Abstract
Rationale: Implementation of intervention strategies to prevent lung damage in early cystic fibrosis (CF) requires objective outcome measures that capture and track lung disease.
Objectives: To define the utility of the Lung Clearance Index (LCI), measured by multiple breath washout, as a means to track disease progression in preschool children with CF.
Methods: Children with CF between the ages of 2.5 and 6 years with a confirmed diagnosis of CF and age-matched healthy control subjects were enrolled at three North American CF centers. Multiple breath washout tests were performed at baseline, 1, 3, 6, and 12 months to mimic time points chosen in clinical care and interventional trials; spirometry was also conducted. A generalized linear mixed-effects model was used to distinguish LCI changes associated with normal growth and development (i.e., healthy children) from the progression of CF lung disease.
Measurements and Main Results: Data were collected on 156 participants with 800 LCI measurements. Although both LCI and spirometry discriminated health from disease, only the LCI identified significant deterioration of lung function in CF over time. The LCI worsened during cough episodes and pulmonary exacerbations, whereas similar symptoms in healthy children were not associated with increased LCI values.
Conclusions: LCI is a useful marker to track early disease progression and may serve as a tool to guide therapies in young patients with CF.
Keywords: cystic fibrosis, disease progression, lung clearance index, lung function
At a Glance Commentary
Scientific Knowledge on the Subject
Implementation of intervention strategies to prevent lung damage early in cystic fibrosis requires objective outcome measures that capture and track lung disease.
What This Study Adds to the Field
In this study we demonstrate that the Lung Clearance Index deteriorates in preschool children with cystic fibrosis over time and during episodes of cough and pulmonary exacerbations, whereas similar symptoms in healthy children did not change the Lung Clearance Index. These findings suggest that Lung Clearance Index may be a useful marker to track early disease progression and guide therapies in young patients with cystic fibrosis.
Cystic fibrosis (CF) lung disease begins early in life and often progresses in the absence of clinical signs and symptoms (1–6). Consequently, there has been an increasing emphasis on early intervention strategies to prevent lung damage during this critical period of disease progression, for which objective outcome measures that capture and track lung disease are needed. Both imaging and functional tests have been proposed as technologies applicable to early lung disease, but these tools are largely limited to the research setting.
One functional measure that is gaining traction is the lung clearance index (LCI). LCI is measured by the multiple breath washout (MBW) test and quantifies the efficiency with which gas mixes in the lungs. A higher LCI value indicates greater ventilation inhomogeneity and disease severity. LCI is more sensitive than spirometry in detecting lung function abnormalities in patients with CF beyond infancy (7, 8). The test requires only tidal breathing and is therefore feasible to perform in preschool (2–6 yr of age) children (9). The LCI is responsive to therapeutic interventions in the school-age population and is being increasingly used in interventional studies (7, 8). However, the ability of LCI to monitor and track clinical symptoms and disease progression in patients with CF in early life is unknown.
Longitudinal studies in older children demonstrate that the LCI worsens with age in school-age children with CF (10, 11). LCI measured during preschool years has been shown to predict both LCI and spirometry outcomes at school age, a time when most children with CF had abnormal LCI values (12). These data further emphasize the importance of the preschool years as an opportunity for early intervention in CF. However, these data were limited to single measurements in the preschool and school-age years, and thereby are not representative of the utility of lung function measurements in clinical care, where tests are typically performed at 3-month intervals. Furthermore, although parental reported cough at the preschool visit was associated with higher LCI values at school age, the study was not designed to assess how changes in clinical status affect the measurement. Preschool children often experience respiratory symptoms triggered by viral infections leading to challenges for physicians to differentiate symptoms that are disease related from those that also occur in healthy children. Thus, current treatment decisions in preschool children with CF are mainly based on symptom duration as a trigger for starting antibiotic therapy rather than relying on objective measures of pulmonary function.
To better define the utility of LCI in preschoolers with CF, we designed a longitudinal 12-month observational study in preschool children. To differentiate disease-related changes from those intrinsic to the technique, the study included parallel measurements in both healthy children and children with CF. We chose study visits that were not only representative of clinical care, but also relevant for interventional studies so that the information gained could be used in both settings. The main aims of the study were to (1) define the trajectory and variability of LCI over time in health and disease, (2) define clinical factors associated with worsening of LCI during the preschool years, and (3) define how LCI changes at times of respiratory symptoms in healthy preschool children and preschool children with CF. Some of the results of these studies have been previously reported in the form of abstracts (13–18).
Methods
The study was designed as a multicenter 12-month longitudinal observational study in preschool children. Children between the ages of 2.5 and 6 years with a confirmed diagnosis of CF, as well as age-matched healthy control subjects were enrolled at three North American CF centers (Chapel Hill, Indianapolis, and Toronto). Details of the inclusion and exclusion criteria are presented in the online supplement.
Participants attended study visits at enrollment, 1 month (± 1 wk), 3 months (± 2 wk), 6 months (± 2 wk), 9 months (± 2 wk), and 12 months (± 2 wk). At each visit a clinical examination, review of past and current symptoms and treatments, MBW, and spirometry were performed. All children were free of acute respiratory infection within 4 weeks of the enrollment visit, whereas symptoms were recorded and outcomes measured at all subsequent visits. Visits were classified as stable, if no respiratory symptoms were present. Respiratory symptoms at the study visit included nasal congestion and/or cough. In CF a pulmonary exacerbation (PEx) was defined as an increase in respiratory symptoms (e.g., cough) and respiratory symptoms combined with a physician-based decision to treat with antibiotics (either oral or intravenous). All PEx events that occurred between consecutive study visits were recorded.
MBW tests were performed with the Exhalyzer D (EcoMedics AG, Duernten, Switzerland), with adaptions for preschool children (see online supplement). Participants were enrolled if they could complete a single MBW trial, whereas a MBW test was considered successful if there were at least two technically acceptable trials. Spirometry was attempted in all subjects according to American Thoracic Society/European Respiratory Society criteria (19).
Statistical Analysis
A generalized linear mixed-effects model was used to distinguish changes in LCI associated with normal growth and development (i.e., healthy children) from the progression of CF lung disease. Age was used as the time-variable and an interaction between disease status and age was used to ascertain whether the rate of change in LCI differed between health and CF. In a similar analysis limited to CF, interactions between time-independent patient factors (sex, genotype, pancreatic status, history of positive microbiology cultures) and age were compared to assess whether the rate of change in LCI differed between groups. For time-dependent clinical measures (anthropometry, symptoms, PEx), absolute difference in LCI at a given age between those with and without the characteristic were assessed. Variability of the LCI was characterized by the coefficient of variation (CV%) and the intraclass correlation coefficient (ICC). All statistical analyses were performed in Stata Statistical Software, Release 14 (StataCorp, College Station, TX).
Results
Study Population
One hundred and fifty-six preschool children (78 healthy and 78 CF) were recruited between January 2013 and June 2015, of which 150 were enrolled (72 healthy; 78 CF) (Figure 1). The average age at enrollment was 4.1 years (range, 2.5–5.9). The healthy children were well matched for sex and age with the CF group, but preschoolers with CF weighed less and were shorter than healthy subjects (Table 1). Six healthy children (8%) had a parental-reported history of wheeze in the preceding 12 months, but did not report any other symptoms or have a physician diagnosis of asthma. Most CF participants were diagnosed by newborn screening (79%). All but three (96%) had class I-III mutations on both alleles and 94% were pancreatic insufficient. In the period between CF diagnosis and the first study visit, 38% (n = 30) had been hospitalized at least once for respiratory causes. More than half of the subjects had grown Staphylococcus aureus (68%), Pseudomonas aeruginosa (60%), or Haemophilus influenza (51%) at least once in their life before enrollment. H. influenza was more common in Canadian versus U.S. participants (63% vs. 39%; P = 0.042), whereas American participants were more likely to have had methicillin-resistant S. aureus (27% vs. 3%; P = 0.003).
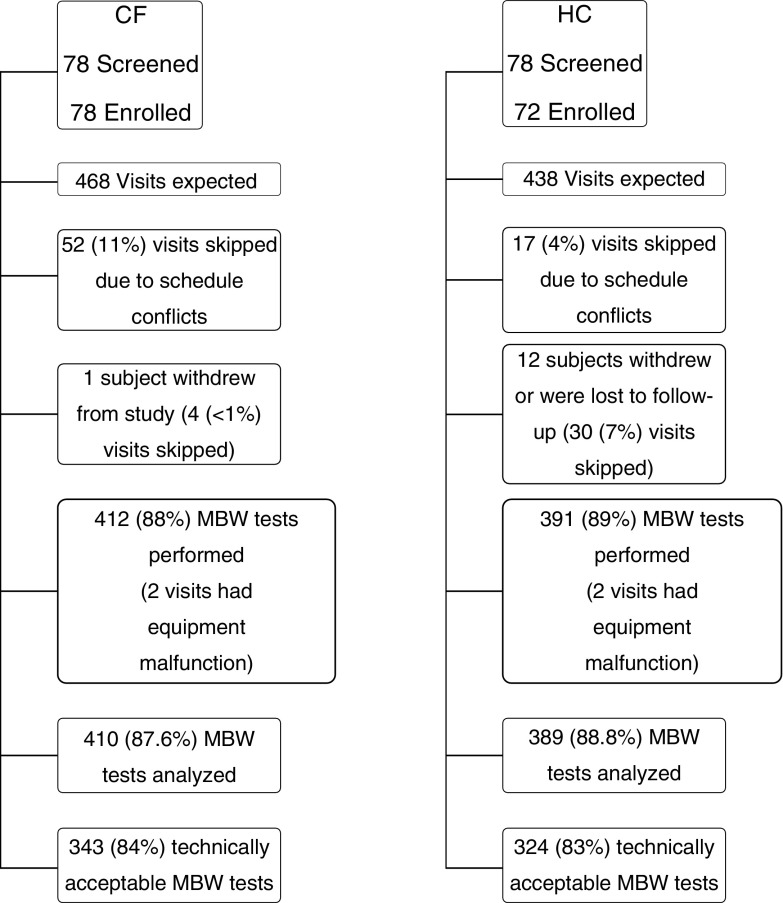
Figure 1.
Summary of study population. Multiple breath washout tests were considered technically acceptable according to quality control review criteria (31) and if there were at least two technically acceptable trials in a test occasion. Percentages are calculated based on the total number of visits expected (468 cystic fibrosis, 438 healthy control subjects), with the exception of the percentage successful, which is calculated as number technically acceptable/number analyzed. CF = cystic fibrosis subjects; HC = healthy control subjects; MBW = multiple breath washout.
Table 1.
Characteristics of the Study Population at the Enrollment Visit
| Demographics | Healthy Controls (n = 78) | CF (n = 78) | P Values |
|---|---|---|---|
| Male, n (%) | 37 (47.4) | 39 (50.0) | 0.749 |
| White, n (%) | 59 (75.6) | 69 (88.5) | 0.037 |
| Age, mean (range) | 4.0 (2.5 to 5.9) | 4.3 (2.6 to 5.9) | 0.097 |
| Height-for-age centile, mean (range) | 61.2 (6.7 to 99.8) | 44.2 (0.8 to 97.5) | 0.0002 |
| Weight-for-age centile, mean (range) | 62.6 (3.8 to 99.8) | 43.1 (2.0 to 94.7) | <0.0001 |
| BMI-for-age centile, mean (range) | 60.9 (1.31 to 99.9) | 47.5 (0.99 to 94.9) | 0.003 |
| MBW enrollment visit | |||
| MBW attempted, n (%) | 78 (100) | 78 (100) | 0.316 |
| MBW successful, n (%) | 52 (66.7) | 51 (65.4) | 0.599 |
| LCI, mean (range), n = 103 (52 HC, 51 CF) | 7.1 (6.1 to 8.1) | 8.8 (6.4 to 13.6) | <0.0001 |
| Spirometry enrollment visit | |||
| Spirometry attempted, n (%) | 71 (91.0) | 74 (94.9) | 0.348 |
| Spirometry successful, n (%) | 51 (71.8) | 51 (68.9) | 0.702 |
| FEV0.75 % predicted, mean (range), n = 71 (27 HC, 44 CF) | 96.9 (69.1 to 147.0) | 90.9 (42.0 to 122.3) | 0.125 |
| FEV1 % predicted, mean (range), n = 89 (44 HC, 45 CF) | 102.0 (73.3 to 136.4) | 92.3 (42.5 to 119.3) | 0.003 |
| FEF25–75 % predicted, mean (range), n = 54 (25 HC, 29 CF) | 96.7 (66.8 to 139.3) | 94.0 (43.5 to 152.9) | 0.687 |
| zFEVt, mean (range), n = 94 (47 HC, 42 CF) | 0.1 (−2.4 to 3.3) | −0.7 (−4.3 to 1.8) | 0.002 |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; FEF25–75% = forced expiratory flow, midexpiratory phase; FEV0.75 = forced expiratory volume in 0.75 s; HC = healthy control subjects; LCI = Lung Clearance Index; MBW = multiple breath washout; zFEVt = z score for forced expiratory volume in t seconds (where t represents either 0.75 or 1).
Values are bold if the difference between health and disease is significant at P < 0.05.
Overall, 412 MBW measurements were performed in preschool children with CF and 391 in healthy children. MBW testing was successful for 66% of participants at the screening visit and 89% of the follow-up visits; further feasibility details are provided in the online supplement. At enrollment LCI was significantly elevated (worse) and FEV1 z scores were significantly lower in the CF group compared with the healthy control group (Table 1). In healthy children, no relationship between LCI and age at the first visit (see Figure E1 in the online supplement) was noted; therefore an age-independent upper limit of normal was defined, and 67% of children with CF had LCI above the upper limit of normal (LCI of 8) at the first study visit.
The CF group had higher LCI values at the first study visit compared with the healthy group (Table 1). To understand the factors that are associated with higher LCI values at the start of the study we conducted univariable linear regression analysis in both health and CF. In healthy children none of the demographic characteristics were statistically associated with LCI measured at the start of the study (Table 2). In the CF group, LCI was higher in older children, those with a history of S. aureus, and in those with two or more hospitalizations before the start of the study (Table 2). In a multivariable analysis only those with a history of hospitalizations (two or more) had worse LCI values. In contrast, none of the clinical variables were associated with lower z scores for forced expiratory volume in t seconds (FEVt) where t represents either 0.75 or 1, in the CF participants at the first study visit (see Table E1).
Table 2.
Factors Associated with LCI at the Enrollment Visit
| Health |
CF |
|||
|---|---|---|---|---|
| n | LCI units/year (95% Confidence Interval) | n | LCI units/year (95% Confidence Interval) | |
| First visit | ||||
| Age | 70 | −0.01 (−0.11 to 0.10) | 78 | 0.58 (0.13 to 1.03) |
| Height-for-age z score | 70 | 0.05 (−0.07 to 0.17) | 78 | −0.39 (−0.87 to 0.08) |
| Weight-for-age z score | 70 | 0.02 (−0.12 to 0.15) | 78 | −0.31 (−0.81 to 0.19) |
| BMI-for-age z score | 70 | −0.02 (−0.14 to 0.11) | 78 | 0.02 (−0.51 to 0.55) |
| Sex | ||||
| Male | 32 | Reference | 39 | Reference |
| Female | 38 | −0.10 (−0.35 to 0.14) | 39 | 0.12 (−0.79 to 1.02) |
| Ethnicity | ||||
| White | 53 | Reference | 69 | Reference |
| Other | 17 | −0.10 (−0.35 to 0.14) | 9 | −0.85 (−2.25 to 0.55) |
| Newborn screening | ||||
| No | 16 | Reference | ||
| Yes | 62 | 0.77 (−0.34 to 1.87) | ||
| Mutation class | ||||
| I–III | 75 | Reference | ||
| IV–V* | 3 | −2.34 (−4.63 to −0.05) | ||
| Pancreatic sufficient* | 5 | Reference | ||
| Pancreatic insufficient | 73 | 1.14 (−0.68 to 2.97) | ||
| Hospitalization history | ||||
| 0 hospitalizations | 48 | Reference | ||
| 1 hospitalizations | 13 | 0.92 (−0.21 to 2.1) | ||
| 2 hospitalization | 10 | 1.9 (0.62 to 3.13) | ||
| 3+ hospitalization | 7 | 2.6 (1.09 to 4.02) | ||
| Microbiology history | ||||
| Pseudomonas aeruginosa | 46 | 0.90 (0.00 to 1.79) | ||
| Staphylococcus aureus | 54 | 1.44 (0.14 to 2.76) | ||
| Methicillin-resistant S. aureus | 11 | −0.001 (−1.30 to 1.30) | ||
| Stenotrophomonas maltophilia | 16 | 0.31 (−0.81 to 1.42) | ||
| Haemophilus influenzae | 40 | −0.38 (−1.29 to 0.51) | ||
| Burkholderia cepacia complex† | 2 | 3.61 (0.87 to 6.35) | ||
| Aspergillus fumigatus | 2 | 1.07 (−1.78 to 3.92) | ||
| Treatment history | ||||
| Hypertonic saline | 34 | 0.69 (−0.20 to 1.59) | ||
| Dornase alfa | 29 | 0.52 (−0.41 to 1.44) | ||
| Chronic inhaled antibiotic therapy | 14 | 1.39 (0.26 to 2.52) | ||
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; LCI = Lung Clearance Index.
Coefficients are derived from a univariable linear regression analysis and represent the slope (LCI units/yr) (95% confidence interval). A positive coefficient indicates higher (worse) LCI, whereas a negative coefficient represents lower (better) LCI. Values are bold if the estimate is significantly different from 0 at the P < 0.05 significance level.
Based on three observations in this category.
Based on two observations in this category.
During the 12-month study period, more than a third of participants (69%) were culture positive for S. aureus, and 14% were positive for P. aeruginosa infection; of these, 10% had acquired P. aeruginosa for the first time during the study period. The proportion of participants receiving chronic maintenance therapies during the study period was similar to that observed before the study; 50% were treated with hypertonic saline, 42% with dornase alfa, and 23% with chronic inhaled antibiotic therapy. One participant was started on ivacaftor during the study period.
Variability of LCI
In healthy participants the intra-test repeatability (%CV) of the LCI was similar to the inter-test reproducibility (Table 3). These observations correspond to a small ICC (0.38), which indicates that the proportion of variance observed between subjects is less than the variance observed between repeated measurements in the same subject.
Table 3.
Measures of Within-Subject and Between-Subject Variability of the Lung Clearance Index Across the Six Study Visits Calculated as the CV (SD/Mean) and the ICC (Proportion of the Variability Explained by the Variability Between-Subjects)
| CV% Median (Range) |
ICC | ||
|---|---|---|---|
| Within-Subject Within-Test | Within-Subject Between-Test | ||
| Health | 3.9 (0.3 to 12.3) | 5.0 (1.5 to 20.1) | 0.38 |
| CF | 4.9 (0.2 to 18.0) | 9.4 (0.8 to 33.7) | 0.57 |
| CF stable visits only | 4.9 (0.2 to 17.6) | 7.2 (0.2 to 23.6) | 0.71 |
Definition of abbreviations: CF = cystic fibrosis; CV = coefficient of variation; ICC = intraclass correlation coefficient.
In the CF group the inter-test %CV of the LCI was greater than the intra-test %CV (Table 3). The ICC in CF was larger than that observed in health (0.57), and increased when the analysis was limited to stable visits (0.71). This suggests that part of the within-subject variability of the LCI may be explained by changes in symptoms or clinical status of the participant. In contrast, inter-test %CV for z scores for FEVt (zFEVt) was 17% in healthy, and 42% in CF, whereas the ICC for zFEVt was much higher in healthy (ICC, 0.76), but comparable in CF (all visits ICC, 0.58; stable visits, ICC 0.64).
Progression of LCI
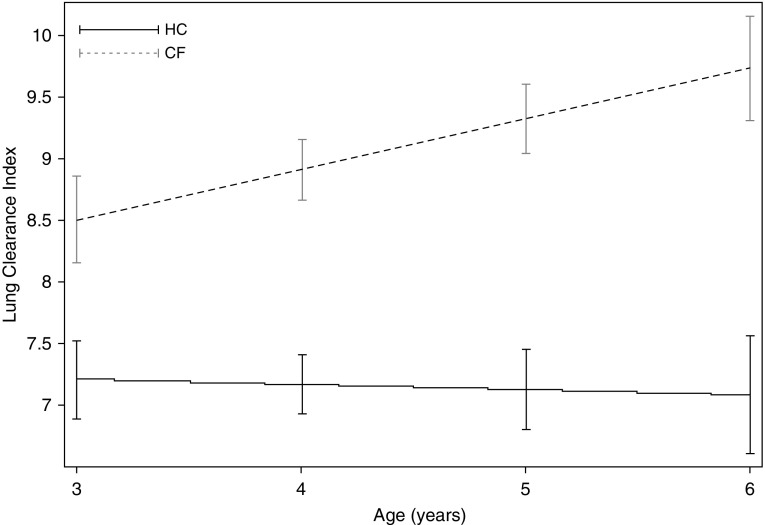
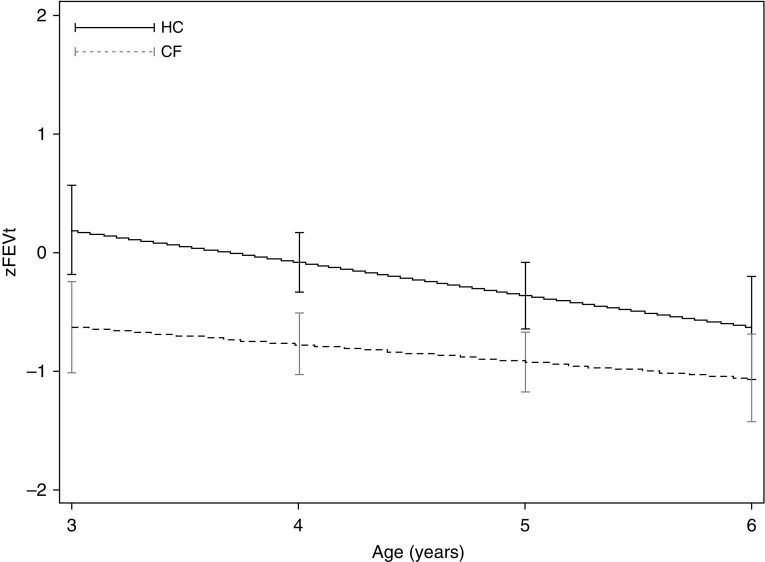
Overall throughout the 12-month study, children with CF had higher LCI values compared with healthy children (Figure 2). Because children entered the study at different ages, and older children had higher LCI values at their first visit, we investigated how LCI changed with age. In healthy children LCI remained stable with age (slope, −0.04 LCI units/yr; 95% confidence interval [CI], −0.12 to 0.04; P = 0.338) (Figure 3), whereas in CF the LCI significantly deteriorated (slope, 0.40 LCI units/yr; 95% CI, 0.14 to 0.66; P = 0.003) (Figure 3). The rate of change of LCI was significantly different between the health and CF group (interaction term, 0.41; 95% CI, 0.12 to 0.70; P = 0.006). LCI also deteriorated significantly when the analyses were limited to asymptomatic visits (slope, 0.34 LCI units/yr; 95% CI, 0.08 to 0.60; P = 0.011); this rate of change was also statistically different from that observed in healthy children (interaction term, 0.38; 95% CI, 0.11 to 0.65; P = 0.007). The rate of change was attenuated when we limited the analysis to MBW measurements before a PEx event (slope, 0.27 LCI units/yr; 95% CI, −0.01 to 0.56; P = 0.058), but was still significantly different from the healthy group (interaction term, 0.31; 95% CI, 0.04 to 0.58; P = 0.026). The deterioration of LCI was independent of demographic and clinical characteristics measured at the start of the study. In other words, the rate of deterioration (slope) was not modified by events that occurred before the study, even though these characteristics were significantly associated with higher values at the first visit (Table 2). In contrast to LCI, the rate of change in FEVt z scores was not different between health and CF (Figure 4).
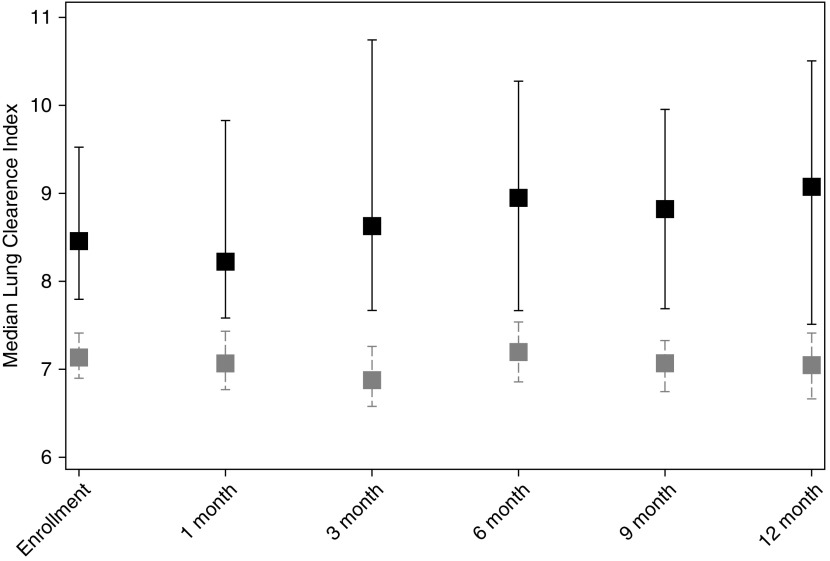
Figure 2.
Median lung clearance index values (interquartile range) for healthy control subjects (gray) and cystic fibrosis subjects (black) at each of the six study visits. Lung clearance index was significantly higher in the cystic fibrosis group compared with the healthy group at each visit.
Figure 3.
The average change in lung clearance index (LCI) in healthy control subjects (HC) and cystic fibrosis subjects (CF). Slope (LCI units/yr) estimates were derived from an interaction term between diagnosis group and age using a mixed-effects model with repeated measures of LCI where random intercepts and random slopes were specified. The rate of deterioration of LCI in children with cystic fibrosis was significantly different from that observed in health.
Figure 4.
The average change in z score for forced expiratory volume in t seconds, where t is either 0.75 or 1 (zFEVt) in health and cystic fibrosis. Slope (zFEVt/yr) estimates were derived from an interaction term between diagnosis group and age using a mixed-effects model with repeated measures of zFEVt where random intercepts only were specified. Although the intercepts differed between health and cystic fibrosis, the rate of decline in zFEVt was similar in both groups. CF = cystic fibrosis subjects; HC = healthy control subjects.
Factors Associated with Higher LCI Values in Subjects with CF
CF participants with at least one positive culture with S. aureus during the study period had higher (worse) LCI values compared with subjects that did not grow S. aureus (Table 4). Similarly, subjects treated with chronic inhaled antibiotics, a proxy for chronic P. aeruginosa infection, during the study period had higher (worse) LCI values compared with those that were not treated with chronic inhaled antibiotics. In contrast, participants with higher weight-for-age z scores had lower (better) LCI values (i.e., for each unit increase in higher the weight-for-age z score, the LCI decreased by 0.41 LCI units (95% CI, −0.77 to −0.05).
Table 4.
Factors Associated with LCI Values in Patients with CF
| CF [LCI units/year (95% Confidence Interval)] | |
|---|---|
| Time-independent factors | |
| Baseline LCI | 0.71 (0.62 to 0.80) |
| Hypertonic saline at enrollment | |
| No | Reference |
| Yes | 0.18 (−0.53 to 0.89) |
| Dornase alfa at enrollment | |
| No | Reference |
| Yes | −0.09 (−0.81 to 0.63) |
| Chronic inhaled antibiotics at enrollment | |
| No | Reference |
| Yes | 0.99 (0.17 to 1.81) |
| Positive microbiology culture during the study compared with reference group (negative culture) | |
| Pseudomonas aeruginosa | 0.5 (−0.7 to 1.7) |
| Staphylococcus aureus | 0.8 (0.04 to 1.6) |
| Methicillin-resistant S. aureus | −0.6 (−1.2 to 0.9) |
| Stenotrophomonas maltophilia | −0.4 (−1.3 to 5.2) |
| Stenotrophomonas influenza | 2.0 (−1.1 to 5.2) |
| Burkholderia cepacia complex* | 3.1 (0.002 to 6.2) |
| Aspergillus fumigatus | 2.2 (−0.8 to 5.2) |
| Time-dependent factors | |
| Increasing height-for-age z score | −0.23 (−0.58 to 0.12) |
| Increasing weight-for-age z score | -0.41 (−0.77 to −0.05) |
| Increasing BMI-for-age z score | −0.27 (−0.59 to 0.06) |
| Symptoms | |
| Pulmonary exacerbations | |
| No | Reference |
| Yes | 1.05 (0.62 to 1.48) |
| Cough | |
| No | Reference |
| Yes | 0.51 (0.13 to 0.89) |
| Nasal congestion | |
| No | Reference |
| Yes | 0.12 (−0.27 to 0.51) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; LCI = Lung Clearance Index.
Coefficients are derived from a univariable generalized mixed effects regression analysis and represent the difference in LCI values (95% confidence interval) relative to the reference group. A positive coefficient indicates higher (worse) LCI, whereas a negative coefficient represents lower (better) LCI relative to the reference. Bold represents values that are significantly different from 0 at a significance level of P < 0.05.
Based on two observations in this category.
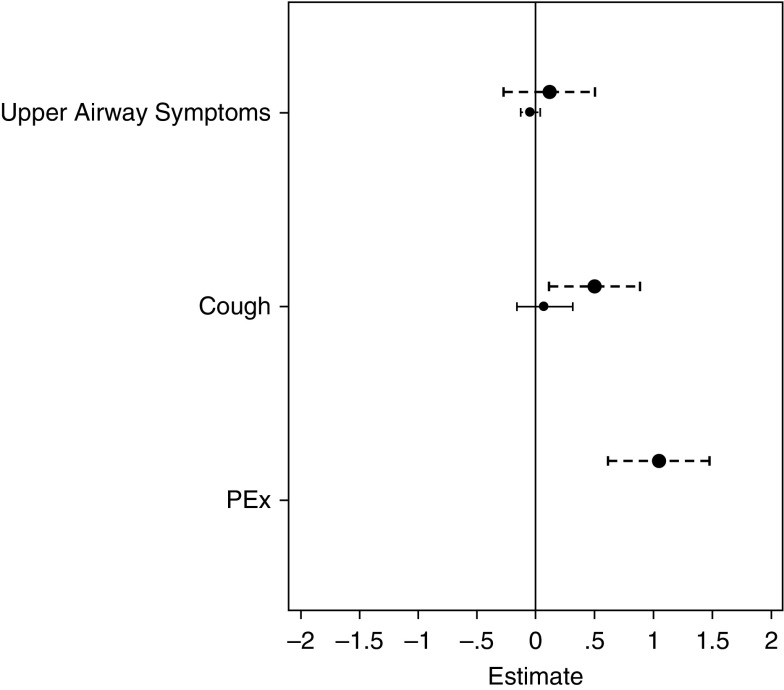
For factors that changed over the study period we evaluated whether absolute values of LCI were elevated at a given age in participants with a specific characteristic compared with those without. Over the 12-month study period, 39 events with new respiratory symptoms (nasal congestion, cough, or both) were recorded in 28 healthy participants, and 133 events in 66 CF participants. Fifty-five (41%) of these events in 41 CF participants were classified as PEx treated with antibiotics; in 80% of these events participants received oral antibiotics. Study visit in children with CF who had respiratory symptoms treated with antibiotics (PEx) had LCI values that were on average 1 unit higher (worse) than those without PEx events (Table 4, Figure 5). CF participants with cough that was not treated with antibiotics also had a significantly elevated LCI, albeit the effect size was smaller than in those with PEx (Table 4, Figure 5). In healthy participants there was no effect of cough on LCI. Nasal congestion was not associated with an elevated LCI value in either the healthy or CF groups (Figure 5).
Figure 5.
Association between pulmonary symptoms and lung clearance index (LCI) in health (solid line) and cystic fibrosis (CF) (dashed line). Estimates (absolute LCI during symptomatic visits relative to asymptomatic visits) derived from a mixed-effects model of LCI, adjusted for age; symptoms were treated as time-varying covariates. Neither upper airway symptoms nor cough were associated with higher LCI values in healthy participants, whereas both cough and pulmonary exacerbation were associated with significantly higher LCI values in CF (Table 4). Upper airways symptoms were not associated with higher LCI values in CF. PEx = pulmonary exacerbation.
The factors found to be significantly associated with LCI in Table 4 (univariable analysis) were then entered into a multivariable analysis; only PEx (estimate, 0.96 LCI units/yr; 95% CI, 0.46 to 1.45) and higher baseline LCI (estimate, 0.71 LCI units/yr; 95% CI, 0.61 to 0.81) remained significantly associated with a higher LCI. In a separate analysis we also evaluated whether LCI was predictive of a PEx event at the next study visit. In a mixed-effects logistic regression analysis, which allowed each participant to have multiple PEx events, the odds of a PEx event were not explained by a participants’ LCI value at the previous visit (odds ratio, 0.99; 95% CI, 0.84 to 1.16).
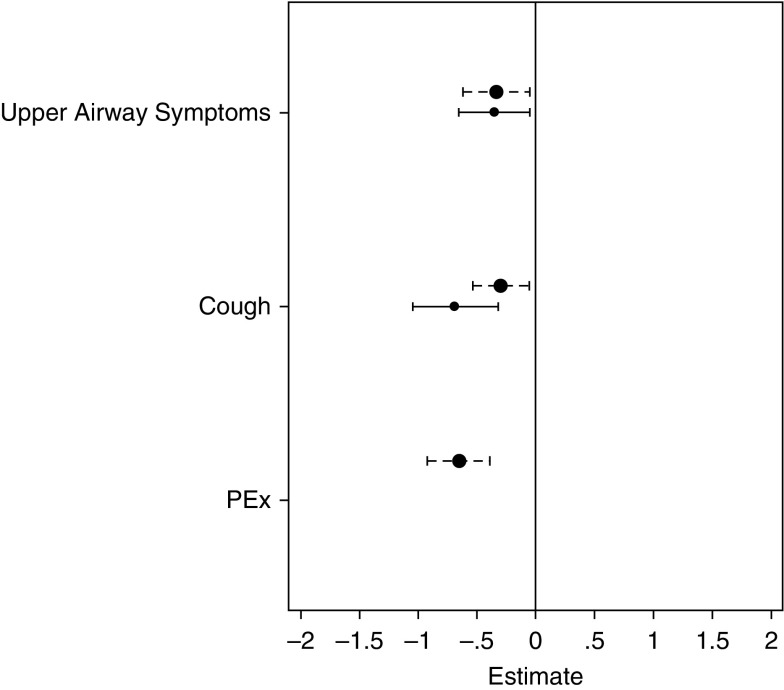
A similar analysis was conducted for FEVt z scores (see online supplement). None of the known risk factors for worse lung function were significantly associated with lower FEVt z scores at the start of the study (see Table E1). Both upper and lower airway symptoms were associated with significantly lower FEVt z scores in healthy and CF participants with no significant differences between the groups (Figure 6; see Table E2). Similar results were observed for forced expiratory flow (FEF)25–75% z scores; both cough and nasal congestion were associated with lower FEF25–75% z scores in health, whereas only cough was associated with lower FEF25–75% z scores in CF (data not shown).
Figure 6.
Association between pulmonary symptoms and z scores for the forced expiratory volume in t seconds, where t is either 0.75 or 1 (zFEVt) in health (solid line) and cystic fibrosis (dashed line). Estimates (absolute zFEVt during symptomatic visits relative to asymptomatic visits) derived from a mixed-effects model of lung clearance index, adjusted for age; symptoms were treated as time-varying covariates. Visits when both healthy and patients with cystic fibrosis had both upper and lower airway symptoms had significantly lower zFEVt values, as did pulmonary exacerbation visits. PEx = pulmonary exacerbation.
Relationship between LCI and FEVt
At each study visit, the correlation between LCI and zFEVt was low but statistically significant (enrollment: r = −0.30, P = 0.02; 1 mo: r = −0.33, P = 0.01; 3 mo: r = −0.51, P < 0.001; 6 mo: r = −0.59, P < 0.001; 9 mo: r = −0.51, P < 0.001; 12 mo: r = −0.37, P < 0.001). In most children with CF, there was a discordance between LCI and zFEVt; LCI values were above the upper limit of normal (“abnormal”) and zFEVt was above the lower limit of normal (“normal”) (see Figure E2). Overall, when the relationship between LCI and zFEVt was modeled longitudinally, there was a significant and inverse relationship between LCI and zFEVt (slope, −0.67 LCI units/FEVt z score; 95% CI, −0.85 to −0.49).
Discussion
This study characterizes clinical factors associated with changes in lung function in preschool children using repeated measures performed at time points relevant for both clinical care and interventional studies. Although LCI and FEVt were equally feasible, and discriminated between health and disease at baseline, only the LCI identified significant deterioration of lung function in the CF population. Furthermore, in this relatively young CF population with mild lung disease, the LCI was able to distinguish between participants based on clinical symptoms. In contrast, zFEVt was significantly lower for clinical symptoms in both healthy and CF participants. Specifically, the LCI was significantly elevated during episodes of cough and PEx events. Respiratory symptoms in healthy children and symptoms of nasal congestion in both health and CF were not associated with higher LCI values, suggesting that the LCI detects changes reflecting worsening CF lung disease. These data support the use of LCI as an objective measure to track lung disease in preschool children with CF.
Our findings further provide evidence that PEx events in children (treated with either oral or intravenous antibiotics) are associated with deterioration in lung function. A history of repeated early PEx events, defined as hospitalizations for respiratory causes, was associated with a significantly elevated LCI at the start of the study and PEx were associated with worse LCI values. These findings are consistent with several studies in older subjects that have shown that PEx have negative consequences for patients with CF (20, 21). Most studies evaluating PEx have focused on events treated with intravenous antibiotics, which is not the treatment course for most events in young children with CF. Our observations support the clinical significance of pulmonary symptoms treated with oral antibiotics, which is consistent with previous findings that these milder events have short- and long-term impact on lung function (22, 23). A recently published study linking changes in LCI during a PEx event to changes in magnetic resonance imaging outcomes suggest that LCI may also be a sensitive outcome to assess response to treatment for PEx events in patients with CF (24). Although these data are encouraging, the recovery after PEx events will likely be heterogeneous with some patients returning to baseline, whereas others may not fully recover (25). Further studies are needed to establish whether LCI is suited to evaluate treatment response during PEx in patients with CF.
Viral infections are common in preschool children, which makes it difficult to determine whether symptoms during these events require treatment in patients with CF. In this study we demonstrate for the first time that upper respiratory symptoms (i.e., nasal congestion) have no impact on lung function as measured by LCI in healthy children and in CF. In contrast, although cough in healthy children was not associated with higher LCI values, LCI was elevated in children with CF with cough. These findings suggest that increases in LCI associated with pulmonary symptoms are indicative of lower airway disease in patients with CF. Cough is the most frequent trigger for defining PEx in interventional studies in this age group. Because this symptom is rather nonspecific, thresholds for duration of symptoms are also often used in clinical practice and in clinical trials (26). Our findings suggest that the LCI may be a sensitive way to identify patients who could benefit from treatment during episodes of acute worsening of respiratory symptoms.
In the current study clinical factors known to be linked to greater disease severity, such as infection with S. aureus were associated with higher LCI values. Our findings are consistent with those from a study of younger patients with CF evaluated by the AREST CF (Australian Respiratory Early Surveillance Team for Cystic Fibrosis), which reported higher LCI values in patients whose bronchoalveolar lavage cultures were positive for CF bacterial pathogens (27). Although most of our cultures were from upper respiratory samples (throat swabs), which may not always reflect lower airway infections (28), these findings nonetheless support airway infection as a major driver of lung disease progression in CF. A minimal number of participants acquired new infections during the observation period, thus the effect of incident and new-onset infections on the LCI could not be established in this study.
Our findings are consistent with previous longitudinal studies that demonstrate that LCI is a sensitive measure of lung function and tracks with disease progression. Even during our relatively short observation period of 12 months the LCI significantly worsened in a group of young patients with CF. The natural rate of deterioration of LCI observed in the current study, both overall and in stable patients, provides a baseline rate of change that can be used to design interventional studies for this age group. As more novel therapeutic interventions become available that directly target the dysfunctional CF transmembrane conductance regulator, LCI may be a functional parameter suitable to establish whether CF transmembrane conductance regulator modulators will impact lung function trajectories over time in young children where early intervention with effective treatments could have the most pronounced long-term effect.
The variability in LCI observed between repeated measurements within the same individual was quite heterogeneous and lower than previously published studies (29). In healthy subjects the ICC, a measure of the variability of an outcome, was quite low, indeed much lower than would be expected of a test that is considered reproducible and appropriate for tracking disease progression (30). However, the low ICC is likely biased by the narrow range of LCI values observed in the healthy children. The ICC in the CF group was higher, and increased further when the analyses were limited to stable visits, which would suggest that part of the within-subject variability of the LCI can be explained by patient symptoms and clinical status. Although this study was designed to elucidate the sources of the variability of the LCI, it remains unclear whether the variability observed during stable visits is an inherent physiologic property of the test (i.e., the ventilation distribution of the lungs is more inhomogeneous in CF compared with health), or if the test is detecting abnormalities in the lung not associated with symptoms. Concurrent measurements of LCI and other sensitive measures of structural lung disease, such as high-resolution computed tomography or magnetic resonance imaging, will help to further elucidate the mechanisms that drive the variability of the LCI.
Strengths and Limitations
The study was designed to include repeated measurements of LCI at time points frequently used for interventional studies and to mimic quarterly clinic visits. Thus, the LCI changes observed over the 12-month study period reflect changes expected in a real-life setting. We applied a mixed-effect model that allows for a varying number of study visits and intervals between visits to maximize the available data for each subject. The lack of association with zFEVt may have been biased for several reasons. Spirometry was not a primary outcome and if children were tired after MBW testing, spirometry was not measured. In addition the healthy group had lower z scores than expected suggesting that the Global Lung Function Initiative may not fit this population.
Symptoms and PEx events that occurred between study visits were captured, and outcomes were measured regardless of symptoms. Although this approach gave a complete account of significant pulmonary events during the study period, the magnitude of change in LCI for a PEx event may have been underestimated. At the extreme, the PEx could have occurred 3 months before the study visit, thus the measured LCI may not have reflected the PEx events. Furthermore, a PEx event was defined based on a physicians’ decision to treat symptoms (i.e., cough). The symptoms that led the physician to treat the symptoms may be independently associated with LCI and our estimate of the effect of PEx may be biased. Finally, all of the outcomes were limited to functional pulmonary function tests, and did not include objective imaging studies that measure structural lung damage (e.g., high-resolution computed tomography, magnetic resonance imaging). Future studies that capture repeated baseline measurements of LCI, and measures at the onset of symptoms and the end of treatment, will be necessary to more accurately define thresholds for changes in LCI that would benefit from treatment.
Conclusions
The LCI demonstrates progression of lung disease in preschool children with CF and corresponds to changes in lower airway symptoms in children with CF. LCI may be a useful marker to track early disease progression and may serve as an endpoint to guide therapies in young patients with CF.
Footnotes
Supported by the NHLBI grant 5R01HL116232-04.
Author Contributions: S.S. and F.A.R. designed the study, interpreted the data, and drafted the manuscript. S.S. performed the statistical analyses. S.D.D., G.R.-B., and P.S. interpreted the data and revised the manuscript. H.W., M.D., R.C.J., M.E.P., M.K., C.C.C., and R.J. collected data, interpreted results, and revised the manuscript. S.S., S.D.D., G.R.-B., H.W., M.D., R.C.J., R.J., M.E.P., M.K., C.C.C., L.S., P.S., and F.A.R. approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201610-2158OC on December 12, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 2.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 3.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 4.Linnane BM, Hall GL, Nolan G, Brennan S, Stick SM, Sly PD, Robertson CF, Robinson PJ, Franklin PJ, Turner SW, et al. AREST-CF. Lung function in infants with cystic fibrosis diagnosed by newborn screening. Am J Respir Crit Care Med. 2008;178:1238–1244. doi: 10.1164/rccm.200804-551OC. [DOI] [PubMed] [Google Scholar]
- 5.Ranganathan SC, Dezateux C, Bush A, Carr SB, Castle RA, Madge S, Price J, Stroobant J, Wade A, Wallis C, et al. London Collaborative Cystic Fibrosis Group. Airway function in infants newly diagnosed with cystic fibrosis. Lancet. 2001;358:1964–1965. doi: 10.1016/s0140-6736(01)06970-7. [DOI] [PubMed] [Google Scholar]
- 6.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 7.Davies J, Sheridan H, Bell N, Cunningham S, Davis SD, Elborn JS, Milla CE, Starner TD, Weiner DJ, Lee PS, et al. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med. 2013;1:630–638. doi: 10.1016/S2213-2600(13)70182-6. [DOI] [PubMed] [Google Scholar]
- 8.Subbarao P, Stanojevic S, Brown M, Jensen R, Rosenfeld M, Davis S, Brumback L, Gustafsson P, Ratjen F. Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med. 2013;188:456–460. doi: 10.1164/rccm.201302-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aurora P, Bush A, Gustafsson P, Oliver C, Wallis C, Price J, Stroobant J, Carr S, Stocks J London Cystic Fibrosis Collaboration. Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2005;171:249–256. doi: 10.1164/rccm.200407-895OC. [DOI] [PubMed] [Google Scholar]
- 10.Kieninger E, Singer F, Fuchs O, Abbas C, Frey U, Regamey N, Casaulta C, Latzin P. Long-term course of lung clearance index between infancy and school-age in cystic fibrosis subjects. J Cyst Fibros. 2011;10:487–490. doi: 10.1016/j.jcf.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Kraemer R, Blum A, Schibler A, Ammann RA, Gallati S. Ventilation inhomogeneities in relation to standard lung function in patients with cystic fibrosis. Am J Respir Crit Care Med. 2005;171:371–378. doi: 10.1164/rccm.200407-948OC. [DOI] [PubMed] [Google Scholar]
- 12.Aurora P, Stanojevic S, Wade A, Oliver C, Kozlowska W, Lum S, Bush A, Price J, Carr SB, Shankar A, et al. Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 183:752–758. doi: 10.1164/rccm.200911-1646OC. [DOI] [PubMed] [Google Scholar]
- 13.Jensen R, Pizarro M, Klingel M, Stanojevic S, Webster H, McDonald N, et al. Progression and variability of LCI in preschool children with cystic fibrosis [abstract] Pediatr Pulmonol. 2015;50(S41):191. [Google Scholar]
- 14.Stanojevic S, Jensen R, Webser H, Kane M, Pizarro ME, Davis S, et al. Tracking pulmonary exacerbations in preschool children using the lung clearance index [abstract] Eur Respir J. 2016;48(Suppl 60):OA1490. [Google Scholar]
- 15.Webster H, Pizarro M, McDonald N, Jensen R, Klingel M, Stanojevic S, et al. Utility of the lung clearance index to monitor pulmonary exacerbations in young children with cystic fibrosis [abstract] Pediatr Pulmonol. 2015;50(S41):242. [Google Scholar]
- 16.Stanojevic S, Jensen R, Webster H, Subbarao P, Pittman J, Davis SD, et al. Defining the variability of the lung clearance index in health and disease for preschool children [abstract] Pediatr Pulmonol. 2015;50(S28):245. [Google Scholar]
- 17.Jensen R, Stanojevic S, Webster H, Gibney K, McDonald N, Stanojevic S, et al. Feasibility of longitudinal multiple breath washout measurements in preschool children [abstract] Am J Respir Crit Care Med. 2014;189:A1032. [Google Scholar]
- 18.Webster H, Jensen R, McDonald N, Subbarao P, Stanojevic S, Ratjen F. Feasibility of longitudinal multiple breath washout measurements in preschool children [abstract] Pediatr Pulmonol. 2014;49(S38):215. [Google Scholar]
- 19.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H, et al. American Thoracic Society/European Respiratory Society Working Group on Infant and Young Children Pulmonary Function Testing. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 20.VanDevanter DR, Elkin EP, Pasta DJ, Morgan WJ, Konstan MW Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Changing thresholds and incidence of antibiotic treatment of cystic fibrosis pulmonary exacerbations, 1995-2005. J Cyst Fibros. 2013;12:332–337. doi: 10.1016/j.jcf.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40:61–66. doi: 10.1183/09031936.00159111. [DOI] [PubMed] [Google Scholar]
- 22.Wagener JS, Rasouliyan L, VanDevanter DR, Pasta DJ, Regelmann WE, Morgan WJ, Konstan MW Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48:666–673. doi: 10.1002/ppul.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanojevic S, McDonald A, Waters V, MacDonald S, Horton E, Tullis E, Ratjen F.Effect of pulmonary exacerbations treated with oral antibiotics on clinical outcomes in cystic fibrosis Thorax[online ahead of print] 9 Sep 2016; DOI: 10.1136/thoraxjnl-2016-208450 [DOI] [PubMed] [Google Scholar]
- 24.Stahl M, Wielpütz MO, Graeber SY, Joachim C, Sommerburg O, Kauczor HU, Puderbach M, Eichinger M, Mall MA. Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med. 2016;195:349–359. doi: 10.1164/rccm.201604-0893OC. [DOI] [PubMed] [Google Scholar]
- 25.Sonneveld N, Stanojevic S, Amin R, Aurora P, Davies J, Elborn JS, Horsley A, Latzin P, O’Neill K, Robinson P, et al. Lung clearance index in cystic fibrosis subjects treated for pulmonary exacerbations. Eur Respir J. 2015;46:1055–1064. doi: 10.1183/09031936.00211914. [DOI] [PubMed] [Google Scholar]
- 26.Anstead M, Saiman L, Mayer-Hamblett N, Lands LC, Kloster M, Goss CH, Rose L, Burns JL, Marshall B, Ratjen F. Pulmonary exacerbations in CF patients with early lung disease. J Cyst Fibros. 2014;13:74–79. doi: 10.1016/j.jcf.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Simpson SJ, Ranganathan S, Park J, Turkovic L, Robins-Browne RM, Skoric B, Ramsey KA, Rosenow T, Banton GL, Berry L, et al. AREST CF. Progressive ventilation inhomogeneity in infants with cystic fibrosis after pulmonary infection. Eur Respir J. 2015;46:1680–1690. doi: 10.1183/13993003.00622-2015. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld M, Bernardo-Ocampo C, Emerson J, Genatossio A, Burns J, Gibson R. Prevalence of cystic fibrosis pathogens in the oropharynx of healthy children and implications for cystic fibrosis care. J Cyst Fibros. 2012;11:456–457. doi: 10.1016/j.jcf.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 29.O’Neill K, Tunney MM, Johnston E, Rowan S, Downey DG, Rendall J, Reid A, Bradbury I, Elborn JS, Bradley JM. Lung clearance index in adults and children with cystic fibrosis. Chest. 2016;150:1323–1332. doi: 10.1016/j.chest.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Chinn S. Statistics in respiratory medicine. 2. Repeatability and method comparison. Thorax. 1991;46:454–456. doi: 10.1136/thx.46.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen R, Stanojevic S, Klingel M, Pizarro ME, Hall GL, Ramsey K, Foong R, Saunders C, Robinson PD, Webster H, et al. A systematic approach to multiple breath nitrogen washout test quality. PLoS One. 2016;11:e0157523. doi: 10.1371/journal.pone.0157523. [DOI] [PMC free article] [PubMed] [Google Scholar]