Abstract
Rationale: Neurocognitive outcome after out-of-hospital cardiac arrest (OHCA) is often poor, even when initial resuscitation succeeds. Lower tidal volumes (Vts) attenuate extrapulmonary organ injury in other disease states and are neuroprotective in preclinical models of critical illness.
Objective: To evaluate the association between Vt and neurocognitive outcome after OHCA.
Methods: We performed a propensity-adjusted analysis of a two-center retrospective cohort of patients experiencing OHCA who received mechanical ventilation for at least the first 48 hours of hospitalization. Vt was calculated as the time-weighted average over the first 48 hours, in milliliters per kilogram of predicted body weight (PBW). The primary endpoint was favorable neurocognitive outcome (cerebral performance category of 1 or 2) at discharge.
Measurements and Main Results: Of 256 included patients, 38% received time-weighted average Vt greater than 8 ml/kg PBW during the first 48 hours. Lower Vt was independently associated with favorable neurocognitive outcome in propensity-adjusted analysis (odds ratio, 1.61; 95% confidence interval [CI], 1.13–2.28 per 1-ml/kg PBW decrease in Vt; P = 0.008). This finding was robust to several sensitivity analyses. Lower Vt also was associated with more ventilator-free days (β = 1.78; 95% CI, 0.39–3.16 per 1-ml/kg PBW decrease; P = 0.012) and shock-free days (β = 1.31; 95% CI, 0.10–2.51; P = 0.034). Vt was not associated with hypercapnia (P = 1.00). Although the propensity score incorporated several biologically relevant covariates, only height, weight, and admitting hospital were independent predictors of Vt less than or equal to 8 ml/kg PBW.
Conclusions: Lower Vt after OHCA is independently associated with favorable neurocognitive outcome, more ventilator-free days, and more shock-free days. These findings suggest a role for low-Vt ventilation after cardiac arrest.
Keywords: out-of-hospital cardiac arrest, cardiac arrest, ventilator-induced lung injury, acute lung injury, cerebral ischemia
At a Glance Commentary
Scientific Knowledge on the Subject
Patients suffering cardiac arrest have several risk factors for lung injury and often experience poor neurocognitive outcome. Low tidal volumes (Vts) attenuate pulmonary and extrapulmonary organ injury in patients at risk of ventilation-induced lung injury. Experimental data suggest low Vt also may be neuroprotective. It is unknown whether low Vt improves neurocognitive outcome postarrest.
What This Study Adds to the Field
In patients suffering nontraumatic out-of-hospital cardiac arrest, lower Vt during the first 48 hours of intensive care unit admission was associated with improved neurocognitive outcome at hospital discharge, more ventilator-free days, and more shock-free days. In context with current understanding of lung–brain crosstalk, these findings suggest low-Vt ventilation may improve neurocognitive outcome after cardiac arrest.
Nontraumatic out-of-hospital cardiac arrest (OHCA) affects an estimated 424,000 people in the United States annually (1). Even when initial resuscitation succeeds, patient outcomes often are poor. More than half of successfully resuscitated patients experiencing OHCA do not survive to hospital discharge (2), and cognitive impairment occurs in half of survivors (3).
After successful resuscitation, cardiovascular dysfunction, global ischemia–reperfusion, and systemic inflammation contribute further to multiorgan dysfunction and brain injury (4). This response, termed post–cardiac arrest syndrome (PCAS) (5), evolves over subsequent hours to days and represents a narrow window within which targeted therapies may be effective.
Mechanical ventilation is a central component of postarrest care, for which neither current routine practice nor optimal management is well defined. Recent international consensus guidelines do not recommend a particular tidal volume (Vt) strategy postarrest, noting a paucity of data on the subject; rather, they urge caution regarding low-Vt strategies owing to potential harm from hypercapnia that could result (5, 6).
In patients with existing lung injury, high Vt ventilation causes further mechanical lung injury that propagates systemic inflammation and contributes to extrapulmonary organ injury (7–12). Among patients with acute respiratory distress syndrome (ARDS), lower Vt increases survival and attenuates both pulmonary and extrapulmonary organ dysfunction (8, 9). Even if overt ARDS is not present, clinical data suggest low Vt may improve outcomes among patients at risk of lung injury (13).
Long-term cognitive impairment occurs in the majority of ARDS survivors and is not fully explained by hypoxemia alone (14–16). Expanding preclinical evidence suggests mechanical lung injury may cause brain injury via several complex pathways (17–21), paralleling clinical trials data that have revealed lung-protective ventilation attenuates other extrapulmonary organ dysfunctions (8, 9).
Patients admitted after OHCA have several risk factors for lung injury, including pulmonary ischemia–reperfusion, pulmonary aspiration, mechanical injury from chest compressions and associated thoracic fractures, and systemic inflammation characteristic of PCAS. Therefore, we reasoned lower Vt may be both lung protective and neuroprotective after successful resuscitation from OHCA. In the present study, we hypothesized that lower Vt is associated with improved neurocognitive outcome at hospital discharge and more rapid resolution of respiratory failure among patients hospitalized after OHCA.
Some of the data from this study have been reported previously in the form of a conference abstract (22).
Methods
Study Population
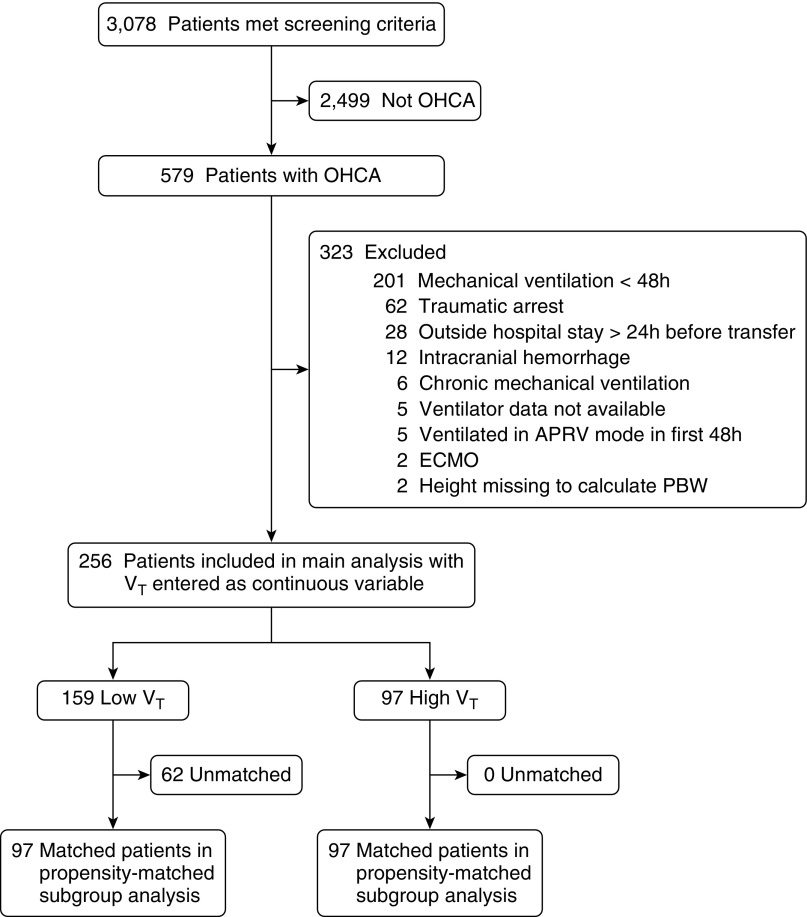
This two-center retrospective cohort study included mechanically ventilated adults aged 18 years or older admitted after nontraumatic OHCA who required conventional mechanical ventilation for at least the first 48 hours of hospitalization. Patients were excluded for outside hospital stay longer than 24 hours before transfer, intracranial hemorrhage, chronic mechanical ventilation, use of airway pressure release mode of ventilation, extracorporeal membrane oxygenation, and missing ventilator data or height (required to calculate predicted body weight) (Figure 1). Each participating hospital’s review board approved the study with waiver of consent.
Figure 1.
Study flow diagram. Of 579 patients with out-of-hospital cardiac arrest (OHCA), 256 were included in the main analyses. Of these, 194 patients (97 pairs) were included in a sensitivity analysis of patients matched by propensity to receive low Vt (≤8 ml/kg predicted body weight [PBW]). APRV = airway pressure release ventilation; ECMO = extracorporeal membrane oxygenation.
Patient records for inclusion were identified via a previously validated approach (23). Potentially relevant admissions between 2008 and 2014 were screened by searching all inpatient records for prespecified International Classification of Diseases 9th revision and Current Procedural Terminology codes corresponding to ventricular arrhythmia, cardiac arrest, hypothermia, and cardiopulmonary resuscitation (23). Each identified chart was reviewed by study physicians to confirm diagnosis of OHCA and eligibility criteria.
Determination of Vt
The primary exposure of interest was time-weighted average Vt in milliliters per kilogram predicted body weight (PBW) during the first 48 hours of intensive care unit (ICU) admission postarrest. Weighted-average Vt was calculated as the area under the Vt-versus-time plot (24), constructed using a last-value-forward step-function to reflect abrupt changes in Vt that would be expected in response to ventilator adjustments (see online supplement). The 48-hour interval was selected based on prior reports that systemic inflammation in PCAS is most pronounced during this time (4), likely increasing lung injury risk. Values for preset and exhaled Vt in volume- and pressure-targeted modes were used, respectively. Vt was scaled to PBW to account for between-patient differences in normal lung volumes; PBW was calculated via the NHLBI ARDS Network equation (9).
Covariates
Cardiac arrest data were collected following revised Utstein template definitions (25). Additional information extracted from medical record review included anthropometrics, illness severity scores (Acute Physiology and Chronic Health Evaluation [APACHE]-II, Sequential Organ Failure Assessment [SOFA]), ventilator and respiratory data, hemodynamic parameters, temperature management strategy, and laboratory data. All data were sourced directly from each patient’s hospital record and extracted for this study.
Primary Outcome
The prespecified primary outcome was favorable neurocognitive outcome at hospital discharge, defined as cerebral performance category (CPC) of 1 or 2 (26–29). CPC scale ranges from 1 to 5, with 1 indicating normal function or minor neurocognitive deficit, 2 moderate disability, 3 severe disability, 4 coma/vegetative state, and 5 death or brain death. CPC was ascertained for all patients via chart review by two physician-investigators blinded to Vt and other respiratory and illness severity measures (see online supplement). Discordant ratings were resolved by consensus.
Secondary Outcomes
Secondary outcomes included ventilator-free days, extrapulmonary organ failure–free days, ICU-free days, and hospital-free days through Day 28, and vital status at hospital discharge. Ventilator-free days were calculated as the time between successful liberation from mechanical ventilation and study Day 28 (30). A value of zero ventilator-free days was assigned for patients not surviving to hospital discharge to avoid misleading conclusions for patients who might get extubated but not survive hospitalization. Extrapulmonary organ failure–free days, ICU-free days, and hospital-free days were calculated similarly. Shock was defined as systolic blood pressure less than or equal to 90 mm Hg or vasopressor use; patients transferred out of intensive care who survived to discharge were assumed to be shock-free for days spent out of ICU. Renal, hepatic, and coagulation failures were ascertained using Brussels score definitions (31) with one modification: patients not requiring dialysis at time of live hospital discharge were considered not to have renal failure after discharge. Patients who received chronic outpatient dialysis before arrest were excluded from analysis of renal failure–free days. When data were unavailable on a particular study day during hospitalization, the last observed value was carried forward, conservatively biasing results toward the null if blood tests were checked less frequently as health improved. Organ failures otherwise were considered resolved after live hospital discharge.
Associations between Vt and mean arterial pressure, arterial oxygen tension, and arterial carbon dioxide tension were evaluated as safety and mechanistic endpoints. Time-weighted average values over the first 48 hours were calculated as above. In addition, we specifically evaluated the association of Vt with hypercapnia, defined as PaCO2 greater than 45 mm Hg (6), given the potential for lower Vt to induce hypercapnia if alveolar ventilation were not maintained. These assessments also sought to determine whether low Vt might simply be a marker of ventilator management more adherent with existing guidelines (6).
Statistics
Descriptive statistics are presented as mean ± SD, median (interquartile range), or number (%). Results were compared with unpaired or paired t test, Wilcoxon rank-sum test, or chi-square test, as appropriate.
Vt strategy assessment
Logistic regression models were developed to identify independent predictors of receiving Vt less than or equal to 8 ml/kg PBW during the first 48 hours. Candidate predictor variables consisted of all those entered in the propensity score as described below. Backward elimination was used to develop the final model, applying threshold P = 0.05 to remain in the model.
Primary analysis of clinical endpoints
Vt in milliliters per kilogram PBW over the first 48 hours, the exposure of interest, was entered as a continuous variable for all primary analyses of clinical endpoints.
Propensity score covariate adjustment was selected as the primary analysis technique for all clinical endpoints to account for the many factors that may influence Vt strategy after OHCA. The propensity score was developed using logistic regression to estimate the probability of receiving low (≤8 ml/kg PBW) compared with high (>8 ml/kg PBW) Vt. This dichotomized Vt threshold was chosen to reflect current standard practice for lung-protective Vt, stemming from the ARDS Network protocol that targeted Vt of 4 to 8 ml/kg PBW (9) and the U.S. Critical Illness and Injury Trials Group Checklist for Lung Injury Prevention recommendation for Vt of 6 to 8 ml/kg PBW in patients at risk for lung injury (32, 33). The final propensity model was chosen to represent current understanding of underlying biology and ensure face validity. Relevant covariates were identified from literature review and consideration of clinical relevance to postarrest biology. In addition, factors known to predict neurocognitive outcome were entered into the propensity model, regardless of their potential association with Vt, to optimize precision of the final effect estimate. Model discrimination and calibration were assessed with the c-statistic and Hosmer-Lemeshow goodness-of-fit test, respectively.
Sensitivity analyses
Several sensitivity analyses were performed for the primary endpoint, neurocognitive outcome at hospital discharge, to confirm results were not dependent on the method of covariate adjustment or formatting of either Vt or CPC scale. Alternative methods used for covariate adjustment included multivariable logistic regression, propensity quintile adjustment (34), inverse-probability-of-treatment weighting (35), and propensity-matched analysis (36–38). To evaluate if results were dependent on entering Vt as a continuous variable, Vt was recoded as high (>8 ml/kg PBW) versus low (≤8 ml/kg PBW) Vt and entered as a categorical predictor in a propensity-adjusted model. Finally, to evaluate if results were dependent on dichotomizing CPC, propensity-adjusted analysis with continuous Vt was repeated using ordinal logistic regression, with CPC scale entered as an ordinal dependent variable. Additional details are provided in the online supplement.
For all hypothesis testing, a two-sided α threshold of 0.05 was considered statistically significant.
Results
Screening identified 579 patients admitted to study hospitals for OHCA during the study period. Of these, 323 were excluded from analysis, the most common reasons being mechanical ventilation less than 48 hours before extubation or death (n = 201), traumatic cause of arrest (n = 62), and initial admission after arrest to a nonstudy hospital lasting more than 24 hours (n = 28). In total, 256 patients were included in the final analysis (Figure 1).
OHCA was witnessed in 76% of included patients, and 51% of patients had an initial rhythm of ventricular tachycardia or ventricular fibrillation. Study patients had high acuity on admission, as evidenced by high APACHE-II and SOFA scores. More than two-thirds had shock in the first 24 hours, and 86% had PaO2:FiO2 less than or equal to 300. Additional patient characteristics are presented in Table 1.
Table 1.
Baseline Characteristics According to High versus Low Vt
| Total Study Population |
Propensity-matched Subgroup Analysis* |
|||||
|---|---|---|---|---|---|---|
| High Vt (n = 97) | Low Vt (n = 159) | SMD | P | Low Vt (n = 97) | SMD | |
| Age, years | 66 ± 17 | 59 ± 17 | 0.389 | 0.001 | 61 ± 17 | 0.249 |
| Height, cm | 165 ± 10 | 177 ± 8 | −1.280 | <0.001 | 173 ± 8 | −0.889 |
| Weight, kg | 81 ± 21 | 88 ± 24 | −0.299 | 0.023 | 84 ± 22 | −0.132 |
| Female | 45 (46) | 31 (19) | 0.597 | <0.001 | 28 (29) | 0.368 |
| Comorbidities | ||||||
| Coronary disease | 31 (32) | 43 (27) | 0.108 | 0.478 | 28 (29) | 0.067 |
| Congestive heart failure | 24 (25) | 35 (22) | 0.065 | 0.648 | 18 (19) | 0.151 |
| Chronic pulmonary disease | 20 (21) | 24 (15) | 0.145 | 0.306 | 16 (16) | 0.106 |
| Arrest characteristics | ||||||
| Witnessed arrest | 77 (79) | 117 (74) | 0.137 | 0.367 | 76 (78) | 0.025 |
| Bystander CPR | 57 (59) | 90 (57) | 0.044 | 0.795 | 55 (57) | 0.042 |
| Time from collapse to CPR initiation, min | 2 (0–8) | 1.5 (0–5) | 0.208 | 0.326 | 1 (0–5) | 0.212 |
| Duration of CPR before sustained ROSC, min | 15 (10–33) | 16 (10–30) | 0.069 | 0.878 | 15 (9–30) | 0.150 |
| Initial rhythm ventricular tachycardia or ventricular fibrillation | 48 (49) | 82 (52) | −0.042 | 0.797 | 51 (53) | −0.062 |
| Comatose after ROSC | 93 (96) | 154 (97) | 0.052 | 0.734 | 93 (96) | 0.000 |
| Therapeutic hypothermia after ROSC | 74 (76) | 139 (87) | −0.292 | 0.025 | 80 (82) | −0.153 |
| Hospital A admission | 53 (55) | 110 (69) | −0.303 | 0.023 | 61 (63) | −0.168 |
| Illness severity | ||||||
| APACHE-II | 34 ± 6 | 34 ± 6 | 0.020 | 0.875 | 34 ± 6 | 0.060 |
| SOFA on Day 1 | 11 ± 3 | 11 ± 3 | −0.016 | 0.692 | 11 ± 3 | −0.083 |
| Shock in first 24 h† | 71 (73) | 109 (69) | 0.102 | 0.482 | 72 (74) | −0.023 |
| Initial lactate, mmol/L | 5.3 ± 4.7 | 4.6 ± 3.7 | 0.173 | 0.247 | 4.5 ± 3.3 | 0.194 |
| Peak lactate in first 24 h, mmol/L | 5.3 ± 4.6 | 4.8 ± 3.7 | 0.126 | 0.357 | 4.8 ± 3.3 | 0.145 |
| Initial respiratory characteristics | ||||||
| Tidal volume, ml | 541 ± 88 | 514 ± 77 | 0.316 | 0.013 | 496 ± 76 | 0.543 |
| Tidal volume, ml/kg PBW | 9.3 ± 1.8 | 7.3 ± 1.0 | 1.401 | <0.001 | 7.4 ± 1.1 | 1.287 |
| Tidal volume averaged over first 48 h, ml/kg PBW | 9.3 ± 1.2 | 7.1 ± 0.6 | 2.294 | <0.001 | 7.2 ± 0.6 | 2.207 |
| Total respiratory rate, breaths/min | 20 ± 6 | 21 ± 6 | −0.138 | 0.287 | 20 ± 7 | −0.084 |
| Minute volume, L/min | 10.4 ± 3.4 | 10.7 ± 3.6 | −0.086 | 0.510 | 10.1 ± 3.5 | 0.073 |
| PEEP, cm H2O | 5 (5–5) | 5 (5–8) | −0.241 | 0.176 | 5 (5–8) | −0.204 |
| Peak inspiratory pressure, cm H2O | 27 ± 7 | 26 ± 8 | 0.186 | 0.154 | 27 ± 8 | 0.068 |
| Mean airway pressure, cm H2O | 12 ± 3 | 11 ± 4 | 0.087 | 0.498 | 11 ± 4 | 0.057 |
| FiO2 | 1.0 (0.6–1.0) | 1.0 (0.6–1.0) | 0.101 | 0.400 | 1.0 (0.6–1.0) | 0.091 |
| pH | 7.23 ± 0.18 | 7.25 ± 0.16 | −0.069 | 0.586 | 7.25 ± 0.16 | −0.099 |
| PaCO2, mm Hg | 47 ± 16 | 49 ± 17 | −0.117 | 0.369 | 48 ± 17 | −0.058 |
| PaO2, mm Hg | 235 ± 133 | 222 ± 150 | 0.089 | 0.497 | 233 ± 159 | 0.013 |
| PaO2:FiO2 | 266 ± 150 | 257 ± 167 | 0.059 | 0.650 | 269 ± 176 | −0.014 |
| PaO2:FiO2 ≤ 300 in first 24 h | 82 (85) | 138 (87) | −0.064 | 0.711 | 84 (87) | −0.059 |
| Lowest PaO2:FiO2 in first 24 h | 165 ± 113 | 166 ± 113 | −0.005 | 0.967 | 173 ± 115 | −0.074 |
Definition of abbreviations: APACHE-II = Acute Physiology and Chronic Health Evaluation-II score; CPR = cardiopulmonary resuscitation; PBW = predicted body weight; PEEP = positive end-expiratory pressure; ROSC = return of spontaneous circulation; SMD = standardized mean difference; SOFA = Sequential Organ Failure Assessment score.
Data presented as mean ± SD, median (interquartile range), or n (%). Data presented with patients grouped according to high Vt (>8 ml/kg PBW) versus low Vt (≤8 ml/kg PBW), the Vt threshold used for developing the propensity score. Primary analyses of all outcomes used the full cohort with Vt entered as a continuous variable and propensity score entered as a model covariate. Propensity matching was performed in a secondary sensitivity analysis to confirm that the main finding was not dependent on method of covariate adjustment.
SMD compared to high-Vt population, with whom matching was performed. Each patient in high-Vt group was successfully matched with a low-Vt patient with specified match criteria.
Shock was defined as systolic blood pressure ≤ 90 mm Hg or vasopressor use.
Vt Strategy
Mean Vt over the first 48 hours was 7.9 ± 1.4 ml/kg PBW (range, 4.9–14.3 ml/kg PBW). Thirty-eight percent of patients received an average Vt greater than 8 ml/kg PBW over the first 48 hours, and just 4% received an average Vt less than or equal to 6 ml/kg PBW during this time. Average Vt over the first 48 hours did not differ significantly from initial Vt at ICU admission (mean difference, 0.1; 95% confidence interval [CI], 0.0–0.3 ml/kg PBW; P = 0.070).
In multivariable analysis, only height (odds ratio [OR], 1.19; 95% CI, 1.13–1.24 per 1-cm increase; P < 0.001), weight (OR, 0.98; 95% CI, 0.97–1.00 per 1-kg increase; P = 0.037), and hospital of admission (OR, 2.89; 95% CI, 1.50–5.59; P = 0.002) were significantly predictive of receipt of low-Vt ventilation. With just these three covariates in a model predicting receipt of low Vt, model discrimination and calibration were high (c-statistic = 0.844; Hosmer-Lemeshow goodness-of-fit P = 0.949).
Propensity Model for Vt over First 48 Hours
The final propensity model predicting Vt included as covariates age, anthropometrics (height, weight, sex), measures of illness severity (APACHE-II, circulatory shock on Day 1), arrest characteristics (witnessed arrest, bystander cardiopulmonary resuscitation, initial shockable rhythm, hospital of admission, receipt of therapeutic hypothermia), and respiratory characteristics (initial pH, PaCO2, and peak inspiratory pressure; lowest PaO2:FiO2 in first 24 h). The model exhibited high discrimination (c-statistic = 0.862) and calibration (Hosmer-Lemeshow goodness-of-fit P = 0.877) for predicting Vt.
Low Vt and Neurocognitive Outcome
In unadjusted analysis, lower Vt was significantly associated with favorable neurocognitive outcome (OR, 1.47; 95% CI, 1.12–1.92 per 1-ml/kg PBW decrease in Vt; P = 0.005). In the prespecified primary analysis, after adjusting for propensity score, lower Vt remained significantly associated with favorable neurocognitive outcome (OR, 1.61; 95% CI, 1.13–2.28 per 1-ml/kg PBW decrease in Vt; P = 0.008).
The association between lower Vt and favorable neurocognitive outcome was robust to method of covariate adjustment and handling of the independent and dependent variables of primary interest. Alternative covariate adjustment techniques used included multivariable regression, propensity quintile adjustment, inverse-probability-of-treatment weighting, and propensity matching (Figure 2).
Figure 2.
Vt and neurocognitive outcome after out-of-hospital cardiac arrest. Sensitivity analyses performed for primary endpoint, favorable neurocognitive outcome at hospital discharge, to determine whether results were dependent on method of covariate adjustment. Odds ratios represent odds of favorable versus unfavorable neurocognitive outcome (cerebral performance category 1–2 vs. 3–5) per 1-ml/kg predicted body weight (PBW) decrease in Vt. Additional sensitivity analyses (not shown in figure) yielded similar results with Vt reentered as a binary variable and with use of ordinal logistic regression to model cerebral performance category as an ordinal outcome. *Indicates prespecified primary outcome analysis. CI = confidence interval.
In the propensity-matched sensitivity analysis, each patient receiving high Vt was successfully matched with a patient receiving low Vt (n = 97 per group), with balance achieved between groups for all available biologically relevant covariates (Table 1). In this cohort, lower Vt again was significantly associated with favorable neurocognitive outcome (OR, 1.68; 95% CI, 1.11–2.55 per 1-ml/kg PBW decrease in Vt; P = 0.014). Figure 3 presents Kaplan-Meier estimated probability of discharge with favorable neurocognitive outcome for patients receiving high versus low Vt in the total study cohort without adjustment (log-rank P = 0.008) and propensity-matched cohort (log-rank P = 0.021).
Figure 3.
Probability of discharge with favorable neurocognitive outcome through Day 28. Kaplan-Meier estimates stratified according to time-weighted average Vt received during the first 48 hours of admission. (A) Entire study cohort (n = 256), unadjusted analysis. (B) Cohort matched by propensity for receiving low-Vt ventilation (n = 194). PBW = predicted body weight.
Reanalysis using ordinal logistic regression, entering CPC as an ordinal dependent variable and adjusting for propensity score, confirmed that the association between Vt and neurocognitive outcome was not dependent on dichotomizing CPC scale (OR, 1.30; 95% CI, 1.02–1.68 per 1-ml/kg PBW decrease in Vt; P = 0.038; score test for proportional odds assumption P = 0.073). Reanalysis entering Vt as a dichotomized variable and adjusting for propensity score similarly found an association between lower Vt and favorable neurocognitive outcome (OR, 2.95; 95% CI, 1.22–7.12; P = 0.016).
Low Vt and Secondary Clinical Outcomes
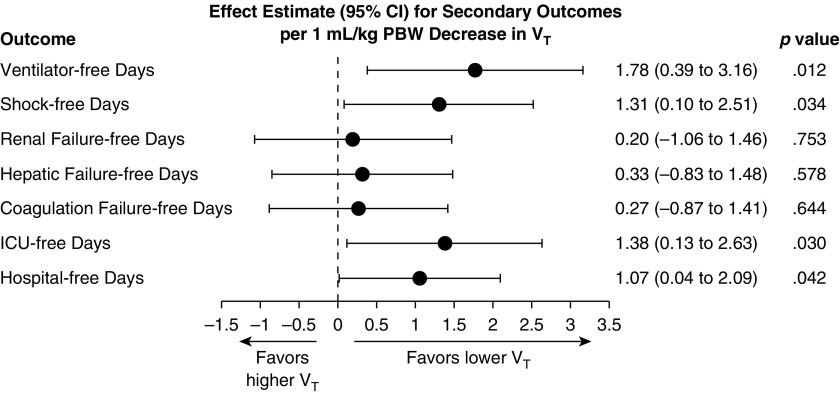
In propensity-adjusted analyses, lower Vt was associated with more ventilator-free days (β = 1.78; 95% CI, 0.39–3.16 for change in ventilator-free days per 1-ml/kg PBW decrease in Vt; P = 0.012) and shock-free days (β = 1.31; 95% CI, 0.10–2.51 for change in shock-free days per 1-ml/kg PBW decrease in Vt; P = 0.034). Vt was not significantly associated with renal, hepatic, or coagulation failure-free days (Figure 4).
Figure 4.
Vt and secondary outcomes. Effect estimates with 95% confidence intervals (CI) for secondary outcomes from linear regression models, propensity score–adjusted analyses. Effect estimate refers to the change in the outcome variable per 1-ml/kg predicted body weight (PBW) decrease in tidal volume. ICU = intensive care unit.
Lower Vt also was associated with more ICU-free days (β = 1.38; 95% CI, 0.13–2.63; P = 0.030) and hospital-free days (β = 1.07; 95% CI, 0.04–2.09; P = 0.042) in propensity-adjusted analyses. Vt was not significantly associated with survival to hospital discharge in propensity-adjusted analysis, although the direction of change favored lower Vt (OR for survival, 1.23; 95% CI, 0.95–1.60 per 1-ml/kg PBW decrease in Vt; P = 0.115).
Alternative Mechanistic and Safety Endpoints
Vt was not associated with mean arterial pressure over the first 48 hours (β = −0.82; 95% CI, −9.25 to 7.60 for change in mean arterial pressure per 1-ml/kg decrease in Vt; P = 0.847). Vt demonstrated no association with arterial oxygen or arterial carbon dioxide tension over the first 48 hours (PaO2: β = −2.23; 95% CI, −6.53 to 2.07 for change in PaO2 per 1-ml/kg PBW decrease in Vt; P = 0.307; PaCO2: β = 0.46; 95% CI, −0.13 to 1.05 for change in PaCO2 per 1-ml/kg PBW decrease in Vt; P = 0.127). Exposure to hypercapnia, defined as average arterial oxygen tension greater than 45 mm Hg during the first 48 hours, was similar between patients receiving higher versus lower Vt (10.7% vs. 10.3%; P = 1.00).
Post Hoc Analyses for Residual Confounding
Therapeutic hypothermia was prescribed more commonly among patients receiving lower Vt in unadjusted analysis, driven by its use in patients without an initial shockable rhythm (84% versus 69% with low versus high Vt, respectively; P = 0.045). After adjusting for propensity score, therapeutic hypothermia was not associated with Vt in the full study cohort (β = 0.14; 95% CI, −0.19 to 0.47 for change in Vt [in ml/kg PBW] associated with use of therapeutic hypothermia; P = 0.413), and its use did not differ by high versus low Vt in the matched cohort (P = 0.375). In addition, therapeutic hypothermia was not associated with favorable neurocognitive outcome in unadjusted analysis (P = 0.757) or in the multivariable regression sensitivity analysis (P = 0.516).
Because hospital of admission predicted receipt of low Vt, additional post hoc analyses were performed to evaluate for residual confounding related to unmeasured hospital-specific aspects of care. Hospital of admission was not associated with favorable neurocognitive outcome in unadjusted analysis (P = 0.747) or in the multivariable regression sensitivity analysis (P = 0.588). Vt remained significantly associated with favorable neurocognitive outcome in separate univariable models constructed for each site (hospital A: OR, 1.50; 95% CI, 1.04–2.17 per 1-ml/kg PBW decrease in Vt; P = 0.022; hospital B: 1.46; 95% CI, 0.98–2.17 per 1-ml/kg PBW decrease in Vt; P = 0.042). Limited sample size precluded separate multivariable analyses by site.
Discussion
In this study, lower Vt during the first 48 hours of admission was independently associated with favorable neurocognitive outcome after OHCA. This finding was consistent across several sensitivity analyses, indicating conclusions were not dependent on statistical approach. Lower Vt also was independently associated with more ventilator-free days and shock-free days. In the context of existing data on lung–brain interaction in critical illness, these results suggest a neuroprotective role for lower Vt in patients hospitalized after OHCA.
Several possible mechanisms could explain a link between low-Vt ventilation and favorable neurocognitive outcome: attenuation of lung injury–mediated systemic inflammation, pulmonary mechanotransduction, lung–brain crosstalk, differences in blood oxygen or carbon dioxide tension, and hemodynamic effects of lower mean airway pressures.
Pulmonary mechanotransduction, independent of tissue hypoxia, produces several responses that may precipitate brain injury. Overdistension lung injury induces local and systemic cytokine release (11) that may heighten systemic inflammation already present from ischemia–reperfusion injury. Peripheral cytokine signaling in turn is transmitted to the brain via vagal afferents, circumventricular organs, and transport of cytokines or downstream molecules across the blood–brain barrier (39–43). Insults ranging from acute myocardial infarction (44, 45) to sepsis (46, 47) have been shown to increase intracerebral proinflammatory cytokine levels and associated neuronal injury. Several studies similarly have implicated lung injury as a precipitant of neuroinflammation and brain injury (17–20). Even absent preexisting lung injury, recent data suggest lung mechanotransduction during high-Vt ventilation may induce vagal afferent-mediated apoptotic pathways in the brain (17, 18).
Such lung–brain communication appears to be bidirectional. Massive brain injury increases susceptibility to lung injury (48). In preclinical studies, bilateral vagotomy exacerbated lung injury from ischemia–reperfusion and high Vt, whereas vagal stimulation attenuated lung injury (49). ARDS is relatively common in brain-injured patients with respiratory failure, in whom high Vt appears to be an independent risk factor for developing lung injury (50, 51). Thus, in PCAS, ventilation-induced lung injury may increase risk of postarrest brain injury and vice versa in a deleterious positive feedback loop mediated in part by the neuroinflammatory reflex (17, 39, 40). Improvements in neurocognitive outcome, ventilator-free days, and shock-free days associated with lower Vt in the present study together support systemic benefits of low-Vt ventilation in PCAS.
Although an important potential safety concern, lower Vt was not associated with hypercapnia in our cohort. Thus, low Vt likely can be prescribed in most patients with PCAS while adhering to treatment guidelines recommending eucapnia by increasing respiratory rate (6). In addition, lower Vt was not simply a marker of improved adherence to guidelines for oxygenation management in our study. The lack of association between Vt and mean arterial pressure argues against differences in cerebral perfusion as an explanatory mechanism. Reduced cerebral venous and lymphatic drainages with higher Vt are potential contributory mechanisms, although their roles in disease pathophysiology and relationship to mechanical ventilation are poorly understood (52–55).
Although our findings support a role for low Vt in PCAS, results are not conclusive. First, causation cannot be inferred from this observational study design. Several features of this study argue against residual confounding: (1) the biological approach used in developing the propensity score, which incorporated several baseline illness severity measures; (2) the finding of height, weight, and hospital as the only independent predictors of low Vt in this cohort; and (3) similarities in intraarrest characteristics, APACHE-II, Day 1 SOFA, and shock incidence in the first 24 hours among patients receiving higher versus lower Vt. Although CPC is a standard outcome in cardiac arrest studies (25), its sensitivity and discriminatory power for mild to moderate brain injury are controversial (56, 57). Such may be limited further by relying on chart review alone to determine CPC. Prospective validation of our findings in a clinical trial with rigorous cognitive testing and functional outcomes measures is warranted.
Receipt of a low-Vt strategy could be an epiphenomenon that marks better care, but similar adherence to oxygenation and eucapnia targets do not support such in this study. Lower Vt was associated with increased use of therapeutic hypothermia in the unmatched, unadjusted analysis due to differences in use for patients with a nonshockable arrest. Postarrest targeted hypothermia remains controversial, is unproven in patients without a shockable rhythm at arrest (27), and was not associated with neurocognitive outcome in our cohort in either unadjusted or multivariable analyses. Vt also differed by hospital of admission, but hospital was not associated with neurocognitive outcome in unadjusted or multivariable analyses. Moreover, in separate hospital-specific models, the association between Vt and neurocognitive outcome remained significant, and ORs were strikingly similar between hospitals. Adjusting for both hypothermia use and admitting hospital via the propensity score and separately as covariates in the multivariable sensitivity analysis did not change the finding that lower Vt predicts favorable neurocognitive outcome, making them unlikely to explain this association.
Finally, precise causal mechanisms cannot be inferred from this analysis. Several pathways of lung–brain crosstalk have been described in other disease states with biological similarities to PCAS (44–47). Additional preclinical and clinical investigations are needed to determine their relevance to cardiac arrest and elucidate other mechanisms that may be unique to the constellation of injuries comprised by PCAS.
In conclusion, lower Vt was independently associated with favorable neurocognitive outcome among patients hospitalized after nontraumatic OHCA. Lower Vt also was associated with earlier liberation from mechanical ventilation and more days free from circulatory shock. These results suggest a role for lung-protective ventilation to improve patient outcomes after OHCA.
Footnotes
Supported in part by NHLBI grant T32-HL007633 (J.R.B.).
Author Contributions: J.R.B. and D.T. conceived and designed the study. J.R.B., T.B.G., S.P.J., A. Mueller, L.H., R.J.A., J.J., and S.T. contributed the primary data. J.R.B. conducted the data analyses. All authors contributed to the interpretation of results. J.R.B. prepared the first draft of the manuscript. All authors revised the draft for important intellectual content. All authors gave approval of the final manuscript submitted for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201609-1771OC on March 7, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fugate JE, Brinjikji W, Mandrekar JN, Cloft HJ, White RD, Wijdicks EFM, Rabinstein AA. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. 2012;126:546–550. doi: 10.1161/CIRCULATIONAHA.111.088807. [DOI] [PubMed] [Google Scholar]
- 3.Moulaert VRMP, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80:297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 5.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 6.Callaway CW, Soar J, Aibiki M, Böttiger BW, Brooks SC, Deakin CD, Donnino MW, Drajer S, Kloeck W, Morley PT, et al. Advanced Life Support Chapter Collaborators. Part 4: Advanced life support: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015;132:S84–S145. doi: 10.1161/CIR.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 7.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 8.Ranieri VM, Giunta F, Suter PM, Slutsky AS. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA. 2000;284:43–44. doi: 10.1001/jama.284.1.43. [DOI] [PubMed] [Google Scholar]
- 9.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 10.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6, discussion 230–232. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 11.Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016;37:633–646. doi: 10.1016/j.ccm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 13.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VGM, Espósito DC, Pasqualucci MdeO, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-LOHR V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-López A, López-Alonso I, Aguirre A, Amado-Rodríguez L, Batalla-Solís E, Astudillo A, Tomás-Zapico C, Fueyo A, dos Santos CC, Talbot K, et al. Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med. 2013;188:693–702. doi: 10.1164/rccm.201304-0691OC. [DOI] [PubMed] [Google Scholar]
- 18.Quilez ME, Fuster G, Villar J, Flores C, Martí-Sistac O, Blanch L, López-Aguilar J. Injurious mechanical ventilation affects neuronal activation in ventilated rats. Crit Care. 2011;15:R124. doi: 10.1186/cc10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heuer JF, Pelosi P, Hermann P, Perske C, Crozier TA, Brück W, Quintel M. Acute effects of intracranial hypertension and ARDS on pulmonary and neuronal damage: a randomized experimental study in pigs. Intensive Care Med. 2011;37:1182–1191. doi: 10.1007/s00134-011-2232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fries M, Bickenbach J, Henzler D, Beckers S, Dembinski R, Sellhaus B, Rossaint R, Kuhlen R. S-100 protein and neurohistopathologic changes in a porcine model of acute lung injury. Anesthesiology. 2005;102:761–767. doi: 10.1097/00000542-200504000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalvo R, Martí-Sistac O, Blanch L, López-Aguilar J. Bench-to-bedside review: brain-lung interaction in the critically ill: a pending issue revisited. Crit Care. 2007;11:216. doi: 10.1186/cc5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghafouri TB, Hsu L, Anderson RJ, Joshua J, Tyagi S, Beitler JR. Lower tidal volume is associated with favorable neurologic outcome following out-of-hospital cardiac arrest [abstract] Am J Respir Crit Care Med. 2016;193:A5239. [Google Scholar]
- 23.De Bruin ML, van Hemel NM, Leufkens HGM, Hoes AW. Hospital discharge diagnoses of ventricular arrhythmias and cardiac arrest were useful for epidemiologic research. J Clin Epidemiol. 2005;58:1325–1329. doi: 10.1016/j.jclinepi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, et al. International Liaison Committee on Resuscitation; American Heart Association; European Resuscitation Council; Australian Resuscitation Council; New Zealand Resuscitation Council; Heart and Stroke Foundation of Canada; InterAmerican Heart Foundation; Resuscitation Councils of Southern Africa; ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 26.Brain Resuscitation Clinical Trial I Study Group. Randomized clinical study of thiopental loading in comatose survivors of cardiac arrest. N Engl J Med. 1986;314:397–403. doi: 10.1056/NEJM198602133140701. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. TTM Trial Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 28.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 29.Mentzelopoulos SD, Zakynthinos SG, Tzoufi M, Katsios N, Papastylianou A, Gkisioti S, Stathopoulos A, Kollintza A, Stamataki E, Roussos C. Vasopressin, epinephrine, and corticosteroids for in-hospital cardiac arrest. Arch Intern Med. 2009;169:15–24. doi: 10.1001/archinternmed.2008.509. [DOI] [PubMed] [Google Scholar]
- 30.Schoenfeld DA, Bernard GR ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Bernard G. The Brussels score. Sepsis. 1997;1:43–44. [Google Scholar]
- 32.Beitler JR, Schoenfeld DA, Thompson BT. Preventing ARDS: progress, promise, and pitfalls. Chest. 2014;146:1102–1113. doi: 10.1378/chest.14-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kor DJ, Talmor DS, Banner-Goodspeed VM, Carter RE, Hinds R, Park PK, Gajic O, Gong MN US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A) Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury. BMJ Open. 2012;2:e001606. doi: 10.1136/bmjopen-2012-001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochran WG. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. 1968;24:295–313. [PubMed] [Google Scholar]
- 35.Rosenbaum PR. Model-based direct adjustment. J Am Stat Assoc. 1987;82:387–394. [Google Scholar]
- 36.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin DB. The use of matched sampling and regression adjustment to remove bias in observational studies. Biometrics. 1973;29:185–203. [Google Scholar]
- 39.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 40.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 41.Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, Chretien F, Sharshar T. Understanding brain dysfunction in sepsis. Ann Intensive Care. 2013;3:15. doi: 10.1186/2110-5820-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002-2003;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- 43.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 44.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286:H2264–H2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 45.Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H791–H797. doi: 10.1152/ajpheart.00099.2004. [DOI] [PubMed] [Google Scholar]
- 46.Semmler A, Hermann S, Mormann F, Weberpals M, Paxian SA, Okulla T, Schäfers M, Kummer MP, Klockgether T, Heneka MT. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.López-Aguilar J, Villagrá A, Bernabé F, Murias G, Piacentini E, Real J, Fernández-Segoviano P, Romero PV, Hotchkiss JR, Blanch L. Massive brain injury enhances lung damage in an isolated lung model of ventilator-induced lung injury. Crit Care Med. 2005;33:1077–1083. doi: 10.1097/01.ccm.0000162913.72479.f7. [DOI] [PubMed] [Google Scholar]
- 49.dos Santos CC, Shan Y, Akram A, Slutsky AS, Haitsma JJ. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med. 2011;183:471–482. doi: 10.1164/rccm.201002-0314OC. [DOI] [PubMed] [Google Scholar]
- 50.Elmer J, Hou P, Wilcox SR, Chang Y, Schreiber H, Okechukwu I, Pontes-Neto O, Bajwa E, Hess DR, Avery L, et al. Acute respiratory distress syndrome after spontaneous intracerebral hemorrhage. Crit Care Med. 2013;41:1992–2001. doi: 10.1097/CCM.0b013e31828a3f4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, Isnardi D, Davi A, Arguis MJ, Berardino M, et al. Brain IT group. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35:1815–1820. doi: 10.1097/01.CCM.0000275269.77467.DF. [DOI] [PubMed] [Google Scholar]
- 52.Zamboni P, Menegatti E, Pomidori L, Morovic S, Taibi A, Malagoni AM, Cogo AL, Gambaccini M. Does thoracic pump influence the cerebral venous return? J Appl Physiol (1985) 2012;112:904–910. doi: 10.1152/japplphysiol.00712.2011. [DOI] [PubMed] [Google Scholar]
- 53.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández Mondéjar E, Vazquez Mata G, Cárdenas A, Mansilla A, Cantalejo F, Rivera R. Ventilation with positive end-expiratory pressure reduces extravascular lung water and increases lymphatic flow in hydrostatic pulmonary edema. Crit Care Med. 1996;24:1562–1567. doi: 10.1097/00003246-199609000-00022. [DOI] [PubMed] [Google Scholar]
- 55.Maybauer DM, Talke PO, Westphal M, Maybauer MO, Traber LD, Enkhbaatar P, Morita N, Traber DL. Positive end-expiratory pressure ventilation increases extravascular lung water due to a decrease in lung lymph flow. Anaesth Intensive Care. 2006;34:329–333. doi: 10.1177/0310057X0603400307. [DOI] [PubMed] [Google Scholar]
- 56.Raina KD, Callaway C, Rittenberger JC, Holm MB. Neurological and functional status following cardiac arrest: method and tool utility. Resuscitation. 2008;79:249–256. doi: 10.1016/j.resuscitation.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiainen M, Poutiainen E, Kovala T, Takkunen O, Häppölä O, Roine RO. Cognitive and neurophysiological outcome of cardiac arrest survivors treated with therapeutic hypothermia. Stroke. 2007;38:2303–2308. doi: 10.1161/STROKEAHA.107.483867. [DOI] [PubMed] [Google Scholar]