A 42-year-old woman with a history of type 2 diabetes, hypertension, dyslipidemia, depression, and anxiety was admitted to the hospital with exacerbation of abdominal pain, nausea, and vomiting of 1 year’s duration. She had initially developed abdominal pain, nausea, and vomiting 15 months earlier and had presented to another hospital. A diagnosis of biliary colic was made, and she underwent cholecystectomy. Her symptoms persisted postoperatively, and over the following 6 months, extensive evaluation was undertaken, including an upper endoscopy, which showed mild esophagitis, and a solid-phase gastric emptying study, which showed markedly delayed gastric emptying, with 84% retention at 3 hours (normal, <30%). A gastrostomy tube was placed for decompression when the patient did not respond to conservative measures but was subsequently removed due to infectious complications. She was ultimately managed with erythromycin and metoclopramide without symptomatic relief. Her course was complicated by recurrent hospital visits for uncontrolled pain, nausea, and vomiting, with an abdominal computed tomography (CT) scan showing no acute abdominal pathology. The patient was given multiple clinical diagnoses, including irritable bowel syndrome, functional abdominal pain, refractory gastroparesis, and narcotic-seeking behavior, and she was discharged from various emergency rooms and hospitals without symptom resolution.
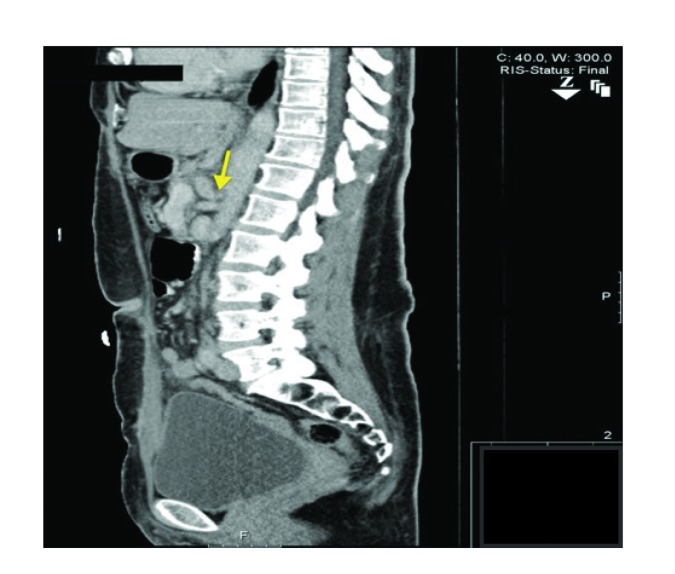
One year later, she was admitted to our institution with intractable nausea and vomiting associated with severe epigastric pain and a 30-lb weight loss over the preceding 6 months. The pain was continuous but was significantly worsened by oral intake. A repeat CT abdomen/pelvis scan revealed no acute intra-abdominal pathology. Despite 3 days of supportive care, her symptoms did not improve. In addition, she was notably hypertensive throughout the hospital stay despite multiple antihypertensive medications. This prompted a renal artery duplex ultrasound to evaluate for renal artery stenosis as a secondary cause of hypertension. No renal artery stenosis was found; however, a high-grade stenosis of celiac artery origin with a velocity of 575 cm/s was noted incidentally. The vascular surgery department was consulted, and her CT images were reviewed again. Sagittal sections through the celiac axis showed upsloping of the celiac artery with associated stenosis at the celiac origin and poststenotic dilation (Figure). The patient also underwent CT angiography; high-grade extrinsic stenosis of the celiac artery was noted with poststenotic dilation as well as worsening of the stenosis with upsloping of the artery during expiration. These findings were thought to be consistent with median arcuate ligament syndrome, and the patient underwent laparoscopic release of the ligament and then celiac ganglionectomy. An intraoperative ultrasound revealed strong flow in the celiac trunk after ligamentous release. The patient’s symptoms improved markedly and remained improved 1 month after discharge.
Figure.
Computed tomography scan showing a sagittal section through the celiac axis. Pictured is upsloping of the celiac artery with associated stenosis at the level of the celiac vessel origin and poststenotic dilation.
Discussion
Celiac axis compression syndrome (CACS), also known as median arcuate ligament syndrome,1 is caused by compression of the celiac artery and associated ganglion by the median arcuate ligament. The syndrome is characterized by the triad of postprandial abdominal pain, weight loss, and occasionally an epigastric abdominal bruit that worsens with expiration. CACS commonly manifests in younger, thin women between the ages of 20 and 40 years. It is usually a diagnosis of exclusion after ruling out more common causes of postprandial abdominal pain and weight loss, including peptic ulcer disease, gastroparesis, upper gastrointestinal malignancy, and chronic pancreatitis.
The presentation of this syndrome varies, with epigastric or upper abdomen pain frequently being the chief presenting complaint. This pain may worsen with meals or exercise, and associated features include nausea, vomiting, bloating, and diarrhea. Weight loss is common secondary to food avoidance, with fear from pain triggered by eating. Epigastric tenderness and abdominal bruit may be found on examination.
Pathogenesis
The median arcuate ligament arises from the base of the diaphragm where the right and left crura meet near the T12 vertebra, forming a fibrous arch uniting them. The ligament forms the anterior aspect of the opening of the diaphragm through which the aorta, thoracic duct, and azygos vein pass. At this vertebral level, the celiac artery plexus branches from the aorta, and the ligament usually passes just above the branch point. A variant is seen in up to 25% of patients with CACS in whom the median arcuate ligament passes inferiorly, compressing the celiac artery and adjacent structures such as the celiac ganglion.2 However, fewer patients experience severe debilitating compression symptoms; others remain asymptomatic from collateral supply from the superior mesenteric vessels.2,3 With inspiration or erect posture, the celiac artery could descend into the abdominal cavity, relieving compression and symptoms. On the other hand, symptoms worsen with expiration. Repeated compression of the celiac artery leads to intimal hyperplasia, elastic lamina proliferation, and adventitial reorganization.4 These effects have been noted on histologic examinations and might explain poor resolution of symptoms in spite of operative release, and they sometimes require further interventions, such as angioplasty and splanchnic revascularization.
Currently, multiple theories have been used to explain the pain, including increased blood demand through the compressed artery leading to foregut ischemia and pain. Typically, at least 2 mesenteric vessels need to be occluded or narrowed to cause chronic mesenteric symptoms; however, in CACS, the superior and inferior mesenteric arteries are widely patent.5 Another possible theory includes midgut ischemia from a steal syndrome, with the superior mesenteric artery blood diverting through collateral vessels to supply the celiac artery distribution. Other than vascular impingement, splanchnic neural plexus compression and delayed gastric emptying have also been postulated to cause these symptoms.6,7 The presence of the ligament impingement in asymptomatic patients makes the cause of pain puzzling.
Evaluation
Common diagnostic modalities include duplex ultrasound with velocity measurement, CT angiogram, and magnetic resonance angiography. Studies have shown celiac artery compression by the median arcuate ligament during expiration following stenotic dilation.3,8 Lateral aortic angiogram has traditionally been the gold standard for the diagnosis of CACS.
Newer imaging, such as thin-section multidetector CT scanners along with 3-dimensional reconstruction software, has negated the need for conventional angiography to diagnose CACS. CT angiogram also helps identify the relationship of the celiac artery with the diaphragm, and the compressed artery can be visualized from various angles.3,9 Sagittal plane images show characteristic focal narrowing with a hooked appearance, which can help distinguish it from other causes, such as atherosclerotic disease. If the narrowing is noted during the inspiratory phase of CT examination, it can be considered diagnostic, as expiratory phase celiac artery compression may be subtle and missed. Narrowing of the collateral vessel with poststenotic dilation would support the diagnosis.3,10
Recently, ultrasound examination of the celiac trunk has been found to be sensitive in detecting median arcuate ligament syndrome in select patients. Maximum expiratory peak velocity over 350 cm/s with a deflection angle greater than 50 degrees was found to be a reliable indicator in a series of 364 patients.11 In patients with a high probability of the condition, functional ultrasound imaging can be used as a screening test. Gastric exercise tonometry can be used to diagnose CACS by measuring intraluminal gastric carbon dioxide levels. This technique has been shown to yield results similar to those of angiography for diagnosis and has potential for follow-up purposes postsurgery.12,13
Management
The main objectives in the management of CACS are restoration of normal celiac axis blood flow and elimination of the neural irritation by the celiac ganglion.14 Different surgical techniques and approaches have been reported in the literature, but open laparotomy through an upper abdominal incision, open division of the median arcuate ligament, and resection of periarterial neural tissue have been the most commonly reported treatments.15-17 Celiac artery release alone has been shown to be effective. In the largest surgical series,15 51 patients had a mean follow-up of 9 years. Sixty-five percent of the patients reported overall improvement in symptoms, with atypical pain patterns, a history of psychiatric disease or alcohol abuse, and elderly age being associated with poorer outcomes.
Other surgical techniques include decompression and celiac ganglion neurolysis, decompression and celiac artery dilation, decompression and celiac artery reconstruction, and celiac artery endovascular stenting.18 To relieve compression, the laparoscopic approach had more successful resolution of symptoms than open surgery methods due to better visualization of the aorta and complete division of the median arcuate ligament fibers.19 Additionally, intraoperative laparoscopic Doppler ultrasound could help determine adequacy of the dissection by monitoring real-time arterial flow characteristics.19,20 Other benefits of the laparoscopic technique include faster recovery, early return to diet, and early discharge. Other vascular procedures, including endovascular stenting and open celiac axis reconstruction, may be required for improvement of symptoms.21,22 Recently, a hybrid approach of laparoscopic median arcuate ligament release followed by endovascular stenting of the celiac artery has produced resolution of symptoms.23
References
- 1.Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 1965;95(3):731–744. doi: 10.2214/ajr.95.3.731. [DOI] [PubMed] [Google Scholar]
- 2.Lindner HH, Kemprud E. A clinicoanatomical study of the arcuate ligament of the diaphragm. Arch Surg. 1971;103(5):600–605. doi: 10.1001/archsurg.1971.01350110102016. [DOI] [PubMed] [Google Scholar]
- 3.Horton KM, Talamini MA, Fishman EK. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005;25(5):1177–1182. doi: 10.1148/rg.255055001. [DOI] [PubMed] [Google Scholar]
- 4.Bech F, Loesberg A, Rosenblum J, Glagov S, Gewertz BL. Median arcuate ligament compression syndrome in monozygotic twins. J Vasc Surg. 1994;19(5):934–938. doi: 10.1016/s0741-5214(94)70021-4. [DOI] [PubMed] [Google Scholar]
- 5.Ghosn PB, Rabbat AG, Trudel J, D’Amico P, Lecours R, Trudel J. Celiac compression syndrome. Can J Surg. 1982;25(4):377–379. [PubMed] [Google Scholar]
- 6.Skeik N, Cooper LT, Duncan AA, Jabr FI. Median arcuate ligament syndrome: a nonvascular, vascular diagnosis. Vasc Endovascular Surg. 2011;45(5):433–437. doi: 10.1177/1538574411406453. [DOI] [PubMed] [Google Scholar]
- 7.Balaban DH, Chen J, Lin Z, Tribble CG, McCallum RW. Median arcuate ligament syndrome: a possible cause of idiopathic gastroparesis. Am J Gastroenterol. 1997;92(3):519–523. [PubMed] [Google Scholar]
- 8.Wolfman D, Bluth EI, Sossaman J. Median arcuate ligament syndrome. J Ultrasound Med. 2003;22(12):1377–1380. doi: 10.7863/jum.2003.22.12.1377. [DOI] [PubMed] [Google Scholar]
- 9.Kopecky KK, Stine SB, Dalsing MC, Gottlieb K. Median arcuate ligament syndrome with multivessel involvement: diagnosis with spiral CT angiography. Abdom Imaging. 1997;22(3):318–320. doi: 10.1007/s002619900199. [DOI] [PubMed] [Google Scholar]
- 10.Patten RM, Coldwell DM, Ben-Menachem Y. Ligamentous compression of the celiac axis: CT findings in five patients. AJR Am J Roentgenol. 1991;156(5):1101–1103. doi: 10.2214/ajr.156.5.2017934. [DOI] [PubMed] [Google Scholar]
- 11.Gruber H, Loizides A, Peer S, Gruber I. Ultrasound of the median arcuate ligament syndrome: a new approach to diagnosis. Med Ultrason. 2012;14(1):5–9. [PubMed] [Google Scholar]
- 12.Mensink PB, van Petersen AS, Kolkman JJ, Otte JA, Huisman AB, Geelkerken RH. Gastric exercise tonometry: the key investigation in patients with suspected celiac artery compression syndrome. J Vasc Surg. 2006;44(2):277–281. doi: 10.1016/j.jvs.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Faries PL, Narula A, Veith FJ, Pomposelli FB, Jr, Marsan BU, LoGerfo FW. The use of gastric tonometry in the assessment of celiac artery compression syndrome. Ann Vasc Surg. 2000;14(1):20–23. doi: 10.1007/s100169910004. [DOI] [PubMed] [Google Scholar]
- 14.Duffy AJ, Panait L, Eisenberg D, Bell RL, Roberts KE, Sumpio B. Management of median arcuate ligament syndrome: a new paradigm. Ann Vasc Surg. 2009;23(6):778–784. doi: 10.1016/j.avsg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Reilly LM, Ammar AD, Stoney RJ, Ehrenfeld WK. Late results following operative repair for celiac artery compression syndrome. J Vasc Surg. 1985;2(1):79–91. [PubMed] [Google Scholar]
- 16.Williams S, Gillespie P, Little JM. Celiac axis compression syndrome: factors predicting a favorable outcome. Surgery. 1985;98(5):879–887. [PubMed] [Google Scholar]
- 17.Tulloch AW, Jimenez JC, Lawrence PF, et al. Laparoscopic versus open celiac ganglionectomy in patients with median arcuate ligament syndrome. J Vasc Surg. 2010;52(5):1283–1289. doi: 10.1016/j.jvs.2010.05.083. [DOI] [PubMed] [Google Scholar]
- 18.Kohn GP, Bitar RS, Farber MA, Marston WA, Overby DW, Farrell TM. Treatment options and outcomes for celiac artery compression syndrome. Surg Innov. 2011;18(4):338–343. doi: 10.1177/1553350610397383. [DOI] [PubMed] [Google Scholar]
- 19.Carbonell AM, Kercher KW, Heniford BT, Matthews BD. Multimedia article. Laparoscopic management of median arcuate ligament syndrome. Surg Endosc. 2005;19(5):729. doi: 10.1007/s00464-004-6010-x. [DOI] [PubMed] [Google Scholar]
- 20.Roayaie S, Jossart G, Gitlitz D, Lamparello P, Hollier L, Gagner M. Laparoscopic release of celiac artery compression syndrome facilitated by laparoscopic ultrasound scanning to confirm restoration of flow. J Vasc Surg. 2000;32(4):814–817. doi: 10.1067/mva.2000.107574. [DOI] [PubMed] [Google Scholar]
- 21.Grotemeyer D, Duran M, Iskandar F, Blondin D, Nguyen K, Sandmann W. Median arcuate ligament syndrome: vascular surgical therapy and follow-up of 18 patients. Langenbecks Arch Surg. 2009;394(6):1085–1092. doi: 10.1007/s00423-009-0509-5. [DOI] [PubMed] [Google Scholar]
- 22.Delis KT, Gloviczki P, Altuwaijri M, McKusick MA. Median arcuate ligament syndrome: open celiac artery reconstruction and ligament division after endovascular failure. J Vasc Surg. 2007;46(4):799–802. doi: 10.1016/j.jvs.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 23.Palmer OP, Tedesco M, Casey K, Lee JT, Poultsides GA. Hybrid treatment of celiac artery compression (median arcuate ligament) syndrome. Dig Dis Sci. 2012;57(7):1782–1785. doi: 10.1007/s10620-011-2019-x. [DOI] [PubMed] [Google Scholar]