Abstract
Purpose
Oxaliplatin and paclitaxel are commonly used chemotherapies associated with acute and chronic neuropathies. There is a need to better understand the similarities and differences of these clinical syndromes.
Methods
Neuropathy data were pooled from patients receiving adjuvant oxaliplatin and weekly paclitaxel or every 3 weeks of paclitaxel. Patients completed daily questionnaires after each chemotherapy dose and the European Organization for Research and Treatment of Cancer quality-of-life questionnaire for patients with chemotherapy-induced peripheral neuropathy before each chemotherapy cycle and for 12 months post-treatment.
Results
Acute neuropathy symptoms from both drugs peaked around day 3. Acute symptoms experienced in cycle 1 predicted occurrence in subsequent cycles. Paclitaxel-induced acute symptoms were similar in intensity in each cycle and largely resolved between cycles. Oxaliplatin-induced acute symptoms were about half as severe in the first cycle as in later cycles and did not resolve completely between cycles. Both drugs caused a predominantly sensory chronic neuropathy (with numbness and tingling being more common than pain). Oxaliplatin-induced neuropathy worsened after the completion of treatment and began to improve 3 months post-treatment. In contrast, paclitaxel-induced neuropathy began improving immediately after chemotherapy cessation. During treatment, the incidence of paclitaxel sensory symptoms was similar in the hands and feet; with oxaliplatin, the hands were affected more than the feet. Both paclitaxel- and oxaliplatin-induced acute neurotoxicity appeared to predict the severity of chronic neuropathy, more prominently with oxaliplatin.
Conclusions
Knowledge of the similarities and differences between neuropathy syndromes may provide insight into their underlying pathophysiology and inform future research to identify preventative treatment approaches.
Keywords: Chemotherapy-induced peripheral neuropathy, Oxaliplatin neuropathy, Paclitaxel neuropathy
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and distressing symptom that lacks many effective preventative or treatment options. Although studies have investigated CIPN characteristics and patterns, few describe agent-specific variations in CIPN signs, symptoms, and recovery patterns. In addition, the lack of a gold-standard CIPN measurement tool leads to difficulties conducting comparisons of neuropathy syndromes. Recent publications regarding the natural history of two commonly used neurotoxic chemotherapy agents, oxaliplatin [1] and paclitaxel [2, 3], used an identical tool for measuring chronic neuropathy, allowing for a comparison of the neuropathies caused by these two drugs.
It has long been known that oxaliplatin is associated with two distinct forms of neurotoxicity. The first is an acute neuropathy characterized by altered sensitivity to touching cold items, sensitivity to swallowing cold items, throat discomfort, and muscle cramps, which has been reported to be reversible [4]. The second is a chronic sensory-predominant neurotoxicity that can last for months after treatment discontinuation [5–8]. Paclitaxel is associated with an acute syndrome characterized by aching pain, which has commonly been referred to as paclitaxel-induced arthralgias and myalgias. However, it has been proposed that this syndrome is more likely a form of acute neurotoxicity, as opposed to injury to joints and/or muscles; this has been referred to as the paclitaxel-associated acute pain syndrome (P-APS) [2, 3, 9]. In addition, paclitaxel is also associated with a sensory-predominant chronic neuropathy.
The purpose of this analysis was to compare the acute neuropathy syndromes as well as the chronic neurotoxicity associated with paclitaxel and oxaliplatin. Specific questions to address included the following: How similar are the acute neurotoxicity syndromes in terms of timing, severity, and incidence? How are the clinical characteristics (numbness versus tingling versus pain) and temporal patterns of the two neurotoxic syndromes similar or different? Does the relationship between the acute and chronic neurotoxicity syndromes differ between paclitaxel and oxaliplatin?
Insight into the similarities and differences between these neurotoxic syndromes may lead to a better understanding of the underlying pathophysiology and thereby allow for more focused investigation for potential treatment/prevention strategies.
Methods
This study utilized data from two previously conducted North Central Cancer Treatment Group (NCCTG) clinical trials, N08C1 and N08CB. NCCTG is now part of the Alliance for Clinical Trials in Oncology. Every participant in each trial signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Data from the NCCTG/Alliance trial N08CB were used to evaluate oxaliplatin neurotoxicity. N08CB was a phase III placebo-controlled, double-blind study designed to test the ability of intravenous (IV) calcium/magnesium (Ca/Mg) to prevent oxaliplatin-induced neurotoxicity [10]. In this trial, patients with colon adenocarcinoma, who had undergone curative-intent resection, were scheduled to receive 12 cycles of adjuvant infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) involving 85 mg/m2 oxaliplatin every 2 weeks. Patients were randomized to receive one of three treatments (approximately 115 patients per group): (1) IV Ca/Mg before and after FOLFOX, (2) IV placebo before and after FOLFOX, or (3) IV Ca/Mg before and IV placebo after FOLFOX. Patients from all three of these study arms were used for the current analysis, as there was no outcome difference seen between the treatment arms. Patients were ineligible if they had pre-existing peripheral neuropathy, a family history of a genetic/familial neuropathy, or had received prior treatment with neurotoxic chemotherapy. Data were not collected on other risk factors for neuropathy, such as diabetes or alcohol use. Other details regarding study methods for this trial have been previously published [10].
In N08CB, patients completed daily questionnaires prior to each cycle of FOLFOX and for 5 consecutive days after the initiation of each 2-week cycle of chemotherapy, to provide data regarding oxaliplatin-associated acute neuropathy. Using a validated methodology [11–13], questions included 0 (“no problem”) to 10 (“major problem”) numeric rating scales addressing “sensitivity touching cold items,” “discomfort swallowing cold items,” “throat discomfort,” and “muscle cramps” during the previous 24 h. Additionally, chronic peripheral neurotoxicity was measured at the initiation of each chemotherapy cycle and at 1, 3, 6, and 12 months after completion of chemotherapy using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire CIPN-20 (EORTC CIPN-20).
The EORTC CIPN-20 is a 20-item self-report questionnaire designed to supplement the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire. It contains nine items assessing sensory function, eight items assessing motor function, and three items assessing autonomic function. Items are scored from 1 to 4 with 1 representing “not at all” and 4 representing “very much.” In the current analysis, the EORTC CIPN-20 subscales were computed according to the standard scoring algorithm and then depicted on a 0–100 quality of life scale, where a high score means less symptom burden and better quality of life.
The EORTC CIPN-20 has been tested in cancer patients receiving a variety of chemotherapy agents and has been shown to be reliable, valid, and responsive to change. Cronbach’s alpha coefficients for the three subscales are 0.82, 0.73, and 0.76, respectively [14]. In addition, the validity of the EORTC CIPN-20 instrument and its relationship with the objective presence of symptoms and signs of CIPN was demonstrated by the CIPN outcome measure standardization study [15]. Recent data also support that the findings from this instrument correlate with clinician tools such as the National Cancer Institute—Common Toxicity Criteria sensory scale and the clinical sensory items of the Total Neuropathy Score [16].
NCCTG N08C1/Alliance, which explored the P-APS and paclitaxel neuropathy, involved different cohorts of patients. Data from two cohorts have been published, including data from a cohort of patients receiving paclitaxel alone at a dose of 70–90 mg/m2 weekly [2] and a cohort of patients receiving paclitaxel at a dose of 175 mg/m2 every 2–4 weeks in combination with carboplatin [3]. Study participants were at least 18 years old, able to provide informed written consent, and able to complete study questionnaires. Eligible patients had a life expectancy greater than 6 months and an Eastern Cooperative Oncology Group performance status of 0 or 1. Exclusion criteria included prior diagnosis of pre-existing neuropathy, fibromyalgia, prior paclitaxel or other neurotoxic chemotherapy, and any plan to receive concurrent neutrophil colony-stimulating factor therapy. The methods for testing neuropathy symptoms in the paclitaxel study group were as follows. P-APS symptoms were measured by asking patients to keep a daily symptom log, comprising 10 items regarding pain symptoms, and the use of pain medications on days 2–7 following each paclitaxel dose. These items asked about aches and pain and anchored these directly to the paclitaxel treatment. A 0–10 numeric analog scale where 0 represented no aches/pains and 10 represented aches/pains “as bad as can be” was utilized. One question asked participants to “Please rate any aches/pains that are new since your last dose of paclitaxel, and that you think might be related to your chemotherapy treatment by circling one number that best describes your aches/pains at its WORST in the last 24 h.” Two similar questions replaced the word “WORST” with “LEAST” and “AVERAGE.” The remaining 7 questions asked about pain mediation use and allowed for other comments. A 22-question summary questionnaire regarding symptom quality, location, alleviating/aggravating factors, and medication use was given on the 8th day following each paclitaxel dose. These instruments used validated methodology [11–13], which defined this syndrome in previous publications [2, 3, 9]. Chronic neuropathy was measured using the EORTC CIPN-20 instrument which was administered at baseline, prior to each dose of chemotherapy, and at monthly intervals for 12 months after the completion of chemotherapy.
In this comparative analysis, descriptive statistics and plots were the primary tools used to summarize the results and findings. Statistical tests were derived from repeated measure models. Statistical analyses were conducted by the Alliance Statistics and Data Center.
Results
The current study presents the data from 337 patients who participated in trial N08CB and 176 patients from N08C1 who had neuropathy data.
Acute neurotoxicity
Acute neuropathy during individual cycles
The acute neuropathy symptoms associated with the first 6 cycles of oxaliplatin are shown in Fig. 1a. Acute neuropathy symptoms appeared within a day after the first dose of oxaliplatin, peaked in severity around day 3, and then improved. Symptoms did not fully resolve between cycles and were still present at the time of the beginning of the second cycle of chemotherapy. In addition, symptoms were more severe in cycle 2; mean severities in subsequent cycles were similar to those observed in cycle 2.
Fig. 1.
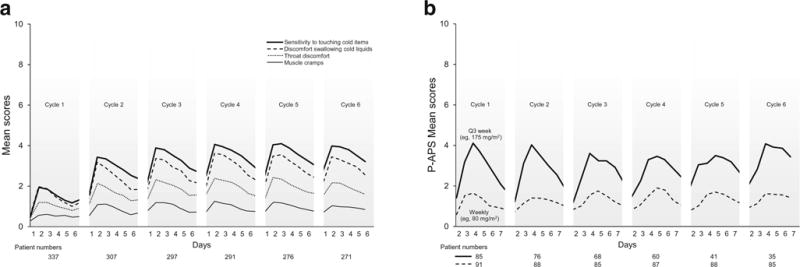
Mean score of the acute neuropathy symptoms during treatment. Higher values indicate more severe symptoms. a Acute neuropathy— oxaliplatin. b Acute neuropathy—paclitaxel. Abreviation: P-APS stands for paclitaxel-acute pain syndrome
Figure 1b demonstrates the P-APS scores for the first 6 cycles for patients receiving every 3 weeks as well as weekly paclitaxel. Similar to with oxaliplatin, symptoms started within a day of receiving paclitaxel and peaked around day 3. Symptoms improved by day 6 and then appeared to return to baseline. Symptoms during cycle 2 were similar to those during cycle 1, as opposed to worsening in cycle 2, as was observed with oxaliplatin. Overall, patients receiving weekly paclitaxel had less severe symptoms of P-APS compared to those receiving larger paclitaxel doses every 3 weeks.
Acute symptoms in cycle 1 predict symptoms with subsequent cycles
We then explored whether symptoms experienced in cycle 1 could predict those experienced in subsequent cycles. In patients receiving oxaliplatin, the level of severity of sensitivity to touching cold items in cycle 1 tended to predict the level of severity of this symptom in subsequent cycles. However, those with no sensitivity to touching cold items in the first cycle, on average, developed mild sensitivity to touching cold items in further cycles. An overall similar trend was seen with discomfort swallowing cold items, throat discomfort, and muscle cramps; however, those patients with no muscle cramps in the first cycle tended to remain without symptoms in the remaining cycles. (Fig. 2a).
Fig. 2.
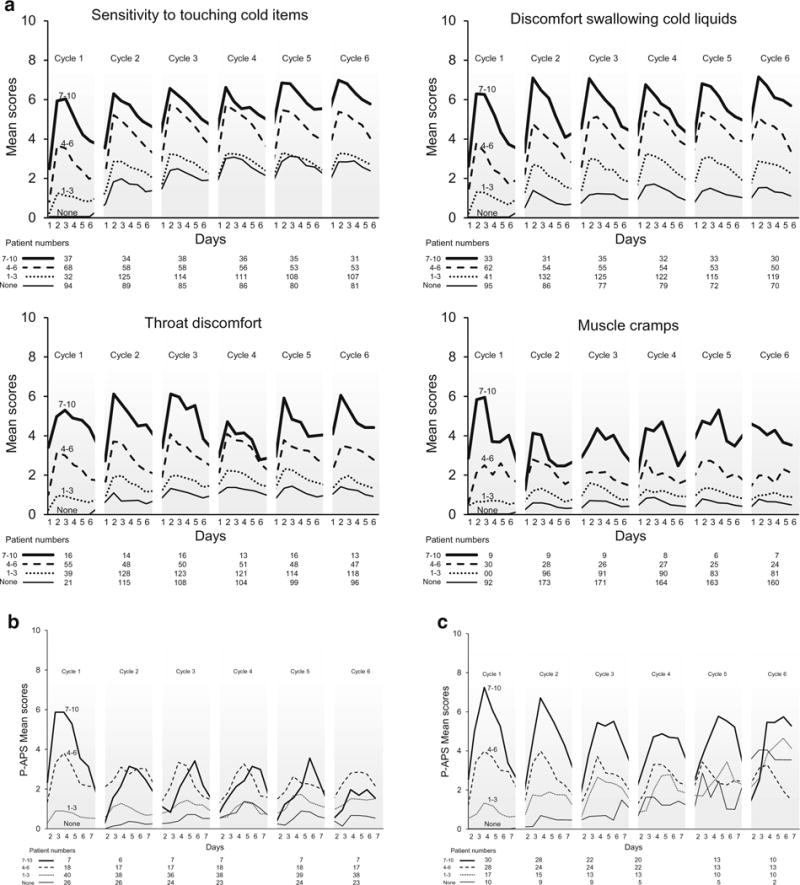
Acute neuropathy symptom scores, segregated by the worst mean maximum acute cycle 1 score [none (0) versus mild (1–3) versus moderate (4–5) versus severe (7–10)] for oxaliplatin acute neuropathy (a); weekly paclitaxel (b); and every 3 weeks of paclitaxel (c). Higher values indicate more severe symptoms. Abreviation: P-APS stands for paclitaxel-acute pain syndrome
Figure 2b demonstrates that with weekly paclitaxel, the few (seven) patients who had severe symptoms during cycle 1 tended to have less severe symptoms in the remaining cycles (potentially due to more opioid use in subsequent cycles, as was allowed by the protocol), while those with moderate or mild symptoms during cycle 1 had similar levels of symptoms with subsequent cycles. Those with no symptoms in cycle 1 tended not to develop symptoms in the remaining cycles. Correspondingly, mean worst pain score severites associated with the first cycle of paclitaxel appeared to predict pain difficulties with subsequent paclitaxel doses in patients receiving paclitaxel every 3 weeks, as demonstrated in Fig. 2c. In addition, similar to oxaliplatin, those with no symptoms in cycle 1 still tended to develop mild symptoms in the remaining cycles.
Chronic neuropathy
EORTC-CIPN 20 score—autonomic, motor, sensory
Figure 3 demonstrates the chronic neurotoxicity associated with both oxaliplatin and paclitaxel, measured with the CIPN-20 questionnaire. This figure illustrates that both oxaliplatin and paclitaxel neurotoxicities are predominantly sensory neuropathies that worsen over the course of treatment. Oxaliplatin sensory neurotoxicity continues to worsen after the completion of treatment, but then starts to improve after about 3 months post-treatment. In contrast, paclitaxel sensory neuropathy does not generally worsen after treatment completion, but, rather, appears to improve soon after drug discontinuation. The follow-up data for every 3-week paclitaxel was not reported due to small patient numbers, as this regimen was usually used in patients with metastatic cancer, as opposed to in an adjuvant chemotherapy setting, for which the other two regimens were used.
Fig. 3.
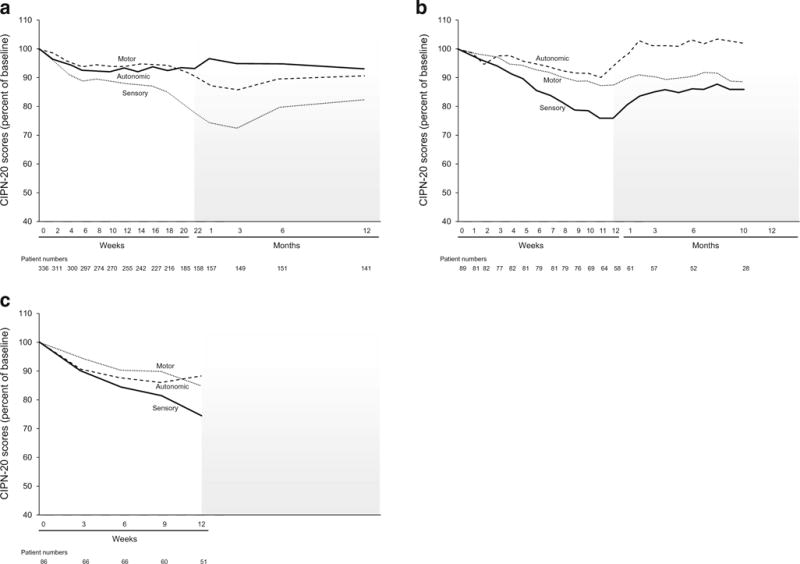
Mean sensory, motor, and autonomic sub-scores from the EORTC QLQ-CIPN20 instrument, during the treatment period and for up to 12 months of follow-up, in terms of percent of baseline over time for oxaliplatin (a), weekly paclitaxel (b), every 3 weeks of paclitaxel (c). Higher values indicate less severe symptoms
CIPN-20 sensory symptoms: numbness, tingling, pain
The sensory impairment was then explored in more detail, with regard to numbness, tingling, and burning pain in the hands and feet; these data are illustrated in Fig. 4. With both oxaliplatin and paclitaxel, pain was a less severe problem than was numbness and/or tingling, during ongoing treatment and during the months following completion of chemotherapy. As with the sensory neuropathy score, numbness and tingling became worse, on average, for the first 3 months after completion of chemotherapy with oxaliplatin, but improved in those 3 months with paclitaxel. With oxaliplatin, all three symptoms were worse in the upper extremities, compared to the lower extremities, during the first few months of treatment, but this was not seen with paclitaxel. With both oxaliplatin and paclitaxel, the upper extremities improved more rapidly than did the lower extremities.
Fig. 4.
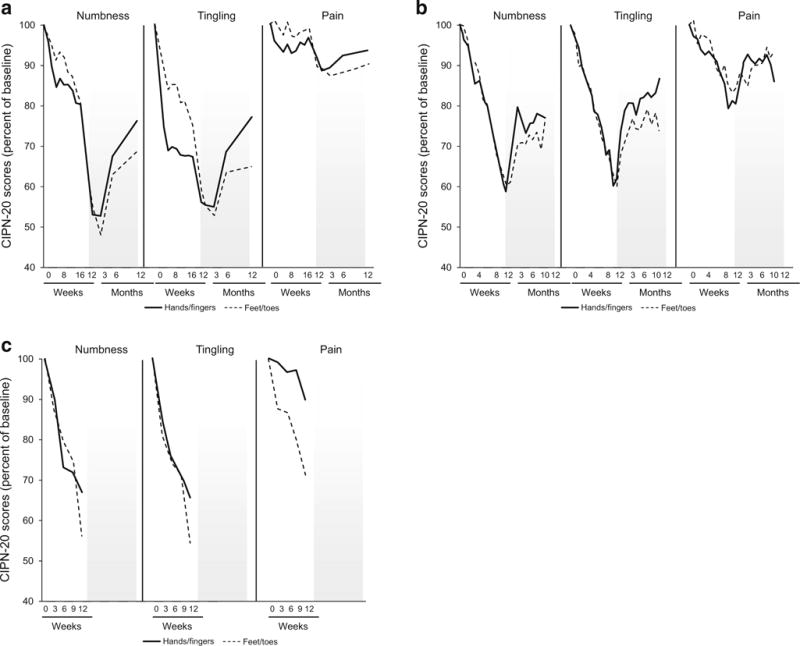
Mean tingling, numbness, and pain scores for the toes/feet and fingers/hands from the EORTC QLQ-CIPN20 tool during treatment period and for up to 12 months of follow-up for oxaliplatin (a), weekly paclitaxel (b), and every 3 weeks of paclitaxel (c). Higher values indicate less severe symptoms
Figure 5 further explores the findings displayed in Figs. 3 and 4; that oxaliplatin neurotoxicity tended to worsen in the 3 months after treatment completion while this did not happen with paclitaxel. This figure illustrates the changes from treatment end until 3 months later for each individual, showing that some patients with both drugs improved during the 3 months following the end of treatment, but, with oxaliplatin, many more initially worsened.
Fig. 5.
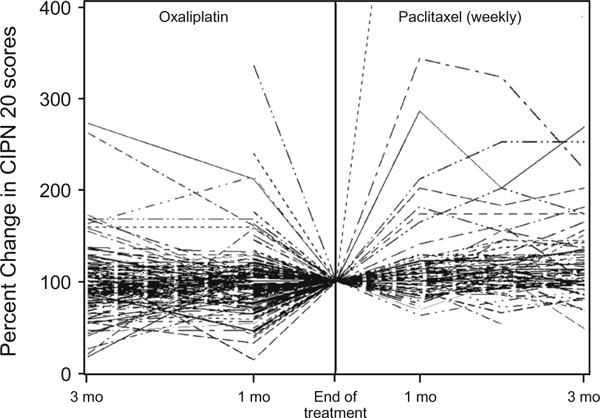
Mean sensory scores for oxaliplatin and weekly paclitaxel after completion of treatment and for 3 months of follow-up, for individual patients. Higher values indicate less severe symptoms
Relationship between acute and chronic neurotoxicity
To determine whether there was any relationship between acute neuropathy and the later appearance of chronic neurotoxicity, EORTC QLQ-CIPN20 sensory scores were compared between those patients who, after the first dose of oxaliplatin or paclitaxel, had acute scores of 0 (none) versus 1–3 (mild) versus 4–6 (moderate) versus severe (7–10) for any of the acute neuropathy symptoms. Figure 6a illustrates that those with more severe acute symptoms during cycle 1 of oxaliplatin tended to have more trouble with chronic neurotoxicity. Figure 6b demonstrates these data for those treated with weekly paclitaxel, showing that those with more severe symptoms with cycle 1 did have more problems with chronic neuropathy; however, this difference did not seem to last through follow-up, as symptoms tended to improve. Figure 6c also illustrates that for patients who received paclitaxel every 3 weeks, the severity of symptoms with cycle 1 predicted the severity of chronic neuropathy symptoms.
Fig. 6.
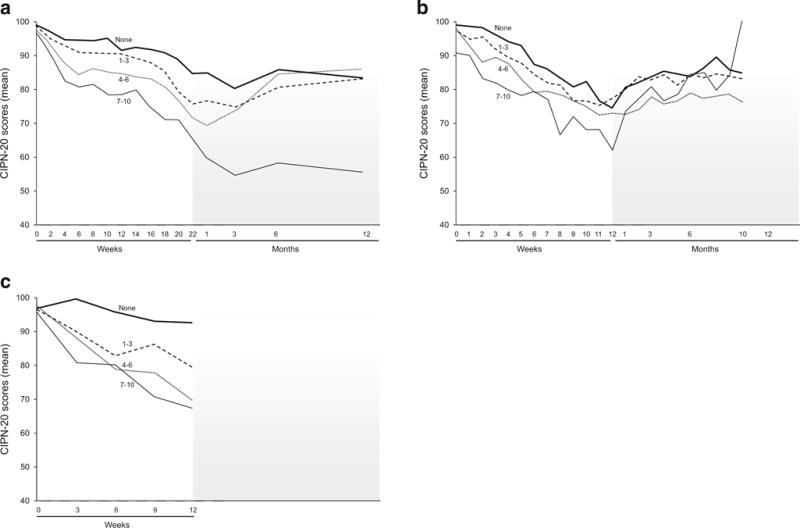
Total sensory neuropathy scores (from EORTC QLQCIPN20 instrument) segregated by the acute neuropathy scores during cycle 1 [none (0) versus mild (1–3) versus moderate (4–5) versus severe (7–10)] for oxaliplatin (a), weekly paclitaxel (b), and every 3 weeks of paclitaxel (c). Higher values indicate less severe symptoms
Discussion
This contrasting and comparing of the acute and chronic sensory involvement associated with the two most commonly utilized neurotoxic chemotherapy drugs enables clinicians to better explain expected neurotoxicities to patients initiating treatment with these drugs and/or patients who are suffering from this toxicity. The findings may allow us to better understand the underlying mechanisms for these symptom complexes.
Acute neuropathies
In trying to understand the reasons for the acute neuropathy similarities and differences for oxaliplatin and paclitaxel, it is worth reviewing the current understanding of the pathologic mechanisms for these toxicities. Oxaliplatin-induced acute neuropathy has been proposed to be due to the presence of an oxalate group that interferes with ion transport/activity by chelating ions, such as Ca++ and Mg++, which could interrupt Na+ channel kinetics [17–20]. This is supported by the fact that acute neuropathy symptoms are not observed in patients receiving other platinum agents, such as cisplatin and carboplatin. Interestingly, oxalate does not appear to have a role in the chronic neurotoxicity since it does not inhibit neurite outgrowth or induce cell death in sensory neurons, which was demonstrated in a study in which dorsal root ganglion neurons were directly exposed to oxalate to varying concentrations through various time courses and these neurons survived with no change in neurite length and an absence of cell death [21].
On the other hand, the acute neuropathy caused by paclitaxel may be related to a pathologic process noted in dorsal root ganglions seen within 24 h of a dose of paclitaxel, which has been demonstrated in an animal model [22]. Substance P, a neurotransmitter that is known to be involved in the regulation of nociception and neuropathic pain, may be related to the acute neuropathy syndrome differences. It has been shown that substance P is released during paclitaxel, but not oxaliplatin, treatment as blocking receptor neurokinin 1 and neurokinin 2 reverses the painful behaviors in neuropathy observed with paclitaxel but not with oxaliplatin [23, 24]. Given these different proposed pathologic mechanisms for the acute neuropathies from these two different drugs, it is not surprising that the clinical manifestations are unique. It is of interest, however, that both acute neuropathy syndrome symptoms peak at about 3 days after each dose.
Chronic neuropathies
In contrast to the acute neuropathies, the manifestations of chronic neurotoxicity are more similar among the two drugs. Both primarily induce a sensory impairment, as opposed to a motor or autonomic neuropathy, and both cause more trouble with numbness and tingling than shooting/burning pain. Their shared common pathophysiological mechanisms of chronic neurotoxicity may include disruption of axonal transport, neuronal injury and inflammation, oxidative stress, and mitochondrial damage [25].
Why oxaliplatin causes more hand toxicity during treatment and paclitaxel causes similar problems with upper and lower extremities is unclear. Interestingly, the hand symptoms improve faster with both drugs, after chemotherapy is discontinued, such that lower extremity neuropathy is a more persistent long-term problem with both drugs.
A potential explanation for why worsening of symptoms after end of treatment is more prominent with oxaliplatin may relate to the properties of oxaliplatin and other platinum compounds that can cause neuronal apoptosis via nuclear DNA damage and mitochondrial damage [26, 27]. In mouse models, it has been demonstrated that cisplatin binds directly to mitochondrial DNA and inhibits mitochondrial gene replication and transcription [28]. This may lead to progressive neurologic damage for several months after a dose of chemotherapy. Additionally, this condition may take a substantially longer time to resolve than might be seen with paclitaxel neuropathy, which likely is mediated by stabilizing microtubules and altering mitochondrial dynamics that can cause a “dying back axonopathy,” but not neuronal cell death [29].
Relationship between acute and chronic neuropathy severities
Although the current analysis illustrates that there is a positive relationship between acute and chronic neuropathies from these two drugs, as has been previously described in the literature [2, 3, 30–33], it has not been established that there is a causal relationship between acute and chronic neuropathies. It may be that the peripheral nervous system of persons who develop more severe acute symptoms is, for some unexplained reason, more prone to be damaged by neurotoxic drugs or that there is a pharmacogenomic issue whereby patients get exposed to more active drug or that some patients are more prone to pain syndromes. The reason that there appears to be a more prominent relationship with paclitaxel given 175 mg/m2 every 3 weeks (Fig. 6c), as opposed to 80 mg/m2 once weekly (Fig. 6b), might be the higher single dose, which is better able to reach a “threshold” for the onset of acute symptoms. This has been proposed with bortezomib in that those treated with IV bortezomib had more severe neuropathy and had higher mean maximum plasma concentrations versus those treated with subcutaneous bortezomib [34].
The findings presented here provide valuable information that can be used to inform patients and families about what to expect regarding neurotoxic side effects of paclitaxel and oxaliplatin. Patients may be comforted to know that if acute neuropathy symptoms are relatively minor in early cycles, they can expect a similar degree of discomfort with subsequent cycles. On the other hand, if they are suffering from more severe acute neuropathy symptoms, then caution will need to be used as they appear at increased risk for more severe chronic neuropathy. This evidence supports the need for further research exploring whether pre-emptive treatments will diminish symptom severity for patients who will likely experience escalating symptoms over time, based on what occurs during cycle 1. In addition, further research is needed comparing other neurotoxic agents, such as cisplatin and bortezomib, utilizing a standardized neuropathy assessment, as this may continue to provide insight into the underlying pathophysiology and inform future research to identify preventative treatment approaches.
Acknowledgments
Dr. Charles Loprinzi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Authors do not have any relevant financial interests, activities, relationships, or affiliations. The authors thank Dr. Nguyet Le-Lindqwister from Heartland Cancer Research NCORP and Dr. Nassim H. Nabbout from Wichita NCI Community Oncology Research Program for accrual of patients to the studies used for this manuscript.
Support Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (to the Alliance for Clinical Trials in Oncology NCORP Grant), and U10CA180882 to the Alliance Statistics and Data Center (Daniel J. Sargent, PhD), and U10CA180858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants U10CA025224, U10CA035090, U10CA035101, U10CA035103, U10CA035113, U10CA035267, U10CA035269, U10CA035272, U10CA035415, U10CA035431, U10CA037404, U10CA037417, U10CA052352, U10CA063844, U10CA063848, and K05CA124477.
Footnotes
Conflict of interest
Dr. Charles Loprinzi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Authors do not have any relevant financial interests, activities, relationships, and affiliations.
References
- 1.Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, Lafky JM, Wagner-Johnston ND, Ruddy KJ, Dakhil S, Staff NP, Grothey A, Loprinzi CL. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance) J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, Le-Lindqwister NA, Soori GS, Jaslowski AJ, Novotny PJ, Lachance DH. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol. 2011;29:1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, Le-Lindqwister NA, Soori GS, Jaslowski AJ, Kelaghan J, Novotny PJ, Lachance DH, Loprinzi CL. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer. 2012;118:5171–5178. doi: 10.1002/cncr.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK. Oxaliplatin-induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve. 2004;29:387–392. doi: 10.1002/mus.10559. [DOI] [PubMed] [Google Scholar]
- 5.Briani C, Argyriou AA, Izquierdo C, Velasco R, Campagnolo M, Alberti P, Frigeni B, Cacciavillani M, Bergamo F, Cortinovis D, Cazzaniga M, Bruna J, Cavaletti G, Kalofonos HP. Long-term course of oxaliplatin-induced polyneuropathy: a prospective 2-year follow-up study. J Peripher Nerv Syst. 2014;19:299–306. doi: 10.1111/jns.12097. [DOI] [PubMed] [Google Scholar]
- 6.Kidwell KM, Yothers G, Ganz PA, Land SR, Ko CY, Cecchini RS, Kopec JA, Wolmark N. Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer. 2012 doi: 10.1002/cncr.27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31:2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 8.Pietrangeli A, Leandri M, Terzoli E, Jandolo B, Garufi C. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol. 2006;56:13–16. doi: 10.1159/000094376. [DOI] [PubMed] [Google Scholar]
- 9.Loprinzi CL, Maddocks-Christianson K, Wolf SL, Rao RD, Dyck PJ, Mantyh P. The paclitaxel acute pain syndrome: sensitization of nociceptors as the putative mechanism. Cancer J. 2007;13:399–403. doi: 10.1097/PPO.0b013e31815a999b. [DOI] [PubMed] [Google Scholar]
- 10.Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P, Seisler D, Qamar R, Lewis GC, Grothey A. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance) J Clin Oncol. 2014;32:997–1005. doi: 10.1200/JCO.2013.52.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer AG, van Lanschot JJ, Stalmeier PF, van Sandick JW, Hulscher JB, de Haes JC, Sprangers MA. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13:311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 12.Hauser K, Walsh D. Visual analogue scales and assessment of quality of life in cancer. J Support Oncol. 2008;6:277–282. [PubMed] [Google Scholar]
- 13.Kindler CH, Harms C, Amsler F, Ihde-Scholl T, Scheidegger D. The visual analog scale allows effective measurement of preoperative anxiety and detection of patients’ anesthetic concerns. Anesth Analg. 2000;90:706–712. doi: 10.1097/00000539-200003000-00036. [DOI] [PubMed] [Google Scholar]
- 14.Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res. 2013;22:2787–2799. doi: 10.1007/s11136-013-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavaletti G, Cornblath DR, Merkies IS, Postma TJ, Rossi E, Frigeni B, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann Oncol. 2013;24:454–462. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberti P, Rossi E, Cornblath DR, Merkies IS, Postma TJ, Frigeni B, et al. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: two sides of the same coin. Ann Oncol. 2014;25:257–264. doi: 10.1093/annonc/mdt409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltagegated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol. 2000;406:25–32. doi: 10.1016/s0014-2999(00)00667-1. [DOI] [PubMed] [Google Scholar]
- 18.Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Dose effects of oxaliplatin on persistent and transient Na+ conductances and the development of neurotoxicity. PLoS One. 2011;6:e18469. doi: 10.1371/journal.pone.0018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakurai M, Egashira N, Kawashiri T, Yano T, Ikesue H, Oishi R. Oxaliplatin-induced neuropathy in the rat: involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain. 2009;147:165–174. doi: 10.1016/j.pain.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Sittl R, Lampert A, Huth T, Schuy ET, Link AS, Fleckenstein J, Alzheimer C, Grafe P, Carr RW. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc Natl Acad Sci U S A. 2012;109:6704–6709. doi: 10.1073/pnas.1118058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27:992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007;1168:46–59. doi: 10.1016/j.brainres.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamieson SM, Liu J, Connor B, McKeage MJ. Oxaliplatin causes selective atrophy of a subpopulation of dorsal root ganglion neurons without inducing cell loss. Cancer Chemother Pharmacol. 2005;56:391–399. doi: 10.1007/s00280-004-0953-4. [DOI] [PubMed] [Google Scholar]
- 24.Tatsushima Y, Egashira N, Kawashiri T, Mihara Y, Yano T, Mishima K, Oishi R. Involvement of substance P in peripheral neuropathy induced by paclitaxel but not oxaliplatin. J Pharmacol Exp Ther. 2011;337:226–235. doi: 10.1124/jpet.110.175976. [DOI] [PubMed] [Google Scholar]
- 25.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev. 2008;34:368–377. doi: 10.1016/j.ctrv.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 28.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, Schlattau A, Lathroum L, Windebank AJ. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41:661–668. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol. 2008;66:218–228. doi: 10.1016/j.critrevonc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Alejandro LM, Behrendt CE, Chen K, Openshaw H, Shibata S. Predicting acute and persistent neuropathy associated with oxaliplatin. Am J Clin Oncol. 2013;36:331–337. doi: 10.1097/COC.0b013e318246b50d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Argyriou AA, Cavaletti G, Briani C, Velasco R, Bruna J, Campagnolo M, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer. 2013;119:438–444. doi: 10.1002/cncr.27732. [DOI] [PubMed] [Google Scholar]
- 32.Park SB, Goldstein D, Lin CS, Krishnan AV, Friedlander ML, Kiernan MC. Acute abnormalities of sensory nerve function associated with oxaliplatin-induced neurotoxicity. J Clin Oncol. 2009;27:1243–1249. doi: 10.1200/JCO.2008.19.3425. [DOI] [PubMed] [Google Scholar]
- 33.Velasco R, Bruna J, Briani C, Argyriou AA, Cavaletti G, Alberti P, et al. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry. 2014;85:392–398. doi: 10.1136/jnnp-2013-305334. [DOI] [PubMed] [Google Scholar]
- 34.Moreau P, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]


