Summary
Background
Pakistan faces huge challenges in eradicating polio due to widespread poliovirus transmission and security challenges. Innovative interventions are urgently needed to strengthen community buy-in, to increase the coverage of oral polio vaccine (OPV) and other routine immunisations, and to enhance immunity through the introduction of inactivated polio vaccine (IPV) in combination with OPV. We aimed to evaluate the acceptability and effect on immunisation coverage of an integrated strategy for community engagement and maternal and child health immunisation campaigns in insecure and conflict-affected polio-endemic districts of Pakistan.
Methods
We did a community-based three-arm cluster randomised trial in healthy children aged 1 month to 5 years that resided within the study sites in three districts of Pakistan at high risk of polio. Clusters were randomly assigned by a computer algorithm using restricted randomisation in blocks of 20 by an external statistician (1:1:1) to receive routine polio programme activities (control, arm A), additional interventions with community outreach and mobilisation using an enhanced communication package and provision of short-term preventive maternal and child health services and routine immunisation (health camps), including OPV (arm B), or all interventions of arm B with additional provision of IPV delivered at the maternal and child health camps (arm C). An independent team conducted surveys at baseline, endline, and after each round of supplementary immunisation activity for acceptability and effect. The primary outcome measures for the study were coverage of OPV, IPV, and routine extended programme on immunisation vaccines and changes in the proportion of unvaccinated and fully vaccinated children. This trial is registered with ClinicalTrials.gov, number NCT01908114.
Findings
Between June 4, 2013, and May 31, 2014, 387 clusters were randomised (131 to arm A, 127 to arm B, and 129 to arm C). At baseline, 28 760 children younger than 5 years were recorded in arm A, 30 098 in arm B, and 29 126 in arm C. 359 clusters remained in the trial until the end (116 in arm A, 120 in arm B, and 123 in arm C; with 23 334 children younger than 5 years in arm A, 26 110 in arm B, and 25 745 in arm C). The estimated OPV coverage was 75% in arm A compared with 82% in arm B (difference vs arm A 6·6%; 95% CI 4·8–8·3) and 84% in arm C (8·5%, 6·8–10·1; overall p<0·0001). The mean proportion of routine vaccine doses received by children younger than 24 months of age was 43% in arm A, 52% in arm B (9%, 7–11) and 54% in arm C (11%, 9–13; overall p<0·0001). No serious adverse events requiring hospitalisation were reported after immunisation.
Interpretation
Despite the challenges associated with the polio end-game in high-risk, conflict-affected areas of Pakistan, a strategy of community mobilisation and targeted community-based health and immunisation camps during polio immunisation campaigns was successful in increasing vaccine coverage, including polio vaccine coverage.
Funding
Bill & Melinda Gates Foundation.
Introduction
Pakistan, Afghanistan, and Nigeria are the only three remaining countries with endemic polio.1 Although the number of polio cases and their geographical spread have reduced, insecurity, poor access to populations, and residual pockets of circulating poliovirus in these three countries remain an important challenge for global polio eradication. Most routine childhood immunisations in Pakistan are delivered at fixed immunisation sites, with some outreach services through vaccinators; however, coverage rates for many routine childhood immunisation vaccines remain low, on average between 65% and 73% for vaccines containing diphtheria, tetanus, and pertussis (DTP).2 Oral polio vaccine (OPV) is delivered differently. Although routine childhood immunisation services should provide four doses of OPV (at birth, 6, 10, and 14 weeks of age), repeated, concerted door-to-door immunisation campaigns, called supplementary immunisation activities, have been used to try to increase OPV coverage.3 The supplementary immunisation activities are household-level campaigns organised by the Pakistan national polio programme and usually last 5 days (3 days to visit all households, with 2 days for return visits to households with initially absent or missed children with door-to-door OPV administration to children younger than 5 years of age by health workers. These children have their fingers marked using permanent ink for ease of identification in post-vaccination mop-up activities and surveys. Supplementary immunisation activities have been particularly challenging in parts of the country affected by insurgency and insecurity, with groups such as the Taliban limiting access to populations,4 disinformation leading to refusal of OPV,5 and attacks targeting polio workers.6 In particular, in the high-risk areas of northwest Pakistan and several slums of Karachi, polio workers have been subjected to several attacks and assassinations, with bans imposed on polio vaccination activities by the Pakistani Taliban and other extremist groups, as well as general insecurity impeding the access of polio programme teams to such communities.7 These barriers to the polio programme activities are compounded by inefficiencies within the routine childhood immunisation programme, the poor state of environmental sanitation, and high burdens of childhood diarrhoea and malnutrition, which make transmission more probable and potentially impair the response to OPV.8, 9, 10 An important issue in many areas is insufficient community buy-in for the polio-focused supplementary immunisation activities and general fatigue with the repeated rounds of household OPV administration (estimated to have exceeded 150 rounds to date).
Research in context.
Evidence before this study
We did a systematic review of available information about strategies for addressing polio eradication in conflict and insecurity settings using the broad terms “polio” and “vaccination strategies”, and MeSH terms “insecurity”, “war”, and “conflict”. The search encompassed Jan 1, 2000, to Dec 31, 2016, and was not restricted to the English language. Our review did not identify any randomised controlled trials or systematic reviews of immunisation strategies addressing polio programme refusals or insecurity. We assessed a large number of reports documenting the temporal association between conflict, insecurity, and risks to polio eradication, as well as re-emergence of polio. These issues have been compounded in the past 5–6 years by attacks on polio and health workers and insecurity had been documented as an important barrier to polio eradication activities in conflict settings. In other instances, negative propaganda by some religious factions and obscurantists have affected population uptake of oral polio vaccine (OPV) and led to refusals. Although a combination of approaches has been suggested to circumvent these issues, the scientific basis for community-based approaches in such settings is very limited. A Cochrane review of strategies to scale up childhood immunisations also did not present any information from studies done in such conflict settings or insecure contexts. Barring one report of mass immunisation in northern Nigeria and other approaches using the military in Angola, we identified no reports of evaluations of strategies to scale up polio or routine immunisations in conflict settings. A systematic review of service delivery platforms for maternal, newborn, and child health strategies in Pakistan did not identify a study of routine immunisations or polio-associated interventions.
Added value of this study
To our knowledge, our study is the first scientifically designed formal evaluation of a community-based strategy to approach high-risk populations in conflict-affected and insecure populations with an integrated package of maternal and child health and immunisation services delivered during routine polio campaigns. The results indicated widespread acceptance, with more than two-thirds of target children and families in these districts accessing these short-term fixed camps during the 4 days of the campaigns. Contrary to initial fears, the coverage of OPV increased 8·5% overall, uptake of inactivated polio vaccine administered alongside OPV was high, and the number of children who were not immunised was reduced overall.
Implications of all the available evidence
Our findings strongly support the adaptation of comparable community-based integrated strategies for community engagement, grass-root mobilisation, and integration of polio, routine immunisation, and maternal and child health services in the residual high-risk population reservoirs with circulating poliovirus in Pakistan and the conflict-affected bordering areas with Afghanistan. These strategies can be integrated with routine health and immunisation services. Our findings suggest that engaging local community members and political leaders is important to secure access and community buy-in. A historical review of the political determinants of polio in five countries affected by Islamist militancy also concluded that rather than military victory against the militants, securing local de-facto political support was crucial for success.
These issues were brought to the fore by a massive upsurge in polio cases in Pakistan (rising from 58 cases in 2012, to 93 cases in 2013, and to 306 cases in 2014; appendix). Additionally, the country faced the challenge of introducing the inactivated polio vaccine (IPV) with the third dose of DTP vaccine (DTP3), as recommended by the WHO Strategic Advisory Group of Experts (SAGE) committee for vaccines.11 The recommendation seeks to address the risk of emergence of circulating vaccine-derived polioviruses (cVDPV), typically cVDPV2, OPV virus type 2, and the coordinated withdrawal of trivalent OPV and its replacement by bivalent OPV.12 This recommendation to introduce IPV was based on evidence from studies documenting enhanced serum antibody response and improved mucosal immunity with a combination of OPV and IPV compared with OPV alone, resulting in a substantial reduction in poliovirus excretion,13, 14, 15 and protecting the population ahead of the withdrawal of trivalent OPV vaccine. The bivalent OPV was introduced in Pakistan in April, 2016.
Given the limited vaccine coverage of the routine childhood immunisation programme, the context of insecurity, and limited community engagement in some populations, introduction of IPV into the national polio eradication strategies in Pakistan posed numerous challenges. In addition to the obvious questions around the probable IPV coverage rates in view of low coverage rates of routine childhood immunisation vaccines, especially DTP3, key concerns were raised about the possible negative consequences of IPV introduction for the acceptance and coverage of the existing OPV programme, especially in areas where refusals of OPV were noted within supplementary immunisation activities.
We hypothesised that in these insecure polio-endemic areas of Pakistan with poorly functioning routine childhood immunisation systems, a strategy of enhanced community engagement together with the provision of some maternal, child health, and immunisation services in time-limited camps alongside planned supplementary immunisation activities would enhance overall OPV coverage. We also hypothesised that this might be a useful mechanism for introducing IPV and enhancing its acceptability in such populations. We aimed to evaluate the effect on vaccine coverage of this enhanced maternal, child health, and immunisation service package, with or without additional IPV, delivered through health camps alongside the regular polio supplementary immunisation activities.
Methods
Study design and participants
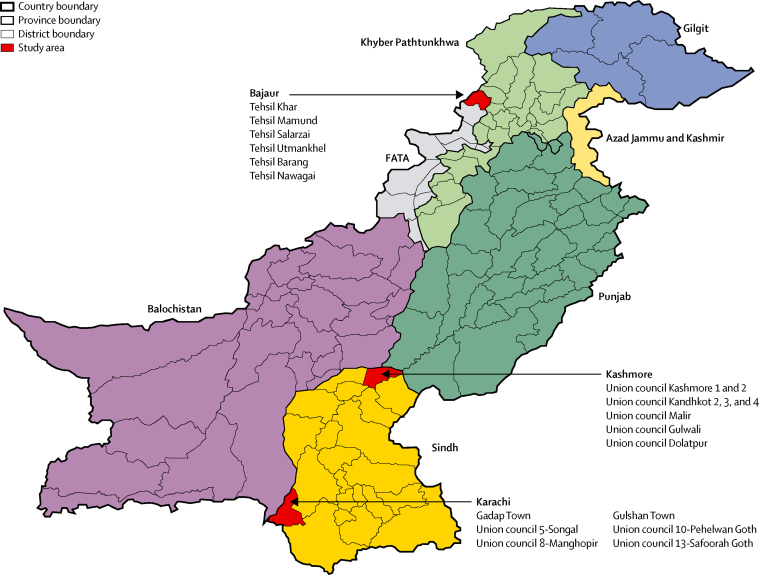
We did this community-based, three-arm cluster randomised trial in three geographical areas in Pakistan reported as high risk for polio by WHO (figure 1). These areas were also selected for risks of ongoing insurgency (Bajaur and Karachi) and general insecurity (Kashmore). Healthy children aged 1 month to 5 years who lived within the study sites, and who did not plan to travel away during entire the study period, were eligible for the study. Children with known thrombocytopenia or bleeding disorders, who were acutely ill or with signs of acute infection (eg, fever ≥38 °C) at the time of supplementary immunisation activity, or who had a diagnosis or suspicion of immunodeficiency disorder were not eligible.
Figure 1.
Map of Pakistan showing study areas
FATA=Federally Administered Tribal Areas. KPK=Khyber Pakhtunkhwa. AJK=Azad Jammu and Kashmir.
We did a baseline census in the three study areas to collect demographic, socioeconomic, routine immunisation, and health-seeking data. The baseline census was done by locally recruited and trained data collection teams, strictly independent of the implementing teams, for 3 months. A structured questionnaire was developed for the purpose and was pretested on 50 households from a locality not included in the trial but having similar sociodemographic conditions (appendix). Double data entry and cleaning were done concurrently.
The ethics review committee of Aga Khan University and the National Bioethics Committee granted approval of the trial. In view of the importance of introduction of IPV through the existing immunisation programme without posing any risks to the existing OPV delivery strategy, we devoted considerable time to achieving consensus among all national and provincial stakeholders. During the inception phase, between October, 2012, and June, 2013, a series of consultative meetings at provincial, national, and international levels were held, resulting in formal approval by the authorities. Life and injury insurance for all participants and staff involved in the trial and institutional indemnification were also provided; because no insurance company in Pakistan was prepared to provide this cover, it was secured from an overseas insurance company (Aon Insurance Managers, Singapore). Individual level consent was taken from the parent of the participating child.
Randomisation and masking
A computer algorithm was used to perform restricted randomisation in blocks of 20 by matching the population size, socioeconomic status, OPV coverage, and extended programme on immunisation (EPI) coverage, to assign (1:1:1) eligible clusters to three groups. An individual cluster was defined as the area assigned to a team for polio vaccination during supplementary immunisation activities. Arm A (control) received the routine immunisation package and additional OPV in supplementary immunisation activities (no special measures such as short message service messages, posters, banners, or mass media promotions were employed in these areas). Arm B received additional interventions with community outreach and mobilisation using an enhanced communication package, and provision of maternal and child health immunisation services through low-cost health camps established to run from day 4 of polio supplementary immunisation activities. The clusters in arm C received the same intervention package as arm B with the addition of IPV (Imovax, Sanofi Pasteur, Lyon, France) delivered at the maternal and child health immunisation strategy camps. The trial was designed to cover four consecutive supplementary immunisation activity rounds over 12 months, with IPV administered in rounds one and three, while the community mobilisation activities continued throughout the duration of the project. An independent team conducted baseline, end line and post-supplementation immunisation strategy surveys at the end of each round (appendix). Because of the nature of the intervention, trial investigators were not masked. The main trial statistician (SC) was not involved in field implementation.
Procedures
A community mobilisation plan and information, education, and communication (IEC) materials—specifically a pictorial booklet and counselling cards—were prepared during the inception phase and ratified by all relevant stakeholders. The IEC materials contained information on maternal health, nutrition, hygiene and sanitation, immunisation, polio, and health camps. Teams of two female and one male community mobilisers were recruited and trained to deliver the information contained in the IEC material and were provided with an IEC booklet and counselling cards as job aids. Each team covered four clusters and delivered these key messages through individual sessions with parents and group sessions with male groups, female groups, and health-care providers at cluster level. Sessions with community and religious leaders, teachers, and other prominent persons were done at union council level. Typically ten to 20 people attended the male and female group sessions; five to ten attended the others. The sessions with health-care providers and influencers were limited to the introduction of the project, with a focus on the importance of routine immunisation and OPV administration (and IPV use as relevant to the specific clusters). We also provided information regarding maternal health, nutrition, hygiene and sanitation, immunisation, and polio during sessions with female members of the community. Although the study team did not make direct contact with local militant leaders and Taliban commanders, community elders were closely consulted who might have communicated with such individuals.
The community mobilisation teams also promoted health camps offering maternal and child health immunisation services during the scheduled supplementation immunisation strategies, including missed OPV doses. Promotion was limited to provision of information regarding services being offered at health camps and encouraging use of those services Additional information about the availability of IPV for coadministration with OPV (as needed) in the health camps was only provided in clusters of arm C. This strategy was agreed with the national polio programme to prevent any disinformation about the existing OPV strategy. Based on instructions from the health department, no large-scale public dissemination was undertaken regarding IPV use and availability. To avoid potential contamination between arms, the community mobilisation teams informing communities in arms B and C about health camps also provided colour-coded invitation cards required for visiting the health camps, and vaccine allocation (additional IPV) was made as per the colour codes. Details of community mobilisation sessions conducted across sites and arms are in the appendix.
During each of the four supplementary immunisation activity rounds, approximately 60 maternal and child health and immunisation camps were held in clusters of arms B and C. The camps generally started on the fourth day of supplementary immunisation activities, lasted for 3–4 days, and covered two adjacent clusters. They were open for 7–8 h per day. In addition to the fixed main camps, a few mobile camps were also organised in some remote clusters to improve community access as needed. Each health camp was staffed by one supervising medical officer, one vaccinator, one paramedic, and two facilitators. Staff provided counselling on hygiene, nutrition, and routine immunisations and undertook general maternal and child health assessments. The health camp staff were trained to provide standard medical care, follow good clinical practices, maintain a strict cold chain, and follow safe injection practices. The health-care providers in the camps delivered nutrition interventions such as micronutrient supplements, routine immunisations, and OPV as needed. Written consent was obtained for IPV administration because it was not included in routine immunisation vaccines at that time. Primary care medications for common illnesses were administered as per WHO and Government of Pakistan guidelines.16 Children receiving IPV were monitored for any adverse reaction for 30 min after vaccination. Camp medical officers provided a contact number for parents to call in the event of any delayed adverse effect of IPV or other injectable vaccines.
Based on the baseline census data, cluster-specific vaccine record books were prepared. These books contained details of children falling in the target age range for vaccination and were used to record information regarding vaccination at the health camps for these children. Vaccination records for children who were born after the census and were vaccinated at health camps were also recorded in the vaccine record books.
At every stage of the project, strict monitoring and supervision was undertaken by both internal and external monitors using checklists. Monitoring supervisors conducted surprise visits to check the project activities and refresher trainings were given to the community mobilisation and health camp teams on the basis of their recommendations.
We established five independent mobile data collection teams of three people per union council or tehsil to do surveys after the vaccination rounds on a subset of households in each cluster to assess OPV and IPV coverage. A list of 25–30 households was computer-generated by the data management unit for each cluster from the existing sampling frame. Within a week of completion of supplementary immunisation activities and conclusion of camps in arms B and C, the data collection teams visited the targeted clusters, identified each household on the list, and, after verbal informed consent, obtained information on OPV or IPV uptake and source from the caregiver of the child.
After four supplementary immunisation activity rounds, an end-line census was performed in all clusters to assess the change in routine immunisation coverage, OPV coverage, and the acceptability of IPV using the same structured questionnaire as for the baseline survey (appendix).
Outcomes
The primary outcome measures for the study were coverage of OPV, IPV, and routine EPI vaccines and changes in the proportion of unvaccinated and fully vaccinated children between baseline and end-line surveys. The original trial protocol also included a primary endpoint of the prevalence of serum neutralising antibodies to all three types of poliovirus in arm B versus arm C, but the risks associated with blood sampling in this region were considered too great to rely on this as a main trial outcome. When registering the trial, we therefore included this as a secondary endpoint, along with estimation of stool shedding of poliovirus and environmental sampling for poliovirus circulation. These results will be reported separately.
Statistical analysis
We estimated that a sample size of 40 clusters per arm per geographical study site with 150 children per cluster would provide greater than 90% power to detect an increase in OPV coverage from 80% to 95% in a given site at the 5% level of significance and a coefficient of variation of 0·14. Across the three sites, an overall sample size of 120 clusters per arm with an average of 150 children per cluster, would provide 90% power to detect an increase in OPV coverage from 80% to 85%.17 To estimate the sample size for the sequential surveys of coverage estimates after supplementary immunisation activity, a conservative OPV coverage of 80% with a 5% increase was assumed. A sample size of 3048 per site (rounded to 3050) was estimated, assuming a design effect of 2, to provide 80% power at the 5% level of significance with 5% attrition. The estimate was revised to 3619 (rounded to 3600) after the first round using 20% attrition to account for non-response.
All data were double-entered using Visual FoxPro and backed up at the data management unit at Aga Khan University, with hard copies archived and stored at the institution's data repository. Demographic, clinical, and vaccination data were merged and analysed using Stata (versions 12 and 14). We calculated area and round-specific vaccination coverage for all arms across baseline, surveys after supplementary immunisation activities, and end-line surveys. We analysed vaccination coverage using a generalised linear model with the binomial probability model and identity link function to produce estimates of the percentage point difference in coverage between arms. We accounted for the clustered nature of the data using Taylor-linearised variance estimates with the three study areas treated as strata and the randomised clusters as primary sampling units. The number of doses administered was the count of all doses of OPV, IPV, and EPI vaccines actually given excluding unused doses and wastage.
This trial is registered with ClinicalTrials.gov, number NCT01908114.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
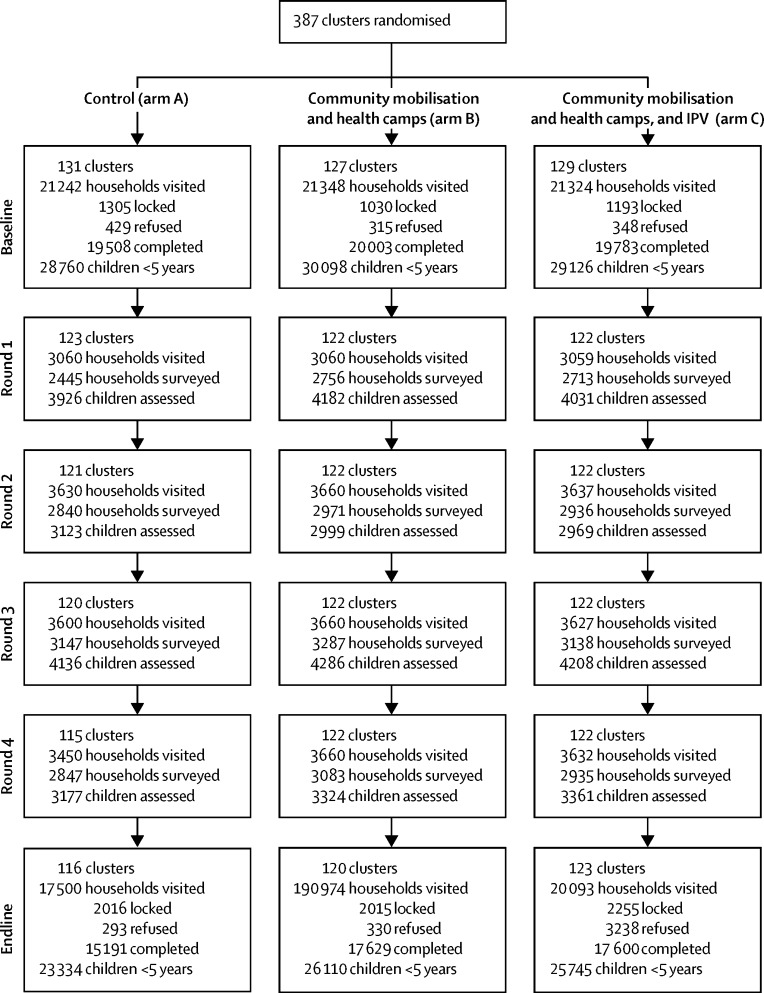
Between June 4, 2013, and May 31, 2014, 387 clusters were randomised (131 to arm A, 127 to arm B, and 129 to arm C). At baseline, 28 760 children younger than 5 years were recorded in arm A, 30 098 in arm B, and 29 126 in arm C. 359 clusters remained in the trial until the end (116 in arm A, 120 in arm B, and 123 in arm C; with 23 334 children younger than 5 years in arm A, 26 110 in arm B, and 25 745 in arm C; figure 2).
Figure 2.
Trial profile
Baseline sociodemographic characteristics of enrolled children and their families were similar (table 1) but with slightly poorer access to an improved water source in arm B. Few children (13%) had vaccination cards available and 42% of children were reported to have received no routine EPI vaccination at all. Most children (81%) were reported to have received OPV during the last round of supplementary immunisation activity and a similar proportion was reported to have received vitamin A drops during the preceding 6 months.
Table 1.
Baseline sociodemographic characteristics and immunisation status of children younger than 5 years and their families
| Control (arm A; n=28 760) | Community mobilisation and health camps (arm B; n=30 098) | Community mobilisation and health camps with IPV (arm C; n=29 126) | |
|---|---|---|---|
| Children <5 years old | |||
| Karachi | 7415 (26%) | 7579 (25%) | 7310 (25%) |
| Bajaur | 11 705 (41%) | 12 481 (41%) | 11 995 (41%) |
| Kashmore | 9640 (34%) | 10 038 (33%) | 9821 (34%) |
| Sex | |||
| Female | 13 787 (48%) | 14 341 (48%) | 13 926 (48%) |
| Male | 14 973 (52%) | 15 757 (52%) | 15 200 (52%) |
| Age (months) | |||
| 0–5 | 2032 (7%) | 2113 (7%) | 2052 (7%) |
| 6–11 | 2493 (9%) | 2493 (8%) | 2474 (8%) |
| 12–23 | 4646 (16%) | 4907 (16%) | 4761 (16%) |
| 24–59 | 19 589 (68%) | 20 585 (68%) | 19 839 (68%) |
| Access to improved water source* | |||
| No | 3517 (12%) | 5339 (18%) | 4398 (15%) |
| Yes | 25 243 (88%) | 24 759 (82%) | 24 728 (85%) |
| Access to a latrine | |||
| No | 7114 (25%) | 6946 (23%) | 7123 (24%) |
| Yes | 21 646 (75%) | 23 152 (77%) | 22 003 (76%) |
| Vaccination card available | |||
| No | 25 050 (87%) | 26 024 (86%) | 25 209 (87%) |
| Yes | 3710 (13%) | 4074 (14%) | 3917 (13%) |
| Immunisation status† | |||
| Full | 6280 (22%) | 6499 (22%) | 6379 (22%) |
| Partial | 10 259 (36%) | 10 916 (36%) | 10 566 (36%) |
| No routine EPI | 12 221 (42%) | 12 683 (42%) | 12 191 (42%) |
| Received OPV during last campaign | |||
| No | 5540 (19%) | 5656 (19%) | 5408 (19%) |
| Yes | 23 220 (81%) | 24 442 (81%) | 23 718 (81%) |
| Received vitamin A during last campaign‡ | |||
| No | 5156 (19%) | 5342 (19%) | 3767 (14%) |
| Yes | 21 572 (81%) | 22 643 (81%) | 23 307 (86%) |
IPV=inactivated polio vaccine. EPI=expanded programme on immunisation. OPV=oral polio vaccine.
Improved source includes government tap, well, or hand pump; bottled water or mineral water; own tap, well, or hand pump; and borehole.
Fully immunised defined as child has received all routine immunisation antigens for his age; partially immunised defined as the child is not fully immunised; and no routine immunisation defined as the child did not receive any routine immunisation antigen.
Of children aged 6 months or older at baseline.
The number of vaccine doses delivered at health camps held during each of the four supplementary immunisation activity rounds are shown in table 2. 23 343 children received 53 359 doses of routine immunisations (including 22 399 OPV doses). 26 867 children younger than 5 years also received 45 515 doses of IPV at the maternal and child health and immunisation camps. No health camps were held in the control arm (arm A). No serious adverse events requiring hospitalisation were reported after immunisation. There were some events of fever and pain at the site of injection that were treated either at health camps or the study team.
Table 2.
Numbers of vaccine doses delivered at health camps
|
Karachi |
Bajaur |
Kashmore |
|||||
|---|---|---|---|---|---|---|---|
| Community mobilisation and health camps (arm B) | Community mobilisation and health camps and IPV (arm C) | Community mobilisation and health camps (arm B) | Community mobilisation and health camps and IPV (arm C) | Community mobilisation and health camps (arm B) | Community mobilisation and health camps and IPV (arm C) | ||
| Children at baseline | 7579 | 7310 | 12 481 | 11 995 | 10 038 | 9821 | |
| BCG | |||||||
| 1 | 51 | 46 | 153 | 287 | 232 | 260 | |
| 2 | 91 | 91 | 229 | 245 | 220 | 238 | |
| 3 | 89 | 46 | 263 | 255 | 238 | 258 | |
| 4 | 94 | 29 | 265 | 274 | 250 | 206 | |
| Total | 325 | 212 | 910 | 1061 | 940 | 962 | |
| OPV | |||||||
| 1 | 458 | 367 | 816 | 1087 | 1224 | 1155 | |
| 2 | 674 | 358 | 977 | 902 | 1310 | 1449 | |
| 3 | 625 | 335 | 939 | 1016 | 1327 | 1456 | |
| 4 | 623 | 408 | 1112 | 1197 | 1257 | 1327 | |
| Total | 2380 | 1468 | 3844 | 4202 | 5118 | 5387 | |
| Pentavalent | |||||||
| 1 | 290 | 572 | 619 | 695 | 826 | 727 | |
| 2 | 251 | 242 | 626 | 576 | 737 | 1138 | |
| 3 | 227 | 248 | 646 | 754 | 912 | 1144 | |
| 4 | 267 | 273 | 564 | 982 | 881 | 1124 | |
| Total | 1035 | 1335 | 2455 | 3007 | 3356 | 4133 | |
| Measles | |||||||
| 1 | 264 | 433 | 490 | 495 | 597 | 806 | |
| 2 | 542 | 616 | 303 | 451 | 778 | 586 | |
| 3 | 111 | 153 | 422 | 415 | 129 | 172 | |
| 4 | 978 | 1040 | 325 | 356 | 447 | 320 | |
| Total | 1895 | 2242 | 1540 | 1717 | 1951 | 1884 | |
| IPV | |||||||
| 1 | NA | 5851 | NA | 7431 | NA | 8469 | |
| 2 | NA | NA | NA | NA | NA | NA | |
| 3 | NA | 6344 | NA | 8318 | NA | 9102 | |
| 4 | NA | NA | NA | NA | NA | NA | |
| Total | NA | 12 195 | NA | 15 749 | NA | 17 571 | |
IPV=inactivated polio vaccine. OPV=oral polio vaccine. NA=not applicable.
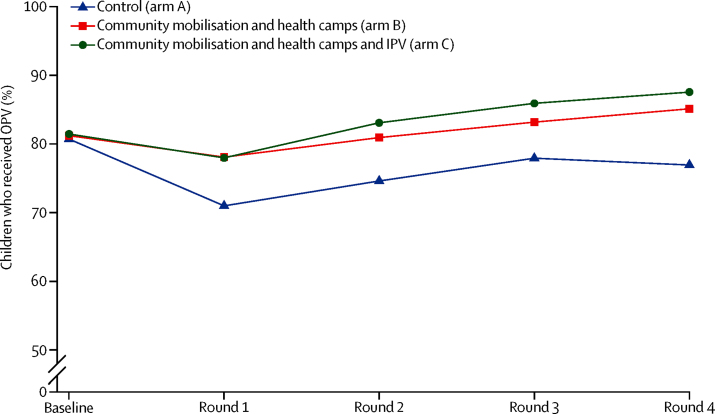
Across all four rounds, the average estimated coverage of OPV was 75% (95% CI 74–77) in arm A, 82% (81–83%) in arm B, and 84% (95% CI 83–85%) in arm C (overall p<0·0001; table 3). On average, across rounds, coverage was 6·6 percentage points (95% CI 4·8–8·3) higher in arm B than in arm A and 8·5 percentage points (6·8–10·1) higher in arm C than in arm A. A consistent pattern across sites and across rounds was observed of higher coverage in the two intervention arms (B and C) than in control arm (arm A; figure 3).
Table 3.
OPV coverage during each round of supplementary immunisation activities
| Control (Arm A) |
Community mobilisation and health camps (arm B) |
Community mobilisation and health camps and IPV (arm C) |
p value* | ||||
|---|---|---|---|---|---|---|---|
| Estimated coverage | Estimated coverage | Coverage difference (95% CI) | Estimated coverage | Coverage difference (95% CI) | |||
| Round 1 | |||||||
| Karachi | 63% (53 to 71) | 81% (74 to 87) | 18·6% (7·2 to 30·1) | 75% (66 to 82) | 12·3% (−0·2 to 24·9) | 0·0072 | |
| Bajaur | 77% (74 to 80) | 79% (77 to 82) | 1·9% (−1·9 to 5·7) | 81% (78 to 83) | 3·5% (−0·4 to 7·4) | 0·21 | |
| Kashmore | 72% (73 to 74) | 76% (74 to 78) | 3·7% (1·0 to 6·4) | 78% (76 to 80) | 5·4% (2·9 to 7·9) | 0·0002 | |
| Total | 71% (68 to 74) | 78% (76 to 80) | 6·6% (3·5 to 9·7) | 78% (76 to 80) | 6·6% (3·4 to 9·8) | <0·0001 | |
| Round 2 | |||||||
| Karachi | 72% (65 to 79) | 80% (74 to 84) | 7·4% (−1·4 to 16·2) | 84% (80 to 88) | 12·0% (3·8 to 20·1) | 0·0142 | |
| Bajaur | 76% (73 to 78) | 83% (80 to 85) | 6·9% (3·4 to 10·1) | 84% (81 to 86) | 7·7% (4·3 to 11·2) | <0·0001 | |
| Kashmore | 75% (72 to 77) | 80% (77 to 82) | 5·2% (2·2 to 7·0) | 82% (78 to 84) | 7·0 (3·5 to 10·4) | 0·0001 | |
| Total | 75% (73 to 76) | 81% (79 to 83) | 6·2% (3·6 to 8·8) | 83% (81 to 85) | 8·4% (5·8 to 11·0) | <0·0001 | |
| Round 3 | |||||||
| Karachi | 77% (67 to 84) | 82% (78 to 86) | 5·5% (−4·0 to 14·9) | 88% (84 to 90) | 10·8% (1·9 to 19·8) | 0·0167 | |
| Bajaur | 77% (75 to 80) | 86% (84 to 88) | 8·3% (5·5 to 11·1) | 87% (84 to 89) | 9·2% (5·4 to 13·0) | <0·0001 | |
| Kashmore | 79% (75 to 82) | 82% (81 to 84) | 3·6% (0·0 to 7·2) | 85% (83 to 86) | 5·8% (2·2 to 9·5) | 0·0054 | |
| Total | 78% (75 to 80) | 83% (82 to 85) | 5·3% (2·3 to 8·2) | 86% (84 to 87) | 7·9% (5·0 to 10·8) | <0·0001 | |
| Round 4 | |||||||
| Karachi | 73% (68 to 77) | 82% (74 to 87) | 8·6% (1·1 to 16·1) | 86% (83 to 89) | 13·1% (7·8 to 18·5) | <0·0001 | |
| Bajaur | 81% (78 to 83) | 88% (86 to 90) | 6·9% (3·8 to 10·0) | 90% (88 to 91) | 8·6% (5·6 to 11·7) | <0·0001 | |
| Kashmore | 76% (74 to 78) | 86% (84 to 87) | 9·5% (6·9 to 12·2) | 87% (85 to 89) | 11·1% (8·3 to 13·9) | <0·0001 | |
| Total | 77% (75 to 79) | 85% (83 to 87) | 8·3% (6·0 to 10·6) | 88% (86 to 89) | 10·7% (8·7 to 12·6) | <0·0001 | |
| Overall (all sites, all rounds) | 75% (74 to 77) | 82% (81 to 83) | 6·6% (4·8 to 8·3) | 84% (83 to 85) | 8·5% (6·8 to 10·1) | <0·0001 | |
OPV=oral polio vaccine.
Testing the null hypothesis of no differences in coverage across the three arms. Numbers in parantheses are 95% CI.
Figure 3.
OPV coverage by rounds
OPV=oral polio vaccine. IPV=inactivated polio vaccine.
The proportion of unvaccinated children according to the routine childhood immunisation schedule was 42% in each arm at baseline (table 4). At end line, this proportion was reduced to 36% (95% CI 32–41%) in arm A, 28% (95% CI 24–31) in arm B, and 27% (24–31) in arm C. Similarly the proportion of fully vaccinated children was about 22% at baseline in all three arms. At end line, this proportion had increased to 25% (22–28) in arm A, 32% (29–35) in arm B, and 34% (31–37) in arm C. A consistent pattern was seen across sites. The proportion of fully vaccinated children increased in the two intervention arms compared with the control arm (7·3% [95% CI 4·5–10·0] increase in arm B vs arm A; 9·5% [6·9–12·0] increase in arm C vs arm A; overall p<0·0001).
Table 4.
Vaccination status of children according to routine EPI schedule
|
Control (arm A) |
Community mobilisation and health camps (arm B) |
Community mobilisation and health camps and IPV (arm C) |
||||
|---|---|---|---|---|---|---|
| Baseline | End line | Baseline | End line | Baseline | End line | |
| Karachi | ||||||
| Not immunised | 30% (24–36) | 25% (21–29) | 27% (22–34) | 16% (12–21) | 25% (21–31) | 11% (9–13) |
| Partially immunised | 29% (26–32) | 26% (24–30) | 29% (27–32) | 30% (27–33) | 33% (31–35) | 34% (30–38) |
| Fully immunised | 41% (36–46) | 49% (44–53) | 43% (37–49) | 54% (48–60) | 41% (37–46) | 56% (51–61) |
| Bajaur | ||||||
| Not immunised | 62% (59–66) | 60% (57–62) | 63% (59–67) | 47% (45–49) | 64% (61–68) | 48% (46–50) |
| Partially immunised | 23% (19–27) | 22% (19–25) | 23% (19–28) | 27% (25–29) | 21% (17–26) | 25% (23–28) |
| Fully immunised | 15% (14–16) | 19% (17–20) | 14% (13–16) | 26% (24–28) | 14% (13–16) | 26% (25–28) |
| Kashmore | ||||||
| Not immunised | 29% (23–35) | 19% (14–25) | 27% (22–34) | 16% (12–21) | 26% (21–33) | 17% (13–21) |
| Partially immunised | 56% (49–63) | 64% (57–71) | 58% (51–65) | 62% (54–69) | 57% (50–64) | 56% (49–63) |
| Fully immunised | 15% (12–20) | 17% (13–21) | 14% (11–19) | 22% (17–27) | 17% (13–21) | 27% (22–32) |
| All sites combined | ||||||
| Not immunised | 42% (38–47) | 36% (32–41) | 42% (38–47) | 28% (24–31) | 42% (38–46) | 27% (24–31) |
| Partially immunised | 36% (32–40) | 39% (34–45) | 36% (32–41) | 40% (36–45) | 36% (32–40) | 39% (35–43) |
| Fully immunised | 22% (19–25) | 25% (22–28) | 22% (19–24) | 32% (29–35) | 22% (19–25) | 34% (31–37) |
EPI=expanded programme on immunisation. IPV=inactivated polio vaccine. Numbers in parantheses are 95% CI.
The mean proportion of routine childhood immunisation doses that each child received was 39% (36–42) in all three arms at baseline and increased to 43% (40–45) in arm A, 52% (49–55) in arm B, and 54% (51–57) in arm C at end line (table 5). The proportion of vaccine doses received increased in all three sites. Children in Karachi received, on average, a higher proportion of vaccine doses than children in the other two sites. The percentage point increase in the proportion of scheduled vaccine doses received by the children versus the control arm A, was estimated to be 9% (95% CI 7–11) for arm B and 11% (9–13) for arm C (table 6).
Table 5.
Mean proportion* of scheduled EPI vaccine doses actually received, by study site and arm
|
Control (arm A) |
Community mobilisation and health camps (arm B) |
Community mobilisation and health camps and IPV (arm C) |
||||
|---|---|---|---|---|---|---|
| Baseline | End line | Baseline | End line | Baseline | End line | |
| Karachi | 59% (53 to 65; 7415) | 60% (57 to 64; 5193) | 61% (54 to 67; 7579) | 70% (65 to 76; 7090) | 61% (56 to 66; 7310) | 74% (70 to 78; 6640) |
| Bajaur | 28% (26 to 30; 11 705) | 32% (30 to 33; 9069) | 27% (25 to 29; 12 481) | 42% (40 to 44; 9641) | 26% (24 to 28; 11 995) | 41% (40 to 43; 9576) |
| Kashmore | 38% (34 to 42; 9640) | 43% (40 to 47; 9072) | 37% (33 to 41; 10 038) | 52% (49 to 56; 9529) | 39% (34 to 43; 9821) | 52% (49 to 56; 9529) |
| All sites combined | 39% (36 to 42; 28 760) | 43% (40 to 45; 23 334) | 39% (36 to 42; 30 098) | 52% (49 to 55; 26 110) | 39% (36 to 42; 29 126) | 54% (51 to 57; 25 745) |
Data are mean percentage (95% CI; n). EPI=expanded programme on immunisation. IPV=inactivated polio vaccine.
58% means that on average children had received 58% of their scheduled EPI vaccinations.
Table 6.
Effect of interventions on proportion of scheduled EPI vaccinations received among children aged <24 months
| Control (arm A) | Community mobilisation and health camps (arm B) | Community mobilisation and health camps and IPV (arm C) | Test of null hypothesis that neither intervention has any effect | |
|---|---|---|---|---|
| Karachi | 0 (control) | 13% (10–16) | 17% (14–20) | p<0·0001 |
| Bajaur | 0 (control) | 10% (8–12) | 10% (9–12) | p<0·0001 |
| Kashmore | 0 (control) | 5% (1–10) | 9% (4–13) | p=0·0003 |
| All sites combined | 0 (control) | 9% (7–11) | 11% (9–13) | p<0·0001 |
Data are the difference in the proportion of EPI vaccine doses received in arms B and C compared with arm A. Numbers in parantheses are 95% CI. EPI=extended programme on immunisation. IPV=inactivated polio vaccine.
The estimated coverage from post-vaccination surveys of IPV in round 1 in arm C was 86·3% (95% CI 81·7–89·9) for Karachi, 90·0% (87·4–92·2) for Bajaur, and 95·9% (93·6–97·3) for Kashmore. The estimated coverage from post-vaccination surveys of IPV in round 3 in arm C was 93·2% (89·9–95·5) for Karachi, 86% (82·6–88·6) for Bajaur, and 98·6% (97·3–99·3) for Kashmore in round 3 (table 7). Coverage with IPV in arm C was higher than that with OPV at both rounds (1 and 3) at which it was administered (greater than 85% at both rounds across all three areas; table 7).
Table 7.
IPV coverage by site for arm C
| Round 1 | Round 3 | |
|---|---|---|
| Karachi | 86·3% (81·7–89·9) | 93·2% (89·9–95·5) |
| Bajaur | 90·0% (87·4–92·2) | 86·0% (82·6–88·8) |
| Kashmore | 95·9% (93·6–97·3) | 98·6% (97·3–99·3) |
| All sites combined | 92·2% (90·6–93·6) | 94·3% (93·0–95·3) |
Data are estimated mean coverage (95% CI). IPV=inactivated polio vaccine.
Completeness of post-supplementary immunisation surveys was consistently very high in Kashmore and, with the exception of round 1 in control arm, in Bajaur as well (appendix). Completeness in Karachi was consistently lower than the other two sites but still reasonably high with the exception of round 2. The low coverage in survey completeness in round 1 of Bajaur and round 2 of Karachi reflects problems of accessibility due to the operations by security forces in some of the areas.
Data for the reasons for non-receipt of OPV and IPV were also collected during the surveys (appendix). For OPV, the most common reasons for non-receipt were that the team did not visit and the child was away at the time of the supplementary immunisation activities and this pattern was consistent across arms, rounds, and sites. For IPV, the most common reason for non-receipt was the child being away from the area; the proportion of refusals across sites was much lower for IPV than for OPV, with some safety concerns largely restricted to Karachi (appendix).
Discussion
The results of our study showed that, despite insecurity due to militancy, and hesitancy in receiving OPV doses at home during supplementary immunisation activities, an innovative approach of community mobilisation combined with delivery of maternal and child health and immunisation interventions through temporary health camps during supplementary immunisation activities was effective in increasing coverage of OPV and other childhood vaccines in Pakistan. Furthermore, the high coverage achieved with IPV in intervention arm C suggested that the strategy of delivering IPV alongside OPV and other vaccines through fixed health camps is both feasible and acceptable. The interventions were well accepted, with the health camps accessed by families with more than 50 000 child visits. The strategy of delivering IPV with maternal and child health and immunisation services resulted in an estimated IPV coverage of about 80% with an excellent safety profile. Contrary to concerns expressed about the possible negative effects of IPV availability and administration on OPV acceptance and uptake, coverage of OPV was 8·5 percentage points higher in the intervention arm that also delivered IPV than in the arm that only delivered OPV. Given the challenges associated with the polio programme in Pakistan, this increase in OPV coverage is important in achieving the threshold needed for stopping poliovirus circulation. The proportion of fully vaccinated children was also increased.
The success and coverage gains in our trial occurred before the initiation of military operations against the Taliban and other militant groups in Federally Administered Tribal Areas (FATA) and inaccessible areas of Karachi.18 The community participation and acceptance of the intervention reflected the widespread unmet needs for maternal and child health and immunisation services in these areas. In fact, the community mobilisation and advocacy strategies focused on promoting general maternal and child health and immunisations without the singular focus on polio vaccination. Our findings support the hypothesis that the provision of polio vaccines as part of a package of health services might be a better way to engage local communities and religious leaders than a polio-specific programme.19 Nigeria has made good progress in reaching difficult populations using a broadly comparable approach,20 although polio has re-emerged after 2 years.21 Nevertheless, the situation is much improved compared with a few years ago.
Our study had considerable challenges in execution. First, at times due to the high demand for services at the health camps, it was difficult for project staff to control the crowds that turned out. To counter this, we used the community leaders and local community volunteers for support. Second, observation of vaccinators revealed some errors in injection technique during the first round—highlighting the need for appropriate training of vaccinators and supervisors. Fortunately, this was not associated with any serious adverse events but was a major source of anxiety for national polio programme managers and local political leadership in FATA where some reports of adverse effects had affected a previous measles vaccination campaign in Khyber Pakhtunkhwa.22 To minimise the risk of problems, we recruited experienced vaccinators from the public sector and provided 5 days of training on standard WHO methods for vaccination. Third, maintenance of the vaccine cold chain in camps was a challenge; to address this, vaccination teams were given adequate volumes of ice packs and back-up thermometers. The vaccines used in the study had vaccine vial monitors and staff were trained in their use. Finally, uniform access and coverage was a challenge: 29 (16 in arm A, seven in arm B, and six in arm C) of the 387 clusters became inaccessible at different times throughout the course of the project due to insecurity and imposition of local curfews. The study was limited by the fact that coverage estimates were largely based on family reports and the relatively lower completeness of post-supplementary immunisation activity surveys in Karachi.
As per the recommendation of the Global Polio Eradication Initiative, IPV should now have been introduced into the routine immunisation schedule of all countries that were previously using OPV alone.23 This decision is now beset with the challenges of a global IPV shortage and prioritisation of supply to some countries.24 Countries such as Pakistan, where the childhood routine immunisation coverage is low among some high-risk populations, need to identify strategies to maximise coverage with OPV and IPV. Our experience indicates that this can be done through short-term camps linked to supplementary immunisation activities. However, this provision comes at a higher cost and with additional needs for human resources, training, and monitoring than routine immunisation services require, and should not be considered a substitute for such services. Nevertheless, in conflict zones and areas with high levels of insecurity where access might be an issue, this could be a pragmatic approach for achieving rapid coverage of routine immunisation and IPV, as has also been shown in refugee populations at risk of outbreaks.25
Our study is the first randomised trial of a strategy to reach at-risk populations in high-risk and insecure populations in Karachi and the border areas of Pakistan and Afghanistan. The findings are consonant with previous approaches to reach similar populations in other regions affected by conflict and insecurity.26, 27 Our experience provides objective evidence that it is possible to gain community trust and achieve gains in immunisation and OPV and IPV coverage using an integrated approach, and has implications for geographies facing resistance and limited community engagement. Polio eradication in Pakistan faces ongoing challenges including insurgency, conflict, and security issues, and mistrust of OPV and its campaigns. The areas of ongoing poliovirus circulation of FATA, Quetta block, and upper Sindh are well known. The military operation by the Pakistan Army in the high insurgency areas of FATA, such as North Waziristan, Khyber Agency, and Tirah valley has opened the opportunity to reach hitherto inaccessible populations.18 Efforts at polio eradication in Pakistan have accelerated rapidly since our study and our findings have been made available to policymakers developing response strategies in high-risk areas. The holistic approach of community mobilisation and establishing regular health camps, focusing on maternal and child health and immunisation services has since been widely implemented in high-risk union councils of Karachi, as well as Khyber Pakhtunkhwa and FATA and is beginning to yield results.28, 29 This approach is now being expanded to high-risk areas in Baluchistan with persistent poliovirus circulation. This experience also has implications for addressing the challenges in the three residual pockets of polio globally, which also happen to be geographies affected by conflict and insecurity.30, 31
Acknowledgments
Acknowledgments
This study was funded by the Bill & Melinda Gates Foundation and would not have been possible without the instrumental support of Waqar Ajmal, Michael Gallway, and Altaf Bosan from the Bill & Melinda Gates Foundation. We acknowledge the contributions of the National Polio Programme, WHO, and UNICEF (Sindh, Khyber Pakhtunkhwa, and FATA) for their cordial support during the planning and implementation phase of the project. We commend the notable contributions that Asghar Ali and his team provided for the legal and administrative responsibilities of the project. We would also like to acknowledge the hard work that the health camp teams, field teams, and data management unit provided during the project. Finally, we thank the communities and participants for being the part of this project as the success of this project would not have been possible without their active involvement.
Contributors
ZAB conceived the study, wrote the protocol along with MAH and SS, and secured funding. MAH, RT, and SS oversaw field operations in Karachi and Kashmore. SA, NA, and NuH supervised Bajaur operations. SC and IA did the statistical analyses. ZAB produced the first draft of the paper with input from SC and all authors contributed to revising the paper and approved the final version.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Global Polio Eradication Initiative Data and monitoring. Polio this week: wild poliovirus list. http://polioeradication.org/wp-content/uploads/2016/12/WPV_2011-2016_28Dec.pdf (accessed Dec 28, 2016).
- 2.National Institute of Population Studies (NIPS) [Pakistan] and ICF International . Pakistan demographic and health survey 2012–13. Demographic and Health Surveys. NIPS and ICF International; Islamabad, Pakistan, and Calverton, Maryland, USA: 2013. https://dhsprogram.com/pubs/pdf/FR290/FR290.pdf (accessed Dec 28, 2016). [Google Scholar]
- 3.Government of Pakistan National Emergency Action Plan for Polio eradication 2015–2016. http://www.polioeradication.org/Portals/0/Document/InfectedCountries/Pakistan/2015-16_NEAP_Pakistan.pdf (accessed April 26, 2016).
- 4.SteelFisher GK, Blendon RJ, Guirguis S. Threats to polio eradication in high-conflict areas in Pakistan and Nigeria: a polling study of caregivers of children younger than 5 years. Lancet Infect Dis. 2015;15:1183–1192. doi: 10.1016/S1473-3099(15)00178-4. [DOI] [PubMed] [Google Scholar]
- 5.Habib MA, Soofi SB, Ali N. Knowledge and perceptions of polio and polio immunization in polio high-risk areas of Pakistan. J Public Health Policy. 2017 doi: 10.1057/s41271-016-0056-6. published online Jan 11. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta ZA. Conflict and polio: winning the polio wars. JAMA. 2013;310:905–906. doi: 10.1001/jama.2013.276583. [DOI] [PubMed] [Google Scholar]
- 7.Alexander JP, Jr, Zubair M, Khan M, Abid N, Durry E. Progress and peril: poliomyelitis eradication efforts in Pakistan, 1994–2013. J Infect Dis. 2014;210(suppl 1):S152–S161. doi: 10.1093/infdis/jiu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patriarca PA, Wright PF, John TJ. Factors affecting immunogenicity of oral polio vaccine in developing countries: a review. Rev Infect Dis. 1991;13:926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 9.Aga Khan University & UNICEF . National Nutritional Survey of Pakistan. Aga Khan University; Islamabad, Pakistan: 2011. [Google Scholar]
- 10.Habib MA, Soofi S, Ali N. A study evaluating poliovirus antibodies and risk factors associated with polio seropositivity in low socioeconomic areas of Pakistan. Vaccine. 2013;31:1987–1993. doi: 10.1016/j.vaccine.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Meeting of the strategic advisory group of experts on immunization, November 2013—conclusions and recommendations. Wkly Epidemiol Rec. 2014;89:1–19. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Introduction of inactivated poliovirus vaccine and switch from trivalent to bivalent oral poliovirus vaccine—worldwide 2013–2016. Morb Mortal Wkly Rep. 2015;64:699–702. [PMC free article] [PubMed] [Google Scholar]
- 13.Estívariz CF, Jafari H, Sutter RW. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6–9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect Dis. 2012;12:128–135. doi: 10.1016/S1473-3099(11)70190-6. [DOI] [PubMed] [Google Scholar]
- 14.John J, Giri S, Karthikeyan AS. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open-label, randomised controlled trial. Lancet. 2014;384:1505–1512. doi: 10.1016/S0140-6736(14)60934-X. [DOI] [PubMed] [Google Scholar]
- 15.Habib MA, Soofi S, Mach O. Effect of booster doses of poliovirus vaccine in previously vaccinated children, clinical trial results 2013. Vaccine. 2016;34:3803–3809. doi: 10.1016/j.vaccine.2016.05.065. [DOI] [PubMed] [Google Scholar]
- 16.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health. Government of Pakistan National Health Policy 2010–2015. Islamabad. https://www.internationalhealthpartnership.net/fileadmin/uploads/ihp/Documents/Country_Pages/Pakistan/PakistanHealthPolicy2010-2015.pdf (accessed Feb 17, 2017).
- 18.Operation Zarbe Azab https://en.wikipedia.org/wiki/Operation_Zarb-e-Azb (accessed Feb 17, 2017).
- 19.Bhutta ZA. Polio eradication hinges on child health in Pakistan. Nature. 2014;511:285–287. doi: 10.1038/511285a. [DOI] [PubMed] [Google Scholar]
- 20.Shuaibu FM, Birukila G, Usman S. Mass immunization with inactivated polio vaccine in conflict zones–Experience from Borno and Yobe States, North-Eastern Nigeria. J Public Health Policy. 2016;37:36–50. doi: 10.1057/jphp.2015.34. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Wild polio and vaccine derived polio in Nigeria. http://www.who.int/csr/don/06-october-2016-polio-nigeria/en/ (accessed Feb 17, 2017).
- 22.Two more children killed due to anti-measles vaccine, DAWN Pakistan. June 1, 2014. http://www.dawn.com/news/1109916 (accessed Dec 28, 2016).
- 23.World Health Organization Introduction of inactivated polio vaccine (IPV) in routine immunizations. http://www.who.int/immunization/diseases/poliomyelitis/inactivated_polio_vaccine/ipv_operational_manual.pdf (accessed Feb 17, 2017).
- 24.Centre for Infectious Diseases Research & Policy WHO Panel recommends dose-sparing strategy for IPV polio vaccine. Oct 21, 2016. http://www.cidrap.umn.edu/news-perspective/2016/10/who-panel-recommends-dose-sparing-strategy-ipv-polio-vaccine (accessed Feb 17, 2017).
- 25.Sheikh MA, Makokha F, Hussein AM. Combined use of inactivated and oral poliovirus vaccines in refugee camps and surrounding communities-Kenya, December 2013. Morb Mortal Wkly Rep. 2014;63:237–241. [PMC free article] [PubMed] [Google Scholar]
- 26.Fekadu L, Okeibunor J, Nsubuga P, Kipela JM, Mkanda P, Mihigo R. Reaching the unreached with polio vaccine and other child survival interventions through partnership with military in Angola. Vaccine. 2016;34:5155–5158. doi: 10.1016/j.vaccine.2016.05.069. [DOI] [PubMed] [Google Scholar]
- 27.Akseer N, Salehi AS, Hossain SM. Achieving maternal and child health gains in Afghanistan: a Countdown to 2015 country case study. Lancet Glob Health. 2016;4:e395–e413. doi: 10.1016/S2214-109X(16)30002-X. [DOI] [PubMed] [Google Scholar]
- 28.End Polio Pakistan Polio cases in provinces. http://www.endpolio.com.pk/polioin-pakistan/polio-cases-in-provinces (accessed Feb 17, 2017).
- 29.Global Polio Eradication Initiative Independent Monitoring Board of the Global Polio Eradication Initiative Fourteenth Report. August, 2016. http://polioeradication.org/wp-content/uploads/2016/09/14IMB_Report_EN.pdf (accessed Feb 17, 2017).
- 30.Garon JR, Orenstein WA. Overcoming barriers to polio eradication in conflict areas. Lancet Infect Dis. 2015;15:1122–1124. doi: 10.1016/S1473-3099(15)00008-0. [DOI] [PubMed] [Google Scholar]
- 31.Hussain SF, Boyle P, Patel P, Sullivan R. Eradicating polio in Pakistan: an analysis of the challenges and solutions to this security and health issue. Global Health. 2016;12:63. doi: 10.1186/s12992-016-0195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.