Abstract
Because of disease chronicity and required shifts in coping and self-management skills over time, it is not surprising that patients with inflammatory bowel disease (IBD) are at increased risk for mental health issues, including depression. Modern conceptualizations of chronic care recognize that the relationship between depression and disease is bidirectional, with (1) poor health leading to poor self-management just as often as poor self-management leads to poor health and (2) inflammation driving depression and depression driving inflammation. Depression in the setting of IBD has been undertreated in this population in the past and, if it remains as such, will continue to pose a significant risk to the current health care system. In this article, we explore these bidirectional relationships and make recommendations for the assessment and treatment of depression in the context of IBD.
Keywords: Depression, inflammatory bowel disease, coping, cognitive behavioral therapy
Self-management of inflammatory bowel disease (IBD), which consists of painful, often disabling, immune-mediated inflammatory conditions of the digestive tract, is of critical importance to successful long-term outpatient management. It is well established across many chronic conditions that when self-management skills decline, patients experience poorer health outcomes.1 Individuals with IBD, including Crohn’s disease and ulcerative colitis, must simultaneously and effectively engage in 3 core self-management tasks: (1) medical management, which includes adherence, decision-making, disease knowledge, and relationships/communication with the medical team; (2) preservation or creation of meaningful life roles and adjustment to any limitations that the disease presents; and (3) acknowledgement and management of the emotional and psychological impacts of IBD.2 Self-management skills are further optimized when the patient is supported by a strong IBD care team that includes gastroenterologists, IBD nurses, dietitians, and psychologists.
Because of disease chronicity and required shifts in coping and self-management skills over time, it is not surprising that patients with IBD are at increased risk for mental health issues, including depression. A recent systematic review of data published between 2005 and 2014 demonstrated that depression was as high as 21.2% in patients with IBD vs 13.4% in healthy controls. Rates of depression rose to 34.7% when IBD was active (although active disease was not consistently defined across studies), with no notable differences between Crohn’s disease and ulcerative colitis.3 Depression, when comorbid with IBD, can negatively impact self-management and associated disease outcomes, including increased risk for hospital readmission within 90 days,4 increased risk of surgery,5 and unnecessary computed tomography scans and colonoscopies.5
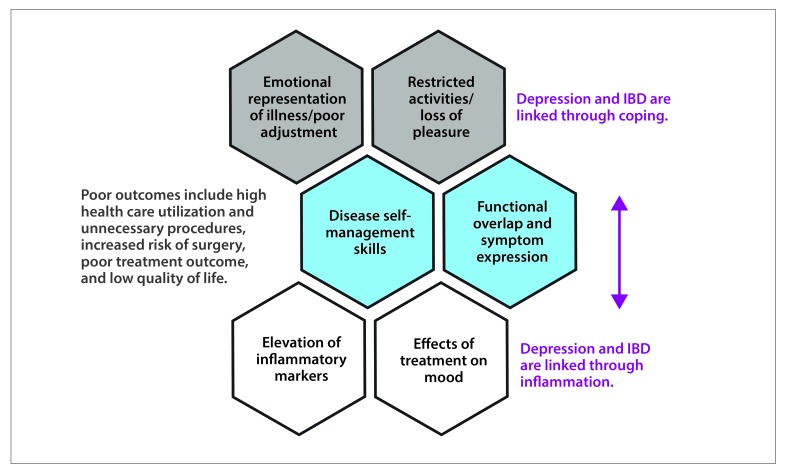
It is not sufficient, however, to view the self-management and coping demands posed by IBD as the sole culprit of depression. Modern conceptualizations of chronic care recognize that the relationship between depression and disease is bidirectional, with (1) poor health leading to poor self-management just as often as poor self-management leads to poor health6 and (2) inflammation driving depression and depression driving inflammation.7 In this article, we explore these bidirectional relationships and make recommendations for the assessment and treatment of depression in the context of IBD (Figure).
Figure.
The bidirectional pathways between depression and inflammatory bowel disease (IBD).
Defining Depression in the Context of IBD
The fifth, and most recent, edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-5) has categorized depression as including 5 or more symptoms over a period of 2 weeks that are distinct from previous functioning and result in impairment in 1 or more areas of a person’s life.8 Although the 2-week time frame is required for diagnostic purposes, in clinical practice, it is much more common for a provider to observe a much longer period of these symptoms, which are fairly pervasive more days than not. One of the 5 symptoms must be either feeling depressed, having a sad or low mood, or anhedonia, which is a loss of interest or pleasure in previously enjoyable activities. The additional 4 or more symptoms can be categorized as somatic, such as weight loss or gain, sleeping too much or too little, psychomotor agitation or slowing, or fatigue or low energy, or can be categorized as cognitive, such as feelings of worthlessness or inappropriate guilt, poor concentration or indecisiveness, or thoughts of death or suicidal ideation. Interestingly, the episode of depression must not be attributable to the physiologic elements of another medication or medical condition, which has implications, especially for active IBD. It is important to point out that depression can be understood as a categorical psychiatric diagnosis (meets criteria vs does not meet criteria), or it can be discussed through a dimensional lens (a threshold of distress or impairment across a range of core symptoms). However, differing definitions of depression make it difficult to draw definitive conclusions regarding its true prevalence and impact on IBD.3
An alternative depressive disorder that was first written about in the DSM-5 is depressive disorder due to another medical condition,8 which is a prominent and persistent period of depressed mood or markedly diminished interest or pleasure in all, or almost all, activities with evidence from patient history, physical examination, or laboratory findings that the disturbance is the direct pathophysiologic consequence of another medical condition. How might an IBD provider determine whether a depressive disorder related to IBD is present? Through a careful multicomponent assessment, the provider could consider (1) whether there is a temporal association between the onset, exacerbation, or remission of the general medical condition and that of the mood disturbance; (2) whether the symptoms are somewhat atypical of traditional depression with respect to the age of onset, course, or family history; or (3) whether depressed mood and its somatic features occur in the absence of other symptoms that typically coexist (eg, feelings of worthlessness or guilt, suicidal ideation). Importantly, because this diagnosis was described only recently in the DSM-5, it is unclear as to whether it will become well validated in clinical practice, particularly with respect to treatment implications. Given the nuances (and time required) to determine the history of depression in a patient with IBD, when possible, an IBD provider should refer the patient for a more complete evaluation by a mental health provider.
One helpful way of thinking about the diagnosis of depression in IBD stems from the work of Szigethy and colleagues, who have been able to identify depression phenotypes from a cohort of 226 depressed pediatric patients with IBD.9 In their study, approximately 75% of the depressed teenagers fit the category of mild depression, with prominent symptoms being fatigue, irritability, and depressed feelings. Another 20% fit the category of somatic depression, with prominent features including anhedonia, change in appetite, fatigue, physical complaints, irritability, and depressed feelings. The third group, which only represented approximately 6% of the sample, fit the category of cognitive despair (its prominent symptom) and also had more traditional depressive symptoms, such as morbid and suicidal ideation, weeping, hopelessness, fatigue, and feelings of sadness. Although these were pediatric patients, it is quite possible that similar categories can be seen in adults.
The Multifactorial Relationship Between Depression and IBD
The relationship between IBD and depression is complex and dynamic with several overlapping pathways. Complicating things further, several somatic symptoms of depression, such as fatigue, difficulty sleeping, and reduced appetite or weight, are also extraintestinal manifestations of IBD and, therefore, may not be that informative in the differential diagnosis. Thus, it is necessary to interpret depression and IBD in the 2 biopsychosocial bidirectional contexts listed below.
Pathway 1: IBD requires a shift in coping. Poor adjustment to IBD increases the risk for depression and negative IBD outcomes.
Behavioral activation theory, one of the most predominant and effective conceptualizations of depression, holds that people become depressed when there is not enough environmental reinforcement (enjoyment of life) or too much environmental punishment (severe illness). Interventions focused on increasing environmental reinforcement (pleasant event scheduling) and reducing punishment (improvement of physical health) are highly effective10 and may be particularly relevant to IBD, which seems to have differential rates of depression based upon disease activity.3 To the extent that IBD symptoms result in a reduction in one’s ability to engage in pleasant activities due to either disease activity or significant changes in one’s lifestyle or coping (restrictions on international travel or enjoyment of food, periods of hospitalization or nutrition support), depression is of increased probability.
Other risk factors for the onset of depression in IBD include the extent to which an individual experiences the diagnosis or change in medical or surgical care as a trauma or sense of loss or the extent to which it affects his or her body image, self-esteem, or perceived disability.11,12 In a small study of patients with IBD, those who had difficulty adapting to disease-related demands (adjustment, also a core self-management task) reported more bowel and systemic symptoms, more pain, less engagement in activities, higher perceived stress, an emotional representation of illness, and higher health care use.13 These findings were supported in a recent review focused on the negative relationship between adjustment and emotion-focused coping.14 In a prospective study of 95 patients with Crohn’s disease, the small set of depressed patients had significantly lower levels of positive reframing and planning and higher levels of denial than their nondepressed counterparts. The depressed patients were also more dependent on others for care and had poorer symptom tolerance,15 suggesting that another way in which depression may affect IBD is through coping and self-care. For example, depression has been associated with reduced adherence to treatment and reduced quality of life,16 and may impact one’s self-management behaviors such as diet, sleep hygiene, smoking, exercise, and maintaining a social support network or even relationships with the treatment team or caregivers.17
Pathway 2: Depression and inflammation are linked.
Another potential pathway through which IBD and depression are related is that depression in and of itself could drive inflammation and subsequent IBD activity. This link has previously been well documented in animal models18,19 and, more recently, in clinical models. Data from the Nurses’ Health Study suggest that depression may increase the risk for the development of Crohn’s disease in women 2-fold.20 There are growing data supporting increased inflammation in depressed individuals compared to healthy controls, with specific elevations in serum levels of interleukin (IL)-1, -6, -12, and tumor necrosis factor-α and possible alterations in IL-12 pathways.7 This seems to be particularly pronounced among those with predominant somatic and vegetative symptoms.7 Elevated levels of C-reactive protein have also been reported in depressed adults21 and can also be used as a marker of depression severity and likeliness of recurrence.22 There is also evidence that depressed individuals are able to activate their immune systems similarly to the immune response seen with an acute infection,7 which may explain the increased propensity to flare in IBD. In a longitudinal study of more than 2000 Swiss patients with IBD, depression was associated with doctor-reported clinical recurrence, with IBD patients who were depressed having a significantly shorter time to a clinical recurrence event, which is a particularly strong finding for Crohn’s disease.23 Similar findings have also been reported previously.24
Treatment of IBD may also impact mood, and mood may affect treatment response. Studies on the psychiatric consequences of corticosteroids indicate that as many as 40% of adults experience mood or anxiety symptoms, with those on high doses or with histories of mood disorders at highest risk.25 In a study of 100 consecutive patients starting infliximab (Remicade, Janssen), patients meeting the criteria for depression according to the Patient Health Questionnaire at baseline had a lower remission rate, and at 4-week follow-up, 88% of these patients needed retreatment.26 Alternatively, treatment with infliximab had a positive impact on depression, potentially by modifying shared inflammatory pathways.27 It is possible that emerging therapeutic paradigms supporting mucosal healing and deep remission28 may have additional benefit on IBD-driven depression.
Another way in which depression could impact response to IBD treatment is through the presentation of functional gastrointestinal disorders, particularly irritable bowel syndrome and centrally mediated abdominal pain syndrome. In a study of 356 patients with IBD, there was a strong association between IBD, depression, and coexisting functional gastrointestinal disorders in the absence of inflammation, suggesting that patient-reported IBD severity may be influenced by depression and pose a risk for overtreatment or poor health care delivery.29,30
In addition to recognizing the multiple ways in which depression and IBD overlap, it is important to consider the impact of treating depression as it relates to IBD outcomes.
Assessment and Treatment of Depression in the Setting of IBD
In a recent review of 79 studies,31 21 different techniques were used to assess depression, with the majority using the Hospital Anxiety and Depression Scale.32 There is some debate as to the cost-effectiveness of screening for depression in primary care33 and higher-risk populations,34 but we still recommend that depression be considered as part of any IBD assessment given its strong, negative impact on outcomes. Depression has been undertreated in this population in the past35 and, if it remains as such, will continue to pose a significant risk to the current health care system.36 Indeed, the recently published guidelines37 for preventive health care in patients with IBD include recommendations regarding the importance of screening for depression.
If depression is identified, mental health interventions, including behavioral therapies and/or medications, can be extremely effective. Behavioral therapies, particularly cognitive behavioral therapy and behavioral activation therapy for depression, are widely accepted and effective.38,39 Trials of cognitive behavioral therapy,40-43 including those adapted specifically to pediatric IBD,44-47 show promise. Online delivery may also be an option for adults with IBD.41 Selective serotonin reuptake inhibitors (SSRIs) have also been widely used in this population with modest effect.48 In one of the only prospective studies,49 depressed patients with Crohn’s disease undergoing 6 months of a range of antidepressant drug treatments (SSRI, serotonin-norepinephrine reuptake inhibitor [SNRI], or a combination of the two) saw improvement in depression, anxiety, quality of life, and sexual functioning scores, as well as an improvement in Crohn’s Disease Activity Index score. In another retrospective study, usage of antidepressants (most commonly citalopram and fluoxetine) to treat concomitant depression in patients with IBD was found to be associated with a decrease in relapse rates, corticosteroid use, and number of endoscopies.50
Conclusion
Depression has been documented in IBD and has a significant impact on disease outcomes, including quality of life, health care use, and efficacy of treatment. The relationship between IBD and depression is likely bidirectional. We recommend that depression be assessed as part of routine care in IBD and that IBD specialists do the following to ensure that the patient’s needs are adequately addressed: (1) spend time on a patient-friendly conversation starter explaining the link between depression and IBD in order to reduce stigma and increase buy-in for treatment if it becomes necessary (Table); (2) identify several local mental health providers to refer patients to when needed and make sure that these providers know that the IBD provider would like to be kept informed of their care plan; and (3) become familiar with 1 or 2 reliable SSRIs/SNRIs that can be used as a backup if mental health care is unavailable.
Table.
Sample Conversation Starters to Use With Patients With Inflammatory Bowel Disease (IBD)
| Explaining the Link Between IBD and Depression |
| We do not fully understand the relationship between IBD and depression, but we know that depression affects between 20% and 30% of individuals with IBD and that the rate of depression may be even higher when disease is active. Experts in this area suspect that the reason that depression and IBD are so intertwined has something to do with both depression and IBD sharing several biologic and inflammatory pathways. Another common reason people with IBD become depressed is that they have to stop doing activities they used to enjoy or were good at because of their illness. For most of my patients, it is a combination of these factors that leads them to feel depressed or have a low mood. Do you feel that any of these things apply to you and your IBD right now? |
| Encouraging Patient Referral |
| You have been going through so much recently with your health, and part of my job is to make sure that you are emotionally managing everything. So many of my patients like you become fatigued, depressed, and down as a result of their IBD and can sometimes benefit from short-term counseling or even medication to figure out how they can move forward in light of everything that has happened. Would you like me to make a referral to see someone in our center who specializes in this? |
References
- 1.Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. 2010;78(3):297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 3.Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22(3):752–762. doi: 10.1097/MIB.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 4.Allegretti JR, Borges L, Lucci M, et al. Risk factors for rehospitalization within 90 days in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(11):2583–2589. doi: 10.1097/MIB.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Gainer VS, Perez RG, et al. Psychiatric co-morbidity is associated with increased risk of surgery in Crohn’s disease. Aliment Pharmacol Ther. 2013;37(4):445–454. doi: 10.1111/apt.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Subero M, Anderson G, Kanchanatawan B, Berk M, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immuneinflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gutbrain pathways. CNS Spectr. 2016;21(2):184–198. doi: 10.1017/S1092852915000449. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington DC: 2013. [Google Scholar]
- 9.Szigethy EM, Youk AO, Benhayon D, et al. Depression subtypes in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58(5):574–581. doi: 10.1097/MPG.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soucy Chartier I, Provencher MD. Behavioural activation for depression: efficacy, effectiveness and dissemination. J Affect Disord. 2013;145(3):292–299. doi: 10.1016/j.jad.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 11.van der Have M, Fidder HH, Leenders M, et al. COIN study group; Dutch Initiative on Crohn and Colitis. Self-reported disability in patients with inflammatory bowel disease largely determined by disease activity and illness perceptions. Inflamm Bowel Dis. 2015;21(2):369–377. doi: 10.1097/MIB.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 12.McDermott E, Mullen G, Moloney J, et al. Body image dissatisfaction: clinical features, and psychosocial disability in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(2):353–360. doi: 10.1097/MIB.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 13.Kiebles JL, Doerfler B, Keefer L. Preliminary evidence supporting a framework of psychological adjustment to inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(10):1685–1695. doi: 10.1002/ibd.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan C, Sin J, Fear NT, Chalder T. A systematic review of the psychological correlates of adjustment outcomes in adults with inflammatory bowel disease. Clin Psychol Rev. 2016;47:28–40. doi: 10.1016/j.cpr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Vigano C, Calzolari R, Marinaccio PM, et al. Unrevealed depression involves dysfunctional coping strategies in Crohn’s disease patients in clinical remission. Gastroenterol Res Pract. 2016;2016:7803262. doi: 10.1155/2016/7803262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Sanromán A, Bermejo F. Review article: how to control and improve adherence to therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(3):45–49. doi: 10.1111/j.1365-2036.2006.03060.x. [DOI] [PubMed] [Google Scholar]
- 17.Trivedi MH, Lin EH, Katon WJ. Consensus recommendations for improving adherence, self-management, and outcomes in patients with depression. CNS Spectr. 2007;12(8) 13:1–27. [PubMed] [Google Scholar]
- 18.Ghia JE, Blennerhassett P, Deng Y, Verdu EF, Khan WI, Collins SM. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136(7):2280–2288. doi: 10.1053/j.gastro.2009.02.069. e1-e4. [DOI] [PubMed] [Google Scholar]
- 19.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118(6):2209–2218. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Khalili H, Pan A, et al. Association between depressive symptoms and incidence of Crohn’s disease and ulcerative colitis: results from the Nurses’ Health Study. Clin Gastroenterol Hepatol. 2013;11(1):57–62. doi: 10.1016/j.cgh.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien SM, Scott LV, Dinan TG. Antidepressant therapy and C-reactive protein levels. Br J Psychiatry. 2006;188:449–452. doi: 10.1192/bjp.bp.105.011015. [DOI] [PubMed] [Google Scholar]
- 22.Liukkonen T, Silvennoinen-Kassinen S, Jokelainen J, et al. The association between C-reactive protein levels and depression: results from the northern Finland 1966 birth cohort study. Biol Psychiatry. 2006;60(8):825–830. doi: 10.1016/j.biopsych.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Mikocka-Walus A, Pittet V, Rossel JB, von Känel R Swiss IBD Cohort Study Group. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14(6):829–835.e1. doi: 10.1016/j.cgh.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 24.Mittermaier C, Dejaco C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. 2004;66(1):79–84. doi: 10.1097/01.psy.0000106907.24881.f2. [DOI] [PubMed] [Google Scholar]
- 25.Ciriaco M, Ventrice P, Russo G, et al. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother. 2013;4(1):S94–S98. doi: 10.4103/0976-500X.120975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persoons P, Vermeire S, Demyttenaere K, et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22(2):101–110. doi: 10.1111/j.1365-2036.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein GR, Bala M, Han C, DeWoody K, Schaible T. Infliximab improves quality of life in patients with Crohn’s disease. Inflamm Bowel Dis. 2002;8(4):237–243. doi: 10.1097/00054725-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Pineton de Chambrun G, Blanc P, Peyrin-Biroulet L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2016;10(8):915–927. doi: 10.1586/17474124.2016.1174064. [DOI] [PubMed] [Google Scholar]
- 29.Gracie DJ, Ford AC. Psychological comorbidity and inflammatory bowel disease activity: cause or effect? Clin Gastroenterol Hepatol. 2016;14(7):1061–1062. doi: 10.1016/j.cgh.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Gracie DJ, Williams CJ, Sood R, et al. Poor correlation between clinical disease activity and mucosal inflammation, and the role of psychological comorbidity, in inflammatory bowel disease. Am J Gastroenterol. 2016;111(4):541–551. doi: 10.1038/ajg.2016.59. [DOI] [PubMed] [Google Scholar]
- 31.Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res. 2016;87:70–80. doi: 10.1016/j.jpsychores.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 33.Thombs BD, Ziegelstein RC. Does depression screening improve depression outcomes in primary care? BMJ. 2014;348:g1253. doi: 10.1136/bmj.g1253. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg D. The value of screening in patient populations with high prevalence of a disorder. BMC Med. 2014;12:14. doi: 10.1186/1741-7015-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennebroek Evertsz’ F, Thijssens NA, Stokkers PC, et al. Do inflammatory bowel disease patients with anxiety and depressive symptoms receive the care they need? J Crohns Colitis. 2012;6(1):68–76. doi: 10.1016/j.crohns.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Angstman KB, Rasmussen NH, Herman DC, Sobolik JJ. Depression care management: impact of implementation on health system costs. Health Care Manag (Frederick) 2011;30(2):156–160. doi: 10.1097/HCM.0b013e318216f8e5. [DOI] [PubMed] [Google Scholar]
- 37.Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG Clinical Guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112(2):241–258. doi: 10.1038/ajg.2016.537. [DOI] [PubMed] [Google Scholar]
- 38.Richards DA, Ekers D, McMillan D, et al. Cost and outcome of behavioural activation versus cognitive behavioural therapy for depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet. 2016;388(10047):871–880. doi: 10.1016/S0140-6736(16)31140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiles NJ, Thomas L, Turner N, et al. Long-term effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: follow-up of the CoBalT randomised controlled trial. Lancet Psychiatry. 2016;3(2):137–144. doi: 10.1016/S2215-0366(15)00495-2. [DOI] [PubMed] [Google Scholar]
- 40.Knowles SR, Monshat K, Castle DJ. The efficacy and methodological challenges of psychotherapy for adults with inflammatory bowel disease: a review. Inflamm Bowel Dis. 2013;19(12):2704–2715. doi: 10.1097/MIB.0b013e318296ae5a. [DOI] [PubMed] [Google Scholar]
- 41.Mikocka-Walus A, Bampton P, Hetzel D, Hughes P, Esterman A, Andrews JM. Cognitive-behavioural therapy for inflammatory bowel disease: 24-month data from a randomised controlled trial. Int J Behav Med. 2017;24(1):127–135. doi: 10.1007/s12529-016-9580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Brink G, Stapersma L, El Marroun H, et al. Effectiveness of disease-specific cognitive-behavioural therapy on depression, anxiety, quality of life and the clinical course of disease in adolescents with inflammatory bowel disease: study protocol of a multicentre randomised controlled trial (HAPPY-IBD) BMJ Open Gastroenterol. 2016;3(1):e000071. doi: 10.1136/bmjgast-2015-000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennebroek Evertsz’ F, Bockting CL, Stokkers PC, Hinnen C, Sanderman R, Sprangers MA. The effectiveness of cognitive behavioral therapy on the quality of life of patients with inflammatory bowel disease: multi-center design and study protocol (KL!C- study) BMC Psychiatry. 2012;12:227. doi: 10.1186/1471-244X-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szigethy E, Whitton SW, Levy-Warren A, DeMaso DR, Weisz J, Beardslee WR. Cognitive-behavioral therapy for depression in adolescents with inflammatory bowel disease: a pilot study. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1469–1477. doi: 10.1097/01.chi.0000142284.10574.1f. [DOI] [PubMed] [Google Scholar]
- 45.Szigethy E, Kenney E, Carpenter J, et al. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1290–1298. doi: 10.1097/chi.0b013e3180f6341f. [DOI] [PubMed] [Google Scholar]
- 46.Szigethy E, Bujoreanu SI, Youk AO, et al. Randomized efficacy trial of two psychotherapies for depression in youth with inflammatory bowel disease. J Am Acad Child Adolesc Psychiatry. 2014;53(7):726–735. doi: 10.1016/j.jaac.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szigethy E, Youk AO, Gonzalez-Heydrich J, et al. Effect of 2 psychotherapies on depression and disease activity in pediatric Crohn’s disease. Inflamm Bowel Dis. 2015;21(6):1321–1328. doi: 10.1097/MIB.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis. 2016;22(6):1509–1522. doi: 10.1097/MIB.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 49.Yanartas O, Kani HT, Bicakci E, et al. The effects of psychiatric treatment on depression, anxiety, quality of life, and sexual dysfunction in patients with inflammatory bowel disease. Neuropsychiatr Dis Treat. 2016;12:673–683. doi: 10.2147/NDT.S106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodhand JR, Greig FI, Koodun Y, et al. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm Bowel Dis. 2012;18(7):1232–1239. doi: 10.1002/ibd.21846. [DOI] [PubMed] [Google Scholar]