Abstract
The management of rotator cuff defects after arthroscopic debridement for calcific tendinitis can be a challenge for physicians. To date, treatment options have included debridement alone, in situ repairs of the tendon, or full-thickness takedown and repair. Each option, however, has been fraught with its own pitfalls and limitations. We propose a technique for the management of rotator cuff defects through the application of a bioinductive collagen implant that may allow for rapid tissue incorporation and regeneration.
The treatment of symptomatic rotator cuff calcific tendinitis can be challenging to both physicians and patients. Initial treatment should focus on conservative treatment modalities including physical therapy, anti-inflammatory medications, activity modification, and subacromial corticosteroid injections. Other considerations include the use of extracorporeal shockwave treatments and ultrasound-guided trephination.
Patients in whom nonoperative measures fail may benefit from arthroscopy and deposit removal. Controversy exists regarding the management of the resultant defect in the rotator cuff created when the calcific deposit is removed. Barber and Cowden1 recommend that defects greater than 5 mm in depth be repaired to optimize outcomes. Consideration must be given to the location of the defect created after debridement of the deposit being addressed. Frequently, these defects are located 1 to 2 cm medial to the tuberosity. As such, the potential risk exists for medialization of the rotator cuff tendon due to insufficient tissue remaining for repair. Attempts to repair these defects with simple side-to-side sutures may be fraught with pitfalls because of the remaining poor tissue. Furthermore, patients with a greater than 50% loss of rotator cuff thickness after debridement often have warranted in situ rotator cuff repairs versus a full-thickness takedown with repair.
Another solution is tissue augmentation. A highly porous and bioinductive implant can stimulate rapid tissue induction and ingrowth.2 Consideration of its use has become a component of our treatment algorithm for the management of rotator cuff defects after arthroscopic debridement of a calcium deposit (Fig 1). This article will discuss the implantation of a specially prepared, bovine Achilles tendon xenograft designed for tendon augmentation and repair (Fig 2). The indications for its use have been outlined in Table 1.
Fig 1.
Treatment algorithm for rotator cuff (RTC) calcific tendinitis. (NSAIDs, nonsteroidal anti-inflammatory drugs.)
Fig 2.
Viewing from the posterior portal of a left shoulder, the bioinductive implant can be seen secured over the top of the native rotator cuff tendon. The arrow denotes the polylactic acid staple through the implant and the rotator cuff tendon. The star identifies the area of rotator cuff defect covered by the implant.
Table 1.
Indications and Contraindications for Use of Bioinductive Implant for Management of Rotator Cuff Defects After Calcific Tendinopathy Debridement
| Indications |
| Low-, medium-, or high-grade symptomatic rotator cuff tissue defects |
| Younger, active patients |
| Contraindications to prolonged shoulder immobilization (i.e., adhesive capsulitis) |
| Defects that would otherwise require medialization of the rotator cuff to repair |
| Patients with poor tissue quality (i.e., smokers and diabetic patients) |
| Contraindications |
| Full-thickness tissue defects that require rotator cuff spanning |
| Far medial or musculotendinous defects that will not allow for the implant to be secured laterally |
| Irreparable rotator cuff tears |
Surgical Technique
Our surgical technique uses the arthroscopic reconstituted collagen scaffold implant from Rotation Medical (Minneapolis, MN). The implant is placed using a disposable scaffold delivery instrument, which will be described later.
We prefer to use an interscalene nerve block in the patient, followed by general anesthesia. The procedure can be performed with the patient in the beach-chair or lateral position. We prefer the lateral position, and 10 lb (4.5 kg) of balanced suspension is used with the arm in flexion and abduction. While viewing through a standard posterior portal and working through a high rotator internal portal, the surgeon performs a systematic standard diagnostic arthroscopy. The articular rotator cuff is carefully inspected. Tendon fraying may be debrided with a mechanical shaver. Any resultant defect or exposed greater tuberosity is measured. Next, the tendon marker is placed percutaneously through the supraspinatus, just posterior to the biceps tendon. A white button on the tendon marker is depressed, allowing for a retractable anchor to expand at the tip. This ensures that the tendon marker does not fall out of position during the remainder of the procedure. Placement of the tendon marker provides demarcation of the anterior edge of the supraspinatus when viewing from the subacromial space, thus ensuring appropriate implant placement. The arthroscope is redirected to the subacromial space, and preparation of the specialized portion of the case may begin (Video 1).
-
1.
A lateral portal is established and a thorough bursectomy is performed to enhance visualization. The lateral portal is positioned slightly superior and anterior in relation to the midportion of the supraspinatus. This will allow for facilitation of the Rotation Medical Tendon Staple placement once the implant has been delivered (Fig 3).
-
2.
An acromioplasty may be performed as needed.
-
3.
A standard probe is used through the lateral portal to palpate the calcium deposit within the supraspinatus. The calcium deposit is identified as irregularly raised, white, and often covered with vessels. An 18-gauge spinal needle is then used percutaneously to perform trephination of the deposit. A shaver is used to debride the toothpaste-like deposit.
-
4.
After debridement of the rotator cuff, the defect is noted within the tissues and measured with a calibrated probe. The appropriately sized implant, medium (20 × 25 mm) or large (25 × 30 mm), is selected. Typically, the medium implant is sufficient. The implant is prepackaged rolled in a cylindrical fashion and is assembled onto a separately packaged scaffold delivery instrument (Fig 4).
-
5.
Attention is next turned toward implant insertion. A spinal needle is used to localize a low lateral starting point 5 mm inferior to the native rotator cuff insertion on the greater tuberosity.
-
6.
A portal is created, and a specialized guidewire instrument is introduced and placed against the lateral humeral head 5 mm inferior to the margin of the supraspinatus tendon.
-
7.
The scaffold delivery instrument is slid over the wire into the subacromial space (Fig 5). A pearl for easier placement entails inserting the mechanical shaver to remove overlying deltoid fascia about the guidewire instrument.
-
8.
After delivery into the subacromial space, the implant is placed over the defect location and deployed by depressing the scaffold delivery trigger (Fig 6). The implant should cover the lateral footprint of the native rotator cuff, as well as the defect. It should not be placed anterior to the previously placed spinal needle marking the location of the supraspinatus anterior edge.
-
9.
A proprietary 5-mm cannula is placed into the original lateral portal. Tendon staples are delivered through the cannula to secure the implant to the rotator cuff. We recommend that the first 2 tendon staples be placed medially to prevent the implant from sliding. After this, 2 additional tendon staples can be placed anteriorly and posteriorly along the border of the implant. For optimal fixation, it is recommended to have the tendon stapler as perpendicular as possible to the tendon. In addition, positive downward pressure should be applied while depressing the trigger on the tendon stapler to allow for the tendon staples to sit flush on the implant.
-
10.
Once the implant has been secured, the scaffold delivery instrument can be removed by gently pulling it laterally out through its portal. The guidewire instrument is then removed by hand.
-
11.
Attention is now turned to the placement of the lateral tuberosity fixation. A bone staple introducer is inserted through the lateral portal used to previously introduce the implant (Fig 7). It is positioned at the lateral margin of the implant over the greater tuberosity.
-
12.
The bone stapler introducer and bone punch are then malleted into the lateral margin of the tuberosity. After these have been malleted flush, the trigger on the bone staple introducer is deployed to eject the bone punch. Next, the Rotation Medical Bone Stapler is inserted and tapped down flush to the tuberosity. We recommend placing 2 to 3 lateral bone staples, depending on the quality of the fixation to the bone and the size of the implant.
-
13.
The shoulder is next taken through a full range of motion to ensure stability of the implant, without impingement.
Fig 3.
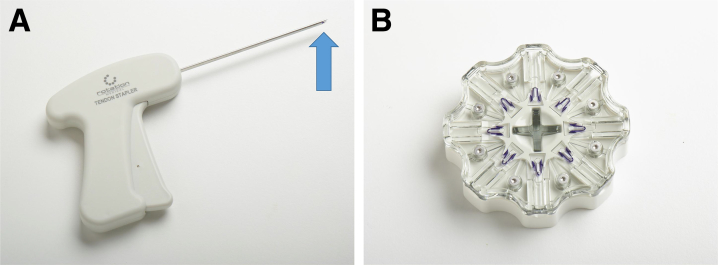
The Rotation Medical Tendon Stapler allows for passage through a 5-mm cannula and placement of the polylactic acid staples through the implant and into the rotator cuff tendon. The polylactic acid staples are loaded at the tip of the stapler (arrow) (A) by inserting the tip into the proprietary “hockey puck” (B) and depressing the trigger.
Fig 4.
The bioinductive implant (blue arrow) is attached to the end of the separately packaged scaffold delivery instrument. The guidewire instrument is slid into the cannulated slot on the inferior aspect of the implant (yellow arrow).
Fig 5.
Outside view of a left shoulder in the lateral position. The bioinductive implant (red arrow) is seen attached to the end of the scaffold delivery instrument (yellow arrow). The implant and delivery instrument are then slid over the top of the guidewire instrument (blue arrow) to facilitate accurate delivery into the subacromial space.
Fig 6.
The bioinductive implant is deployed by depressing the trigger on the scaffold delivery instrument and held in place within the subacromial space by the flexible metal wings (arrow).
Fig 7.
The Rotation Medical Bone Stapler Implant allows for placement of the PEEK (polyether ether ketone) lateral bone staples. After being introduced into the subacromial space, the prongs of the stapler (blue arrow) are malleted flush against the bone. The black trigger is depressed, ejecting the punch (yellow arrow). The PEEK Staple Inserter (red arrow) is then placed into the stapler and malleted flush through the implant, securing it to the tuberosity.
Postoperative Course
-
1.
The patient is discharged home in a sling for 24 to 48 hours. Range-of-motion exercises of the elbow and pendulum exercises for the shoulder may begin on postoperative day 1.
-
2.
Unrestricted active motion begins when normal neurologic function has returned.
-
3.
Patients are instructed to lift no more than 5 lb (2.3 kg) for the initial 6 weeks after surgery.
-
4.
Full range of motion is permitted as tolerated.
-
5.
Physical therapy, focusing on range of motion and periscapular stabilization, is begun approximately 7 to 10 days postoperatively.
-
6.
Of note, if a concomitant biceps tenodesis is performed, we recommend still following the aforementioned postoperative course to avoid stiffness.
Discussion
Arthroscopic treatment of rotator cuff calcific tendinitis has produced mixed results. Good to excellent results have been reported after simple arthroscopic debridement. However, when compared with the contralateral extremity, strength deficits may remain.2, 3, 4, 5 Debridement and the concomitant addition of a tissue-augmenting bioinductive implant may improve postoperative strength. Tissue growth stimulated by the implant may decrease strain in the remaining articular tendon. Early studies have shown a reduction in peak strains across the bursal-sided rotator cuff defect of approximately 47% after placement of the bio-inductive implant.6 In our patient population, magnetic resonance imaging evaluation and patient-reported outcomes have pointed toward a very favorable alternative to traditional methods for arthroscopic treatment of calcific tendinitis of the rotator cuff (Fig 8, Fig 9, Fig 10).
Fig 8.
T2-weighted magnetic resonance images of a 47-year-old female patient's left shoulder. The coronal image (A) and sagittal image (B) show a 17-mm × 11-mm calcific deposit (arrows) occupying a large volume of the supraspinatus tendon.
Fig 9.
Coronal T2-weighted magnetic resonance image of the patient's left shoulder 6 months after calcific tendinopathy debridement with defect augmentation using the bio-inductive implant. A clear layer of tendon-like tissue is noted over the defect area (arrow).
Fig 10.
Coronal T2-weighted magnetic resonance image of the patient's left shoulder 12 months after placement of the bioinductive implant for the rotator cuff defect. Thickening of the tendon overlying the area of initial defect shows further integrity of the tendon (arrow).
The pros and cons of using a bioinductive implant for the treatment of a rotator cuff defect must be weighed on a patient-by-patient basis (Table 2). However, the ability to allow for an accelerated rehabilitation protocol and the ability to avoid medialization of the rotator cuff defect are 2 of the principal reasons we prefer this technique. The understanding of the surgical technique, as described, and the use of the surgical pearls will allow for rapid acclimation to the procedure and optimization of patient outcomes (Table 3).
Table 2.
Advantages and Disadvantages of Use of Bioinductive Implant for Management of Rotator Cuff Defects After Calcific Tendinopathy Debridement
| Advantages |
| The technique allows ease of technique reproducibility. |
| Surgical time is decreased. |
| The procedure may be performed supine or lateral with changing technique. |
| Rotator cuff medialization is avoided. |
| Accelerated postoperative rehabilitation is allowed compared with transtendinous repair or full-thickness takedown and repair. |
| Disadvantages |
| There are insurance coverage limitations with Medicare and Medicaid patients. |
| The implant is not intended for irreparable tears. |
| The implant is friable and can rip if skiving occurs with the tendon stapler. |
| Separate implants may be needed for multitendon involvement. |
| Lateral greater tuberosity bone cysts may prevent adequate fixation of PEEK (polyether ether ketone) lateral bone staples. |
Table 3.
Surgical Pearls for Arthroscopic Placement of Bioinductive Implant for Management of Rotator Cuff Defects After Calcific Tendinopathy Debridement
| When the surgeon is debriding the calcific deposit from the bursal side, care should be taken to not violate the articular fibers. |
| Appropriate placement of the guidewire instrument 5 mm below the insertion of the supraspinatus will allow for the implant to lie flat on the tendon. |
| Positioning the rotator interval and initial lateral working portal high will allow them to be used for placement of the polylactic acid (PLA) tendon staples. |
| An acromioplasty should be performed if needed to allow working room for the cannula. |
| Use of the mechanical shaver to debride the deltoid fascia around the guidewire will facilitate easy entrance of the implant into the subacromial space. |
| When the surgeon is introducing the implant into the subacromial space, care should be taken to avoid implant medialization. This will allow for adequate fixation of the lateral PEEK (polyether ether ketone) staples. |
| Placement of the PLA staples perpendicular to the tendon will optimize the fixation of the implant to the native tendon. |
| The medial PLA staples should be placed in an anterior-to-posterior orientation on the implant to avoid the occurrence of staple failure. |
| After final fixation is obtained, the shoulder should be taken through a full range of motion under arthroscopic visualization to ensure the implant does not impinge upon the undersurface of the acromion. |
In summary, the addition of a bioinductive implant for arthroscopic treatment of calcific tendinopathy defects may improve postoperative outcomes. Consideration of using a bioinductive implant for this pathology offers an approach for a difficult case.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: S.M. receives support from Rotation Medical as a consultant (however, this technique paper was not funded or paid for by the company).
Supplementary Data
Arthroscopic repair of a rotator cuff tendon defect after debridement of a calcium deposit. The patient is in the lateral decubitus position with the arthroscope viewing from the posterior portal of the left shoulder within the subacromial space. The field of view is orientated such that the acromion is superior, the rotator cuff is inferior, and the lateral portal is to the left of the field of view. A high rotator interval portal is also used anteriorly for some of the polylactic acid staple placement. After the calcium deposit has been debrided, the bioinductive implant is secured over the top of the resultant tendon defect, allowing for bioinductive restoration of the native tissue.
References
- 1.Barber F.A., Cowden C.H., III Arthroscopic treatment of calcific tendonitis. Arthrosc Tech. 2014;3:e237–e240. doi: 10.1016/j.eats.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klean K., Bishai S. Rotation Medical; Minneapolis, MN: 2015. Clinical experience with the Rotation Medical bioinductive implant for rotator cuff repair with biological augmentation. [white paper] [Google Scholar]
- 3.Rebuzzi E., Coletti N., Schiavetti S., Giusto F. Arthroscopy surgery versus shock wave therapy for chronic calcifying tendinitis of the shoulder. J Orthop Traumatol. 2008;9:179–185. doi: 10.1007/s10195-008-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ark J.W., Flock T.J., Flatow E.L. Arthroscopic treatment of calcific tendinitis of the shoulder. Arthroscopy. 1992;8:183–188. doi: 10.1016/0749-8063(92)90034-9. [DOI] [PubMed] [Google Scholar]
- 5.Maier D., Jaeger M., Izadpanah K. Rotator cuff preservation in arthroscopic treatment of calcific tendinitis. Arthroscopy. 2013;29:824–831. doi: 10.1016/j.arthro.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Van Kampen C., Arnoczky S., Parks P. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: A histological evaluation in sheep. Muscles Ligaments Tendon J. 2013;3:229–235. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arthroscopic repair of a rotator cuff tendon defect after debridement of a calcium deposit. The patient is in the lateral decubitus position with the arthroscope viewing from the posterior portal of the left shoulder within the subacromial space. The field of view is orientated such that the acromion is superior, the rotator cuff is inferior, and the lateral portal is to the left of the field of view. A high rotator interval portal is also used anteriorly for some of the polylactic acid staple placement. After the calcium deposit has been debrided, the bioinductive implant is secured over the top of the resultant tendon defect, allowing for bioinductive restoration of the native tissue.