Abstract
The past century has witnessed a transformative shift in lung cancer from a rare reportable disease to the leading cause of cancer death among men and women worldwide. This historic shift reflects the increase in tobacco consumption worldwide, spurring public health efforts over the past several decades directed at tobacco cessation and control. Although most lung cancers are still diagnosed at a late stage, there have been significant advances in screening high-risk smokers, diagnostic modalities, and chemopreventive approaches. Improvements in surgery and radiation are advancing our ability to manage early-stage disease, particularly among patients considered unfit for traditional open resection. Arguably, the most dramatic progress has occurred on the therapeutic side, with the development of targeted and immune-based therapy over the past decade. This article reviews the major shifts in the lung cancer landscape over the past 100 years. Although many ongoing clinical challenges remain, this review will also highlight emerging molecular and imaging-based approaches that represent opportunities to transform the prevention, early detection, and treatment of lung cancer in the years ahead.
It is hard to argue that any pulmonary disease has undergone a more dramatic transformation over the past century than lung cancer. More than 100 years ago, lung cancer was an extremely rare disease, with malignant lung tumors representing only 1% of all cancers seen at autopsy at the Institute of Pathology of the University of Dresden in Germany in 1878 (1). Today, lung cancer is the leading cause of cancer death in both men and women worldwide, accounting for 1.8 million new cases and 1.6 million deaths annually (2). More than 158,000 Americans died from lung cancer in 2016, accounting for ∼27% of all cancer deaths (2). The 5-year survival rate for lung cancer is only 17.7%, strikingly lower than other common cancers, including colon (64.4%), breast (89.7%), and prostate (98.9%) (3). This review summarizes the rapidly changing landscape for lung cancer over the past 100 years, with a particular focus on the evolution in our understanding of the epidemiology of the disease, attempts to prevent it, strategies for screening/early detection, and the evolving approaches to treating those with early- or advanced-stage disease. Although the clinical challenges remain significant, this review also highlights future opportunities for impacting the prevention, diagnosis, and treatment of this leading deadly cancer in the years ahead.
Epidemiology and Prevention
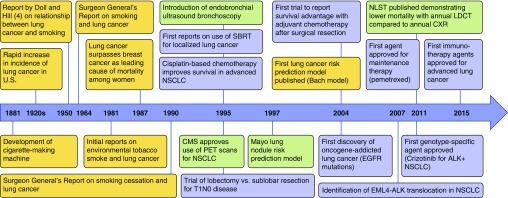
Over the past 100 years, lung cancer has transitioned from a reportable disease to the leading cause of cancer death among men and women worldwide (Figure 1). The rapid increase of the deadliest cancer in the last century can be attributed directly to the increase in tobacco smoking over that same period. Although burning tobacco and inhaling the smoke is believed to have begun as early as 5000 to 3000 bc, it was not until the cigarette-making machine was developed in 1881 that cigarette smoking became widespread. In 1950, Doll and Hill were the first to report the epidemiological evidence on the association of tobacco smoking with lung cancer (4). This was followed by the U.S. Surgeon General’s report in 1964, which unequivocally linked smoking to negative health outcomes and directed public health efforts toward quitting and abstinence. Since that report, smoking rates in the United States have declined significantly, from a peak prevalence of 42% to approximately 15% of adults reporting daily cigarette use in 2015. This decline has been largely attributed to the impact of anti-smoking advertising campaigns, cigarette taxes, smoking bans, and advances in tobacco dependence treatment with behavioral counseling and pharmacotherapy. There have, however, been concerns raised regarding the recent increase over the past decade in electronic cigarette (e-cigarette) use, the long-term effects of which are unknown (5). It is also concerning that tobacco smoking continues to be highly prevalent in many parts of the developing world, a particular concern in China, where there are more than 350 million active smokers.
Figure 1.
Timeline depicting historical milestones in lung cancer epidemiology, screening, diagnosis, and treatment. Events related to epidemiology are in yellow; screening and diagnosis are in green; treatment is in blue. ALK = anaplastic lymphoma kinase; CMS = Centers for Medicaid and Medicare Services; CXR = chest X-ray; EGFR = epidermal growth factor receptor; EML4 = echinoderm microtubule–associated protein-like 4; LDCT = low-dose computed tomography; NLST = National Lung Screening Trial; NSCLC = non–small-cell lung cancer; PET = positron emission tomography; SBRT = stereotactic body radiation therapy.
Although tobacco smoking is the dominant risk factor for lung cancer, it is estimated that 10 to 15% of cases in the United States are caused by factors other than smoking, making lung cancer in never smokers among the top causes of cancer mortality in the United States (6). Lung cancer that develops in never-smokers has distinct molecular alterations and response to targeted therapy (see Targeted Therapy). Risk factors for never-smoker lung cancer include exposure to radon, environmental tobacco smoke, and other indoor air pollutants; however, a significant fraction of lung cancers occurring in never smokers cannot be definitively associated with established environmental risk factors.
The primary tool for lung cancer prevention remains smoking cessation and avoidance of other environmental risk factors. In a large prospective study, smokers who successfully quit had a 55% reduction in lung cancer risk (7). Unlike the rapid reduction in cardiovascular risk after smoking cessation, the lung cancer risk from smoking fades more slowly, with several studies showing that an ex-smoker who quit 10 to 15 years earlier is still several times more likely to die of lung cancer than someone who has never smoked (8). Whether lung cancer risk ever returns to the levels seen in never smoker may depend on age at time of quitting; studies have reported that those who quit before age 30 years are no more likely to die from lung cancer than never smokers (9).
Although smoking cessation is clearly the most effective intervention to reduce the incidence of lung cancer, a majority of cases are now diagnosed among former smokers, given the relatively slow decrease in lung cancer risk after cessation. As a result, there has been considerable effort over the past three decades to develop chemoprevention strategies, using dietary or pharmacological interventions to slow or reverse the progression to invasive lung cancer (10). These efforts, by and large, have fallen short, and there is currently no effective chemopreventive agent for lung cancer. Early chemoprevention trials focused on vitamin A and beta carotene failed to show a protective effect, and some trials suggested a potential increase in lung cancer risk (11–13). Furthermore, a recent phase III trial failed to demonstrate efficacy for selenium supplementation (14). There are, however, a number of agents, including iloprost (15), myoinositol (16), and cyclooxygenase-2 inhibitors, that are currently being explored in early-phase trials.
The generally disappointing history over the last several decades of lung cancer chemoprevention studies suggests that there remain a number of critical barriers to developing effective interventions in this space. First and foremost, we struggle with identifying the patients at highest risk who should be evaluated in these trials. Identifying higher-risk populations would enable intervening with agents that have potentially higher toxicity. Ultimately, all of these trials struggle with the identification of appropriate clinical endpoints to demonstrate efficacy of the interventions. Although tertiary prevention trials (among those with history of lung cancer) use incident lung cancers, given the relative high rate in this population, primary and secondary prevention trials lack well-established surrogate endpoints or biomarkers. Finally, similar to the recent paradigm shift in the systemic therapy of lung cancer, there is a clear need to personalize the interventions on the basis of the molecular pathways driving lung carcinogenesis within a given individual.
Screening and Early Detection
In the 1970s, the main efforts to develop screening tests for lung cancer were based on chest radiography and sputum cytology (17–19). Randomized controlled trials assessing these modalities failed to show a reduction in lung cancer mortality. Because of concerns regarding limited power and contamination of the control arms, screening with chest radiography was further evaluated as a part of the Prostate, Lung, Colorectal, and Ovarian (PLCO) trial, which randomized more than 154,000 patients, including both smokers and nonsmokers, to annual radiography or usual care. Annual chest radiographic screening for up to 4 years did not have an effect on lung cancer mortality with 13 years of follow-up in the trial (20).
Although the PLCO trial was ongoing, interest in lung cancer screening turned to use of low-dose computed tomography (LDCT), which provided a more sensitive method for the detection of pulmonary nodules than chest radiography. After the publication of the initial experience with LDCT in 1999 (21), multiple single-arm studies went on to demonstrate two key findings: although LDCT identified a large number of noncalcified lung nodules, most of which were not malignant, it resulted in a higher proportion of stage I or II lung cancers compared with historical data. Given these promising results, the National Cancer Institute launched the National Lung Screening Trial (NLST) in 2002, randomizing 53,454 patients at high risk (defined as age 55–74 yr; tobacco pack-years > 30; and, if a previous smoker, quit within 15 yr) to either annual LDCT or chest radiography for 3 years. After 7 years of follow-up, the LDCT arm demonstrated a reduction in lung cancer–specific mortality from 1.66 to 1.33% (a 20% relative reduction) (22).
Although the NLST represents a landmark advance in lung cancer screening, implementation of screening programs has been slow, in part due to concerns regarding the high false-positive rate and potential for harms to individuals without lung cancer. Among all LDCT scans, 24% of were classified as positive (i.e., identified at least one nodule > 4 mm in size), with 96% ultimately proven to be falsely positive (22). Although the subsequent evaluation after a positive finding was most commonly surveillance imaging, and the use of invasive procedures was relatively infrequent, 4.0% of LDCT participants underwent surgical lung biopsy after the baseline scan, identifying benign disease in 29% of these procedures (23). In their evaluation of LDCT, the U.S. Preventative Services Task Force recognized the concerns regarding false positive and potential for harm and provided a B-level recommendation for its use (24). The Centers for Medicaid and Medicare Services also requires a shared decision-making visit to allow patients to understand their lung cancer risk and weigh the potential benefits and harms of undergoing screening (25).
The enthusiasm for implementation has been further diminished by negative results of several other randomized trials evaluating LDCT screening. Both the Italian DANTE (Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays) trial and the Danish Lung Cancer Screening Trial (DLCST) failed to show a difference in mortality benefit with LDCT (26, 27). Although these trials were considerably smaller and underpowered to show a mortality benefit, their findings have raised concerns regarding the generalizability of results from NLST. Although implementation efforts are proceeding in the United States, most other regions around the world are awaiting the results of the NELSON trial (the Dutch-Belgian Randomized Lung Cancer Screening trial), a collaborative Dutch-Belgian study that has randomized 15,822 participants to LDCT screening versus no screening (28).
Recent efforts have focused on improving the ability to identify patients who are more likely to benefit from LDCT screening (29–31). Studies have now shown that the use of enhanced risk-based approaches is potentially more efficient than identifying patients simply on the basis of age and smoking history. Whether guidelines and coverage decisions will be altered to incorporate these enhanced risk-based approaches for patient selection remains uncertain. Additional future challenges include translating the adherence in annual screening and maintaining the high quality of care seen in the NLST to clinical practice.
One potential approach to improve the balance between benefits and harms with LDCT screening is the development of novel markers measured in biological samples (e.g., blood, urine) or imaging-based approaches that improve the ability to predict lung cancer risk. Biomarker discovery and validation have proven far more difficult than initially anticipated. The primary challenges relate to tumor heterogeneity and the variable natural history of cancer that slowed progress. Despite these challenges, a few biomarkers have now emerged that have reported sufficiently high levels of performance in early-phase studies and are currently undergoing prospective evaluation. In a randomized screening trial sponsored by National Health Service Scotland, investigators are testing whether screening high risk populations with seven serum autoantibodies can reduce the incidence of patients with late-stage lung cancer compared to standard practice (32). Other approaches under evaluation include a serum microRNA profile (33) and a nasal gene-expression profile (34).
Diagnosis and Staging
Methods for establishing the diagnosis of lung cancer have evolved considerably over the last century and are increasingly focused on the use of minimally invasive approaches. Before the introduction of computed tomography (CT) scans in the 1970s, plain chest radiograph was the only means of assessing lung cancers. CT provides considerably greater anatomic information regarding the location and extent of the tumor and involvement of adjacent structures, such as major vessels, the mediastinum, and the chest wall, as well as assessment of the liver and adrenal glands.
In current practice, most lung cancers are initially identified incidentally on chest imaging performed for another indication, during an evaluation for respiratory symptoms, or on a screening LDCT. The rapid increase in the use of thoracic CT imaging has resulted in an extraordinary increase in the identification of incidentally detected pulmonary nodules. One recent report estimates that approximately 4.8 million individuals undergo at least one chest CT scan, with 1.2 million identified with a new lung nodule (35). Although most incidentally detected pulmonary nodules are benign, some may represent lung cancer, influenced primarily by nodule size, patient age, and smoking history (36–38).
The uncertainty for any given nodule results in the need for a thoughtful evaluation regarding the prior probability of malignancy and any subsequent evaluation, either additional surveillance imaging, fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging, or an invasive evaluation (39, 40). FDG-PET scanning, based on the principle that cancer cells have increased cellular uptake of glucose and use of 18-FDG as the tracer results in greater uptake into neoplastic cells, which can then be identified by PET imaging, was first approved for lung nodule assessment in 1995. Although this has improved the ability to assess the likelihood of malignancy, particularly in patients with intermediate risk of disease, PET is still hampered by a high rate of false-negative and -positive findings. This, coupled with both patient and physician anxiety, results in an excessively high rate of invasive evaluation, including surgical lung biopsy, for many patients with indeterminate lung nodules in whom the likelihood of malignancy does not support such an aggressive approach (41–43).
In appropriately selected patients with a high probability of early-stage disease on the basis of imaging, the initial diagnostic procedure may be a surgical resection to both establish the diagnosis and treat the cancer. In most other circumstances, establishing the diagnosis and stage of lung cancer largely relies on the use of minimally invasive procedures, such as bronchoscopy, transthoracic needle aspiration, or image-guided biopsy of an extrathoracic lesion. Diagnostic bronchoscopy has undergone two major paradigm shifts in the last 40 years (44). First, the advent of flexible bronchoscopy gave chest physicians improved access to the tracheobronchial tree compared with rigid bronchoscopy. The second major advance has evolved over the last 20 years with the advent and refinement of new technologies that have greatly improved the capabilities of flexible bronchoscopy. At the forefront of these new technologies is endobronchial ultrasound (45). In its various forms, endobronchial ultrasound has improved diagnostic yield for pulmonary masses, nodules, and intrathoracic adenopathy, thereby reducing the need for more invasive surgical interventions. Various navigational bronchoscopy systems have also become available, allowing greater access to parenchymal lesions, especially peripherally located nodules that could not previously have been considered for bronchoscopy (46, 47).
Strategies to improve our ability to assess and manage indeterminate lung nodules in the future include the use of molecular biomarkers (48), quantitative imaging techniques (49, 50), and improvements in biopsy technique (51). Recent reports have demonstrated that a gene-expression biomarker in cytologically normal bronchial epithelium collected from brushings of the mainstem bronchus improves the sensitivity of bronchoscopy for lung cancer (52, 53), whereas a number of candidate biomarkers in the peripheral blood, nasal epithelium, urine, sputum, and exhaled breath are being evaluated in this space (34, 54–57).
Surgery
Although thoracic surgery emerged as a separate specialty during World War II, use of surgical resection for lung cancer did not become a commonly used approach for treatment until the 1960s, when multiple studies reported the use of lobectomy and segmentectomy. Over the next 2 decades, there was debate over the adequacy of sublobar resection for curative treatment of lung cancer in terms of long-term survival (58–60). This controversy ultimately led to the first randomized clinical trial in thoracic surgery; the Lung Cancer Study Group randomized 247 patients with stage T1N0 lesions to lobectomy or sublobar resection between 1982 and 1988 (61). Locoregional recurrence was significantly higher, but death from cancer did not differ between groups. The results from this trial have been used to support the use of lobectomy for all patients with stage I and II non–small-cell lung cancer (NSCLC) who are medically fit for surgical resection (61). However, in the current era of diagnosis and staging with CT and PET scanning, there is uncertainty if the results of this study apply to all patients with early-stage disease or if there are some patients who may benefit from a limited resection.
Over the last 20 years, efforts have largely focused on the development of minimally invasive approaches to lung resection. After the introduction of anatomic lung resection by video-assisted thoracoscopic surgery (VATS) in the early 1990s (62, 63), there was considerable debate regarding the role of this technique in achieving an adequate resection and assessment of lymph nodes. Although randomized trial data are lacking, recent studies have now demonstrated equivalent efficacy with less morbidity—fewer complications, lower perioperative mortality and pain, shorter hospital length of stay—with VATS for the management of early-stage disease (64–66). Although there is some controversy regarding the ability of VATS to allow for adequate nodal sampling, this may reflect differences based on surgeon as opposed to inherent limitations of this technique (67, 68).
Robotic-assisted surgery, performed by controlling surgical instruments from a console, has been proposed as an alternative to VATS for lung cancer resection. Potential advantages of robotic surgery over VATS include three-dimensional visualization and allowing natural movements of the surgeon’s hands and wrists to be translated by the computer-assisted robotic arms inside the thorax (69, 70). However, these advantages are potentially offset by the steep learning curve, the loss of tactile feedback, and concerns regarding the management of intraoperative bleeding.
Radiotherapy
Radiotherapy (RT) plays a key role in both curative and palliative treatments for lung cancer. In the modern era of radiation, curative-intent RT is well established for patients with locally advanced and early-stage NSCLC. Perhaps the most impactful recent advance is the emergence of stereotactic ablative body radiotherapy (SABR) for treatment of early-stage lung cancer in medically inoperable or high surgical risk patients. SABR, initially described in the mid-1990s (71), is a method for delivering external beam radiotherapy with a high degree of accuracy to a target using high doses of radiation in one to eight treatment fractions (72, 73).
The success of SABR depends significantly on appropriate patient selection and appropriate diagnostic and staging evaluation before RT initiation. All patients with early-stage lung cancer considered for SABR should undergo appropriate clinical and invasive staging to assure the full extent of disease. Many patients treated with SABR have poor pulmonary reserve and significant comorbidities, and so the risks of biopsy can outweigh the benefits. In addition, many patients have tumors that are not safely accessible to either transthoracic needle aspiration or transbronchial needle biopsy. Given these considerations, SABR is now increasingly used without a histologic diagnosis of lung cancer (i.e., empiric SABR), raising concern regarding the potential for treatment of benign disease. Although generally very well tolerated, SABR carries a low risk of adverse events, including pneumonitis, chest wall toxicity, and rib fracture for peripheral lesions, and concern for great vessel or airway injury for central tumors (74). This underscores the importance of performing empiric treatment only in patients with a high certainty of malignancy.
There is considerable debate regarding the use of SABR in patients who are appropriate candidates for surgery. Although randomized trials to address this question have been attempted, progress has been hampered by poor accrual resulting in study closure. A recent pooled analysis of two these studies suggests a higher 3-year survival with SABR than with surgery, largely due to a higher rate of perioperative mortality in the surgical group (75). Several trials of SABR in both high-risk and medically fit patients are ongoing and should help to further define the role of this approach in early-stage disease.
Medical Treatment of Advanced Disease
The last few years have brought unprecedented breakthroughs in the tools available to treat advanced lung cancer. In a short time, the number of available Food and Drug Administration (FDA)-approved drugs has more than doubled, and treatments have become more personalized and more tolerable. Oncologists are able to categorize lung cancers in a more sophisticated manner and use biomarkers to tailor treatment to histological cell type, driver mutation, and immune biomarker status (Table 1).
Table 1.
Summary of Commonly Used Standard Medical Therapies for Advanced Non–Small-Cell Lung Cancer in 2017
| Patient Cohort | First Line | Second Line | Third Line and Beyond |
|---|---|---|---|
| Nonsquamous, wild type for driver mutations, PDL1 < 50% | Platinum + pemetrexed, followed by maintenance pemetrexed (some add bevacizumab) | PD-1 or PDL1 inhibitor | Salvage chemotherapy |
| Nonsquamous, wild type for driver mutations, PDL1 ≥ 50% | Pembrolizumab | Platinum + pemetrexed, followed by maintenance pemetrexed (some add bevacizumab) | Salvage chemotherapy |
| Squamous cell, PDL1 < 50% | Platinum + taxane or gemcitabine (some add necitumumab to gemcitabine) | PD-1 or PDL1 inhibitor | Salvage chemotherapy |
| Squamous cell, PDL1 ≥ 50% | Pembrolizumab | Platinum + taxane or gemcitabine (some add necitumumab to gemcitabine) | Salvage chemotherapy |
| Nonsquamous, EGFR mutation positive | Erlotinib, afatinib, or gefitinib | Osimertinib if T790M positive, platinum + pemetrexed, followed by maintenance pemetrexed (some add bevacizumab) if T790M negative | Salvage chemotherapy |
| Nonsquamous, ALK rearrangement positive | Crizotinib | Alectinib or ceritinib | Platinum + pemetrexed, followed by maintenance pemetrexed (some add bevacizumab) |
| Nonsquamous, ROS1 rearrangement positive | Crizotinib | Platinum + pemetrexed, followed by maintenance pemetrexed (some add bevacizumab) | Salvage chemotherapy |
Definition of abbreviations: ALK = anaplastic lymphoma kinase; EGFR = epidermal growth factor receptor; PD-1 = programmed death 1; PDL1 = programmed death ligand-1; ROS1 = ROS proto-oncogene.
Clinical trials should also be considered if available, regardless of line of therapy. Approved PD-1 inhibitors include nivolumab and pembrolizumab. Approved PDL1 inhibitor is atezolizumab. Salvage chemotherapy options include docetaxel ± ramucirumab, paclitaxel, nanoparticle albumin-bound paclitaxel, vinorelbine, and gemcitabine, and these are often given sequentially according to patient tolerance. Other rare driver mutations often have specific targeted therapies available as well, either used off-label or via clinical trials. Only Food and Drug Administration–approved indications for targeted therapies are listed here.
Chemotherapy
Medical oncologists believed chemotherapy advancement for NSCLC had reached a plateau at the turn of the century, when a number of studies suggested two-drug combination regimens were all equivalent, with median overall survival (OS) of 7 to 8 months, and three-drug regimens offered no additional benefit (76). However, since that time there have been three major advances in the approach, resulting in longer survival for patients: (1) the recognition that certain chemotherapeutics work better in one histology compared with another, (2) the introduction of maintenance chemotherapy as a strategy, and (3) the combination of chemotherapy with biologics.
Pemetrexed was initially FDA approved in 2004 as a salvage chemotherapeutic, but subsequent studies revealed that it was particularly active in patients with nonsquamous NSCLC, a classification primarily consisting of adenocarcinoma. A randomized study of front-line chemotherapy regimens showed that in the nonsquamous group, median OS was improved from 10.9 months with cisplatin/gemcitabine to 12.6 months for cisplatin/pemetrexed (77). Furthermore, in nonsquamous NSCLC, continuing pemetrexed as a “maintenance” therapy after completion of four to six cycles of platinum/pemetrexed doublet increased median OS from 10.6 to 13.4 months (78). Pemetrexed also modernized the patient experience of front-line chemotherapy, as it does not cause alopecia or neuropathy, and febrile neutropenia is rarer than in older regimens.
The term biologics usually refers to monoclonal antibodies directed at specific markers on the cancer cell. Biologics are often given in combination with chemotherapy. The first biologic approved for NSCLC was the vascular endothelial growth factor–targeted bevacizumab, which improved OS from 10.3 to 12.3 months for patients with nonsquamous disease when added to carboplatin/paclitaxel chemotherapy but later failed to further improve outcomes when added to carboplatin/pemetrexed (79, 80). Because of increased risk of life-threatening hemoptysis, bevacizumab should not be used in patients with squamous cell cancer. However, another vascular endothelial growth factor–targeted biologic, ramucirumab, is approved regardless of histology, in combination with second-line docetaxel (81).
Patients with squamous cell carcinoma are preferentially treated in the front line with platinum doublet regimens including gemcitabine or taxanes, with a novel nanoparticle albumin-bound form of paclitaxel (NAB-paclitaxel) having particularly high response rates among patients with squamous disease (82). The final biologic approved in lung cancer at this time, necitumumab, is solely for patients with squamous carcinoma and targets epidermal growth factor receptor (EGFR). A randomized trial showed the addition of necitumumab to cisplatin/gemcitabine improved overall survival from 9.9 to 11.5 months (83).
Unfortunately, chemotherapeutics for small-cell lung cancer have not shown similar advances, and the standard of care remains as it has been for the last few decades—platinum and etoposide for initial treatment and topoisomerase inhibitors as salvage therapy.
Targeted Therapy
NSCLC was the first solid tumor to enter the targeted therapy revolution with the 2004 discovery that somatic mutations in EGFR were present in a subset of adenocarcinoma and convey an oncogene-addicted biology in which targeting the driver oncogene with a specific oral tyrosine kinase inhibitor (TKI) yields dramatic and durable responses (median progression-free survival of about 1 yr) (84). Since that initial discovery, several other driver oncogenes have been discovered in lung adenocarcinoma, clustering mainly within patients with low or never-smoking histories. In fact, patients with adenocarcinoma have a 60 to 90% chance of harboring a mutation, depending on the region of the world in which they live and their smoking history; genotyping is now a standard part of the diagnostic work-up for lung adenocarcinoma (85, 86).
Multiple randomized trials have been conducted showing that genotype-specific TKIs are superior to front-line chemotherapy in terms of response, quality of life, and progression-free survival, including the EGFR drugs gefitinib, erlotinib, and afatinib, and the anaplastic lymphoma kinase inhibitor crizotinib, all of which are approved as initial treatments in genotype-selected patients (87–90). However, because these agents have strong efficacy even in the salvage setting, OS advantages are often only apparent when comparing against historical data. For example, a recent study showed that modern-day patients with ALK have a median OS of 4 years, a significant improvement from the era preceding the advent of targeted therapy (91).
For patients with EGFR mutations and ALK rearrangements, the two most common driver mutations in lung adenocarcinoma, we now have multiple drugs approved for the setting of acquired resistance to first-line drugs. Osimertinib is a novel drug that inhibits the most common resistance mutation to arise on first-line EGFR TKI therapy, a point mutation called T790M (92), whereas ceritinib and alectinib are second-generation ALK inhibitors that are more potent than crizotinib in patients with ALK rearrangements (93, 94). Through studying such patients and developing such drugs, several principles about the mechanisms of acquired TKI resistance have been uncovered, and hence current trials are focusing on reexamining our overall strategy by putting some of the drugs that thwart resistance in the front-line setting and/or looking at up-front combinations of targeted therapy. Other areas of active investigation include the best strategies for patients with brain metastases and whether and how to combine targeted therapies with chemotherapy or immunotherapy.
In addition to EGFR and ALK, there are active therapies for lung cancers with driver mutations in MET proto-oncogene (MET), B-Raf proto-oncogene (BRAF), and human epidermal growth factor receptor 2 (HER2), rearrangements in ROS proto-oncogene (ROS1), RET proto-oncogene (RET), and tyrosine receptor kinase (TRK), and amplifications of MET and HER2 (95). Although these mutations each make up a small percentage of all lung cancer cases diagnosed each year (1–2%), there are a fair absolute number of patients with rarer driver mutations, because lung cancer is so prevalent overall. More research is needed still, especially about mutations such as KRAS, which are known to be frequently recurrent in lung cancer, but from whom a successful targeted therapeutic strategy has thus far eluded us.
Immunotherapy
The newest class of therapeutics for lung cancer is immunotherapy (IO), with three different checkpoint inhibitors FDA approved for lung cancer since 2015 and likely more on the way. These drugs are monoclonal antibodies against either the programmed death 1 (PD1) or programmed death ligand-1 (PDL1) proteins, which are key modulators of the immune regulation system, important for self-tolerance. Some tumors, including lung cancers, can co-opt this mechanism to encourage immune tolerance to the cancer. IO treatments are active in both adenocarcinoma and squamous cell carcinoma. They are generally very well tolerated, without the typical myelosuppression, nausea, and other toxicities associated with chemotherapy. However, IO drugs can rarely induce autoimmune side effects, which can be severe or life threatening, including pneumonitis, colitis, vasculitis, and a host of other inflammatory conditions. Patients with clinically significant autoimmune diseases at baseline are excluded from this family of therapies. The most promising aspect of the field of IO is that a small portion of patients seem to have unusually long periods of disease quiescence, sometimes lasting for multiple years after the IO agent is stopped—a long “tail of the curve,” which has led to speculation that if patients could be rationally selected, cure may be a future possibility. For example, one of the earliest phase I studies started in NSCLC using the checkpoint inhibitor nivolumab among heavily pretreated patients has now shown that 18% of subjects are still alive at 3 years, a departure from historical outcomes for salvage phase I trials in unselected patients (96).
The putative biomarker for selecting patients for IO is PDL1 tumor staining by immunohistochemistry. However, it is in some ways a suboptimal test, as each specific IO drug has been tested using a separate PDL1 staining antibody and distinct analysis methods. Currently, the PD-1 inhibitor pembrolizumab is approved in the front-line setting for patients with NSCLC with PDL1 expression greater than 50% (which includes approximately 30% of patients with NSCLC) on the basis of a randomized trial in which median progression-free survival for pembrolizumab was 10.3 months compared with 6.0 months for the investigator’s choice of platinum-doublet chemotherapy (97). In the second-line setting, nivolumab (PD-1 inhibitor) and atezolizumab (PDL1 inhibitor) are both approved for any histology, without the need for biomarker selection, on the basis of successful randomized trials against docetaxel with OS as the primary endpoint (98–100). Pembrolizumab is approved in the second-line setting for those with PDL1 expression greater than 1% (which is approximately 66% of patients) (101).
Areas of future research in IO include combining PD-1 or PDL1 inhibitors with chemotherapy or with other types of immune inhibitors like cytotoxic T-lymphocyte–associated protein-4 blockers and increasing our understanding of response and resistance. There was already a promising randomized phase II study showing front-line platinum-pemetrexed chemotherapy plus pembrolizumab in unselected patients with nonsquamous disease had a progression-free survival of 13.0 months, which is on par with the overall survival seen in most front-line chemotherapy studies (102). Finally, early research indicates that another potential method for predicting benefit from IO therapy may have to do with mutational load—the more mutations a given cancer harbors, the more likely it may be to respond to IO (103).
Future Directions
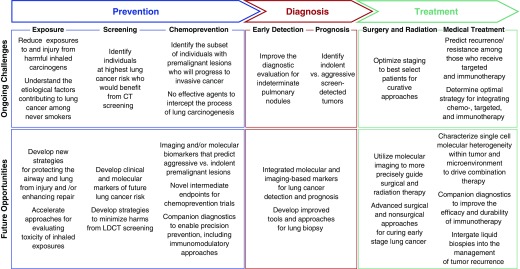
Although the last century has witnessed transformative changes in all aspects of lung cancer care, a number of critical unmet challenges remain. With the rapid evolution in molecular and imaging-based technologies, there are a number of opportunities for the clinical and translational research community to directly impact these unmet needs in the years ahead (Figure 2). Techniques for more rapidly evaluating the potential carcinogenicity of a number of emerging environmental exposures are being developed, as are strategies for protecting the pulmonary epithelium from damage secondary to those exposures. Although LDCT screening is arguably the main advance over the past several decades for early lung cancer detection, clinical and molecular approaches for selecting those at highest risk who would benefit from annual surveillance are emerging. Furthermore, attempts to develop a premalignant cancer genome atlas (104) for both squamous and adenocarcinoma of the lung will provide insights into the earliest cellular and molecular events associated with lung carcinogenesis, which can lead to: (1) molecular biomarkers to identify those premalignant lesions likely to progress to invasive cancer, (2) novel targets for precision-based chemoprevention trials, and (3) intermediate endpoints for evaluating therapeutic efficacy in those trials. Understanding the role of the microenvironment in early lung carcinogenesis will also lead to novel immune-based strategies for lung cancer prevention. Advances in quantitative imaging, molecular biomarkers, and biopsy techniques will accelerate the diagnostic evaluation of the increasing number of pulmonary nodules that are being detected incidentally and on LDCT screening. These same imaging and molecular modalities promise to yield prognostic markers to identify those early-stage tumors that are indolent versus aggressive, addressing the potential concern regarding overdiagnosis of lung cancer in the screening era. On the therapeutic side, advances in molecular imaging alongside new surgical and nonsurgical approaches promise to improve cure rates for those with early-stage lung cancer. Liquid biopsies hold the potential to intercept disease recurrence and impact management of advanced-stage disease. Finally, single-cell approaches to characterize cellular and molecular heterogeneity within the tumor and its microenvironment will impact strategies for combining targeted treatment and immunotherapy to overcome resistance and provide precision approaches to lung cancer treatment.
Figure 2.
Future opportunities to impact the prevention, detection, and treatment of lung cancer. CT = computed tomography; LDCT = low-dose computed tomography.
Footnotes
Supported by the National Institutes of Health grants R01CA210360 (A.S.), U01CA214182 (A.S.), and P30ES013508 (A.V.).
CME will be available for this article at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Heuvers M, Hegmans JP, Stricker BH, Aerts JG. Improving lung cancer survival: time to move on. BMC Pulm Med. 2012;12:77. doi: 10.1186/1471-2466-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; 2016. [Google Scholar]
- 4.Doll R, Hill AB. Smoking and carcinoma of the lung: preliminary report. BMJ. 1950;2:739–748. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinakar Ch, O’Connor GT. The health effects of electronic cigarettes. N Engl J Med. 2016;375:2608–2609. doi: 10.1056/NEJMc1613869. [DOI] [PubMed] [Google Scholar]
- 6.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, Rudin CM. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. ClinCancer Res. 2009;15:5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. J Natl Cancer Inst. 1993;85:457–464. doi: 10.1093/jnci/85.6.457. [DOI] [PubMed] [Google Scholar]
- 9.Pirie K, Peto R, Reeves GK, Green J, Beral V Million Women Study Collaborators. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133–141. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keith RL. Lung cancer chemoprevention. Proc Am Thorac Soc. 2012;9:52–56. doi: 10.1513/pats.201107-038MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 12.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 13.van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–986. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 14.Karp DD, Lee SJ, Keller SM, Wright GS, Aisner S, Belinsky SA, Johnson DH, Johnston MR, Goodman G, Clamon G, et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J Clin Oncol. 2013;31:4179–4187. doi: 10.1200/JCO.2013.49.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keith RL, Blatchford PJ, Kittelson J, Minna JD, Kelly K, Massion PP, Franklin WA, Mao J, Wilson DO, Merrick DT, et al. Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prev Res (Phila) 2011;4:793–802. doi: 10.1158/1940-6207.CAPR-11-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam S, Mandrekar SJ, Gesthalter Y, Allen Ziegler KL, Seisler DK, Midthun DE, Mao JT, Aubry MC, McWilliams A, Sin DD, et al. Cancer Prevention Network. A randomized phase IIb trial of myo-inositol in smokers with bronchial dysplasia. Cancer Prev Res (Phila) 2016;9:906–914. doi: 10.1158/1940-6207.CAPR-15-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR. Lung cancer screening: the Mayo program. J Occup Med. 1986;28:746–750. doi: 10.1097/00043764-198608000-00038. [DOI] [PubMed] [Google Scholar]
- 18.Kubik A, Parkin DM, Khlat M, Erban J, Polak J, Adamec M. Lack of benefit from semi-annual screening for cancer of the lung: follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int J Cancer. 1990;45:26–33. doi: 10.1002/ijc.2910450107. [DOI] [PubMed] [Google Scholar]
- 19.Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer: results of the Memorial Sloan-Kettering study in New York. Chest. 1984;86:44–53. doi: 10.1378/chest.86.1.44. [DOI] [PubMed] [Google Scholar]
- 20.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, Crawford ED, Fouad MN, Isaacs C, Reding DJ, et al. PLCO Project Team. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 21.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 22.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, Fagerstrom RM, Gareen IF, Gierada DS, Jones GC, et al. National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Medicare and Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N); 2015 [accessed 2017 Feb 26]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.
- 26.Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, Brambilla G, Angeli E, Aranzulla G, Chiti A, et al. DANTE Study Group. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191:1166–1175. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 27.Wille MM, Dirksen A, Ashraf H, Saghir Z, Bach KS, Brodersen J, Clementsen PF, Hansen H, Larsen KR, Mortensen J, et al. Results of the randomized Danish Lung Cancer Screening Trial with focus on high-risk profiling. Am J Respir Crit Care Med. 2016;193:542–551. doi: 10.1164/rccm.201505-1040OC. [DOI] [PubMed] [Google Scholar]
- 28.Horeweg N, Scholten ET, de Jong PA, van der Aalst CM, Weenink C, Lammers JW, Nackaerts K, Vliegenthart R, ten Haaf K, Yousaf-Khan UA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol. 2014;15:1342–1350. doi: 10.1016/S1470-2045(14)70387-0. [DOI] [PubMed] [Google Scholar]
- 29.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315:2300–2311. doi: 10.1001/jama.2016.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tammemägi MC, Church TR, Hocking WG, Silvestri GA, Kvale PA, Riley TL, Commins J, Berg CD. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. Plos Med. 2014;11:e1001764. doi: 10.1371/journal.pmed.1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan FM, Farmer E, Mair FS, Treweek S, Kendrick D, Jackson C, Robertson C, Briggs A, McCowan C, Bedford L, et al. Detection in blood of autoantibodies to tumour antigens as a case-finding method in lung cancer using the EarlyCDT®-Lung Test (ECLS): study protocol for a randomized controlled trial. BMC Cancer 2017;17:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montani F, Marzi MJ, Dezi F, Dama E, Carletti RM, Bonizzi G, Bertolotti R, Bellomi M, Rampinelli C, Maisonneuve P, et al. miR-Test: a blood test for lung cancer early detection. J Natl Cancer Inst. 2015;107:djv063. doi: 10.1093/jnci/djv063. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Rogers JF, Gerrein J, Anderlind C, Porta K, Whitney D, Johnson WE, Elashoff D, Dubinett D, Brody J, Spira A, Lenburg M. AEGIS study team. Shared gene expression alterations in nasal and bronchial epithelium for lung cancer detection. J Natl Cancer Inst. 2017;109:djw327. doi: 10.1093/jnci/djw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, Kosco AE, Di Fiore JL, Suh DE. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192:1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 36.Gould MK, Ananth L, Barnett PG Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383–388. doi: 10.1378/chest.06-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, Yasufuku K, Martel S, Laberge F, Gingras M, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules: application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 39.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012;185:363–372. doi: 10.1164/rccm.201104-0679CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanner NT, Aggarwal J, Gould MK, Kearney P, Diette G, Vachani A, Fang KC, Silvestri GA. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148:1405–1414. doi: 10.1378/chest.15-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanner NT, Porter A, Gould M, Li XJ, Vachani A, Silvestri GA. Physician assessment of pre-test probability of malignancy and adherence with guidelines for pulmonary nodule evaluation. Chest. doi: 10.1016/j.chest.2017.01.018. [online ahead of print] 20 Jan 2017; DOI: 10.1016/j.chest.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiener RS, Gould MK, Slatore CG, Fincke BG, Schwartz LM, Woloshin S. Resource use and guideline concordance in evaluation of pulmonary nodules for cancer: too much and too little care. JAMA Intern Med. 2014;174:871–880. doi: 10.1001/jamainternmed.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas AR, Vachani A, Sterman DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med. 2010;182:589–597. doi: 10.1164/rccm.201002-0186CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dooms C, Muylle I, Yserbyt J, Ninane V. Endobronchial ultrasound in the management of nonsmall cell lung cancer. Eur Respir Rev. 2013;22:169–177. doi: 10.1183/09059180.00001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, Greenhill S, Toth J, Feller-Kopman D, Puchalski J, et al. AQuIRE Bronchoscopy Registry. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions: results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193:68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142:385–393. doi: 10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassanein M, Callison JC, Callaway-Lane C, Aldrich MC, Grogan EL, Massion PP. The state of molecular biomarkers for the early detection of lung cancer. Cancer Prev Res (Phila) 2012;5:992–1006. doi: 10.1158/1940-6207.CAPR-11-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Balagurunathan Y, Atwater T, Antic S, Li Q, Walker RC, Smith GT, Massion PP, Schabath MB, Gillies RJ. Radiological image traits predictive of cancer status in pulmonary nodules. Clin Cancer Res. 2017;23:1442–1449. doi: 10.1158/1078-0432.CCR-15-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dooms C, Seijo L, Gasparini S, Trisolini R, Ninane V, Tournoy KG. Diagnostic bronchoscopy: state of the art. Eur Respir Rev. 2010;19:229–236. doi: 10.1183/09059180.00005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, Parsons E, Mitra N, Brody J, Lenburg ME, et al. AEGIS Study Team. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373:243–251. doi: 10.1056/NEJMoa1504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vachani A, Whitney DH, Parsons EC, Lenburg M, Ferguson JS, Silvestri GA, Spira A. Clinical utility of a bronchial genomic classifier in patients with suspected lung cancer. Chest. 2016;150:210–218. doi: 10.1016/j.chest.2016.02.636. [DOI] [PubMed] [Google Scholar]
- 54.Li XJ, Hayward C, Fong PY, Dominguez M, Hunsucker SW, Lee LW, McLean M, Law S, Butler H, Schirm M, et al. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Sci Transl Med. 2013;5:207ra142. doi: 10.1126/scitranslmed.3007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Massion PP, Healey GF, Peek LJ, Fredericks L, Sewell HF, Murray A, Robertson JF. Autoantibody signature enhances the positive predictive power of computed tomography and nodule-based risk models for detection of lung cancer. J Thorac Oncol. 2017;12:578–584. doi: 10.1016/j.jtho.2016.08.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pecot CV, Li M, Zhang XJ, Rajanbabu R, Calitri C, Bungum A, Jett JR, Putnam JB, Callaway-Lane C, Deppen S, et al. Added value of a serum proteomic signature in the diagnostic evaluation of lung nodules. Cancer Epidemiol Biomarkers Prev. 2012;21:786–792. doi: 10.1158/1055-9965.EPI-11-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hubers AJ, Prinsen CF, Sozzi G, Witte BI, Thunnissen E. Molecular sputum analysis for the diagnosis of lung cancer. Br J Cancer. 2013;109:530–537. doi: 10.1038/bjc.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennett WF, Smith RA. Segmental resection for bronchogenic carcinoma: a surgical alternative for the compromised patient. Ann Thorac Surg. 1979;27:169–172. doi: 10.1016/s0003-4975(10)63261-4. [DOI] [PubMed] [Google Scholar]
- 59.Jensik RJ, Faber LP, Milloy FJ, Monson DO. Segmental resection for lung cancer: a fifteen-year experience. J Thorac Cardiovasc Surg. 1973;66:563–572. [PubMed] [Google Scholar]
- 60.Thomas P, Rubinstein L Lung Cancer Study Group. Cancer recurrence after resection: T1 N0 non-small cell lung cancer. Ann Thorac Surg. 1990;49:242–246. [Discussion, pp. 246–247. doi: 10.1016/0003-4975(90)90145-v. [DOI] [PubMed] [Google Scholar]
- 61.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer:Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. [Discussion, pp. 622–613. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 62.Lewis RJ, Caccavale RJ, Sisler GE, Mackenzie JW. One hundred consecutive patients undergoing video-assisted thoracic operations. Ann Thorac Surg. 1992;54:421–426. doi: 10.1016/0003-4975(92)90431-3. [DOI] [PubMed] [Google Scholar]
- 63.McKenna RJ., Jr Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg. 1994;107:879–881. [Discussion, pp. 881–872. [PubMed] [Google Scholar]
- 64.Paul S, Sedrakyan A, Chiu YL, Nasar A, Port JL, Lee PC, Stiles BM, Altorki NK. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg. 2013;43:813–817. doi: 10.1093/ejcts/ezs428. [DOI] [PubMed] [Google Scholar]
- 65.Whitson BA, Andrade RS, Boettcher A, Bardales R, Kratzke RA, Dahlberg PS, Maddaus MA. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;83:1965–1970. doi: 10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 66.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 67.Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, Rizk NP, Bains M, Downey RJ, Rusch VW. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11–18. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 68.Ilonen IK, Räsänen JV, Knuuttila A, Salo JA, Sihvo EI. Anatomic thoracoscopic lung resection for non-small cell lung cancer in stage I is associated with less morbidity and shorter hospitalization than thoracotomy. Acta Oncol. 2011;50:1126–1132. doi: 10.3109/0284186X.2011.555780. [DOI] [PubMed] [Google Scholar]
- 69.Cao C, Manganas C, Ang SC, Yan TD. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg. 2012;1:3–10. doi: 10.3978/j.issn.2225-319X.2012.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kent M, Wang T, Whyte R, Curran T, Flores R, Gangadharan S. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236–242. [Discussion, pp. 242–234. doi: 10.1016/j.athoracsur.2013.07.117. [DOI] [PubMed] [Google Scholar]
- 71.Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator: clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 72.Baker S, Dahele M, Lagerwaard FJ, Senan S. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiat Oncol. 2016;11:115. doi: 10.1186/s13014-016-0693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ricardi U, Badellino S, Filippi AR. Stereotactic body radiotherapy for early stage lung cancer: history and updated role. Lung Cancer. 2015;90:388–396. doi: 10.1016/j.lungcan.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 74.Simone CB, II, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med. 2015;3:172. doi: 10.3978/j.issn.2305-5839.2015.07.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 77.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 78.Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 79.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 80.Patel JD, Hensing TA, Rademaker A, Hart EM, Blum MG, Milton DT, Bonomi PD. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009;27:3284–3289. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 81.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 82.Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 83.Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy G, et al. SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 84.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 85.Li C, Fang R, Sun Y, Han X, Li F, Gao B, Iafrate AJ, Liu XY, Pao W, Chen H, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. Plos One. 2011;6:e28204. doi: 10.1371/journal.pone.0028204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kris M, Johnson B, Kwiatkowski DJ, Iafrate AJ, Wistuba I, Aronson SL, Engelman JA, Shyr Y, Khuri F, Rudin CM, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: the NCI’s Lung Cancer Mutation Consortium (LCMC) [abstract] J Clin Oncol 2011(suppl);CRA7506 [Google Scholar]
- 87.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 88.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 89.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 90.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al. PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 91.Gainor JF, Tan DS, De Pas T, Solomon BJ, Ahmad A, Lazzari C, de Marinis F, Spitaleri G, Schultz K, Friboulet L, Yeap BY, Engelman JA, Shaw AT. Progression-free and overall survival in ALK-Positive NSCLC patients treated with sequential crizotinib and ceritinib. Clin Cancer Res. 2015;21:2745–2752. doi: 10.1158/1078-0432.CCR-14-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et al. AURA3 Investigators. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:2537–2539. doi: 10.1056/NEJMc1404894. [DOI] [PubMed] [Google Scholar]
- 94.Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T, et al. Study Investigators. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirsch FR, Suda K, Wiens J, Bunn PA., Jr New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388:1012–1024. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 96.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 98.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial Lancet2017389255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 102.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al. KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Campbell JD, Mazzilli SA, Reid ME, Dhillon SS, Platero S, Beane J, Spira AE. The case for a Pre-Cancer Genome Atlas (PCGA) Cancer Prev Res (Phila) 2016;9:119–124. doi: 10.1158/1940-6207.CAPR-16-0024. [DOI] [PubMed] [Google Scholar]