Abstract
Context:
The differential cortical function obtained by Tc-99m EC is comparable to that of Tc-99m DMSA. However, identification of scars on Tc-99m EC images needs to be studied.
Aims:
The aim of the study is to evaluate role of Tc-99m EC for detection of scarring and differential cortical function by comparing with Tc-99m DMSA.
Settings and Design:
Prospective observational study of recurrent UTI; minimum 6 weeks after acute episode; when urine examination is negative for pus cells.
Materials and Methods:
Forty-seven children with normal positioned kidneys underwent Tc-99m EC and DMSA scintigraphy. The DRF and cortical phase images of both studies in the same image matrix size were evaluated by two independent observers for scarring; Tc-99m DMSA was considered as the gold standard.
Statistical analysis used:
MS Excel 2007 and GraphPad Instat V3.1 and ROC analysis.
Results:
There was no significant difference in the detection of scarring using two studies with Cohen's kappa coefficient (κ) 0.932. The sensitivity and specificity of Tc-99m EC for detection of scarring was 98.75% and 99.15%, respectively. There was good agreement between the differential cortical function calculated using two studies.
Conclusions:
The summed Tc-99m EC images with an acceptable high image contrast allow detection of cortical scarring in patients with normal kidney positions. It is an excellent single-modality comprehensive investigational agent for renal parenchymal defects, function, and excretion evaluation with the added advantages of lower cost, convenience, and low radiation exposure to the child.
Key Messages: As the differential renal function and cortical scarring detected using Tc-99m EC is comparable to the gold standard of Tc-99m DMSA; Tc-99m EC can be used as a single modality for obtaining multiple information reducing time, cost, and radiation exposure to the child.
Keywords: cortical scarring, differential renal function, DMSA, EC, UTI
Introduction
Complicated urinary tract infection (UTI) is defined on the basis of clinical and biological criteria, to treat this entity in a more aggressive way than in the case of simple cystitis, to prescribe a prophylactic treatment to protect the kidney from further deterioration and to treat dysfunctional bladders. The workup involves micturating cystourethrography (MCU) to diagnose the grade of vesico-ureteric reflux (VUR). UTI may affect the urinary bladder (cystitis), the kidneys, and the collecting systems or both. Bacterial infection of the lower urinary tract can be asymptomatic and limited to urinary bladder. But the lower urinary tract infection has a risk of spreading to the kidneys. Infected urine stimulates immunologic and inflammatory response resulting in renal injury and scarring. With grade III, IV, or V VUR along with an episode of febrile UTI, there is 90% chance of acute pyelonephritis on renal scintigraphy.[1] The diagnosis of pyelonephritis and subsequent scarring of renal cortex is made on Tc-99m dimercaptosuccinic acid (DMSA) scanning and is routinely done in complicated UTI.[1,2]
Tc-99m mercapto acetyl triglycine (MAG3) scintigraphy is indicated whenever the collecting systems are dilated on ultrasound to rule out obstruction. Tc-99m DMSA and MAG3 scans provide information about the differential renal function (DRF). The DRF provided by two studies has been found to be comparable. However, the detection of scarring on Tc-99m MAG3 imaging during the cortical uptake phase (1-3 minutes) was variable in the studies performed.[3,4,5,6,7]
Tc-99m-N,N-ethylenedicysteine (EC) is another tubular secreted agent used for the renogram study. The greatest advantage of Tc-99mEC over Tc-99mMAG3 appears to be the negligible accumulation in the liver.[8] Thus, it can be used in patients with high creatinine with better delineation of kidney cortex. The DRF and detection of scarring using this agent was compared with the same on Tc-99m DMSA. There was good agreement between the DRF obtained using two radiopharmaceuticals. However, the confidence level of detection of areas of scarring was variable.[9,10,11,12,13,14,15]
The main disadvantage of Tc-99m DMSA is its slightly higher radiation dose in comparison with other renal agents because of tubular fixation of DMSA.[16,17,18] However, about 70% of EC is excreted from the cortex with no tubular fixation.[9]
The techniques used for the Tc-99m EC and Tc-99m DMSA acquisitions are different, the first being a dynamic acquisition and the second being the static acquisition, causing difficulty in selection of comparable images of the cortical phase. The cortical scars are detected qualitatively and need good quality images to identify scars. Most of the studies performed in the past comparing the Tc-99m EC or MAG3 cortical phase images with Tc-99m DMSA images used variable acquisition parameters.
The purpose of the study was to compare Tc-99m EC cortical phase images to that of Tc-99m DMSA using the same image matrix size during acquisition. This exercise provided comparable image quality of Tc-99m EC to the same of Tc-99m DMSA to identify cortical scars.
Materials and Methods
Forty-seven children with mean age of 4.44 years (range 20 days to 10 years) were included in the prospective study conducted in the Department of Nuclear Medicine, over a period of 2 years from June 2014 to July 2016. All the children with urinary tract infection (UTI) with negative urine examinations for pus cells for minimum 6 weeks were included in the study. The children with known ectopic, horseshoe or mal-rotated kidneys, renal agenesis, post-renal transplant, and parents unwilling to sign informed consent were excluded. After obtaining ethical approval from the local ethics committee, an informed consent document was obtained from parents/guardians.
The two studies, i.e., Tc-99m DMSA and Tc-99m EC scintigraphy, were performed on two separate days, maximum 10 days apart. The Tc-99m EC dynamic images were acquired in posterior projection using the 128 × 128 image matrix size after administering a mean activity of 74 MBq/2 mCi (minimum of 15MBq/400 µCi), and Tc99m DMSA static images were acquired in anterior and posterior projection using 128 ×128 as well as the 256 × 256 image matrix 3 hours after intravenous injection of mean 55.5 MBq/1.5 mCi (minimum dose of 18.5MBq /500 µCi).[19,20] The 2-3 minute dynamic images of EC were processed and reframed as 1 minute static cortical phase image set for comparison of cortical scarring with that of DMSA. The wedge shape defect in the cortex with distortion of the cortical contour was considered as the scar. The regions of interest (ROIs) around the kidney contours were drawn manually, and each was divided in three sub-regions (upper, median, and lower segments) for easier and accurate analysis [Figure 1a]. On visual analysis, a photopenic defect > 50% area in a segment was defined as the large scar and ≤50% of cortex in a segment was defined as the small scar. The renal cortical contour was also assessed visually to locate the distortion, which is an indicator of scarring. The Tc-99m DMSA images in 128 × 128 and 256 × 256 matrix were compared to detect scarring with a higher matrix size being considered as the gold standard. The Tc-99m EC and Tc-99m DMSA images in the 128 × 128 matrix size were compared with to identify cortical scars, and the same scarred areas were confirmed on 256 × 256 matrix size images of DMSA (considered as the gold standard). Both images of EC and DMSA were analyzed on a high-resolution monitor meant for gray and color scale presentations, with positive and negative variants. The images were reviewed independently by a two nuclear medicine physicians to eliminate bias due to inter-observer variability and obtain the receiver operator curve (ROC) analysis.
Figure 1.
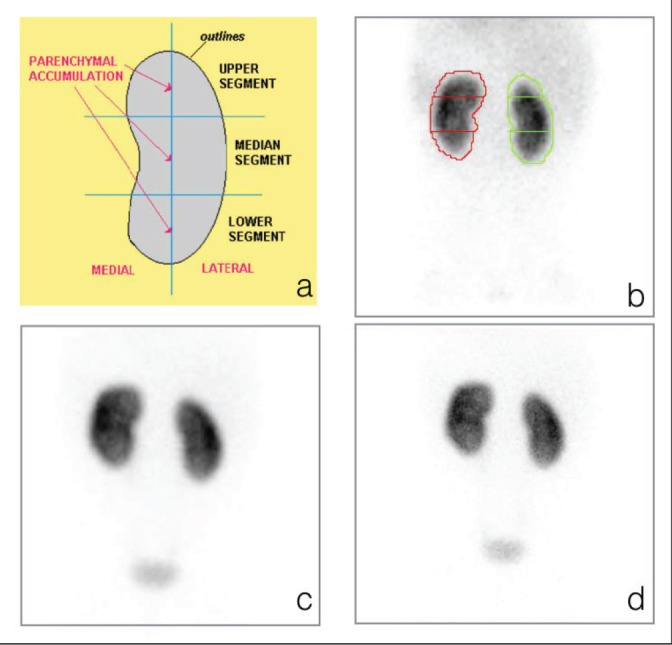
(a) Division of the renal cortex in upper, median, and lower segments, (b) segments drawn on the Tc-99m EC cortical phase image, (c) comparison with Tc-99m DMSA in the 128 × 128 image matrix size, (d) and Tc-99m DMSA in the 256 × 256 image matrix size. There are no cortical scarring bilaterally with normal contour and DRF
The DRF of both kidneys was calculated using Tc-99m EC and Tc-99m DMSA quantification protocols provided with the gamma camera. All statistical analysis was done using MS Excel 2007 and Graph Pad Instat V3.1.
Results
The 47 patients evaluated for UTI had known posterior urethral valves (post-radiofrequency fulguration) in 12 patients, VUR (15 unilateral and 6 bilateral) in 21 patients, pyonephrosis in 7 patients, and recurrent UTI without dilated collecting system in 7. Out of 94 kidneys evaluated in 47 patients; 42 kidneys did not show evidence of scarring with normal contour on the Tc-99m DMSA images with a higher matrix.
Detection of scarring
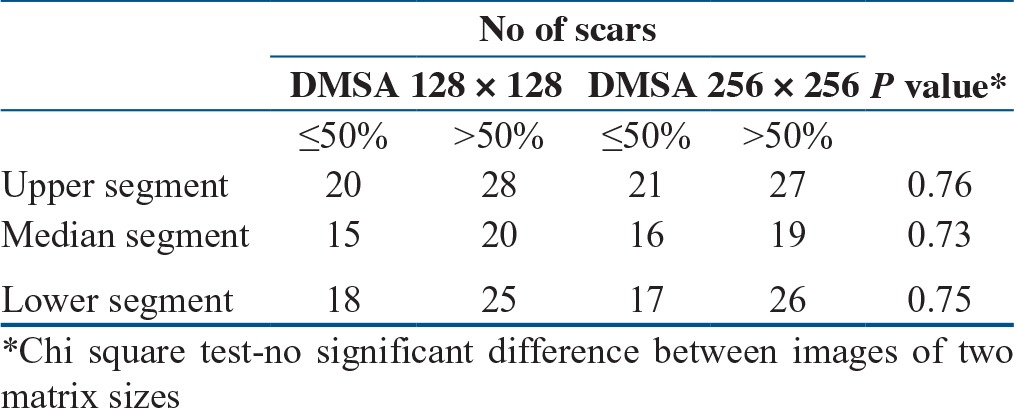
In 47 patients, 94 kidneys were evaluated to detect the extent of scarring in upper, Median, and lower segments of kidney [Figure 1b–Figure 1d; Figure 2]. There was no significant difference noted in the detection of extent of scars (≤ 50% and >50%) using Tc-99m EC in each cortical segment when evaluated with Tc-99m DMSA using the same image matrix size [Table 1].
Figure 2.
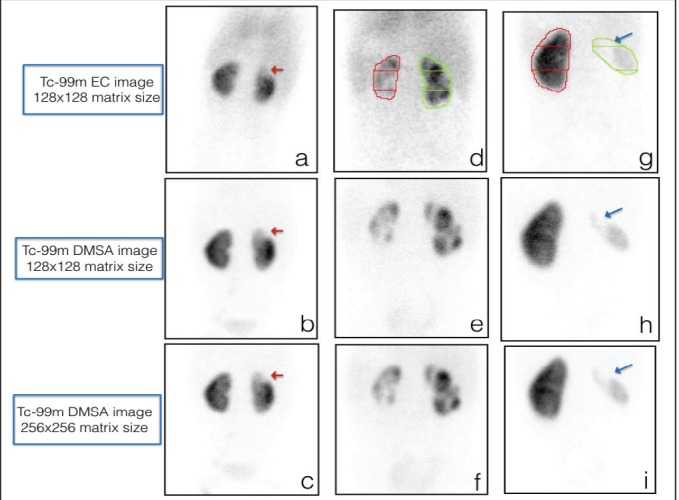
Comparison of scars using Tc-99m EC, Tc-99m DMSA 128 × 128 image matrix size, and Tc-99m DMSA 256 × 256 image matrix size: ≤ 50% scar at the upper pole of the right kidney (a, b, c red arrow), detection of multiple ≤50% and >50% size scars bilaterally (d, e, f), and identification of large scar involving entire right kidney (g, h, i blue arrow)
Table 1.
Comparison of detection of scarring in the image of two matrix sizes of Tc-99m DMSA
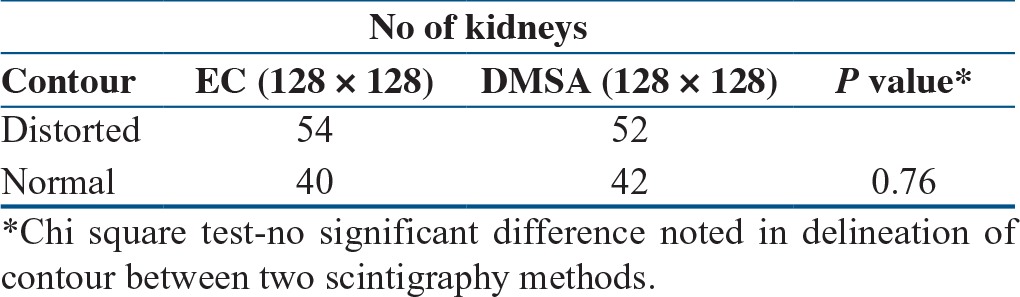
The distorted cortical contour is representative of the scarring. In total, 42 kidneys had normal contour and 52 had distorted contour that was identified on Tc-99m EC images. There was no significant difference (P = 0.76) noted in the characterization of contour by Tc-99m EC and Tc-99m DMSA imaging [Table 2].
Table 2.
Comparison of delineation of contour by Tc-99m EC and Tc-99m DMSA
The static images of Tc-99m DMSA acquired using the higher image matrix (256 × 256) was considered as the gold standard. The comparison of these images with the lower image matrix was considered to be equivalent by demonstrating no statically significant difference in detection of scarring in each segment of the renal cortex [Table 3].
Table 3.
Comparison between Tc-99m EC and Tc-99m DMSA for identification of cortical scarring
When Tc-99m EC was compared to gold standard Tc-99m DMSA for detection of cortical scarring, the sensitivity was 98.75% (93.23-99.97%), specificity was 99.15 % (95.33-99.98%), positive predictive value was 98.75% (93.23-99.97%), and negative predictive value was 99.15% (95.33-99.98%).
The ROC analysis of Tc-99m EC and Tc-99m DMSA images showed Cohen's kappa coefficient (κ) within the range of 0.81 to 1.0 suggesting almost perfect agreement between the two observers for detection of scarring by Tc-99m EC and Tc-99m DMSA imaging [Figure 3].
Figure 3.
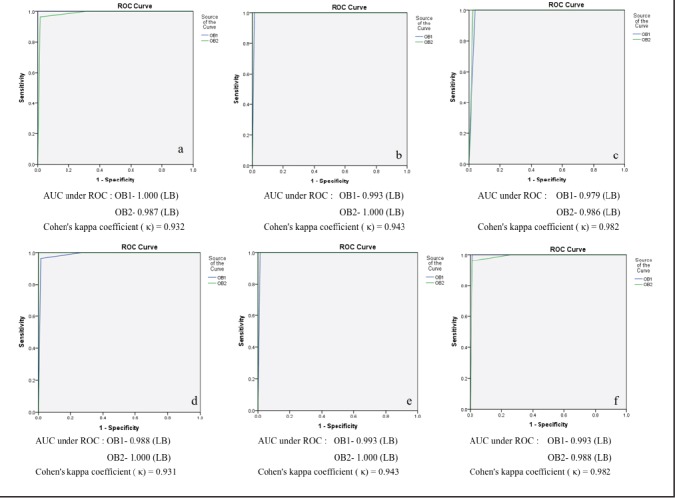
ROC curves for agreement between two independent observers for identification of scarring: upper segment (a), median segment (b), and lower segment (c) on the 128 × 128 image matrix size of Tc-99m EC and Tc-99m DMSA and upper segment (d), median segment (e), and lower segment (f) on Tc-99m DMSA (128 × 128) and Tc-99m DMSA (256 × 256)
Comparison of DRF
The DRF calculated by the Tc-99m EC and Tc-99m DMSA for 47 patients and 94 kidneys was comparable as shown by no statistically significant difference (P = 0.99) between the two [Table 4].
Table 4.
Comparison between Tc-99m EC and Tc-99m DMSA for DRF
Discussion
The DRFs calculated using Tc-99m DMSA and Tc-99m EC were almost similar. This finding was in agreement with the existing literature.[9,10,11,12,13,14] Kibar M et al,[9] Raja S et al,[10] Atasever T et al,[12] and Buyukdereli G et al[13] suggested that the DRF obtained by the Tc-99m EC and Tc-99m DMSA were comparable; however, Tc-99m DMSA was found to be gold standard for the identification of scarring. The Tc-99m EC cortical images were not enough for identifying scarring in these studies. The image acquisition parameters being used for Tc-99m EC were variable and not comparable with Tc-99m DMSA in these studies. The use of the lower image matrix size for Tc-99m EC scintigraphy reduced the resolution of images. Also, the use of pin hole collimator for acquisition of Tc-99m DMSA improved the resolution of image.[10,21,22] The pinhole magnification imaging increased the level of certainty with which renal cortical scintigraphy was interpreted but do not significantly increased the number of studies whose findings were considered abnormal on the basis of high-resolution planar imaging.[21,22]
The ROC analysis in this study showed excellent agreement between the two observers for identification of scarring. Thus, a simple modification during the acquisition of Tc-99m EC study with use of 128 × 128 image matrix size extended the use of single scintigraphy for obtaining multiple reliable information.[23] The higher matrix size enhanced the image quality to detect the scars even in kidneys with hydronephrosis and impaired cortical function. Narayana et al[11] also noted good agreement between Tc-99m EC and Tc-99m DMSA images for detection of scarring in the presence of raised creatinine and impaired renal cortical function in addition to the DRF. Thus, Tc-99m EC can be used as a preferred scintigraphy technique in UTI even with a dilated collecting system.
This study has evaluated kidneys positioned in the normal location in the clinical scenario of UTI. However, further studies are required for evaluation of scarring in ectopically located kidneys with hydronephrosis as well as impaired renal cortical function.
Conclusion
The DRF provided by Tc-99m EC was comparable with that provided by Tc-99m DMSA. There was excellent agreement between these two studies for detection of scarring in cases of UTI (average κ-0.932) by two independent observers. The summed Tc-99m EC images with an acceptable high image contrast allowed detection of cortical scarring in patients with kidney in normal anatomical positions. Thus, Tc-99m EC was found to be an excellent single-modality comprehensive investigational agent for renal parenchymal defects, function, and outflow tract evaluation with the added advantages of lower cost, convenience, and low radiation exposure to the child.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgement
Nil
References
- 1.Kliegman RM, Staton BF, St Geme JW, III, Schor NF. Nelson textbook of pediatrics. 20 ed. Philadelphia: Elsevier; 2016. pp. 2556–61. [Google Scholar]
- 2.Treves ST, Harmon WE, Packard AB, Kuruc A. Pediatric nuclear medicine/PET. 3rd ed. New York: Springer; 2007. pp. 239–306. [Google Scholar]
- 3.Ardela Díaz E, Miguel Martínez B, Gutiérrez Dueñas JM, et al. Comparative study of differential renal function by DMSA and MAG-3 in congenital unilateral uropathies. Cir Pediatr. 2002;15:118–21. [PubMed] [Google Scholar]
- 4.Ritchie G, Wilkinson AG, Prescott RJ. Comparison of differential renal function using technetium-99m mercaptoacetyltriglycine (MAG3) and technetium-99m dimercaptosuccinic acid (DMSA) renography in a paediatric population. Pediatr Radiol. 2008;38:857–62. doi: 10.1007/s00247-008-0908-8. [DOI] [PubMed] [Google Scholar]
- 5.Sfakianakis GN, Cavagnaro F, Zilleruelo G, Abitbol C, Montane B, Georgiou M, et al. Diuretic MAG3 scintigraphy (F0) in acute pyelonephritis: regional parenchymal dysfunction and comparison with DMSA. J Nucl Med. 2000;41:1955–63. [PubMed] [Google Scholar]
- 6.Grbac-Ivanković S, Smokvina A, Girotto N, Licul V. Initial presentation of scintigraphic changes during the first episode of acute pyelonephritis in children: simultaneous evaluation with MAG3 and DMSA. Nuklearmedizin. 2007;46:129–34. [PubMed] [Google Scholar]
- 7.Smokvina A, Grbac-Ivanković S, Girotto N, Dezulović MS, Saina G, Barković MM. The renal parenchyma evaluation: MAG3 vs DMSA. Coll Antropol. 2005;29:649–54. [PubMed] [Google Scholar]
- 8.Kabasakal L. Technetium-99m ethylenedicysteine: a new renal tubular function agent. Eur J Nucl Med. 2000;27:351–7. doi: 10.1007/s002590050045. [DOI] [PubMed] [Google Scholar]
- 9.Kibar M, Yapar Z, Noyan A, Anarat A. Technetium-99m-N N-ethylenedicysteine and Tc-99m DMSA scintigraphy in the evaluation of renal parenchymal abnormalities in children. Ann Nucl Med. 2003;17:219–25. doi: 10.1007/BF02990025. [DOI] [PubMed] [Google Scholar]
- 10.Raja S, Pareek V, Singh B, Sharma S, Rao KLN, Mittal BR. Comparison of 99mTc-ethylene dicysteine and 99mTc-dimercaptosuccinic acid scintigraphy for the Evaluation of Cortical Scarring and Differential Renal Function in Children with recurrent urinary tract infection. J Postgrad Med Edu Res. 2012;46:183–6. [Google Scholar]
- 11.Narayana C, Tripathi M, Kumar A, Gowda NK, Phom H, Chandra P, et al. Technetium-99m-L,L-ethylenedicysteine renal scan as a single-modality investigation for the evaluation of renal morphology and function: a comparative study with technetium-99m-dimercaptosuccinic acid. Nucl Med Commun. 2004;25:743–7. doi: 10.1097/01.mnm.0000131133.07279.60. [DOI] [PubMed] [Google Scholar]
- 12.Atasever T, Ozkaya O, Abamor E, Söylemezoğlu O, Buyan N, Unlü M. 99mTc ethylene dicysteine scintigraphy for diagnosing cortical defects in acute pyelonephritis: a comparative study with 99mTc dimercaptosuccinic acid. Nucl Med Commun. 2004;25:967–70. doi: 10.1097/00006231-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Buyukdereli G1, Guney IB. Role of technetium-99m N,N-ethylenedicysteine renal scintigraphy in the evaluation of differential renal function and cortical defects. Clin Nucl Med. 2006;31:134–8. doi: 10.1097/01.rlu.0000200460.41091.13. [DOI] [PubMed] [Google Scholar]
- 14.Çelik T, Yalçin H, Günay EC, Özen A, Özer C. Comparison of the relative renal function calculated with 99mTc-diethylenetriaminepentaacetic acid and 99mTc-dimercaptosuccinic acid in children. World J Nucl Med. 2014;13:149–53. doi: 10.4103/1450-1147.144812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammadi-Fallah M1, Alizadeh M, Ahmadi Lavin T. Comparison of DMSA scan 99 m and EC scan 99 m in diagnosis of cortical defect and differential renal function. Glob J Health Sci. 2014;6:38–43. doi: 10.5539/gjhs.v6n7p38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith T, Veall N, Altman G. Dosimetry of renal radiopharmaceuticals: the importance of bladder radioactivity and a simple aid for its estimation. Br J Radiol. 1981;54:961–4. doi: 10.1259/0007-1285-54-647-961. [DOI] [PubMed] [Google Scholar]
- 17.Johansson L, Mattsson S, Nosslin B. Effective dose equivalent from radiopharmaceuticals. Eur J Nucl Med. 1984;9:485–9. doi: 10.1007/BF00263250. [DOI] [PubMed] [Google Scholar]
- 18.Verboven M, Ham HR, Josephson S. How inaccurate are the 5 h measurements? Nucl Med Commun. 1987;8:45–7. doi: 10.1097/00006231-198701000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Gordon I, Piepsz A, Sixt R. Guidelines for standard and diuretic renogram in children. Eur J Nucl Med Mol Imaging. 2011;38:1175–88. doi: 10.1007/s00259-011-1811-3. [DOI] [PubMed] [Google Scholar]
- 20.Dosage Card (Version 12, 2014) http://www.eanm.org/docs/EANM_Dosage_Card_040214.pdf .
- 21.Applegate KE1, Connolly LP, Davis RT, Zurakowski D, Treves ST. A prospective comparison of high-resolution planar, pinhole, and triple-detector SPECT for the detection of renal cortical defects. Clin Nucl Med. 1997;22:673–8. doi: 10.1097/00003072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Connolly LP, Treves ST, Davis RT, Zimmerman RE. Pediatric applications of pinhole magnification imaging. J Nucl Med. 1999;40:1896–901. [PubMed] [Google Scholar]
- 23.Shulkin BL, Mandell GA, Cooper JA, Leonard JC, Parisi MT, et al. Procedure guideline for diuretic renography in children 30. J Nucl Med Technol. 2008;36:162–8. doi: 10.2967/jnmt.108.056622. [DOI] [PubMed] [Google Scholar]


