority of mice in the FV group (10 of 12) and SF mice (7 of 8) made an audible vocalization during the procedure. All of the FV group mice that vocalized did so during positioning or scruffing; no vocalizations coincided with actual facial vein puncture. In addition, 3 of the SF mice vocalized exclusively during scruffing, whereas 4 of the SF mice vocalized during scruffing and during sham blood collection or transfer to the observation cage. No mice tested produced ultrasonic vocalizations (see Methods). There were no significant differences between groups in regard to body flinching, defecation, and aggressive behaviors (yes–no scoring), but there was a difference in the probability of urination (Kruskal–Wallis test, H4,46 = 15.549, P = 0.004). Specifically, 5 of the 12 FV mice urinated during the procedure (2 mice with one venipuncture attempt, one mouse with 2 attempts, and 2 mice with 3 venipuncture attempts), whereas no mice in any other group urinated.
Postprocedural epochs of inactivity.
During the 10-min observation period immediately after the phlebotomy or sham procedure, phlebotomy group had a significant effect on the number of inactive epochs (periods of at least 15 s during which a mouse was either largely or completely immobile). The FV group differed from all other groups and exhibited the most inactive epochs (Figure 3; F4,41 = 6.347, P < 0.001; Tukey post hoc tests comparing FV with all other groups, P < 0.01 in all cases; no other intergroup differences).
Figure 3.
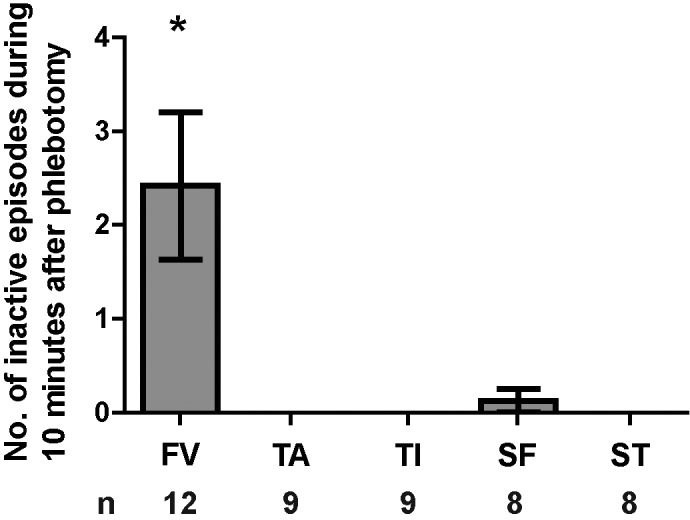
Mice in the FV phlebotomy group were more often inactive than all other groups. The number of inactive episodes, defined as being largely immobile or freezing for 15 s or more, was recorded for 10 min after the phlebotomy or sham procedures. Although the range varied from 0 to 8 inactive periods per mouse, the number of inactive periods observed per mouse (mean ± SEM) in the FV group was significantly greater than that in the other treatment groups. (one-way ANOVA with Tukey post hoc tests: F4,41 = 6.347, P < 0.001). No inactive periods were observed for any tail manipulation group, and only one inactive period was observed in the SF group. Error bars represent SEM. *, P < 0.05.
Effect of phlebotomy techniques on grooming behavior.
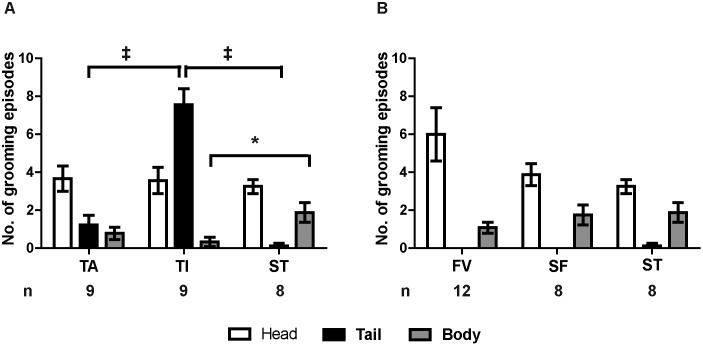
During the 10-min observation period immediately after the phlebotomy or sham procedure, grooming events were recorded and categorized as grooming of the head, tail, or body (any area other than head, tail, or limbs). Because mice of all groups groomed their head much more frequently than their tail, facial and tail grooming data were analyzed separately. Specifically, the TI, TA, and ST (tail techniques) groups were compared using one MANOVA; FV, SF, and ST (facial techniques) groups were compared in another MANOVA. Phlebotomy method had a significant effect on grooming across the 3 tail groups (Figure 4 A; Wilks λ, F6,42 = 10.242, P < 0.001). Between-subjects effects testing showed no difference in the number of head grooming events among groups (F2,23 = 0.121, P = 0.886), but there was a highly significant effect on the number of tail grooming events (F2,23 = 45.769, P < 0.001). Specifically, the TI group exhibited more tail grooming events than either TA or ST groups (Tukey post hoc test, P < 0.001 for both pairwise tests). There was no difference between TA and ST in the number of tail grooming events (Tukey post hoc test, P = 0.416). In addition, group significantly affected body grooming events (F2,23 = 4.582, P = 0.021), with the TI group exhibiting significantly fewer body grooming events than the ST group (Tukey post hoc test, P = 0.019). No other pairwise differences were significant.
Figure 4.
Phlebotomy by tail incision, but not tail tip amputation, evoked significantly more tail grooming. Grooming behavior was recorded during the 10 min after the observation period and was categorized according to body location (head, tail, or body) and averaged by treatment group. (A) TA, TI, and ST groups were compared using MANOVA with Tukey post hoc tests. The TI group exhibited significantly more tail grooming (mean ± SEM) than did either the ST (P < 0.001) or TA groups (P < 0.001). The TA group did not differ from the ST group in tail grooming frequency. In addition, TI mice exhibited significantly fewer body grooming episodes than did ST animals (P = 0.019). (B) The grooming behavior of FV, SF, and ST groups were compared in the same manner; there was no difference in the frequency or distribution of grooming behaviors among these groups ‡, P < 0.001; P < 0.05.
Among the 3 facial phlebotomy groups (FV, SF, ST), phlebotomy method had no effect on the number of head, body, or tail grooming events (Figure 4 B; Wilks λ: F6,46 = 1.374, P = 0.245). Mice always spent significantly more time grooming the head than the body and more time grooming the body than the tail, regardless of phlebotomy group (grooming times: F2,75 = 29.773, P < 0.001; P < 0.05 for all Tukey pairwise comparisons; interaction with phlebotomy method: F4,75 = 2.267, P = 0.070). Overall, only the tail incision method evinced a significant increase in grooming at the phlebotomy site.
Activity in the open field.
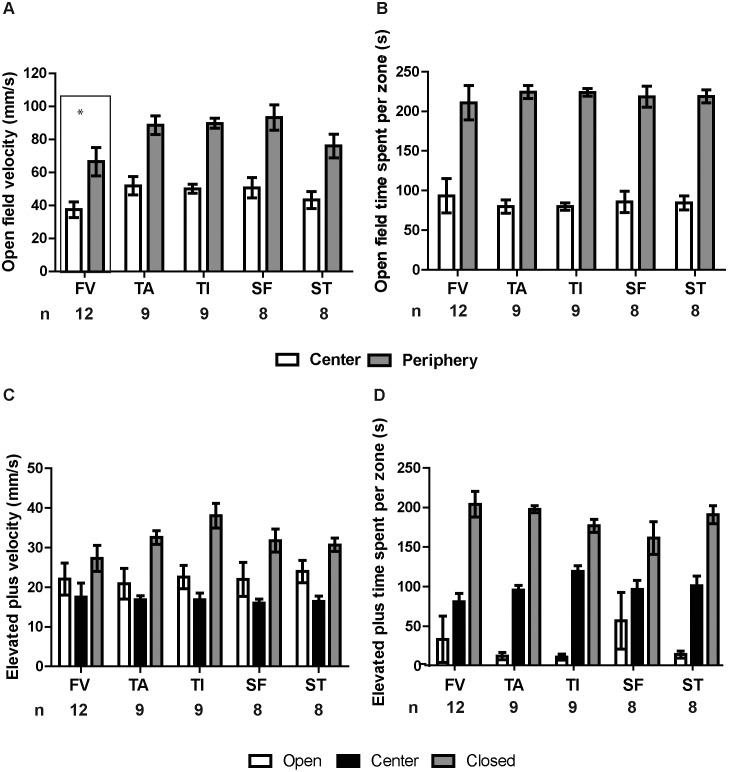
Within the open field arena, zone (central compared with peripheral) had a highly significant effect on mean velocity (mm/s; this measure was averaged over the total time spent in a given zone and therefore conflates velocity of movement with the proportion of time spent moving). On average, mice moved less in the center of the open field than along its edges (Figure 5 A; F1,82 = 84.692, P < 0.001). In addition, phlebotomy group had a significant effect on mean velocity (F4,82 = 4.415, P = 0.003), with the FV group exhibiting a slightly but significantly lower velocity than the TA, TI, or SF groups (Tukey test, P < 0.025 in each case); a similar effect of FV phlebotomy has been previously reported.30 No other pairwise differences or interactions were significant.
Figure 5.
The FV group exhibited a significantly lower average speed in the open field test relative to all other groups. Mice were placed into an open field arena for 5 min, during which their movements were recorded and analyzed with CleverSys TopScan software. (A) Velocity and (B) time spent in the center and peripheral zones were analyzed. (A) Region (center compared with periphery, P < 0.001) and treatment (P = 0.003) had significant effects on velocity; mice moved more slowly in the center than along the periphery. Between phlebotomy groups, the FV group exhibited a significantly lower velocity overall (regardless of zone) than any of the other phlebotomy groups (P < 0.05 for all) except ST (P > 0.05); there were no other significant pairwise differences between groups. (B) Mice spent more time along the periphery (P < 0.001) than in the center of the open field, but treatment had no effect on the times spent in either region (P = 0.862). After the open field test, mice were placed into the observation cage for 5 min, then placed in an elevated plus maze for 5 min. Maze zone significantly influenced (C) mean velocity and (D) the time spent in each zone (P < 0.001), but phlebotomy group did not affect performance in the elevated plus maze (P > 0.05). Error bars represent SEM.
As expected, the times spent in each zone differed significantly (Figure 5 B; F1,82 = 202.482, P < 0.001), with mice spending more time in the peripheral zone than in the center of the open field. Phlebotomy group did not significantly affect the distribution of times spent in each zone (zone×phlebotomy group interaction: F4,82 = 0.323, P = 0.862).
Elevated plus maze performance.
Maze zone (open arm, closed arm, or center) had a significant effect on mean velocity and the total time spent in each zone (Figure 5 C and D; Wilks λ: F4,224 = 75.406, P < 0.001). Between-subjects effects analysis indicated that both dependent variables were significantly affected by zone (velocity: F2,113 = 38.43, P < 0.001; time spent: F2,113 = 147.522, P < 0.001), as expected for normal rodent behavior. The interaction between zone and phlebotomy group had no effect on velocity (P > 0.05), although it had a marginal effect on the time spent in each zone (F8,113 = 2.091, P = 0.042; Figure 5 D).
Nest building.
Phlebotomy groups did not differ regarding nest-building scores (Figure 6; Kruskal–Wallis test, H4,42 = 3.519, P = 0.475) or nest location (corner compared with central; Kruskal–Wallis test, H4,42 = 4.271, P = 0.371).
Figure 6.
Nest-building scores did not differ significantly between phlebotomy groups. A modified version of a published nest-scoring system9 was used to score nests built within a 4-h period. Mice were moved to a clean cage with a new cotton square about 6 h after phlebotomy and removed from the cage 4 h later for euthanasia. Pictures of the nests then were scored by an assessor who was blind to phlebotomy group. (A) Examples of nests receiving each indicated score. (B) Quantification of average nest score by group. The FV group seemed to exhibit a lower average nest score, but the difference was not significant (Kruskal–Wallis test: H4,42 = 3.519, P = 0.475). Error bars represent SEM.
Histology.
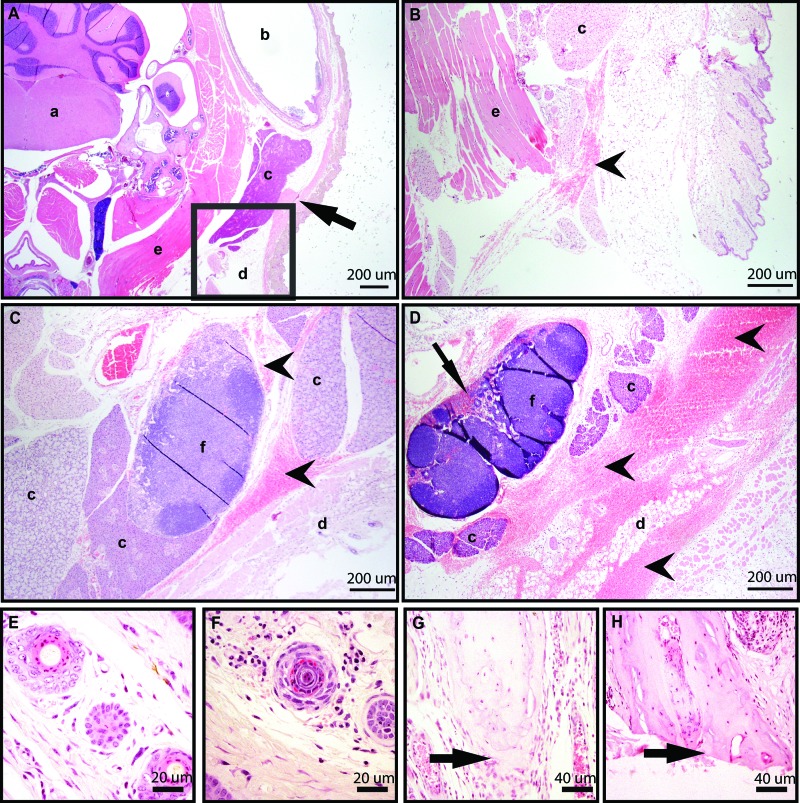
Of 11 FV group mice scored histologically, 6 had no significant findings (3 mice with one puncture attempt and 3 mice with 3 puncture attempts; Figure 7 A); the remaining 5 FV mice exhibited some degree of hemorrhage. Of these 5 FV mice, 3 had focal hemorrhage (one mouse each with 1, 2, or 3 puncture attempts; Figure 7 B), 1 mouse with 3 attempts had locally extensive hemorrhage (Figure 7 C), and 1 mouse with 3 attempts had extensive hemorrhage (Figure 7 D). Areas of hemorrhage included neck muscle, around the trigeminal ganglion, submandibular and parotid salivary glands, brown fat, and the mandibular lymph nodes. Two of the 11 FV mice exhibited myodegeneration (one mouse each with 1 and 3 attempts). Of the 4 randomly chosen TI mice, 2 had no significant findings, whereas the remaining 2 exhibited mild neutrophilic infiltration into the deep dermis (Figure 7 E and F). Finally, 5 randomly chosen TA mice were examined. Three had the last caudal vertebra transected, while the last vertebra was intact in 2 (Figure 7 G and H). All 5 had fibrin present at the tail tip, 4 had mild neutrophil infiltration, and 1 had moderate neutrophil infiltration.
Figure 7.
Tissue sections stained with hematoxylin and eosin showed histologic changes associated with phlebotomy via (A–D ) the facial vein, (E and F) tail vein incision, or (G and H) tail tip amputation. (A) Transverse section of the head including the facial vein (arrow) and showing no significant histologic findings from a mouse with a single attempt at facial vein puncture. (B) Transverse section of the head, showing an enlarged version of the boxed region in A, from a mouse with a single attempt at facial vein puncture showing mild hemorrhage (arrowhead). (C) Transverse section of the head showing moderate hemorrhage in the subcutaneous tissue and surrounding the mandibular lymph node (arrowheads). (D) Transverse section of head showing extensive hemorrhage around the parotid gland, brown fat, and mandibular lymph node (arrow heads), with RBC in lymph node sinuses (arrow) . (E) Cross section of midtail with no significant findings in a TI mouse. (F) Cross section of mid-tail in a mouse from the TI group showing mild neutrophilic infiltrate in the deep dermis of cross section. (G) Longitudinal section of the tail tip showing an intact vertebra (arrow) with fibrin and mild neutrophilic infiltrate in a mouse from the TA group. (H) Longitudinal section of the tail tip showing the last caudal vertebra transected (arrow) with fibrin and moderate neutrophilic infiltrate in a mouse from the TA group. a, brainstem; b, external ear canal; c, salivary gland; d, adipose tissue; e, skeletal muscle; f, lymph node.
Discussion
This study compared the physical and behavioral effects of tail tip amputation, a phlebotomy method not commonly used at our institution, with those from the 2 commonly used methods of facial vein puncture and lateral tail vein incision. Blood glucose levels, performance on an elevated plus maze, and nest-building scores did not differ significantly among groups. No mice in any group produced ultrasonic vocalizations during the procedures. Mice in the FV and SF groups had significantly more audible vocalizations than the other groups, and mice in the FV group had significantly more inactive epochs during the 10-min postprocedural observation than all other groups. Mice in the TI group exhibited significantly more tail grooming events during the 10-min observation period than did TA or ST mice, whereas the FV, SF, and ST groups showed no differences in the grooming of the head, body, or tail. Compared with the other groups, mice in the FV group had a significantly lower average velocity in the open field test and more extensive histologic tissue trauma. The findings support tail tip amputation as a potentially superior method of phlebotomy in mice.
We found tail tip amputation to be technically easier to perform than facial vein phlebotomy for several reasons. Gently manipulating mice by their tail requires less dexterity and is likely less stressful for novice and even experienced handlers. The tail tip is easily visualized, and in our experience failed blood collection via tail tip amputation is extremely rare: all 9 trials in this study were successful. In contrast, the facial vein is not visible, and phlebotomy involves the use of landmarks to puncture the vein. Therefore, this technique is more likely to require repeated puncture attempts. In addition, we found tail tip amputation to be more feasible than lateral tail vein incision. Depending on skin pigmentation, the lateral tail vein may take longer to visualize than the tail tip, and the operator must judge an appropriate depth of incision. We also found it easier to gently milk the tail to collect blood drops in the TA group than the TI group, where the incision–collection site was partway down the tail and had to be avoided by the fingers applying gentle tail restraint. We found that all 3 methods could be used to collect the appropriate blood volume based on body weight.
The lack of a difference in blood glucose levels between the 3 phlebotomy methods (measured as an acute physiologic stress response) may reflect an inadequate amount of time for activation of the hypothalamic–pituitary–adrenal axis during the phlebotomy procedures, which required 2 min or less to complete. This lack of difference may indicate that when blood is to be collected once only, all methods might be acceptable if performed before hypothalamic–pituitary–adrenal axis activation can occur, in regard to the goal of minimizing variability in samples due to stress. Activation of the hypothalamic–pituitary–adrenal axis would likely play a more important role in sample characteristics based on repeated blood sampling. Moreover, hematologic parameters, including blood glucose, hemolysis, WBC counts, and serum chemistry values, can vary significantly between sites of blood collection.1,2,7,25
The significantly increased number of audible vocalizations by the FV and SF groups suggests that those procedures were more painful or stressful to the mice. The vocalization proportions were similar between the SF and FV groups, and the vocalizations generally coincided with handling procedures rather than actual phlebotomy. Very few vocalizations were heard in the other 3 groups, where the only restraint was gentle manipulation of the tail. This finding is in agreement with other reports, emphasizing that handling may have greater effects than tissue sampling procedures themselves in mice.8 Although the production of ultrasonic vocalizations is associated with aversive stimuli in rats22 and chronic pain in mice,17 our findings suggest that neither audible nor ultrasonic vocalizations are reliably associated with acute pain in laboratory mice, in agreement with a previous study.34 However, our data do demonstrate that scruffing results in vocalization almost 100% of the time, whereas gentle tail manipulation rarely incites vocalization, suggesting that tail manipulation is less stressful or disruptive to mice than are any blood collection methods requiring scruffing.
The increased number of epochs of inactivity during the 10 min after blood collection further suggests that the FV phlebotomy method has a greater effect on mouse wellbeing than the other methods tested. This finding is in agreement with a previous study, which yielded lower activity scores in mice who had undergone facial vein or anesthetized jugular vein bleeding than in mice that had undergone retrobulbar, saphenous, or tail vein blood collection.30 We suggest that the reduced activity of the FV group might be a result of increased pain or distress.
Phlebotomy group had no significant effect on the grooming of the head, body, or tail when FV, SF, and ST groups were compared. This finding may indicate there was no significant difference in pain or discomfort after phlebotomy between the groups. However, mice in the TI group groomed their tail significantly more than did mice in the TA or ST groups, suggesting that TI is more painful or disruptive than TA. In previous studies, when grooming was factored in to a total impact score, removal of the distal 5 mm of tail had a significant effect;6,15 these previous results resemble the effects on our TI mice but contrast sharply with our TA group. We did, however, observe a wicking effect of blood along the skin in the TI group which was not present in the TA group. This slight wicking of blood along the skin might have contributed to the increased tail grooming in addition to, or instead of, actual pain or discomfort in this group. The lack of a difference in tail grooming behavior between the TA and ST groups supports tail tip amputation as a humane method of blood collection in awake mice.
The mice exhibited normal behavior in the open field test and elevated plus maze, spending less time and moving more slowly in the open regions of both tests. The reduced mean velocity of the FV group in the open field test supports the interpretation of greater pain or distress (or both) associated with this method that was also suggested by the decreased activity during the 10 min observation period in this group. In addition, this finding resembles the open field results reported in a previous study, in which mice in facial vein and jugular vein blood collection groups traveled significantly less distance in the open field test than did mice that had undergone retrobulbar, saphenous, or tail vein blood collection.30 Behavioral responses might have been affected by moving mice to single housing 24 h prior to experimental manipulation; however, because this procedure was identical across treatment groups, it is unlikely to influence comparative results. In addition, performance might generalize across repeated testing instances, but this generalization is unlikely to influence outcomes in our study because the tests were simple exploration metrics and were performed in the same order and on the same timescale among groups.
We noted a nonsignificant trend toward decreased nest complexity in the FV group. If facial vein puncture caused pain in the masticatory muscles, nesting pad shredding might be expected to decrease, given that mice use their teeth to shred bedding material. Although important differences between the phlebotomy groups might not have emerged during the 4-h window in which the nests were built, it also is possible that nest scoring is not a sufficiently sensitive method with which to detect the effects of phlebotomy. Analyzing nest complexity at different time points or with different nesting material might have yielded different results, given that nesting material has been shown to influence nest complexity.23
The most severe histologic changes were found in mice from the FV phlebotomy group. Although 6 of the 11 FV group mice scored for histologic changes had no significant findings, 5 had hemorrhage ranging from local to extensive and surrounding several important anatomic structures. There was no clear pattern between the number of phlebotomy attempts and the severity of the histologic changes, given that mice that experienced 3 attempts ranged from no significant findings to extensive hemorrhage, whereas focal hemorrhage and myodegeneration were seen in a mouse that underwent a single phlebotomy attempt. However, the most extensive hemorrhage was seen in a mouse that had 3 facial vein phlebotomy attempts. These histologic changes of hemorrhage and muscle damage after facial vein puncture are similar to those observed in previous reports.29
Work evaluating the effect of removing the distal 5 mm of tail for genotyping revealed an increase in dermal and subcutaneous inflammation.6 Mild neutrophilic infiltration and fibrin accumulation are considered a normal response to mild trauma and occurred in 2 of the 4 TI mice scored and in 4 of the 5 TA mice scored. The lack of hemorrhage and other deleterious changes in either the TI or TA group likely reflect the less extensive vasculature in the tail compared with the face, the smaller distance between the target vessel and the surface of the skin, and a greater degree of accuracy achieved by the ability to visualize the target vessel in the tail. Of the 5 TA group mice, 3 had the last caudal vertebra transected, whereas it was intact in the other 2. Ideally transecting vertebrae would be avoided—and perhaps could be avoided with greater development of this phlebotomy method—but the lack of behavioral responses overall in the TA group suggest that transection did not cause noteworthy pain or distress, and there were no histologic differences between the mice that did or did not have vertebrae transected. These histologic findings indicate that greater trauma risk was incurred when phlebotomy was attempted via the facial vein than when it was attempted through lateral tail vein incision or tail tip amputation, although all phlebotomy methods caused some level of tissue response.6,12
The adverse effects of facial vein puncture may be reduced when performed by a highly experienced phlebotomist, for whom the need for multiple venipuncture attempts is rare. However, often research personnel who have been appropriately trained but have not yet gained extensive experience will perform phlebotomy. Our results emphasize that facial vein phlebotomy is less practical for such personnel than tail tip amputation and increases the likelihood of pain and distress.
In summary, we found no evidence that tail tip amputation causes more pain or stress to mice than a sham manipulation of the tail or than 2 already approved methods in awake, adult C57BL/6J mice according to the metrics we used. The increased number of audible vocalizations, increased number of inactive epochs in the 10-min observation period, reduced velocity in the open field test, and increased severity of postprocedural histologic findings suggest that facial vein phlebotomy may be the least desirable of the 3 tested methods in terms of animal welfare, despite requiring less total handling time. Furthermore, we find no evidence to suggest the need for analgesia or anesthesia to accompany tail tip amputation, given the lack of differences between the TA and ST groups. However, alternative methods of assessing pain and distress or evaluating additional time points might reveal some adverse effects of these phlebotomy methods in mice. Animals were acclimated to the environmental conditions of the behavior testing suite for 1 wk; additional acclimatization might further reduce experimental variation. Finally, the length of time the tail is milked for blood collection may alter the influence of the procedure on the animal. This method may not be appropriate in situations in which multiple temporally spaced blood samples need to be collected or in mice from which tail biopsies have previously been collected for genotyping. These circumstances should be experimentally evaluated before adoption of tail tip amputation as a standard method of blood collection under these conditions. Future studies could evaluate the feasibility and animal welfare consequences of repeated tail tip amputation and the feasibility of blood-clot disruption for repeated sampling within a short time frame. From an animal welfare standpoint, we suggest that tail tip amputation is a superior phlebotomy method in experimental design.
Acknowledgments
We thank Matthew Einhorn for his assistance with behavioral testing and Courtney Hale and Haley Szczublewski for their assistance with analyses of vocalization data.
References
- 1.Aasland KE, Skjerve E, Smith AJ. 2010. Quality of blood samples from the saphenous vein compared with the tail vein during multiple blood sampling of mice. Lab Anim 44:25–29. [DOI] [PubMed] [Google Scholar]
- 2.Abatan OI, Welch KB, Nemzek JA. 2008. Evaluation of saphenous venipuncture and modified tail-clip blood collection in mice. J Am Assoc Lab Anim Sci 47:8–15. [PMC free article] [PubMed] [Google Scholar]
- 3.American Association for Laboratory Animal Science. [Internet] 2017. Position Statement Humane Care and Use of Laboratory Animals. Federal Register: Office of Science and Technology Policy; [Cited 5 May 2016]. Available at: https://www.aalas.org/about-aalas/position-papers/humane-care-and-use. [Google Scholar]
- 4.Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. 2001. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci 115:443–454. [PubMed] [Google Scholar]
- 5.Arras M, Rettich A, Seifert B, Kasermann HP, Rulicke T. 2007. Should laboratory mice be anaesthetized for tail biopsy? Lab Anim 41:30–45. [DOI] [PubMed] [Google Scholar]
- 6.Braden GC, Brice AK, Hankenson FC. 2015. Adverse effects of vapocoolant and topical anesthesia for tail biopsy of preweanling mice. J Am Assoc Lab Anim Sci 54:291–298. [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen SD, Mikkelsen LF, Fels JJ, Bodvarsdottir TB, Hansen AK. 2009. Quality of plasma sampled by different methods for multiple blood sampling in mice. Lab Anim 43:65–71. [DOI] [PubMed] [Google Scholar]
- 8.Cinelli P, Rettich A, Seifert B, Burki K, Arras M. 2007. Comparative analysis and physiologic impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184. [DOI] [PubMed] [Google Scholar]
- 9.Crawley JN. 2003. Behavioral phenotyping of rodents. Comp Med 53:140–146. [PubMed] [Google Scholar]
- 10.Deacon RMJ. 2006. Assessing nest building in mice. Nat Protoc 1:1117–1119. [DOI] [PubMed] [Google Scholar]
- 11.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C, European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods 2001. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23. [DOI] [PubMed] [Google Scholar]
- 12.Fried JH, Worth DB, Brice AK, Hankenson FC. 2015. Type, duration, and incidence of pathologic findings after retroorbital bleeding of mice by experienced and novice personnel. J Am Assoc Lab Anim Sci 54:317–327. [PMC free article] [PubMed] [Google Scholar]
- 13.Gärtner K, Büttner D, Döhler K, Friedel R, Lindena J, Trautschold I. 1980. Stress response of rats to handling and experimental procedures. Lab Anim 14:267–274. [DOI] [PubMed] [Google Scholar]
- 14.Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR. 2013. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp 82:51012 doi.org/10.3791/51012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankenson FC, Braden-Weiss GC, Blendy JA. 2011. Behavioral and activity assessment of laboratory mice (Mus musculus) after tail biopsy under isoflurane anesthesia. J Am Assoc Lab Anim Sci 50:686–694. [PMC free article] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 17.Kurejova M, Nattenmuller U, Hildebrandt U, Selvaraj D, Stosser S, Kuner R. 2010. An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer pain and neuropathic pain. Mol Pain 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kvetnansky R, Sun CL, Lake CR, Thoa N, Torda T, Kopin IJ. 1978. Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-β-hydroxylase. Endocrinology 103:1868–1874. [DOI] [PubMed] [Google Scholar]
- 19.Naggert JK, Svenson KL, Smith RV, Paigen B, Peters LL, [Internet] 2003. Diet effects on bone mineral density and content, body composition, and plasma glucose, leptin, and insulin levels. Online Mouse Phenome Database; [Cited 12 January 2016]. Available at: http://phenome.jax.org/ [Google Scholar]
- 20.Pecoraro N, Dallman MF, Warne JP, Ginsberg AB, Laugero KD, la Fleur SE, Houshyar H, Gomez F, Bhargava A, Akana SF. 2006. From Malthus to motive: How the HPA axis engineers the phenotype, yoking needs to wants. Prog Neurobiol 79:247–340. [DOI] [PubMed] [Google Scholar]
- 21.Pellow S, Chopin P, File SE, Briley M. 1985. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167. [DOI] [PubMed] [Google Scholar]
- 22.Portfors CV. 2007. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci 46:28–34. [PubMed] [Google Scholar]
- 23.Robinson-Junker A, Morin A, Pritchett-Corning K, Gaskill BN. 2016. Sorting it out: bedding particle size and nesting material processing method affect nest complexity. Lab Anim 51:170–180. [DOI] [PubMed] [Google Scholar]
- 24.Rock ML, Karas AZ, Rodriguez KB, Gallo MS, Pritchett-Corning K, Karas RH, Aronovitz M, Gaskill BN. 2014. The time-to-integrate-to-nest test as an indicator of wellbeing in laboratory mice. J Am Assoc Lab Anim Sci 53:24–28. [PMC free article] [PubMed] [Google Scholar]
- 25.Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM. 2002. Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther 13:155–161. [DOI] [PubMed] [Google Scholar]
- 26.Sutanto W, de Kloet ER. 1994. The use of various animal models in the study of stress and stress-related phenomena. Lab Anim 28:293–306. [DOI] [PubMed] [Google Scholar]
- 27.Tabata H, Kitamura T, Nagamatsu N. 1998. Comparison of effects of restraint, cage transportation, anaesthesia and repeated bleeding on plasma glucose levels between mice and rats. Lab Anim 32:143–148. [DOI] [PubMed] [Google Scholar]
- 28.Takao K, Kobayashi K, Hagihara H, Ohira K, Shoji H, Hattori S, Koshimizu H, Umemori J, Toyama K, Nakamura HK, Kuroiwa M, Maeda J, Atsuzawa K, Esaki K, Yamaguchi S, Furuya S, Takagi T, Walton NM, Hayashi N, Suzuki H, Higuchi M, Usuda N, Suhara T, Nishi A, Matsumoto M, Ishii S, Miyakawa T. 2013. Deficiency of schnurri 2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology 38:1409–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teilmann AC, Nygaard Madsen A, Holst B, Hau J, Rozell B, Abelson KSP. 2014. Physiologic and pathologic impact of blood sampling by retrobulbar sinus puncture and facial vein phlebotomy in laboratory mice. PLoS One 9:e113225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai PP, Schlichtig A, Ziegler E, Ernst H, Haberstroh J, Stelzer HD, Hackbarth H. 2015. Effects of different blood collection methods on indicators of welfare in mice. Lab Anim (NY) 44:301–310. [DOI] [PubMed] [Google Scholar]
- 31.Tuli JS, Smith JA, Morton DB. 1995. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Lab Anim 29:90–95. [DOI] [PubMed] [Google Scholar]
- 32.Tuli JS, Smith JA, Morton DB. 1995. Stress measurements in mice after transportation. Lab Anim 29:132–138. [DOI] [PubMed] [Google Scholar]
- 33.van Herck H, Baumans V, Brandt CJ, Hesp AP, Sturkenboom JH, van Lith HA, van Tintelen G, Beynen AC. 1998. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab Anim 32:377–386. [DOI] [PubMed] [Google Scholar]
- 34.Williams WO, Riskin DK, Mott AK. 2008. Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci 47:8–10. [PMC free article] [PubMed] [Google Scholar]