Abstract
How nuclear morphology is regulated during development and disease remains poorly understood. In this issue of Developmental Cell, using a pronuclear assembly assay, Xue et al. (2013) demonstrate that Dppa2, a chromatin-bound microtubule regulator, controls both the morphology and function of the pronucleus by fine-tuning microtubule dynamics.
The shape and size of eukaryotic nuclei vary widely among different cell types and during development. Extensive studies have shown a strong connection between nuclear morphology and genome organization in the context of development and disease (Zink et al., 2004), yet how a nucleus attains and maintains its size and shape and how the size and shape influence nuclear function are poorly understood. In this issue of Developmental Cell, Xue et al. (2013) have used nuclear size and shape as readouts to discover the role of micro-tubules in sperm pronuclear formation and function in Xenopus egg extracts.
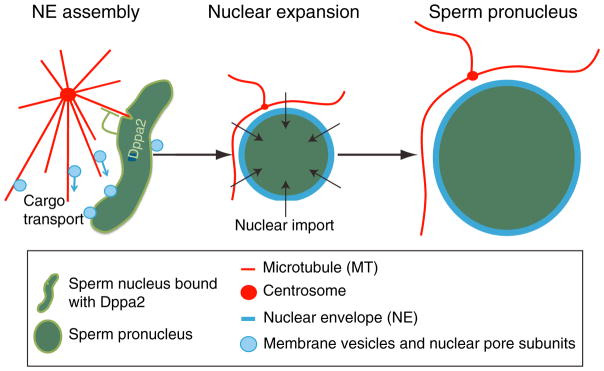
It is well established that during cell division, chromatin provides signals to promote spindle assembly and chromosome alignment (Carmena et al., 2012; Clarke and Zhang, 2008). At the end of mitosis, chromosomal signals also induce the assembly of the nuclear envelope (NE) (Wandke and Kutay, 2013). Considering that microtubules can transport nuclear proteins and NE membranes, regulated microtubule assembly near the decondensing chromatin during postmitotic nuclear assembly could be important for nuclear assembly. To gain insights, Xue and colleagues (2013) took advantage of a robust nuclear assembly assay using Xenopus sperm. Upon addition into interphase Xenopus egg extracts, the highly condensed sperm chromatin undergoes decondensation, and the decondensing chromatin recruits membrane vesicles that fuse to form the NE with the associated nuclear pores. Further import of nuclear proteins leads to the expansion of the pronucleus that is competent to undergo DNA replication (Figure 1). Among the novel chromatin-bound proteins that the authors identified using mass spectrometry analyses of the pronuclear-associated proteins, they focused on Dppa2, which is known to be essential for Xenopus embryogenesis (Siegel et al., 2009).
Figure 1. Dppa2 Regulates the Shape and Size of Sperm Pronuclei by Fine-Tuning the Dynamics of Microtubules.
During the early stage of pronuclear assembly, Dppa2 dampens microtubule assembly, which could prevent the tearing of the nascent nuclear structures.
By depleting Dppa2 in Xenopus egg extracts, Xue et al. found that compared to controls, the sperm pronuclei had a distorted shape, smaller size, and excessive assembly of microtubules near the chromatin during the early phase of pronuclear formation. Addition of purified Dppa2 rescued the defects in pronuclear and microtubule assembly, demonstrating the involvement of this chromatin-bound protein in both processes. Strikingly, the pronuclear assembly defects were also rescued by either codepleting the chromosome passenger complex (CPC), which is known to enhance microtubule assembly (Carmena et al., 2012), or adding nocodazole to inhibit microtubule polymerization. Only a low nocodazole dose, which dampens but does not completely prevent microtubule assembly when added during early pronuclear assembly, can rescue nuclear assembly upon Dppa2 depletion as judged by the nuclear size, shape, and DNA replication. Based on this result, Dppa2 appears to regulate nuclear assembly by fine-tuning microtubule polymerization near the chromatin (Figure 1). Consistently, both the DNA-binding and microtubule regulation activities of Dppa2 are required for the assembly of pronuclei from the sperm in Xenopus egg extracts. These findings establish an important role for microtubules in regulating pronuclear assembly in vitro.
Like any pioneering studies, the findings presented by Xue et al. raise a number of important and exciting questions for further investigation. One critical issue is whether Dppa2, which appears to be expressed only during early embryogenesis (Siegel et al., 2009), is indeed required for the assembly of sperm pronuclei and early embryonic nuclei in vivo. Although many important cell biological functions defined initially in Xenopus egg extract have been shown to also occur in vivo, the lack of proper cell and tissue contexts may render some egg extract-based assays less representative of the in vivo processes. For example, recent lamin-knockout studies in embryonic stem cells and mice (Kim et al., 2012, 2013) have shown that the role of nuclear lamins in controlling nuclear size, as implicated by egg extract-based assays (Newport et al., 1990; Levy and Heald, 2010), may not be broadly applicable in vivo. Morpholino-based protein reduction in fertilized Xenopus eggs should help to answer whether Dppa2 is indeed required for pronuclear and postmitotic nuclear assembly during early embryogenesis. Considering that Xue and colleagues have identified many chromatin-bound proteins in Xenopus egg extracts, further study of these proteins using the pronuclear assembly assay, in conjunction with in vivo validation, should allow the discovery of additional regulators of nuclear assembly.
Another interesting and unanswered question is how Dppa2 reduces microtubule assembly near the sperm chromatin. Since Dppa2 is not detected on microtubules assembled around the sperm chromatin, it may regulate microtubule assembly indirectly. However, purified Dppa2 is able to inhibit microtubule assembly from purified tubulin, which seems to suggest a direct role. Nonetheless, by identifying Dppa2 as a chromatin-bound microtubule regulator, Xue et al. have opened the door to further dissecting how the microtubule cytoskeleton regulates the assembly of NE, nuclear lamina, and nuclear pore complexes around the chromatin. As the authors have suggested, the fine-tuning of microtubule assembly dynamics near chromatin may allow microtubules to efficiently transport NE, nuclear lamina proteins, and nuclear pore subunits to the forming nuclei while preventing the microtubule-based forces from tearing and rupturing the nascent nuclei (Figure 1).
One of the exciting questions of eukaryotic biology is how genome organization is established in differentiated cells. Repeated cell division should offer progenitor cells the opportunity to sculpt and fold chromatin in 3D so that the terminally differentiated cells can attain a unique genome organization to support lineage-specific gene expression. From this perspective, the discovery of Dppa2 should provide the field a strong impetus to further study whether and how by binding to specific regions of the genome, proteins like Dppa2 may function along with the cytoskeleton, nuclear pores, and NE to regulate the epigenetic landscape and mold the genome within the nucleus during differentiation and tissue building.
Acknowledgments
The work in the authors’ laboratory is supported by the Ellison Medical Foundation, NIH R01-GM056312, and R01-GM106023.
References
- Carmena M, Wheelock M, Funabiki H, Earnshaw WC. Nat Rev Mol Cell Biol. 2012;13:789–803. doi: 10.1038/nrm3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PR, Zhang C. Nat Rev Mol Cell Biol. 2008;9:464–477. doi: 10.1038/nrm2410. [DOI] [PubMed] [Google Scholar]
- Kim Y, McDole K, Zheng Y. Nucleus. 2012;3:256–262. doi: 10.4161/nucl.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Zheng X, Zheng Y. Cell Res. 2013 doi: 10.1038/cr.2013.118. Published online August 27, 2013 http://dx.doi.org/10.1038/cr.2013.118. [DOI] [PMC free article] [PubMed]
- Levy DL, Heald R. Cell. 2010;143:288–298. doi: 10.1016/j.cell.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport JW, Wilson KL, Dunphy WG. J Cell Biol. 1990;111:2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D, Schuff M, Oswald F, Cao Y, Knöchel W. Mech Dev. 2009;126:974–989. doi: 10.1016/j.mod.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Wandke C, Kutay U. Cell. 2013;152:1222– 1225. doi: 10.1016/j.cell.2013.02.046. [DOI] [PubMed] [Google Scholar]
- Xue JZ, Woo EM, Postow L, Chait BT, Funabiki H. Dev Cell. 2013;27:47–59. doi: 10.1016/j.devcel.2013.08.002. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D, Fischer AH, Nickerson JA. Nat Rev Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]