Abstract
Background:
Exposure to endogenous hormones such as estrogen is known as a lifetime Breast Cancer (BC) risk factor. Polymorphisms in genes that are involved in the steroidogenic process, such as Cytochrome P450c17alpha (CYP17), affect individuals’ susceptibility to BC. In Iran, the highest incident of BC is among young women. This study aimed to find prevalence of Single Nucleotide Polymorphisms (SNPs) in genes such as CYP17 and significant correlation with age-oriented group of breast cancer.
Methods:
In 2016, a case series study was conducted on a total population of 205 patients suffering from breast cancer referred to Cancer Institute, Imam Khomeini Hospital Complex, Tehran, Iran. This population consisted of 104 cases less than 40 yr old and 101 cases over 40. The genotype variants of CYP17 MspA1 were determined using PCR, followed by RFLP. The association of CYP17 MspA1 polymorphisms with the risk of BC in two different age groups was evaluated by calculating odds ratio and 95% confidence intervals using unconditional logistic regression.
Results:
Carriers of at least one A2 allele may have higher risk of developing breast cancer at younger age compared to patients with A1/A1 genotype (Odds Ratio: 1.99, 95% Confidence Interval: 1.11–3.57, P=0.02).
Conclusion:
CYP17gene polymorphisms may have influence on the early onset of breast cancer.
Keywords: Breast cancer, CYP17 gene, Early onset breast cancer, Estrogen, Late-onset breast cancer
Introduction
Breast Cancer (BC) is the most prevalent malignancy and is known as the second leading cause of cancer death among women all around the world (1). In Iran, it has the highest cancer rate among women and accounts for 24.4% of all cancers affecting women (2, 3).
Mixture of genetic susceptibility and environmental exposure are widely accepted as causes of breast cancer. Over the last decades, several different factors such as early menarche, late menopause, post menopause obesity, hormone replacement therapy (HRT), and drinking alcohol have been identified and are strongly linked to increased risk of breast cancer (1, 4, 5).
As an environmental risk factor, exposure to endogenous hormones such as estrogen is known as the lifetime BC risk factor. Although the exact mechanism is not well understood, however, there are two proposed hypotheses. The first one is based on the intrinsic characteristic of the estrogen, as it is a cell proliferation driver. Increase in the number of cells can lead to increase in the number of errors during DNA replication and ultimately increase in the incident of random mutations. The second hypothesis explains that estrogen can be metabolized into quinone derivatives that are involved in DNA depurination which can make DNA prone to more errors and generate carcinogenic mutations (6, 7).
In addition, factors which have influence on the activity and/or metabolism of steroid hormones are of high importance since they potentially affect the incidence of breast cancer. A good example of this is the cytochrome P450c17alpha (CYP17) gene. The location of this gene is on chromosome 10 at q24.3 (8). It encodes steroid 17-alpha-hydroxylase, also known as steroid 17-alpha-monooxygenase. It performs both 17-alpha-hydroxylase and 17, 20-lyase activity. Functions of this enzyme allow the adrenal glands and gonads to synthesize both 17-alpha-hydroxylated glucocorticoids (via 17-alpha-hydroxylase activity) and sex steroids (via 17, 20-lyase activity) (9–12). The level of endogenous estrogen varies in different people, which is the result of polymorphism in the steroidogenic genes that CYP17 is one of them (13, 14). Currently, three important polymorphisms have been identified in CYP17 gene. One of which is a single base polymorphism (SNP) T-> C (rs743572) in the promoter region of CYP17 gene. This specific SNP can create an additional SP1 binding site (CCACC box) which in turn may enhance the gene expression that ultimately may lead to elevated level of estrogen (15, 16).
In Iran, highest incident of BC is among women aged between 40 to 49 yr old. The early age breast cancer is associated with worse prognosis, rapid disease progression and poorer response to treatment necessitates early screening tests and treatments base on the age of the patients (2). Altogether, an unopposed prolonged lifetime exposure of estrogen enhances the risk of breast cancer (17, 18). Studying the prevalence of SNPs in genes such as CYP17 shows a significant correlation with age oriented group of breast cancer patients that suggests a screening marker for risk group can be developed.
This study aimed to find prevalence of Single Nucleotide Polymorphisms (SNPs) in genes such as CYP17 and significant correlation with age-oriented group of breast cancer.
Materials and Methods
Study population
In this case series project conducted in 2016, 205 individuals suffering from breast cancer referred to Imam Khomeini Hospital (Tehran, Iran) were studied. All the subjects included in this study gave their informed consent.
The Ethical Committee of the Tehran University of Medical Sciences approved this study. Patients were categorized according to the age of onset into two groups of younger onset (<40 yr) and elder onset (≥40 yr). All the patients’ breast cancers were confirmed based on their positive histopathology results. A validating questionnaire was used to collect demographic information including age at time of diagnosis, marital and occupational status, educational level, hometown and residence and clinical information such as tumor characteristics, tumor location, type of breast cancer, age at menarche (under 12, 12 yr old or more) and family history of cancer (first to third degree relatives with history of cancer).
Blood Collection and DNA Extraction
Five milliliters of peripheral blood were collected from each patient and later used for DNA isolation. Genomic DNA extraction was performed using Gentra Puregene Blood Kit (Qiagen).
Genotyping
Genotyping of patients was determined by polymerase chain reaction (PCR) followed by restriction fragment length polymorphism analysis (RFLP). Briefly, 50 ng of genomic DNA was used in PCR reaction containing 50 pmol of forwarding primer as 5′-GGCTCCAGGAGAATCTTTC-3′ and reverse primer as 5′-GGGCCAAAACAAATAAGCTA-3′ (19), 100umol of dNTP, 1× Taq buffer and 1 unit of Taq polymerase in total volume of 25μl. The PCR procedure was performed with initiation temperature of 94 °C for 5 min followed by 30 cycles of 94 °C for 1 min for denaturing, 57 °C for 1 min for annealing and 72 °C for 1 min for elongation time. The final cycle was followed by 72 °C for 5 min for extension. PCR products were digested with MspAI restriction enzyme (CutSmart, New England BioLab, USA) at 37 °C for 15 min for investigating the presence of A1 (not digested) and A2 (T->C with MspAI digestion recognition site) genotypes. Digested fragments were stained with RedSafe Nucleic Acid Staining Solution (iNtRON Biotechnology) and further analyzed on 3% agarose gel. Digestion of the mutant genotype creates 86 and 123 bp products, while an uncut product indicate the wild type genotype of 209 bp.
Statistical Analysis
Data were registered into excel file and transferred to SPSS Ver. 16.0 (Chicago, IL, USA) for analysis. Student t-test was used for statistical analysis (P-value). The risk of breast cancer in either age subgroups of population was estimated by calculating Odds Ratio (OR). The X2 test was done to evaluate the consistency of the frequency of CYP17 genotypes variants with Hardy-Weinberg equilibrium.
Results
Out of 205 patients participated in this study, 104 cases (50.7%) were younger than 40 yr old and 101 cases (49.3%) were older than 40.
Age range in young onset group was between 19 to 39 yr old and the average was 33.7 (±4.6 standard deviation). In late onset group, the age range was between 40 and 82 and was average of 51.4 (±9 standard deviation). Only 1 case (1%) was male and belonged to late onset group. Majority of patients were married, homemaker and educated in both young and late onset groups. Demographic data of 2 age subgroups of breast cancer patients has shown in Table 1.
Table 1:
Demographic and disease condition data of 2 age subgroups of breast cancer patients
| Variables |
Under 40 Total=104 n (%) |
Over 40 Total=101 n (%) |
|---|---|---|
| Gender | ||
| Female | 104 (100) | 100 (99) |
| Male | 0 (0) | 1(1) |
| Marital status | ||
| Single | 15 (14) | 4 (4) |
| Married | 88 (85) | 94 (93) |
| Missing | 1 (1) | 3 (3) |
| Occupational level | ||
| Housewife | 75 (72) | 76 (75) |
| Working | 24 (23) | 24 (24) |
| Missing | 5 (5) | 1 (1) |
| Educational status | ||
| Educated | 91 (87) | 78 (77) |
| Uneducated | 5 (5) | 17 (17) |
| Missing | 8 (8) | 6 (6) |
| Hometown | ||
| Tehran | 32 (31) | 47 (46%) |
| Other cities | 72 (69) | 54 (53%) |
| Residence | ||
| Tehran | 51(49) | 77 (76) |
| Other cities | 53(51) | 24 (24) |
| Type of BC | ||
| Invasive ductal carcinoma | 59 (57) | 59 (58) |
| Other types | 19 (18) | 23 (23) |
| Missing | 26 (25) | 19 (19) |
| Breast Involvement | ||
| Unilateral | 100 (96) | 91 (90) |
| Bilateral | 4 (4) | 9 (9) |
| Missing | 0 (0) | 1 (1) |
| Tumor Location (Laterality) | ||
| Left side | 48 (46) | 53 (52) |
| Right side | 48(46) | 35 (35) |
| Both | 4 (4) | 11 (11) |
| Missing | 4 (4) | 2 (2) |
| Age at Menarche | ||
| 12 yr old or under | 25 (24) | 18 (18) |
| Over 12 | 51 (49) | 53 (52) |
| Missing | 28 (27) | 30 (30) |
In regards to menarche age, recorded data was available for 147 patients. The rest of the 58 patients were unable to recall their exact age at menarche (Table 1).
Concerning the family history of cancer, majority of patients in both subsets of population had no family history of cancer (Table 2).
Table 2:
Family history of cancer of 205 patients included in this study
| Family History of Cancer |
Under 40 n (%) |
Over 40 n (%) |
|---|---|---|
| First Degree relative | ||
| 1 person | 16 (15) | 22 (22) |
| 2 persons | 5 (5) | 7 (7) |
| 3 persons or more | 0 (0) | 4 (4) |
| No History | 83 (80) | 68 (67) |
| Missing | 0 (0) | 0 (0) |
| Second Degree relative | ||
| 1 person | 19 (18) | 17 (17) |
| 2 persons | 6 (6) | 12 (12) |
| 3 persons or more | 3 (3) | 1 (1) |
| No History | 75 (72) | 71 (70) |
| Missing | 1 (1) | 0 (0) |
| Third Degree relative | ||
| 1 person | 18 (17) | 18 (18) |
| 2 persons | 4 (4) | 2 (2) |
| 3 persons or more | 1 (1) | 1 (1) |
| No History | 81 (78) | 80 (79) |
| Missing | 0 (0) | 0 (0) |
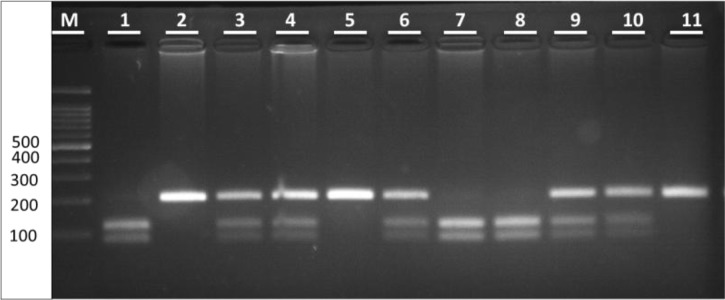
In case of genotype variation in the whole population, 72 cases (35.1%) were A1/A1 (209 bp), 104 cases (50.7%) were A1/A2 (209, 123 and 86 bp) and 29 cases (14.1%) were A2/A2 (123 and 86 bp) (Fig. 1). The frequency of A1 and A2 alleles in the total population was 60.48% and 39.51%, respectively. The frequency of CYP17 alleles in the total population did not deviate from Hardy-Weinberg equilibrium (X2=0.77, P=0.37).
Fig. 1:
CYP17 MspA1 digested products separated on 3% agarose gel electrophoresis Lane1 100bp ladder; Lane 2, 5 & 11 Homozygous A1 genotype (A1/A1); Lane 1, 3, 4, 6, 9 & 10 Heterozygous genotype (A1/A2); Lane 7 & 8 Homozygous A2 genotype (A2/A2).
Among the population of patients younger than 40 yr 27.8% had A1/A1, 55.7% had A1/A2, and 16.3% had A2/A2 genotype respectively (X2= 1.76, P=0.18) while it was 42.5%, 45.4% and 11.8% (X2=0.003, P=0.95) for elder group. No significant association was found between CYP17 genotype variants and age of the patients. However, a significant correlation was found between the presence of at least one A2 allele and increased risk of breast cancer in patients age under 40 (P=0.02) (Table 3). In younger subset, the frequency of A1 and A2 allele was 55.76% and 44.23%, respectively while this was 65.34% and 34.65% for elder subset in that order. Comparing two subsets of groups in regards to laterality, type of BC and family history of cancer no significant association was found (Table 4). Regarding the menarche age, majority of young patients with at least one A2 allele had their first menstrual cycle at age 12 and this was statistically significant (P=0.04, OR: 0.35, 95% Confidence Interval: 0.12–0.98) (Table 4).
Table 3:
Distribution of CYP17 MSPA1 polymorphism in 2 subgroups of population
| Variables |
Under 40 n (%) |
Over 40 n (%) |
OR | 95%CI | P |
|---|---|---|---|---|---|
| Genotypes | |||||
| Homozygous | 29 (28) | 43 (43) | 0.88 | ||
| A1/A1 | |||||
| Heterozygous | 58 (56) | 46 (46) | 2.101 | 0.875–5.046 | 0.97 |
| A1/A2 | |||||
| Homozygous | 17 (16) | 12 (12) | 1.124 | 0.488–2.587 | 0.784 |
| A2/A2 | |||||
| Combined polymorph | 75(72) | 58 (57) | 1.996 | 1.116–3.572 | 0.02 |
| A1/A2 + A2/A2 |
Table 4:
Association between CYP17 MspA1 polymorphism and potential BC risk factors such as laterality, type of breast cancer, family history of cancer and menarche age
| Variables | Patients Under 40 | Patients over 40 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Combined genotype A1/A2 & A2/A2 n (%) | Genotype A1/A1 n (%) | OR | 95%CI | P value | Combined genotype A1/A2 & A2/A2 n (%) | Genotype A1/A1 n (%) | OR | 95%CI | P value | |
| Unilateral | 71 (71) | 29 (29) | 0 | 0 | 0.998 | 51 (56) | 40 (44) | 0.348 | 0.069– | 0.203 |
| Bilateral | 4 (100) | 0 (0) | 7 (77.8) | 2 (22.2) | 1.769 | |||||
| Invasive ductal carcinoma | 47 (79.7) | 12 (20.3) | 2.848 | 0.939- | 0.064 | 30 (50.8) | 29 (49.2) | 0.796 | 0.302- | 0.644 |
| Other types | 8 (42.1) | 11 (57.9) | 8.640 | 14 (60.9) | 9 (39.1) | 2.098 | ||||
| Family history of cancer | 37 (69.8) | 16 (30.2) | 0.791 | 0.335- | 0.594 | 38 (63.3) | 22 (36.7) | 2 | 0.892- | 0.093 |
| No family history of | 38 (74.5) | 13 (25.5) | 1.871 | 20 (48.8) | 21 (51.2) | 4.486 | ||||
| cancer | ||||||||||
| Menarche age under 12 | 14 (56) | 11 (44) | 0.35 | 0.124- | 0.047 | 9 (50) | 9 (50) | 0.963 | 0.330- | 0.945 |
| Menarche age 12 or over | 40 (78.4) | 11 (21.6) | 0.984 | 28 (52.8) | 25 (47.2) | 2.806 | ||||
Discussion
Endogenous exposure to circulating steroid hormones is known as a risk factor in developing breast cancer. There are different factors that have influence on the exposure to endogenous hormones and may affect susceptibility of the individuals to breast cancer. Polymorphisms in genes that are involved in the steroidogenic process, such as CYP17, are thought to be as one of these factors. Up to now, several studies have investigated the correlation of CYP17 MspA1 polymorphism and risk of breast cancer but there is still a controversy in the results (20). In this case-series study, we demonstrated that carriers of at least one A2 allele might have higher risk of developing breast cancer at younger age compared to patients with A1/A1 genotype.
The frequencies of 34%, 52%, and 14% were reported for A1/A1, A1/A2, and A2/A2, respectively, in patients with polish origin (21). A prevalence of 41.5% for A1/A1, 47.4% was reported for A1/A2 and 11.1% for A2/A2 genotypes in Finnish population which were in accordance with our findings (22). Similar frequencies were also reported for different variants of CYP17 MspA1 polymorphism (23).
Genotypes other than A1/A1 which may have more activity for CYP17 enzyme for estrogen metabolism (A1/A2, A2/A2) were more frequent among younger breast cancer patients compared to older ones and this was statistically significant (P=0.02). Carriers of at least one A2 allele may have higher risk of developing breast cancer at younger age compared to patients with A1/A1 genotype (OR: 1.99, 95% CI: 1.11–3.57). In 1996, the first research group brought up the association of A2 allele and increased risk of breast cancer (18). Higher risk of breast cancer was reported among younger patients who were Hetero- and Homozygote for A2 allele and our results are in line with their findings (19). Statistically significant association between A2 allele and early age breast cancer were reported (24). On the other hand, two meta-analyses, showed lack of correlation between CYP17 polymorphisms and overall risk of breast cancer (20, 25–28). A reason that can be brought for the existing controversy is that different studies used different subgroups of populations defined by ethnicity, age (24, 29, 30), tumor stage/aggressiveness and menopausal status which may vary from one study to the other (31, 32). A second reason could be due to this fact that early onset of breast cancer is different with late onset in terms of pathological characteristics, diagnosis, and biological origin. This could explain the difference between 2 subgroups of population and controversy in different studies (19).
The relationship between estrogen exposure and risk of breast cancer were evaluated. Early menarche and late menopause, which means longer exposure to estrogen, increase the risk of breast cancer; however, in the present study, a significant relationship was found between young patients harboring A2 allele and late menarche. Several other studies including a meta-analysis also failed to show any association between CYP17 MspA1 polymorphism and early menarche (33, 34). Twin and Family studies have shown that genetic factors account for 53–72% of menarche age variation (34). Thus, CYP17 polymorphism may not be the only effect modifier in this regards and other genes may contribute to this trait variation.
Analyzing the CYP17 MspA1 polymorphism in two subsets of population did not show any association with family history of cancer. Other genes are participating in steroidogenic pathways that may have greater roles in familial cases.
Conclusion
Polymorphism in CYP17 is possibly age specific and in addition to other risk factors may have influence on increased risk of breast cancer in early age. CYP17 MspA1 polymorphism can be used as a potential genetic marker for identification of high-risk groups. However, a larger population is needed to confirm the major influence of CYP17 MspA1 variants on early onset breast cancer among Iranian women.
Ethical Consideration
Ethical issues (including plagiarism, obtaining informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This study was supported by Cancer Research Center, Cancer Institute of Iran (Tehran University of Medical Sciences). The authors declare that there is no conflict of interest.
References
- 1.Antognelli C, Del Buono C, Ludovini V, Gori S, Talesa VN, Crinò L, Barberini F, Rulli A. (2009). CYP17, GSTP1, PON1 and GLO1 gene polymorphisms as risk factors for breast cancer: an Italian case-control study. BMC Cancer, 20;9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu GR, Samari G, Cohen SP, Mahapatra T, Wahbe RM, Mermash S, Galal OM. (2011). Breast cancer screening among females in Iran and recommendations for improved practice: a review. Asian Pac J Cancer Prev, 12:1647–1655. [PubMed] [Google Scholar]
- 3.Harirchi I, Kolahdoozan S, Karbakhsh M, Chegini N, Mohseni S, Montazeri A, Momtahen A, Kashefi A, Ebrahimi M. (2011). Twenty years of breast cancer in Iran: downstaging without a formal screening program. Ann Oncol, 22:93–97. [DOI] [PubMed] [Google Scholar]
- 4.Yager JD, Davidson NE. (2006). Estrogen carcinogenesis in breast cancer. N Engl J Med, 354:270–282. [DOI] [PubMed] [Google Scholar]
- 5.Dumitrescu R, Cotarla I. (2005). Understanding breast cancer risk - where do we stand in 2005? J Cell Mol Med, 9:208–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue W, Santen R, Wang J-P, Li Y, Verderame M, Bocchinfuso W, Korach K, Devanesan P, Todorovic R, Rogan E. (2003). Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem Mol Biol, 86:477–486. [DOI] [PubMed] [Google Scholar]
- 7.De Bont R, Van Larebeke N. (2004). Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis, 19:169–185. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimoto FK, Auchus RJ. (2015). The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1). J Steroid Biochem Mol Biol, 151:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid AH, Attard G, Barrie E, de Bono JS. (2008). CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol, 5:610–620. [DOI] [PubMed] [Google Scholar]
- 10.van den Akker EL, Koper JW, Boehmer AL, Themmen AP, Verhoef-Post M, Timmerman MA, Otten BJ, Drop SL, De Jong FH. (2002). Differential inhibition of 17α-hydroxylase and 17, 20-lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J Clin Endocrinol Metab, 87:5714–5721. [DOI] [PubMed] [Google Scholar]
- 11.Blackburn HL, Ellsworth DL, Shriver CD, Ellsworth RE. (2015). Role of cytochrome P450 genes in breast cancer etiology and treatment: effects on estrogen biosynthesis, metabolism, and response to endocrine therapy. Cancer Causes Control, 26:319–332. [DOI] [PubMed] [Google Scholar]
- 12.Setiawan VW, Schumacher FR, Haiman CA, et al. (2007). CYP17 genetic variation and risk of breast and prostate cancer from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium (BPC3). Cancer Epidemiol Biomarkers Prev, 16:2237–2246. [DOI] [PubMed] [Google Scholar]
- 13.Rannala B. (2001). Finding genes influencing susceptibility to complex diseases in the post-genome era. Am J Pharmacogenomics, 1:203–221. [DOI] [PubMed] [Google Scholar]
- 14.Sharp L, Cardy AH, Cotton SC, Little J. (2004). CYP17 gene polymorphisms: prevalence and associations with hormone levels and related factors. a HuGE review. Am J Epidemiol, 160:729–740. [DOI] [PubMed] [Google Scholar]
- 15.Ye Z, Parry JM. (2002). The CYP17 MspA1 polymorphism and breast cancer risk: a meta-analysis. Mutagenesis, 17:119–126. [DOI] [PubMed] [Google Scholar]
- 16.Rai R, Sharma KL, Misra S, Kumar A, Mittal B. (2014). CYP17 polymorphism (rs743572) is associated with increased risk of gallbladder cancer in tobacco users. Tumour Biol, 35:6531–6537. [DOI] [PubMed] [Google Scholar]
- 17.Mense SM, Remotti F, Bhan A, Singh B, El-Tamer M, Hei TK, Bhat HK. (2008). Estrogen-induced breast cancer: alterations in breast morphology and oxidative stress as a function of estrogen exposure. Toxicol Appl Pharmacol, 232:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feigelson HS, Coetzee GA, Kolonel LN, Ross RK, Henderson BE. (1997). A polymorphism in the CYP17 gene increases the risk of breast cancer. Cancer Res, 57:1063–1065. [PubMed] [Google Scholar]
- 19.Bergman - Jungeström M, Gentile M, Lundin AC, Wingren S. (1999). Association between CYP17 gene polymorphism and risk of breast cancer in young women. Int J Cancer, 84:350–353. [DOI] [PubMed] [Google Scholar]
- 20.Mao C, Wang X-W, He B-F, Qiu L-X, Liao R-Y, Luo R-C, Chen Q. (2010). Lack of association between CYP17 MspA1 polymorphism and breast cancer risk: a meta-analysis of 22,090 cases and 28,498 controls. Breast Cancer Res Treat, 122:259–265. [DOI] [PubMed] [Google Scholar]
- 21.Jakubowska A, Gronwald J, Menkiszak J, Górski B, Huzarski T, Byrski T, Tołoczko-Grabarek A, Gilbert M, Edler L, Zapatka M. (2010). BRCA1-associated breast and ovarian cancer risks in Poland: no association with commonly studied polymorphisms. Breast Cancer Res Treat, 119:201–211. [DOI] [PubMed] [Google Scholar]
- 22.Mitrunen K, Jourenkova N, Kataja V, Eskelinen M, Kosma V-M, Benhamou S, Vainio H, Uusitupa M, Hirvonen A. (2000). Steroid metabolism gene CYP17 polymorphism and the development of breast cancer. Cancer Epidemiol Biomarkers Prev, 9:1343–1348. [PubMed] [Google Scholar]
- 23.Kristensen VN, Haraldsen EK, Anderson KB, Lønning P, Erikstein B, Kåresen R, Gabrielsen OS, Børresen-Dale A-L. (1999). CYP17 and Breast Cancer Risk The Polymorphism in the 5′ Flanking Area of the Gene Does Not Influence Binding to Sp-1. Cancer Res, 59:2825–2828. [PubMed] [Google Scholar]
- 24.Chakraborty A, Murthy N, Chintamani C, Bhatnagar D, Mohil R, Sharma P, Saxena S. (2007). CYP17 gene polymorphism and its association with high-risk north Indian breast cancer patients. J Hum Genet, 52:159–165. [DOI] [PubMed] [Google Scholar]
- 25.Cristina Tote Franco Pinotti S, da Silva IDCG, Carvalho CV, Nazário ACP. (2012). MspA1 polymorphism of the CYP17 gene in breast cysts. Gynecol Endocrinol, 28:443–446. [DOI] [PubMed] [Google Scholar]
- 26.Chang J-H, Gertig DM, Chen X, Dite GS, Jenkins MA, Milne RL, Southey MC, McCredie M, Giles GG, Chenevix-Trench G. (2005). CYP17 genetic polymorphism, breast cancer, and breast cancer risk factors: Australian Breast Cancer Family Study. Breast Cancer Res, 7:R513–R521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos SS, Jácome GPO, Koifman R, Koifman S. (2014). CYP17, CYP19, and NQO1 Genetic Polymorphisms and Breast Cancer Susceptibility in Young Women in Brazil. Br J Med Med Res, 4:68. [Google Scholar]
- 28.Yao L, Fang F, Wu Q, Yang Z, Zhong Y, Yu L. (2009). No association between CYP17 T-34C polymorphism and breast cancer risk: a meta-analysis involving 58,814 subjects. Breast Cancer Res Treat, 122:221–227. [DOI] [PubMed] [Google Scholar]
- 29.Spurdle AB, Hopper JL, Dite GS, Chen X, Cui J, McCredie MR, Giles GG, Southey MC, Venter DJ, Easton DF. (2000). CYP17 promoter polymorphism and breast cancer in Australian women under age forty years. J Natl Cancer Inst, 92:1674–1681. [DOI] [PubMed] [Google Scholar]
- 30.Shin MH1, Lee KM, Yang JH, Nam SJ, Kim JW, Yoo KY, Park SK, Noh DY, Ahn SH, Kim B, Kang D. (2005). Genetic polymorphism of CYP17 and breast cancer risk in Korean women. Exp Mol Med, 37:11–17. [DOI] [PubMed] [Google Scholar]
- 31.TüZüNER BM, Öztürk T, Kisakesen HI, Ilvan Ş, Zerrin C, Öztürk O, Isbir T. (2010). CYP17 (T-34C) and CYP19 (Trp39Arg) polymorphisms and their cooperative effects on breast cancer susceptibility. In Vivo, 24:71–74. [PubMed] [Google Scholar]
- 32.Verla-Tebit E, Wang-Gohrke S, Chang-Claude J. (2005). CYP17 5’-UTR MspA1 polymorphism and the risk of premenopausal breast cancer in a German population-based case-control study. Breast Cancer Res, 7:R455–R464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waqar-ul-Haq Dvornyk., V1 (2012). Genetics of age at menarche: a systematic review. Hum Reprod Update, 18:198–210. [DOI] [PubMed] [Google Scholar]
- 34.Pei Y-F, Zhang L, Deng H-W, Dvornyk V. ( 2008 . ). CYP17 MspA1 polymorphism and age at menarche: a meta-analysis . Dis Markers, 25 : 87 –95. [DOI] [PMC free article] [PubMed] [Google Scholar]