Abstract
Background
A variety of agents have been used to treat female pattern hair loss (FPHL), including topical minoxidil, topical 17α-estradiol, oral anti-androgen agents, and mineral supplements. Compared with these single agent regimens, combination therapies could be a better therapeutic option in expectation of superior treatment outcome.
Objective
This study was designed to determine the efficacy of a combination therapy consisting of topical 0.025% 17α-estradiol and 3% minoxidil in Korean patients with FPHL.
Methods
Therapeutic efficacy was evaluated in 34 women who applied topical 0.025% 17α-estradiol and 3% minoxidil once daily for more than 6 months. Phototrichogram analysis was performed before and after therapy. The efficacy was evaluated with respect to total hair count, hair caliber (as assessed by phototrichogram analysis), and photographic assessment.
Results
Total hair count and hair caliber both increased from baseline to 6 months in patients treated with the combination therapy of topical 0.025% 17α-estradiol and 3% minoxidil (p<0.001). Photographic assessment also revealed significant disease improvement, thus supporting the therapeutic efficacy.
Conclusion
A combination therapy consisting of topical 0.025% 17α-estradiol and 3% minoxidil can be tried as an effective treatment for FPHL.
Keywords: 17a-estradiol, Androgenetic alopecia, Combination therapy, Female pattern hair loss, Minoxidil
INTRODUCTION
Androgenetic alopecia (AGA), also known as pattern hair loss, is a relatively common disease in adults over 40 years old, with a prevalence of up to 73% in the general Asian population1.
Although AGA itself does not cause serious health-related issues, it may cause personal, social, and work-related problems, all of which can affect life quality2,3. Thus, AGA should not be regarded simply as an isolated disease and treatments should be holistic and comprehensive.
Miniaturization of the hair follicles and changes in hair cycle dynamics are crucial elements of AGA pathogenesis. Unlike male pattern hair loss, female pattern hair loss (FPHL) is characterized by a reduction in the number of terminal fibers per follicular unit over the crown and frontal scalp with retention of the frontal hairline4. Various treatments have been used for AGA. The medical therapies commonly used in FPHL, in addition to topical minoxidil solution, are cyproterone acetate, spironolactone and flutamide, finasteride, topical 17α-estradiol (alfa-tradiol), mineral supplements, and prostaglandin analogs, as well as surgical therapy and light therapy5,6,7,8,9,10.
However, combination therapies are often preferred in clinical settings, since they are expected to yield superior treatment outcomes compared with single agent regimens. The aim of the current study was to determine the efficacy of a commonly used combination therapy consisting of topical 0.025% 17α-estradiol and 3% minoxidil in Korean patients with FPHL.
MATERIALS AND METHODS
Study design and subjects
This was a retrospective, noncomparative, single institution study. Patients diagnosed with FPHL between March 2010 and December 2015 at Wonju Severance Christian Hospital who had concurrently applied minoxidil and 17α-estradiol for more than 6 months were considered. Of the patients who visited our institution with FPHL, only patients with both gross photos and phototrichograms, before and after treatment, were enrolled. The patient age range was 18 to 71 years old and all patients were diagnosed with F type FPHL according to the basic and specific (BASP) classification11. Patients with any dermatological disorder, systemic disease, or abnormal laboratory finding that required additional treatment and evaluation that could have affected the results of the study were excluded.
In addition to age, family history was also obtained, and the patients were categorized and stratified according to the severity indices suggested by the BASP classification scheme.
In the BASP classification nomenclature11, LF1 is defined as the mild group, LF2 as the moderate group, and LF3 as the severe group.
This study was approved by the Institutional Review Board of Wonju College of Medicine, Yonsei University (IRB no. CR316011).
Drug treatment
For treatment, 0.025% 17α-estradiol (Ell-Cranell® alpha 0.025%; Galderma Korea, Co., Seoul, Korea) and 3% minoxidil (Minoxyl® 3%; Hyundai Pharm, Co., Seoul, Korea) were used. Using a predosed applicator, 3% minoxidil and 17α-estradiol were applied once a day (1 ml/application and 3 ml/application, respectively), and the head was massaged for approximately one minute to facilitate drug absorption. Topical 17α-estradiol was applied in the morning, whereas minoxidil was applied in the evening.
Efficacy evaluation
All subjects used the 0.025% 17α-estradiol and 3% minoxidil combination for 6 to 12 months. Efficacy was first evaluated by examining the change in the number of hairs per unit area as assessed by phototrichogram analysis at 6 months using a Folliscope® instrument (Lead M Co., Seoul, Korea).
For the second efficacy evaluation, the change in the number of hairs per unit area after 12 months since the initial application and the changes in hair thickness at 6 months and 12 months were assessed.
The subjects were subdivided according to family history and the changes of hair count at 6 months were compared between the two groups. The results were also compared among the groups stratified according to FPHL severity.
Clinical photograph evaluation
Prior to photographic documentation, each patient’s hair was combed in order to expose all areas of hair loss. Paired baseline photos were analyzed and compared with paired photos taken at 6 months or one year since the beginning of treatment. Photographs were evaluated on a scale ranging from ‘greatly improved’ to ‘not improved.’ The classifications were defined as follows: ‘greatly improved,’ more than 75% improvement; ‘moderately improved,’ 50%~75% growth; ‘slightly improved,’ 25%~50% improvement; and ‘not improved,’ less than 25% improvement.
Evaluation by phototrichogram analysis
The changes in hair thickness and density after 6 and 12 months of application were assessed by phototrichogram analysis using constant contrast and exposure.
Even though we had not done tattooing on the scalp, we had tried to improve the accuracy of taking phototrichogram from the same area by using the device developed by Canfield Scientific Inc. (Fairfield, NJ, USA), which could fix the forehead and chin.
The number of total hairs in a unit area (hair density) was measured by calculating the number of all hairs within the 70 mm2 circle. The diameters of the thickest five hairs were measured using a 200× lens and their average is presented as the average hair thickness.
Statistical analysis
Changes in the number of hairs and the hair diameters were assessed by phototrichogram analysis. Differences in data were analyzed by the paired t-test or the Wilcoxon signed rank test. Unless stated otherwise, the significance level for statistical analysis was 0.05. All data were analyzed with the SPSS statistics program ver. 23.0 (IBM Co., Armonk, NY, USA).
RESULTS
Subject information
1) Subject age and disease history of FPHL
A total of 34 subjects were recruited. The average age of the subjects was 43.15 (±14.39) years. Patients aged between 30 and 59 years comprised more than 2/3 of the overall subjects. Approximately 55% of all subjects had a family history of AGA and most patients (55%) were classified into the moderate BASP classification group, followed by the mild and severe groups (Table 1).
Table 1. Patient demographic and hair loss features at baseline.
| Variable | Patients (n=34) |
|---|---|
| Age (yr) | |
| Mean±SD | 43.15±14.39 |
| Min, max | 18, 71 |
| Age group (yr) | |
| 18~29 | 7 (20.6) |
| 30~39 | 6 (17.6) |
| 40~49 | 9 (26.5) |
| 50~59 | 8 (23.5) |
| >59 | 4 (11.8) |
| Family history of AGA | |
| Yes | 19 (55.9) |
| No | 15 (44.1) |
| Severity of hair loss as defined by the BASP classification | |
| Mild | 11 (32.4) |
| Moderate | 19 (55.9) |
| Severe | 4 (11.8) |
| Average amounts of drugs prescribed for daily (ml)* | |
| Minoxidil | 1.03±0.46 |
| 17α-estradiol | 2.62±0.79 |
Values are presented as mean±standard deviation (SD), number only, or number (%). AGA: androgenetic alopecia, BASP: basic and specific. *Average amounts of drugs prescribed for daily=(prescribed bottles×volume of 1 bottle/treatment period).
Efficacy evaluation
1) First efficacy evaluation
(1) Change in hair number as assessed by phototrichogram analysis after 6 months of treatment
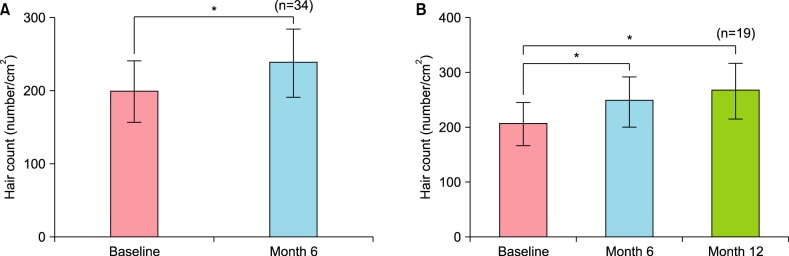
Each subject applied 17α-estradiol and minoxidil for 6 months. The changes in hair count in a constant area are shown in Fig. 1A. The average number of baseline hairs was 199.03±41.19 hairs/cm2, whereas the average number of hairs after 6 months of drug application was 237.68±45.76 hairs/cm2. The mean change over the 6 months was 38.65±39.64 hairs/cm2, which was statistically significant (p<0.0001).
Fig. 1. Changes in the number of hairs per unit area as assessed by phototrichogram analysis after 6 months and 12 months of treatment. (A) Changes after 6 months (n=34), (B) changes after 12 months (n=19). Results are shown as the mean±standard error of the mean. *p<0.001.
2) Second efficacy evaluation
(1) Change in the number of hairs as assessed by phototrichogram analysis after 12 months of treatment
Efficacy was next compared among the 19 patients who applied 0.025% 17α-estradiol and minoxidil for more than 12 months. Their hair counts were as follows: baseline, 205.69±37.90 hairs/cm2; 6 months, 246.46±43.89 hairs/cm2; and 12 months, 265.77±49.25 hairs/cm2. The average changes at 6 months and 12 months were 40.77±36.77 and 60.08±39.35 hairs/cm2, respectively; both these changes were statistically significant (p<0.001; Fig. 1B).
(2) Changes in hair thickness as assessed by phototrichogram analysis after 6 months and 12 months of treatment
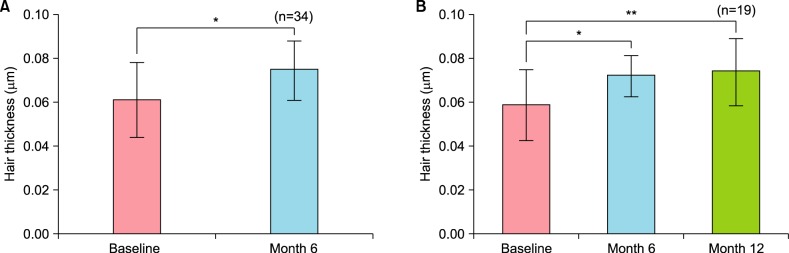
The changes in hair thickness were assessed by phototrichogram analysis after 6 months and one year of treatment, as shown in Fig. 2. The average baseline hair thickness was 0.061±0.017 µm, whereas the average hair thickness after 6 months of drug application was 0.074±0.013 µm (n=34, p<0.01). Similar results were observed in the 19 patients who applied the treatment for more than 1 year. The thicknesses were as follows: baseline, 0.058±0.016 µm; 6 months, 0.072±0.009 µm; and 12 months, 0.073±0.015 µm. Each of these changes was statistically significant when compared with the baseline value (p<0.01, p<0.05, respectively).
Fig. 2. Changes in hair thickness as assessed by phototrichogram analysis after 6 months and 12 months of treatment. (A) Changes after 6 months (n=34), (B) changes after 12 months (n=19). Results are shown as the mean±standard error of the mean. *p<0.05, **p<0.01.
(3) Changes in hair count after treatment stratified according to family history
The treatment responses were next compared according to family history. The average hair counts of the 19 patients with a positive family history were 195.68±38.42 hairs/cm2 at baseline and 242.95±52.57 hairs/cm2 at 6 months; this difference was significant (p< 0.05). The hair counts of the 15 patients who did not have a family history were 203.27±44.08 hairs/cm2 at baseline and 231.00±34.14 hairs/cm2 after 6 months, which was also statistically significant (p<0.01; Fig. 3).
Fig. 3. Changes in hair count after treatment according to disease severity and the presence of family history of androgenetic alopecia (AGA). FHx: family history of AGA, FPHL: female pattern hair loss. Results are shown as the mean±standard error of the mean. *p<0.05, **p<0.01.
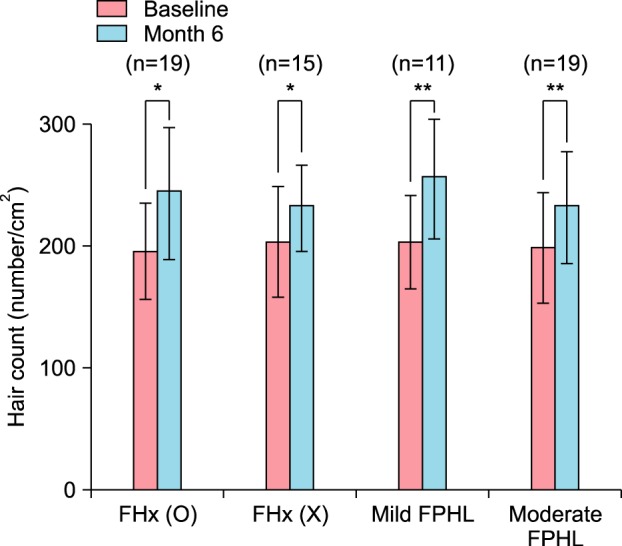
(4) Changes of hair count after treatment according to disease severity
Subjects were categorized into mild (11), moderate (19), and severe (4) groups. The severe group was excluded from this analysis due to an insufficient number of subjects. In the mild group, the baseline hair count was 203.09±36.43 hairs/cm2 and the 6-month count was 255.18±46.62 hairs/cm2. In the moderate group, the baseline hair count was 198.35±44.10 hairs/cm2 and the 6-month count was 231.40±44.81 hairs/cm2. These changes were both significant (*p<0.05, **p<0.01; Fig. 3).
(5) Overall photographic investigation
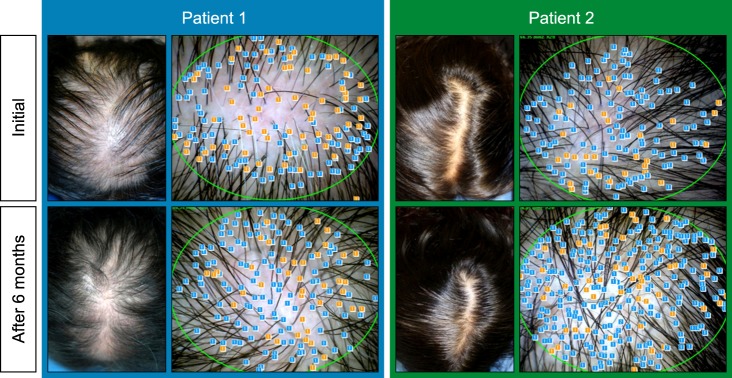
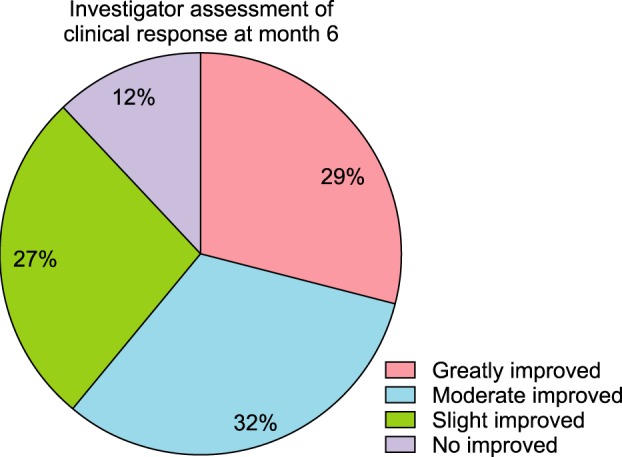
Clinical photos were also compared after 6 months of treatment. By comparing the clinical photos of the patients at baseline and after 6 months, more than 88% of the overall patients exhibited improvement; similarly, 61% of the patients with greater than moderately severe disease exhibited improvement (Fig. 4). In contrast, 12% of the patients showed either no change or aggravation of symptoms (Fig. 5).
Fig. 4. Effectiveness of hair regrowth in 2 patients with female pattern hair loss after 6 months of treatment.
Fig. 5. Overall photographic evaluation of improvement.
3) Safety evaluation
Pruritus and irritation are known adverse events associated with the application of topical minoxidil and 17α-estradiol. All patients were given full instructions for medication application, including the appropriate management if irritation occurred. Except for the patients who switched to another treatment due to irritation, only a minority of patients complained of mild discomfort during the study. No other adverse events occurred to cause the withdrawal of any subject.
DISCUSSION
AGA in females is also called FPHL. Compared with male pattern hair loss, FPHL tends to present in relatively less severe forms. However, these cosmetic issues still may seriously affect the quality of life2,3.
Various medical therapies and surgical procedures are currently used in the treatment of FPHL. However, only limited medications are available with proven efficacy, and those that are found to be effective may not always result in satisfactory treatment outcomes.
Minoxidil for FPHL has been proven to be effective in numerous studies12,13.
The efficacy of this medication was also confirmed in recent meta-analyses in both sexes, which reported a large amount of supporting evidence14.
However, the pathophysiology of minoxidil is still not fully understood. Minoxidil is converted to minoxidil sulfate after application, which is believed to shorten the telogen phase and cause the hair follicle to enter the anagen stage. Some evidence also suggests that minoxidil prolongs anagen and increases hair follicle size. In in vitro studies of various skin and hair follicle cell types, minoxidil has been shown to stimulate cell proliferation, enhance vascular endothelial growth factor and prostaglandin synthesis, and inhibit collagen synthesis, all of which may be relevant to hair growth15.
Minoxidil may also promote hair growth via its action as an adenosine triphosphate-sensitive potassium channel opener, which in turn causes relaxation of vascular smooth muscle, leading to increased cutaneous blood flow16.
Recent studies have suggested that minoxidil also enhances hair growth by increasing the production of prostaglandin E2 through stimulation of prostaglandin endoperoxide synthase-117.
Besides minoxidil, 17α-estradiol has also been commonly used in Europe, South America, and Korea for the past decades.
17α-estradiol is a stereoisomer of the female hormone 17β-estradiol, which suppresses 5α-reductase activity and inhibits 17β-dehydrogenase activity. This inhibition slows the conversion of androstenedione to testosterone and increases the conversion of testosterone to 17β-estradiol and the conversion of androstendione to estrone, thus improving hair growth. However, 17α-estradiol does not exhibit estrogen activity or exhibit only very weak activity18,19,20. Kim et al.21 reported that the hair count and thickness steadily increased after application of topical 17α estradiol when compared with the baseline.
In a double-blind randomized controlled trial by Orfanos and Vogels22, 63% of all patients treated with 17α-estradiol had decreased telogen hair, which was comparable with the level (37%) in the control group.
Due to study by Wozel et al.23, alfa-tradiol is involved more in stabilization or deceleration of hair loss, which is consistent with the results found in the previous placebo-controlled study of 51 patients (9 women) over 6 months by Van Neste and Rushton24.
Combinations of medications with different mechanisms are broadly used in many medical fields in anticipation of the possibility of achieving greater efficacy than with single medications.
Since this was a noncomparative study, however, our data do not provide definitive information as to whether single use of either minoxidil or 17α-estradiol is more effective than the combination.
After 6 months of application, the majority of patients exhibited increased hair count and increased hair thickness. Also, hair count and hair thickness both constantly increased in patients who had continuously applied the medication for 12 months. These changes were also conspicuously observable with the naked eye (Fig. 4).
Furthermore, after 6 months of application, most of patients with or without a family history and with mild type FPHL or moderate type FPHL exhibited increased hair count. When we compare the differences between patients with or without a family history and between patients with mild type FPHL or moderate type FPHL, both of them were not statistically significant. Taken together, combination therapy consisting of topical 0.025% 17α-estradiol and 3% minoxidil shows overall clinical efficacy regardless of symptom severity or family history. In addition to the satisfactory clinical efficacy observed during this study, no major side effects were observed in any patient.
As mentioned previously, treatment with minoxidil can induce an increase in hair density and hair thickness, whereas treatment with 17α-estradiol results in deceleration or stabilization of hair loss. The different mechanisms of the two medications may have contributed to the high effectiveness observed here.
Nonetheless, the clinical photos and phototrichograms had not been always performed on the same areas due to the absence of tattooing on the scalp. And the data were reviewed retrospectively, which may have created some limitations with respect to comparing the efficacy.
Additionally, we have to consider seasonal variations such as increase of hair follicle number in spring season; however, our patients were enrolled randomly regardless of season and majority of patients were not enrolled at spring season. So we assumed that the influence of seasonal variation was insignificant.
Further controlled studies are needed to definitively determine the effectiveness of the simultaneous use of these two medications.
Despite these limitations of our study, we found that the combination therapy of topical 0.025% 17α-estradiol and topical 3% minoxidil had clear clinical efficacy. Thus, this combination therapy is an effective and safe treatment modality for FPHL that can be used promptly in a clinical setting.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Lee WS, Lee HJ. Characteristics of androgenetic alopecia in asian. Ann Dermatol. 2012;24:243–252. doi: 10.5021/ad.2012.24.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Güleç AT, Tanriverdi N, Dürü C, Saray Y, Akçali C. The role of psychological factors in alopecia areata and the impact of the disease on the quality of life. Int J Dermatol. 2004;43:352–356. doi: 10.1111/j.1365-4632.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 3.Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005;331:951–953. doi: 10.1136/bmj.331.7522.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189–199. [PMC free article] [PubMed] [Google Scholar]
- 5.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 6.Kim WJ, Song M, Ko HC, Kim BS, Kim MB. Efficacy of finasteride 1.25 mg on female pattern hair loss; pilot study. Ann Dermatol. 2012;24:370–372. doi: 10.5021/ad.2012.24.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss) J Investig Dermatol Symp Proc. 2003;8:20–23. doi: 10.1046/j.1523-1747.2003.12167.x. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998;317:865–869. doi: 10.1136/bmj.317.7162.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trüeb RM. New and established methods in therapy of hair diseases. Hautarzt. 2003;54:732–740. doi: 10.1007/s00105-003-0558-6. [DOI] [PubMed] [Google Scholar]
- 10.Park HY, Lee WS, Park J, Kim DW, Ahn SY, Jung YJ, et al. An open label, multi-center clinical trial of topical 5% minoxidil solution for the treatment of male androgenetic alopecia (a phase IV study) Korean J Dermatol. 2009;47:295–302. [Google Scholar]
- 11.Lee WS, Ro BI, Hong SP, Bak H, Sim WY, Kim DW, et al. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007;57:37–46. doi: 10.1016/j.jaad.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 12.van Zuuren EJ, Fedorowicz Z, Carter B. Evidence-based treatments for female pattern hair loss: a summary of a Cochrane systematic review. Br J Dermatol. 2012;167:995–1010. doi: 10.1111/j.1365-2133.2012.11166.x. [DOI] [PubMed] [Google Scholar]
- 13.Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011;65:1126–1134.e2. doi: 10.1016/j.jaad.2010.09.724. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Charrette A. Topical minoxidil: systematic review and meta-analysis of its efficacy in androgenetic alopecia. Skinmed. 2015;13:185–189. [PubMed] [Google Scholar]
- 15.Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Marubayashi A, Nakaya Y, Fukui K, Arase S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J Invest Dermatol. 2001;117:1594–1600. doi: 10.1046/j.0022-202x.2001.01570.x. [DOI] [PubMed] [Google Scholar]
- 17.Colombe L, Vindrios A, Michelet JF, Bernard BA. Prostaglandin metabolism in human hair follicle. Exp Dermatol. 2007;16:762–769. doi: 10.1111/j.1600-0625.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann R, Niiyama S, Huth A, Kissling S, Happle R. 17alpha-estradiol induces aromatase activity in intact human anagen hair follicles ex vivo. Exp Dermatol. 2002;11:376–380. doi: 10.1034/j.1600-0625.2002.110413.x. [DOI] [PubMed] [Google Scholar]
- 19.Smart RC, Oh HS. On the effect of estrogen receptor agonists and antagonists on the mouse hair follicle cycle. J Invest Dermatol. 1998;111:175. doi: 10.1046/j.1523-1747.1998.00257.x. [DOI] [PubMed] [Google Scholar]
- 20.Whitaker WL, Baker BL. A comparison of the direct action of estrogen and adrenal cortical extracts on growth of hair in the rat. J Invest Dermatol. 1951;17:69–77. doi: 10.1038/jid.1951.66. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Lee SY, Lee HJ, Yoon NY, Lee WS. 17α-Estradiol (Ell-Cranell® alpha 0.025%) solution on female pattern hair loss: single center, open-label, non-comparative, phase IV study. Ann Dermatol. 2012;24:295–305. doi: 10.5021/ad.2012.24.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orfanos CE, Vogels L. Local therapy of androgenetic alopecia with 17 alpha-estradiol. A controlled, randomized double-blind study (author's transl) Dermatologica. 1980;161:124–132. [PubMed] [Google Scholar]
- 23.Wozel G, Naranayan S, Jäckel A, Lutz G. Alfatradiol (0,025%)-Eine wirksame und sichere Therapieoption zur Behandlung der androgenetischen Alopezie bei Frauen und Männern. Akt Dermatol. 2005;31:553–560. [Google Scholar]
- 24.Van Neste DJ, Rushton DH. Hair problems in women. Clin Dermatol. 1997;15:113–125. doi: 10.1016/s0738-081x(96)00114-9. [DOI] [PubMed] [Google Scholar]