Abstract
Chronic cancer pain is a serious complication of malignancy or its treatment. Currently, no comprehensive, universally accepted cancer pain classification system exists. Clarity in classification of common cancer pain syndromes would improve clinical assessment and management. Moreover, an evidence-based taxonomy would enhance cancer pain research efforts by providing consistent diagnostic criteria, ensuring comparability across clinical trials. As part of a collaborative effort between the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) and the American Pain Society (APS), the ACTTION-APS Pain Taxonomy initiative worked to develop the characteristics of an optimal diagnostic system. After the establishment of these characteristics, a working group consisting of clinicians and clinical and basic scientists with expertise in cancer and cancer-related pain was convened to generate core diagnostic criteria for an illustrative sample of 3 chronic pain syndromes associated with cancer (ie, bone pain and pancreatic cancer pain as models of pain related to a tumor) or its treatment (ie, chemotherapy-induced peripheral neuropathy). A systematic review and synthesis was conducted to provide evidence for the dimensions that comprise this cancer pain taxonomy. Future efforts will subject these diagnostic categories and criteria to systematic empirical evaluation of their feasibility, reliability, and validity and extension to other cancer-related pain syndromes.
Keywords: Cancer pain, taxonomy, bone pain, chemotherapy-induced peripheral neuropathy, pancreatic cancer
One significant barrier to better understanding the growing dilemma of chronic cancer pain is the lack of consistent diagnostic criteria that can be used in research and clinical settings. A common taxonomy would provide a foundation for studies of the prevalence, as well as the consequences of these pain syndromes for people with cancer, present evidence for the significance of this problem, support the need for improvements in management, and increase research efforts.153 This standardized classification system would enhance research efforts by ensuring greater homogeneity in pain conditions across clinical trials and support the development of animal models to replicate these cancer pain conditions. Ultimately, valid and reliable diagnostic criteria would facilitate clinical assessment and management and potentially guide prognostic accuracy.59,65
Current systems to classify cancer pain provide general clinical utility. Cancer pain is often organized by its intensity (eg, mild, moderate, or severe), its expected time course (eg, acute vs chronic), its presumed underlying pathophysiology (eg, nociceptive vs neuropathic), its location (eg, head and neck pain), or its putative mechanisms (eg, tumor-related, treatment-related, pain unrelated to tumor or treatment).126 Although these general categories are useful, more specific diagnostic criteria would allow more precise diagnosis with therapeutic implications and would enhance research efforts.
This lack of a unified taxonomy is not specific to cancer pain. Currently, there is an absence of evidence-based classification systems for most chronic pain conditions.65 To meet this need, the Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations Opportunities and Networks, a public-private partnership with the US Food and Drug Administration and the American Pain Society collaborated to develop the ACTTION-APS Pain Taxonomy (AAPT). The initiative worked to develop the characteristics of an ideal diagnostic system that would be biologically plausible, exhaustive, mutually exclusive, reliable, clinically useful, and simple through consensus conferences. The resulting diagnostic system includes 5 dimensions: 1) core diagnostic criteria; 2) common features; 3) common medical comorbidities; 4) neurobiological, psychological, and functional consequences; and 5) putative neurobiological and psychosocial mechanisms, risk factors, and protective factors.65
After the establishment of these 5 dimensions, a working subgroup of clinical and basic scientists and clinicians with expertise in cancer pain was convened by the AAPT organizers. The aim of their effort was to apply the ideal framework of 5 dimensions developed during the original AAPT conference to cancer pain. The objectives included: 1) to identify chronic pain syndromes seen in oncology with high prevalence and significant effects; and 2) to generate a classification system of diagnostic criteria for several of these syndromes on the basis of these originally proposed 5 dimensions.
Methods
A working group of clinicians and clinical and basic scientists with expertise in cancer pain met during a consensus meeting held in July 2014. Group members from the United States and the United Kingdom were carefully selected on the basis of their contributions to the science and management of cancer pain, representing multiple disciplines (basic scientists, physicians, and nurses) with significant achievements in cancer-related epidemiology, research, and clinical care.
Before this meeting, a systematic review was conducted by 2 of the working group members (M.M., M.B.) using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting system.99 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses is an evidence-based minimum set of items for reporting systematic reviews and meta-analyses. The databases searched included the following: the Cumulative Index to Nursing and Allied Health Literature from 1981 to June 2014, the Cochrane Library of Systematic Reviews and the Cochrane Library of Controlled Trials from inception to June 2014, Database of Abstracts of Reviews of Effect from inception to April 2014, Embase Classic and Embase from 1947 to June 2014, Ovid MEDLINE 1946 to June 2014, and OVID MEDLINE In-Process and Other Non-Indexed Citations on June 11, 2014. These databases were searched for review articles (including summary reports, systematic reviews, and meta-analyses), as well as observational and experimental studies published in English. Articles were excluded if they did not describe the clinical characteristics of chronic cancer pain, including those that described animal studies, or were studies of acute or breakthrough cancer pain. Data were extracted and summarized descriptively with respect to the 5 AAPT diagnostic dimensions: 1) core diagnostic criteria; 2) common features; 3) common medical comorbidities; 4) neurobiological, psychosocial, and functional consequences; and 5) putative neurobiological and psychosocial mechanisms, risk factors, and protective factors. Figure 1 shows the process used in this systematic review. Key words included cancer pain, malignancy, chemotherapy, surgery, radiotherapy, and neuropathy.
Figure 1.
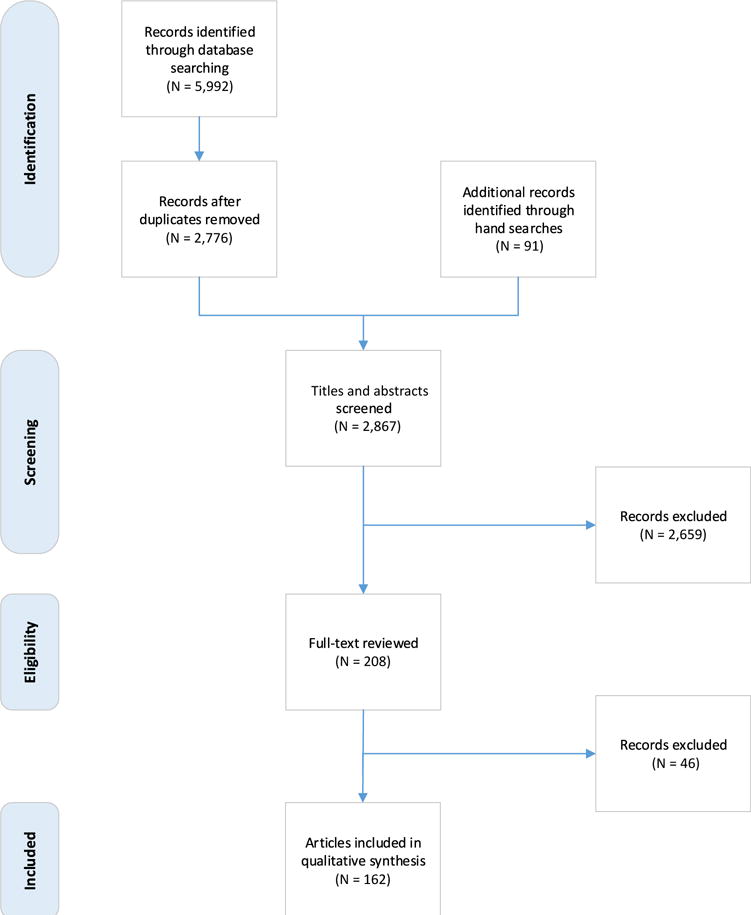
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram. Preferred Reporting Items for Systematic Reviews and Meta-Analyses is an evidence-based minimum set of items for reporting systematic reviews.
Bone pain, postmastectomy pain, head and neck pain, neuropathic pain (disease-related), and chemotherapy-induced peripheral neuropathy (CIPN) were most frequently referenced. These results were correlated with prevalence data of pain reported according to tumor site, that generally show a higher rates of pain reports in people with pancreatic, lung, genitourinary, breast, and prostate cancers.20 The expert panel considered these and other pain syndromes aiming to initiate the process of taxonomy development with 3 syndromes before extending to the full range of cancer-related pain syndromes at a later time. The challenge in narrowing the selection to 3 syndromes was to achieve a balance between the most prevalent cancer pain syndromes versus areas that have received the most study and thus have the largest body of evidence. Ultimately guiding the selection was the aim to identify syndromes with clinical or research utility (ie, the painful conditions that are most homogeneous in terms of mechanism or presentation and offer the most relevant targets for further research).
Through working group discussion, debate, and verbal consensus, 3 chronic pain syndromes seen as a result of cancer (ie, bone pain and pancreatic cancer pain (PCP) as a model of pain related to the tumor) or its treatment (CIPN) were unanimously selected. Head and neck pain was omitted because the source of this pain may be multifactorial (eg, tumor, treatment-related, or both) and diverse in relationship to specific anatomic location. Similarly, the working group agreed that disease-related neuropathic pain was too broad. Although postsurgical pain syndromes, including postmastectomy pain, were initially included, this group of pain conditions was omitted from the cancer-related pain taxonomy to avoid overlap with a broader discussion of postsurgical pain syndromes being conducted concurrently by a separate working group classifying neuropathic pain.
Then the cancer pain working group generated a classification for these syndromes on the basis of the AAPT multidimensional framework. This work was conducted during the consensus meeting and later refined through online discussion. To ensure that the references were timely, the entire literature review was updated as of January 2015. The report on these findings was written by several members (J.A.P., M.M., M.B.) and reviewed, edited, and approved by all members of the working group.
Results
On the basis of this qualitative review and after extensive discussion, the diagnostic criteria for 3 representative chronic cancer-related pain syndromes were developed collectively by the working group, including cancer-induced bone pain (CIBP), CIPN, and PCP.
Cancer-Induced Bone Pain
It is estimated that 50 to 95% of all patients who die of cancer have bone involvement.49,132 The most common cancers to originate in the bone are osteosarcomas.84 Cancers that frequently metastasize to bone include prostate, breast, lung, and myeloma.6,7,14,29,38,42,47,50,52,75,87,94,106,136 Of those with bone metastases, approximately 85% experience pain, with resultant immobility and reduced quality of life.54 CIBP is a specific pain state with overlapping but distinct features of acute nociceptive, inflammatory, and neuropathic pain processes.61
Dimension 1. Core Diagnostic Criteria of CIBP
The AAPT core diagnostic criteria for CIBP are summarized in Table 1. The history must include a cancer diagnosis and imaging evidence of bone disease consistent with primary or metastatic cancer.
Table 1.
Dimension 1: Core Diagnostic Criteria for Cancer-Induced Bone Pain
Criteria
|
Dimension 2. Common Features of CIBP
The cardinal feature of CIBP is a mixture of continuous background pain (usually described as annoying, dull, gnawing, aching, and/or nagging) punctuated by evoked or spontaneous pain (often described as electric or shock-like) in 1 or more locations generally consistent with the given known distribution of bone lesions, associated with weight-bearing or movement or can occur spontaneously.14,22,40,43,48,61,63,72,73,85,88,92,95,113,123,125,130,132,133,140 Generalized bone pain can occur because of the presence of multiple bone lesions or resultant expansion of bone marrow from bone metastases.26,61 The distribution of the pain can be localized, radicular, or both.73
Typical sites of CIBP include the vertebrae (lower thoracic spine and lumbar regions are most prevalent), pelvis, long bones, and ribs.26,48 Bone lesions on the skull can result in headache pain due to calvarial, maxillary, or medullary lesions, as well as cranial nerve palsies such as mental nerve numbness or visual difficulties.25,26 Chest wall pain can occur as a result of bone lesions in the ribs.26
As the disease and tumor mass progress, the background pain increases in intensity and interference61 and is generally responsive to opioid analgesics, alone or in conjunction with nonsteroidal anti-inflammatory drugs.10,22,63,71,79,112 Conversely, spontaneous pain (without specific eliciting stimuli) and evoked pain (ie, in response to standing, weight-bearing, movement, touch, or other stimuli) associated with CIBP are difficult to treat from the onset because of their intermittent nature, which tends to be very rapid in onset, intense, and of short duration.10,22,63,71,79,112 Terminology commonly used in the clinical setting to describe evoked or spontaneous pain include breakthrough pain, incident pain, or a pain flare; however, definitions for these terms often lack precision and may overlap. For example, breakthrough pain is defined as a transitory flare of pain in the setting of chronic pain managed with opioid drugs,127 yet the evoked pain seen in CIBP can occur during weight-bearing without current opioid use. The International Association for the Study of Pain Taxonomy, the principal resource for definitions related to chronic pain, does not currently address evoked, breakthrough, incident, flare, or spontaneous pain.
Dimension 3. Common Medical Comorbidities for CIBP
The primary comorbidity for CIBP is the presence of skeletal-related events. These skeletal complications include pathological fractures of long bones, vertebrae, pelvis, rib, and other sites11,24–26,28,49,51,154 and in some cases spinal cord compression.56,63,73,132
Dimension 4. Neurobiological, Psychosocial, and Functional Consequences of CIBP
The consequences of CIBP can be serious because they affect biological, psychological, and functional aspects of the patient’s life.38,73,137 Although some pathologic fractures produce limited pain, primary or metastatic bone lesions affecting the femur, pelvis, or spine are likely to cause significant pain during standing or ambulation, resulting in reduced mobility.63 Reduced quality of life and diminished activities of daily living are associated with CIBP.41,44,48,49,91,118,132,134,160 Functional impairment is strongly associated with evoked or breakthrough pain.92,113 Fatigue is frequently reported in patients with metastatic bone disease and resultant pain.133
Depression is common in people with painful bone metastases and has been reported to be significantly associated with impaired quality of life.134 Additionally, the meaning of pain as a sign of advancing disease in individuals with CIBP has been shown to be correlated with increased pain intensity.134 Anxiety in men with advanced prostate cancer is associated with increased pain intensity and number of metastatic bone lesions.86 These studies reveal that the consequences of CIBP are not unlike those seen in chronic noncancer pain syndromes, including impaired function, mood, and quality of life.60,155
Dimension 5. Putative Neurobiological and Psychosocial Mechanisms, Risk Factors, and Protective Factors for CIBP
CIBP is a mixed mechanism condition that includes elements of acute nociceptive pain, inflammatory pain, and neuropathic pain.58,61,70,103,125,132 Distinctive peripheral modifications to bone and nervous tissues occur as well as neurobiological changes at the level of the spinal cord.61,70
Acute nociceptive pain occurs because of localized bone destruction, which leads to loss of structural integrity and a decrease in pH. Cancer cells do not destroy bone directly, but rather they express the receptor activator of nuclear factor κB ligand (RANKL), which binds to its receptor, RANK. Activation of the RANKL/RANK pathway stimulates the production of bone-destroying osteoclasts.103 Osteoclasts resorb bone by forming a highly acidic environment between the osteoclast and the bone. This stimulates the TRPV1 or ASIC3 channels expressed by a significant population of nociceptors that ultimately leads to the perception of incident pain with movement and weight-bearing activities.61
Inflammatory pain develops when peripheral nerve endings in bone marrow and bone matrix are sensitized by localized inflammatory mediators stimulated by the cancer cells or their associated stromal cells. Locally released factors include bradykinin, endothelins, interleukin-6, granulocyte-macrophage colony-stimulating factor, nerve growth factor, proteases, and tumor necrosis factor −α.103 This change is generally associated with steady background pain.61
Neuropathic pain results from compression, distension, increase in sprouting, or denervation of nerve endings and/or axonal structures caused by expansion of the tumor. These changes lead to spontaneous pain and associated altered sensations.61
Nociceptive and neuropathic mechanisms work in concert to produce a complex mixture of ongoing acute, inflammatory, and neuropathic processes. These processes lead to a hyperexcitable state within the spinal cord, which itself is associated with amplification and modification of noxious and non-noxious peripheral stimuli.61,70,156
Interventions to protect against CIBP have been developed on the basis of current understanding of the underlying neurobiology. Bisphosphonates bind to bone, interfering with osteoclast function, later resulting in osteoclast apoptosis.103 Osteoprotegerin or denosumab, therapies that interfere with RANKL binding to RANK deplete activated osteoclasts, reduce signs of bone resorption, and diminish bone cancer pain.146 This is a rapidly evolving area of research and numerous studies are underway to examine compounds that might block CIBP.146 Regarding putative psychosocial mechanisms associated with CIBP or other risk factors, little is currently understood and additional research is warranted.
Chemotherapy-Induced Peripheral Neuropathy
CIPN is a serious treatment-induced toxicity that can limit function, impair quality of life, and in some cases, diminish the potential for cure when chemotherapy doses need to be reduced.9 This condition is increasing in prevalence as greater numbers of neurotoxic agents are introduced and as patients live longer with the consequences of neuropathy. In a recent systematic review of 31 studies with data from 4,179 patients, CIPN prevalence was 68.1% in the first month after chemotherapy, 60.0% at 3 months, and 30.0% at 6 months or later.141
Dimension 1. Core Diagnostic Criteria of CIPN
CIPN occurs in oncology patients when treatment involves a neurotoxic agent. A temporal relationship exists between the onset of symptoms and the starting, stopping, and duration of therapy.34,37,46,62,78,148 Peripheral sensory and motor nerve damage or dysfunction are the putative mechanisms for CIPN.36,37,62,101,121,148,151,162
CIPN poses a significant challenge for the patient and clinician in terms of diagnosis, management, and associated reductions in function and quality of life, particularly in patients with coexisting conditions or disorders that involve the peripheral nervous system (eg, diabetes, HIV).2,33,68,78,80,81,104,120,121,148,151 Table 2 shows the core diagnostic criteria for CIPN.
Table 2.
Dimension 1: Core Diagnostic Criteria for Chemotherapy-Induced Peripheral Neuropathy
Criteria
|
Dimension 2. Common Features of CIPN
The cardinal feature of dose-limiting CIPN is a gradually progressive distal symmetrical sensory neuropathy (stocking/glove distribution), which may be associated with diminished motor function. However, more often than not motor symptoms are absent until later stages of CIPN.3,13,36,37,62,78,80,101,121,148,151,162 Neuropathy in the feet without involvement in the hands is common. In addition to descriptors such as “tingling” or “burning,” patients often describe these sensory abnormalities with terms such as “discomfort” or “unpleasant.” Cramping, more common in the lower limbs, may be reported.151
Clinical examination reveals sensory loss to one or more sensory modalities and/or evoked pain in a stocking and glove distribution. These findings include hypoesthesia (a bilateral increase in detection thresholds to tactile, vibration, or non-noxious warm or cool stimuli),53,57,78 or hypoalgesia (a bilateral increase in pain detection thresholds to blunt pressure or pinprick stimuli),53,57,78 or hyperalgesia (a bilateral decrease in pain detection threshold to noxious heat or cold stimuli).18,53 The anatomic distribution of these physical examination findings may not correspond exactly to the sensory symptoms.2,30,46,53,78,80,109,115,120,148,152,158,162
Signs and symptoms of CIPN, including pain, commonly begin in the lower extremities followed by the upper extremities and progress proximally.78 However, not all go on to experience neuropathy in the upper extremities. The temporal features of CIPN are rapid onset (hours or days) of sensory abnormalities after initiation of neurotoxic chemotherapy.78,101 In most cases, the onset of CIPN symptoms and signs is progressive; beginning with mild paresthesias in the lower extremities, becoming progressively more intense, and advancing proximally with cumulative dose exposure.78,101
Some patients may experience a reduction in the intensity of symptoms between treatment cycles—sometimes referred to as a waxing and waning effect.122 In some cases, symptoms and signs of CIPN may continue or worsen after treatment hasended, a phenomenon known as ‘coasting.’37,78,101 Increasing evidence suggests that pre-existing sensory deficits (clinical or subclinical neuropathy) are associated with the onset of more extensive and severe CIPN symptoms and signs.18,53 The prevalence of autonomic changes associated with CIPN is poorly understood, but can include serious complications such as falls related to orthostatic hypotension.1
Agents most likely to result in CIPN include platinum-based drugs (eg, cisplatin, carboplatin, and oxaliplatin), vinca alkaloids (eg, vincristine, vinblastine), taxanes (eg, paclitaxel, docetaxel), bortezomib, thalidomide, lenalidomide, eribulin, and ixabepilone. The frequency and severity of CIPN is generally related to the specific drug, dose, schedule (eg, more prevalent with weekly vs every 3 weeks dosing of paclitaxel), speed of administration, and duration of therapy.111 In the case of bortezomib, route of delivery affected the prevalence of CIPN. Peripheral neuropathy of any grade was significantly less common with subcutaneous bortezomib administration compared with intravenous delivery.114
Sensations described by patients vary with the administered chemotherapeutic agent. In a recent prospective study that compared the experience of patients receiving docetaxel versus oxaliplatin, tingling was the most common symptom experienced by both groups, yet pain and discomfort associated with cold was uniquely reported by those who received oxaliplatin.157
Motor weakness, with a similar peripheral distribution to sensory alterations, can occur in CIPN, but overall is observed less frequently than sensory abnormalities.62,101,141 However, patients frequently show a decrease in mechanosensory function, measured using a timed pegboard test or the time taken to button a shirt.16,17,128 Importantly, impaired proprioception is reported by many patients with CIPN, described as feeling unbalanced, particularly in the absence of visual cues when walking or standing (eg, in dark settings, when closing one’s eyes in the shower).78,116 Clinical examination may reveal a positive Romberg sign and generalized ataxia in more severe cases.124 Symmetrical loss of deep tendon reflexes (Achilles or bra-chioradialis) is a sign of more advanced CIPN.78
Dimension 3. Common Medical Comorbidities of CIPN
People at greatest risk for CIPN are believed to include those with comorbid conditions known to contribute to neuropathy, including diabetes, obesity, and HIV.81,128,141 Pharmacogenetic profiling of genetic polymorphisms has been conducted to identify susceptibility to CIPN on the basis of genetic polymorphisms. For example, polymorphisms in the CYP2C8 and CYP3A5 genes that encode for paclitaxel-metabolizing enzymes were found to be associated with CIPN.31,82 Although pharmacogenetic profiling may one day identify patients at greater risk for severe CIPN, the data so far are insufficient to draw any definitive conclusions.
Dimension 4. Neurobiological, Psychosocial, and Functional Consequences of CIPN
Terminal axonal degeneration and axonal microtubule disruption are the most common pathophysiologic consequences observed in CIPN.78 Psychosocial consequences of CIPN include depression, anxiety, impaired sleep, and other mood changes.69,83,151,157 The functional outcomes of CIPN range from mild symptoms that do not interfere with activities of daily living to moderate and severe dose-limiting sensory and motor alterations that interfere with activities of daily living.32,34–37,131 The need to limit doses of chemotherapy because of CIPN can lead to shortened survival. In the most severe cases sensory and motor alterations are disabling, resulting in paralysis, complete loss of function, or both.101,104,147
Dimension 5. Putative Neurobiological and Psychosocial Mechanisms, Risk Factors, and Protective Factors for CIPN
The underlying pathophysiologic mechanism(s) that lead to the development of CIPN are not completely understood. Nevertheless, the similarity in the pattern and spectrum of clinical symptoms and signs of CIPN caused by different chemotherapeutic agents is apparent. Common underlying mechanisms purported to be involved in the development of CIPN are5,12,23,62,77,119,122,152,161,162:
Disruption of axoplasmic microtubule-mediated transport causing distal axonopathy, a known cellular effect of many chemotherapy agents;
Distal axonal degeneration;
Direct damage to sensory nerve cell bodies of the dorsal root ganglia;
Mitochondrial dysfunction;
Activation of protein kinases and extracellular kinases (associated with cisplatin- induced CIPN);
Oxaliplatin is associated with actual nerve cell death and decreased epidermal nerve fiber density with each cycle, as well as decreased conduction velocity and amplitude;
Alteration of gene expression thought to be involved in pain mediation in spinal cord dorsal horn (associated with vincristine exposure);
Decrease in the density of gray matter after 1 month in women with breast cancer experiencing CIPN;
Central sensitization as a consequence of long-term peripheral nerve injury.
A recent systematic review explored risk factors for CIPN and reported the following elements: baseline neuropathy, smoking, abnormal creatinine clearance, and distinct sensory changes during chemotherapy treatment, including cold allodynia and cold hyperalgesia.141 Sensory changes during chemotherapy treatment, including increased pain and neuronal hyperexcitability, are also predictors of CIPN.141 A prospective study of patients receiving oxaliplatin and followed for 1 year reported that patients with elevated heat detection thresholds (higher temperature levels were needed to perceive heat) before receiving chemotherapy were more likely to experience intense CIPN.128
Few protective factors for CIPN have been identified. A recent investigation used large Medicare claims data and reported that a history of autoimmune disease was associated with reduced risk of CIPN.81 Regarding prevention of CIPN, a recent clinical practice guideline from the American Society of Clinical Oncology reviewed existing evidence. After extensive analysis, the authors were unable to recommend any agents to prevent this syndrome because of the lack of high-quality evidence.80
Pancreatic Cancer Pain
The estimated incidence of pancreatic cancer for 2016 is more than 53,000 in the United States, with approximately 42,000 dying from this disease.144 Risk factors for pancreatic cancer include family history, obesity, smoking, and chronic pancreatitis.89,145,149 Upper abdominal pain is a common presenting symptom of pancreatic cancer. The prevalence of pain associated with pancreatic cancer ranges from 72 to 100%.15
Dimension 1. Core Diagnostic Criteria of PCP
PCP occurs in the presence of a diagnosis of pancreatic cancer confirmed by imaging evidence of an epigastric mass and/or biopsy that establishes the diagnosis. Table 3 shows the core diagnostic criteria.
Table 3.
Dimension 1: Core Diagnostic Criteria for Pancreatic Cancer Pain
Criteria
|
Dimension 2. Common Features of PCP
The cardinal features of PCP are upper abdominal pain with frequent extension to the back, either to the low back or the region between the scapulae spreading laterally, and unexplained weight loss.143 Less frequently PCP is diffuse within the abdomen or referred to the lower abdominal quadrants.27,90 The pain is often described as dull, aching, gnawing, or spasmodic27,143 and the intensity can fluctuate throughout the day with position (eg, exacerbated by supine positioning) and food ingestion.143 Pain intensity usually increases with disease severity. However, because this cancer is often diagnosed late, 20 to 30% of patients report moderate to severe pain at diagnosis.27 The back pain associated with PCP may be worse when the patient is supine and eased by sitting forward.27,90,143
Dimension 3. Common Medical Comorbidities of PCP
Jaundice and dark urine can be a presenting symptom in cancers of the pancreatic head.143 Unexplained weight loss, anorexia, diabetes, and other sequelae of pancreatic cancer or its treatment are common.15,90,143 To date, few medical comorbidities of PCP have been identified.
Dimension 4. Neurobiological, Psychosocial, and Functional Consequences of PCP
Because pancreatic cancer is highly associated with pain, it is difficult to discern whether other symptoms are related to the cancer or pain. Several studies have documented a very high prevalence of depressed mood in patients with pancreatic cancer, higher than other cancers with similar prognoses.8,27,45 Symptom burden in general is high in this population, notably including disturbed sleep and fatigue90 as well as nausea and vomiting associated with obstruction or delayed gastric emptying.15
Dimension 5. Putative Neurobiological and Psychosocial Mechanisms, Risk Factors, and Protective Factors for PCP
Pain occurs in 90% of patients with cancer of the head of the pancreas and is much less common in cancer of pancreatic body or tail.27,64,143 Back pain often indicates that retroperitoneal or celiac plexus infiltration has occurred.143 Putative mechanisms include compression or infiltration (perineural invasion) of splanchnic nerves in the celiac plexus by direct local tu-mor expansion,8,19,117,143 as well as compression of surrounding tissues and organs. Celiac plexus block has been reported to be effective in relieving PCP.4,107,135 It is unclear if relief signifies extension of tumor into the plexus, or interruption of visceral afferent neurons that are also found in the plexus. No other risk factors or protective factors for PCP could be identified.
Discussion
Cancer-related pain remains a complex, multidimensional phenomenon. The exercise of developing a standardized, rigorous, valid taxonomy for just 3 common cancer pain syndromes revealed the limitations in our existing nomenclature. Current studies attempting to characterize and establish prevalence rates for specific cancer pain syndromes are hampered by the absence of explicit definitions. A clear example is the large, prospective study by Ventzel and colleagues comparing neuropathy characteristics in patients receiving oxaliplatin versus docetaxel.157 Although the prevalence of pain in the hands and feet was approximately equal between the 2 groups, on further analysis, a significant percentage of those treated with docetaxel reported less burning and numbness, suggesting the pain was not consistent with CIPN. The investigators postulated this difference may be related to the use of adjuvant endocrine therapies, such as the aromatase inhibitors, often prescribed for those who have received docetaxel. These endocrine therapies are known to cause arthralgias, myalgias, and carpal tunnel syndrome.110,142 The investigators used a variety of questionnaires to determine these differences, yet not all clinicians or researchers will be able to use such an extensive battery of measures. It is our hope that the core diagnostic criteria will help future investigators to not only better characterize cancer pain syndromes, as was done in this study, but to differentiate them from related phenomenon to avoid inaccurate interpretations.100
The consequences of cancer pain can be significant, including deleterious effects on function, mood, sleep, fatigue, and ultimately, quality of life.60,155 Additionally, increased intensity of cancer pain is associated with heightened suffering in those at end of life.159 Finally, studies support the association between pain and reduced survival, demanding more urgent attention to this symptom.76,129 More research is warranted to discern the neurobiological, psychological, and functional consequences of each of these and other cancer pain syndromes.
Another area that demands additional study is the determination of the mechanisms of cancer pain. Although important work has begun in the area of CIBP,39,102,103,105 CIPN,66,74,97,98 and PCP,55,93 additional research is needed to elucidate the neurobiological factors responsible for cancer pain. An exciting line of investigation is the interactions among the cancer microenvironment, the primary afferent nociceptor, and the immune system.138,139
An additional area of clarification relates to risk factors and protective determinants for cancer pain. Early research exploring cancer pain focused on clinical or biological factors, such as cancer diagnosis, stage of disease, or treatment. In recent important work, Miaskowski and colleagues reported that cancer patients with the highest symptom burden were significantly younger, more likely to be female and nonwhite, had lower levels of social support, lower socioeconomic status, poorer functional status, and a higher level of comorbidity.108 In a large study of people with breast cancer, colorectal cancer, or prostate cancer, Lewis et al reported that factors associated with more severe CIPN included colon versus other cancers, the duration and type of therapy, poor socioeconomic status, and black race.96 Factors influencing cancer pain must be expanded in future studies, including psychosocial factors60 and overlapping chronic pain conditions and comorbidities.100 Additionally, identification of genetic polymorphisms might allow for the identification of those at risk for these painful syndromes, as well as direct prevention and treatment innovations.
Finally, an emerging area of research that requires further investigation is the development of phenotypic profiles of cancer pain syndromes on the basis of symptoms and clinical signs. Recent studies in noncancer chronic pain syndromes (such as diabetic peripheral neuropathy) suggest that stratification of patients into homogeneous groups on the basis of symptom profiles may be advantageous for analgesic drug trials and ultimately lead to a more targeted approach to cancer pain management.67,150
This proposed taxonomy presents early work in developing a classification system for cancer-related pain conditions. Current classification systems focusing on duration (acute vs chronic) or presumed etiology (related to the cancer, related to treatment, or unrelated) do not provide the specificity needed to clearly define distinct cancer pain syndromes. There were numerous challenges in the development process, notably limitations in existing research related to cancer pain. Studies are often hampered by a wide array of weaknesses, including heterogeneous populations, small sample sizes, dissimilar assessment tools and techniques, and inadequate duration of investigations. Although the working group strove to identify diagnostic criteria that were absolutely necessary to describe each painful syndrome, when these criteria are applied more broadly, controversy will arise and modification will likely be indicated. Future work will now be required to validate this proposed taxonomy in populations of people with cancer and determine the feasibility of its use in clinical as well as research settings. Investigators studying these 3 syndromes should incorporate the core diagnostic criteria when using research methods. Clinicians may find the use of the criteria of benefit when considering the differential diagnosis of complex cancer pain syndromes. This current undertaking classified just 3 syndromes; much additional work is needed to characterize the many other painful syndromes that occur in individuals diagnosed with cancer.
Conclusions
Three cancer pain syndromes, 2 related to cancer, and 1 related to a common cancer treatment, were classified using the AAPT multidimensional chronic pain taxonomy. Future work will show the validity and reliability of these proposed diagnostic criteria.21 As our understanding of these cancer pain conditions matures, it is expected that the taxonomy will expand and evolve. It is the hope of this working group that classification of these cancer pain syndromes will ultimately strengthen clinical, scientific, and educational efforts around cancer pain. Transforming our understanding of cancer pain is urgently needed to improve its management as well as improve patients’ relief and survivors’ quality of life.
Perspective.
The ACTTION-APS chronic cancer pain taxonomy provides an evidence-based classification for 3 prevalent syndromes, namely malignant bone pain, pancreatic cancer pain, and chemotherapy-induced peripheral neuropathy. This taxonomy provides consistent diagnostic criteria, common features, comorbidities, consequences, and putative mechanisms for these potentially serious cancer pain conditions that can be extended and applied with other cancer-related pain syndromes.
Acknowledgments
Support was provided by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks public-private partnership with the US Food and Drug Administration (FDA), which has received contracts, grants, and other revenue for its activities from the FDA, multiple pharmaceutical and device companies, and other sources. A complete list of current Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks sponsors is available at: http://www.acttion.org/partners.
Footnotes
The views expressed in this article are those of the authors, none of whom has financial conflicts of interest relevant to the issues discussed in this article. No official endorsement by the FDA should be inferred.
The authors have no conflicts of interest to declare.
References
- 1.Adams SC, Schondorf R, Benoit J, Kilgour RD. Impact of cancer and chemotherapy on autonomic nervous system function and cardiovascular reactivity in young adults with cancer: A case-controlled feasibility study. BMC Cancer. 2015;15:414. doi: 10.1186/s12885-015-1418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti P, Rossi E, Cornblath DR, Merkies IS, Postma TJ, Frigeni B, Bruna J, Velasco R, Argyriou AA, Kalofonos HP, Psimaras D, Ricard D, Pace A, Galie E, Briani C, Dalla Torre C, Faber CG, Lalisang RI, Boogerd W, Brandsma D, Koeppen S, Hense J, Storey D, Kerrigan S, Schenone A, Fabbri S, Valsecchi MG, Cavaletti G, CI-PeriNomS Group Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: Two sides of the same coin. Ann Oncol. 2014;25:257–264. doi: 10.1093/annonc/mdt409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen KG, Jensen MB, Kehlet H, Gartner R, Eckhoff L, Kroman N. Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer: Cyclophosphamide, epirubicin and fluorouracil compared with docetaxel 1 epirubicin and cyclophosphamide. Acta Oncol. 2012;51:1036–1044. doi: 10.3109/0284186X.2012.692884. [DOI] [PubMed] [Google Scholar]
- 4.Arcidiacono Paolo G, Calori G, Carrara S, McNicol Ewan D, Testoni Pier A. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011:CD007519. doi: 10.1002/14651858.CD007519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Bae HM, Lee SH, Kim TM, Kim DW, Yang SC, Wu HG, Kim YW, Heo DS. Prognostic factors for non–small-cell lung cancer with bone metastasis at the time of diagnosis. Lung Cancer. 2012;77:572–577. doi: 10.1016/j.lungcan.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 7.Bagi CM. Skeletal implications of prostate cancer. J Musculoskelet Neuronal Interact. 2003;3:112–117. [PubMed] [Google Scholar]
- 8.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 9.Bhatnagar B, Gilmore S, Goloubeva O, Pelser C, Medeiros M, Chumsri S, Tkaczuk K, Edelman M, Bao T. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: A single-center experience. Springerplus. 2014;3:366. doi: 10.1186/2193-1801-3-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchi R, Gilardini A, Rodriguez-Menendez V, Oggioni N, Canta A, Colombo T, De Michele G, Martone S, Sfacteria A, Piedemonte G, Grasso G, Beccaglia P, Ghezzi P, D’Incalci M, Lauria G, Cavaletti G. Cisplatin-induced peripheral neuropathy: Neuroprotection by erythropoietin without affecting tumour growth. Eur J Cancer. 2007;43:710–717. doi: 10.1016/j.ejca.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg. 2009;91:1503–1516. doi: 10.2106/JBJS.H.00175. [DOI] [PubMed] [Google Scholar]
- 12.Bilinska M, Usnarska-Zubkiewicz L, Pokryszko-Dragan A. Bortezomib-induced painful neuropathy in patients with multiple myeloma. Wspolczesna Onkologia. 2013;17:421–426. doi: 10.5114/wo.2013.37214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binda D, Cavaletti G. Chemotherapy induced peripheral neuropathy outcome measures standardisation (CI-PERI-NOMS) study. J Peripher Nerv Syst. 2011;16:S12. [Google Scholar]
- 14.Boland E, Eiser C, Ezaydi Y, Greenfield DM, Ahmedzai SH, Snowden JA. Living with advanced but stable multiple myeloma: A study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J Pain Symptom Manage. 2013;46:671–680. doi: 10.1016/j.jpainsymman.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ. 2012;344:e2476. doi: 10.1136/bmj.e2476. [DOI] [PubMed] [Google Scholar]
- 16.Boyette-Davis JA, Cata JP, Driver LC, Novy DM, Bruel BM, Mooring DL, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol. 2013;71:619–626. doi: 10.1007/s00280-012-2047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain. 2011;12:1017–1024. doi: 10.1016/j.jpain.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyette-Davis JA, Eng C, Wang XS, Cleeland CS, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Zhang H, Dougherty PM. Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clin Cancer Res. 2012;18:3180–3187. doi: 10.1158/1078-0432.CCR-12-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brant JM. Cancer-related neuropathic pain. Nurse Pract Forum. 1998;9:154–162. [PubMed] [Google Scholar]
- 20.Breivik H, Cherny N, Collett B, de Conno F, Filbet M, Foubert AJ, Cohen R, Dow L. Cancer-related pain: A pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420–1433. doi: 10.1093/annonc/mdp001. [DOI] [PubMed] [Google Scholar]
- 21.Bruehl S, Ohrbach R, Sharma S, Widerstrom-Noga E, Dworkin RH, Fillingim RB, Turk DC. Approaches to demonstrating the reliability and validity of core diagnostic criteria for chronic pain. J Pain. 2016;17:T118–T131. doi: 10.1016/j.jpain.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Buga S, Sarria JE. The management of pain in metastatic bone disease. Cancer Control. 2012;19:154–166. doi: 10.1177/107327481201900210. [DOI] [PubMed] [Google Scholar]
- 23.Burakgazi AZ, Messersmith W, Vaidya D, Hauer P, Hoke A, Polydefkis M. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology. 2011;77:980–986. doi: 10.1212/WNL.0b013e31822cfc59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callstrom MR, Charboneau JW, Goetz MP, Rubin J, Atwell TD, Farrell MA, Welch TJ, Maus TP. Image-guided ablation of painful metastatic bone tumors: A new and effective approach to a difficult problem. Skeletal Radiol. 2006;35:1–15. doi: 10.1007/s00256-005-0003-2. [DOI] [PubMed] [Google Scholar]
- 25.Caraceni A. Evaluation and assessment of cancer pain and cancer pain treatment. Acta Anaesthesiol Scand. 2001;45:1067–1075. doi: 10.1034/j.1399-6576.2001.450903.x. [DOI] [PubMed] [Google Scholar]
- 26.Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. Pain. 1999;82:263–274. doi: 10.1016/S0304-3959(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 27.Caraceni A, Portenoy RK. Pain management in patients with pancreatic carcinoma. Cancer. 1996;78:639–653. doi: 10.1002/(SICI)1097-0142(19960801)78:3<639::AID-CNCR45>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Caraceni A, Weinstein SM. Classification of cancer pain syndromes. Oncology (Williston Park) 2001;15:1627–1640. 1642. [PubMed] [Google Scholar]
- 29.Carla R, Fabio T, Gloria B, Ernesto M. Prevention and treatment of bone metastases in breast cancer. J Clin Med. 2013;2:151–175. doi: 10.3390/jcm2030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cata JP, Weng H, Burton AW, Villareal H, Giralt S, Dougherty PM. Quantitative sensory findings in patients with bortezomib-induced pain. J Pain. 2007;8:296–306. doi: 10.1016/j.jpain.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Cavaletti G, Alberti P, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity in the era of pharmacogenomics. Lancet Oncol. 2011;12:1151–1161. doi: 10.1016/S1470-2045(11)70131-0. [DOI] [PubMed] [Google Scholar]
- 32.Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, Parma G, Lissoni A, Fei F, Cundari S, Zanna C. Gradingofchemotherapy-inducedperipheral neurotoxicityusing the Total Neuropathy Scale. Neurology. 2003;61:1297–1300. doi: 10.1212/01.wnl.0000092015.03923.19. [DOI] [PubMed] [Google Scholar]
- 33.Cavaletti G, Cornblath DR, Merkies IS, Postma TJ, Rossi E, Frigeni B, Alberti P, Bruna J, Velasco R, Argyriou AA, Kalofonos HP, Psimaras D, Ricard D, Pace A, Galie E, Briani C, Dalla Torre C, Faber CG, Lalisang RI, Boogerd W, Brandsma D, Koeppen S, Hense J, Storey D, Kerrigan S, Schenone A, Fabbri S, Valsecchi MG. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: From consensus to the first validity and reliability findings. Ann Oncol. 2013;24:454–462. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavaletti G, Frigeni B, Lanzani F, Mattavelli L, Susani E, Alberti P, Cortinovis D, Bidoli P. Chemotherapy-induced peripheral neurotoxicity assessment: A critical revision of the currently available tools. Eur J Cancer. 2010;46:479–494. doi: 10.1016/j.ejca.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Cavaletti G, Frigeni B, Lanzani F, Piatti M, Rota S, Briani C, Zara G, Plasmati R, Pastorelli F, Caraceni A, Pace A, Manicone M, Lissoni A, Colombo N, Bianchi G, Zanna C. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: Comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12:210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 36.Cavaletti G, Jakubowiak AJ. Peripheral neuropathy during bortezomib treatment of multiple myeloma: A review of recent studies. Leuk Lymphoma. 2010;51:1178–1187. doi: 10.3109/10428194.2010.483303. [DOI] [PubMed] [Google Scholar]
- 37.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6:657–666. doi: 10.1038/nrneurol.2010.160. [DOI] [PubMed] [Google Scholar]
- 38.Cetin K, Christiansen CF, Jacobsen JB, Norgaard M, Sorensen HT. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: A Danish population-based cohort study. Lung Cancer. 2014;86:247–254. doi: 10.1016/j.lungcan.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Chartier SR, Thompson ML, Longo G, Fealk MN, Majuta LA, Mantyh PW. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain. 2014;155:2323–2336. doi: 10.1016/j.pain.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen E, Khan L, Zhang L, Nguyen J, Cramarossa G, Tsao M, Danjoux C, Barnes E, Sahgal A, Holden L, Jon F, Dennis K, Culleton S, Chow E. Symptom clusters in patients with bone metastases - a reanalysis comparing different statistical methods. Support Care Cancer. 2012;20:2811–2820. doi: 10.1007/s00520-012-1403-1. [DOI] [PubMed] [Google Scholar]
- 41.Cheng EY. Prospective quality of life research in bony metastatic disease. Clin Orthop Relat Res (415S) 2003:S289–S297. doi: 10.1097/01.blo.0000093054.96273.20. [DOI] [PubMed] [Google Scholar]
- 42.Chow E, Doyle M, Li K, Bradley N, Harris K, Hruby G, Sinclair E, Barnes EA, Danjoux C. Mild, moderate, or severe pain categorized by patients with cancer with bone metastases. J Palliat Med. 2006;9:850–854. doi: 10.1089/jpm.2006.9.850. [DOI] [PubMed] [Google Scholar]
- 43.Chow E, Fan G, Hadi S, Filipczak L. Symptom clusters in cancer patients with bone metastases. Support Care Cancer. 2007;15:1035–1043. doi: 10.1007/s00520-007-0241-z. [DOI] [PubMed] [Google Scholar]
- 44.Chow E, Hruby G, Davis L, Holden L, Schueller T, Wong R, Hayter C, Szumacher E, Loblaw A, Danjoux C. Quality of life after local external beam radiation therapy for symptomatic bone metastases: A prospective evaluation. Support Cancer Ther. 2004;1:179–184. doi: 10.3816/SCT.2004.n.009. [DOI] [PubMed] [Google Scholar]
- 45.Clark KL, Loscalzo M, Trask PC, Zabora J, Philip EJ. Psychological distress in patients with pancreatic cancer–an understudied group. Psychooncology. 2010;19:1313–1320. doi: 10.1002/pon.1697. [DOI] [PubMed] [Google Scholar]
- 46.Cleeland CS, Farrar JT, Hausheer FH. Assessment of cancer-related neuropathy and neuropathic pain. Oncologist. 2010;15(Suppl 2):13–18. doi: 10.1634/theoncologist.2009-S501. [DOI] [PubMed] [Google Scholar]
- 47.Clemons M, Dranitsaris G, Cole D, Gainford MC. Too much, too little, too late to start again? Assessing the efficacy of bisphosphonates in patients with bone metastases from breast cancer. Oncologist. 2006;11:227–233. doi: 10.1634/theoncologist.11-3-227. [DOI] [PubMed] [Google Scholar]
- 48.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 49.Coleman RE. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 50.Conti G, La Torre G, Cicalese V, Micheletti G, Ludovico MG, Vestita GD, Cottonaro G, Introini C, Cecchi M. Prostate cancer metastases to bone: Observational study for the evaluation of clinical presentation, course and treatment patterns. Presentation of the METAURO protocol and of patient baseline features. Arch Ital Urol Androl. 2008;80:59–64. [PubMed] [Google Scholar]
- 51.Cook RJ, Major P. Methodology for treatment evaluation in patients with cancer metastatic to bone. J Natl Cancer Inst. 2001;93:534–538. doi: 10.1093/jnci/93.7.534. [DOI] [PubMed] [Google Scholar]
- 52.Coombes RC, Dady P, Parsons C. Assessment of response of bone metastases to systemic treatment in patients with breast cancer. Cancer. 1983;52:610–614. doi: 10.1002/1097-0142(19830815)52:4<610::aid-cncr2820520406>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 53.de Carvalho Barbosa M, Kosturakis AK, Eng C, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Wang XS, Cleeland CS, Dougherty PM. A quantitative sensory analysis of peripheral neuropathy in colorectal cancer and its exacerbation by oxaliplatin chemotherapy. Cancer Res. 2014;74:5955–5962. doi: 10.1158/0008-5472.CAN-14-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delaney A, Fleetwood-Walker SM, Colvin LA, Fallon M. Translational medicine: Cancer pain mechanisms and management. Br J Anaesth. 2008;101:87–94. doi: 10.1093/bja/aen100. [DOI] [PubMed] [Google Scholar]
- 55.Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12:649–659. doi: 10.1038/nrgastro.2015.166. [DOI] [PubMed] [Google Scholar]
- 56.Donovan PJ, Achong N, Griffin K, Galligan J, Pretorius CJ, McLeod DS. PTHrP-mediated hypercalcemia: Causes and survival in 138 patients. J Clin Endocrinol Metab. 2015;100:2024–2029. doi: 10.1210/jc.2014-4250. [DOI] [PubMed] [Google Scholar]
- 57.Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage. 2007;33:166–179. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Dubey A, Koul R. Bone metastasis – an overview. Internet J Pain, Symptom Contr Palliat Care. 2010;8:6. [Google Scholar]
- 59.Dworkin RH, Bruehl S, Fillingim RB, Loeser JD, Terman GW, Turk DC. Multidimensional diagnostic criteria for chronic pain: Introduction to the ACTTION-American Pain Society Pain Taxonomy (AAPT) J Pain. 2016;17:T1–T9. doi: 10.1016/j.jpain.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. 2016;17:T70–T92. doi: 10.1016/j.jpain.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falk S, Dickenson AH. Pain and nociception: Mechanisms of cancer-induced bone pain. J Clin Oncol. 2014;32:1647–1654. doi: 10.1200/JCO.2013.51.7219. [DOI] [PubMed] [Google Scholar]
- 62.Farquhar-Smith P. Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care. 2011;5:1–7. doi: 10.1097/SPC.0b013e328342f9cc. [DOI] [PubMed] [Google Scholar]
- 63.Farrell C. Bone metastases: Assessment, management and treatment options. Br J Nurs. 2013;22:S4, S6, S8–S11. doi: 10.12968/bjon.2013.22.Sup7.S4. [DOI] [PubMed] [Google Scholar]
- 64.Fasanella KE, Davis B, Lyons J, Chen Z, Lee KK, Slivka A, Whitcomb DC. Pain in chronic pancreatitis and pancreatic cancer. Gastroenterol Clin North Am. 2007;36:335–364. doi: 10.1016/j.gtc.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 65.Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, Widerstrom-Noga E, Arnold L, Bennett R, Edwards RR, Freeman R, Gewandter J, Hertz S, Hochberg M, Krane E, Mantyh PW, Markman J, Neogi T, Ohrbach R, Paice JA, Porreca F, Rappaport BA, Smith SM, Smith TJ, Sullivan MD, Verne GN, Wasan AD, Wesselmann U. The ACTTION-American Pain Society Pain Taxonomy (AAPT): An evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain. 2014;15:241–249. doi: 10.1016/j.jpain.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flatters SJ. The contribution of mitochondria to sensory processing and pain. Prog Mol Biol Transl Sci. 2015;131:119–146. doi: 10.1016/bs.pmbts.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155:367–376. doi: 10.1016/j.pain.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 68.Geber C, Breimhorst M, Burbach B, Egenolf C, Baier B, Fechir M, Koerber J, Treede RD, Vogt T, Birklein F. Pain in chemotherapy-induced neuropathy–more than neuropathic? Pain. 2013;154:2877–2887. doi: 10.1016/j.pain.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 69.Golan-Vered Y, Pud D. Chemotherapy-induced neuropathic pain and its relation to cluster symptoms in breast cancer patients treated with paclitaxel. Pain Pract. 2013;13:46–52. doi: 10.1111/j.1533-2500.2012.00554.x. [DOI] [PubMed] [Google Scholar]
- 70.Gordon-Williams RM, Dickenson AH. Central neuronal mechanisms in cancer-induced bone pain. Curr Opin Support Palliat Care. 2007;1:6–10. doi: 10.1097/SPC.0b013e328133f5e9. [DOI] [PubMed] [Google Scholar]
- 71.Gough N, Miah AB, Linch M. Nonsurgical oncological management of cancer pain. Curr Opin Support Palliat Care. 2014;8:102–111. doi: 10.1097/SPC.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 72.Gralow J, Tripathy D. Managing metastatic bone pain: The role of bisphosphonates. J Pain Symptom Manage. 2007;33:462–472. doi: 10.1016/j.jpainsymman.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Grant R, Papadopoulos SM, Greenberg HS. Metastatic epidural spinal cord compression. Neurol Clin. 1991;9:825–841. [PubMed] [Google Scholar]
- 74.Griffiths LA, Flatters SJ. Pharmacological modulation of the mitochondrial electron transport chain in paclitaxel-induced painful peripheral neuropathy. J Pain. 2015;16:981–994. doi: 10.1016/j.jpain.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hage WD, Aboulafia AJ, Aboulafia DM. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin North Am. 2000;31:515–528. vii. doi: 10.1016/s0030-5898(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 76.Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, Small EJ. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–2549. doi: 10.1200/JCO.2007.15.0367. [DOI] [PubMed] [Google Scholar]
- 77.Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN) Front Pharmacol. 2013;4:156. doi: 10.3389/fphar.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33:15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 79.Herdon CM. Pharmacologic management of cancer pain. J Neurosci Nurs. 2003;35:321–326. [PubMed] [Google Scholar]
- 80.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL, American Society of Clinical Oncology Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 81.Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, Minasian LM, Unger J. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group clinical trials. J Clin Oncol. 2016;34:3014–3022. doi: 10.1200/JCO.2015.66.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hertz DL, Roy S, Motsinger-Reif AA, Drobish A, Clark LS, McLeod HL, Carey LA, Dees EC. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann Oncol. 2013;24:1472–1478. doi: 10.1093/annonc/mdt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong JS, Tian J, Wu LH. The influence of chemotherapy-induced neurotoxicity on psychological distress and sleep disturbance in cancer patients. Curr Oncol. 2014;21:174–180. doi: 10.3747/co.21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janjan NA, Payne R, Gillis T, Podoloff D, Libshitz HI, Lenzi R, Theriault R, Martin C, Yasko A. Presenting symptoms in patients referred to a multidisciplinary clinic for bone metastases. J Pain Symptom Manage. 1998;16:171–178. doi: 10.1016/s0885-3924(98)00069-4. [DOI] [PubMed] [Google Scholar]
- 86.Johanes C, Monoarfa RA, Ismail RI, Umbas R. Anxiety level of early- and late-stage prostate cancer patients. Prostate Int. 2013;1:177–182. doi: 10.12954/PI.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kagohashi K, Satoh H, Ishikawa H, Ohtsuka M, Sekizawa K. Bone metastasis as the first manifestation of lung cancer. Int J Clin Pract. 2003;57:184–186. [PubMed] [Google Scholar]
- 88.Kirou-Mauro AM, Hird A, Wong J, Sinclair E, Barnes EA, Tsao M, Danjoux C, Chow E. Has pain management in cancer patients with bone metastases improved? A seven-year review at an outpatient palliative radiotherapy clinic. J Pain Symptom Manage. 2009;37:77–84. doi: 10.1016/j.jpainsymman.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 89.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S, Hruban RH. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 90.Krech RL, Walsh D. Symptoms of pancreatic cancer. J Pain Symptom Manage. 1991;6:360–367. doi: 10.1016/0885-3924(91)90027-2. [DOI] [PubMed] [Google Scholar]
- 91.Kurosaka S, Satoh T, Chow E, Asano Y, Tabata K, Kimura M, Tsumura H, Matsumoto K, Ishiyama H, Inoue Y, Hayakawa K, Baba S. EORTC QLQ-BM22 and QLQ-C30 quality of life scores in patients with painful bone metastases of prostate cancer treated with strontium-89 radionuclide therapy. Ann Nucl Med. 2012;26:485–491. doi: 10.1007/s12149-012-0598-z. [DOI] [PubMed] [Google Scholar]
- 92.Laird BJ, Walley J, Murray GD, Clausen E, Colvin LA, Fallon MT. Characterization of cancer-induced bone pain: An exploratory study. Support Care Cancer. 2011;19:1393–1401. doi: 10.1007/s00520-010-0961-3. [DOI] [PubMed] [Google Scholar]
- 93.Lam DK, Dang D, Flynn AN, Hardt M, Schmidt BL. TMPRSS2, a novel membrane-anchored mediator in cancer pain. Pain. 2015;156:923–930. doi: 10.1097/j.pain.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lam MG, De Klerk JM, Van Rijk PP. 186Re-HEDP for metastatic bone pain in breast cancer patients. Eur J Nucl Med Mol Imaging. 2004;31:S162–S170. doi: 10.1007/s00259-004-1539-4. [DOI] [PubMed] [Google Scholar]
- 95.Langford DJ, Tripathy D, Paul SM, West C, Dodd MJ, Schumacher K, Miaskowski C. Trajectories of pain and analgesics in oncology outpatients with metastatic bone pain. J Pain. 2011;12:495–507. doi: 10.1016/j.jpain.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lewis MA, Zhao F, Jones D, Loprinzi CL, Brell J, Weiss M, Fisch MJ. Neuropathic symptoms and their risk factors in medical oncology outpatients with colorectal vs. breast, lung, or prostate cancer: Results from a prospective multi-center study. J Pain Symptom Manage. 2015;49:1016–1024. doi: 10.1016/j.jpainsymman.2014.11.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM. The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci. 2015;35:13487–13500. doi: 10.1523/JNEUROSCI.1956-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Zhang H, Kosturakis AK, Cassidy RM, Zhang H, Kennamer-Chapman RM, Jawad AB, Colomand CM, Harrison DS, Dougherty PM. MAPK signaling downstream to TLR4 contributes to paclitaxel-induced peripheral neuropathy. Brain Behav Immun. 2015;49:255–266. doi: 10.1016/j.bbi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 100.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: Implications for diagnosis and classification. J Pain. 2016;17:T93–T107. doi: 10.1016/j.jpain.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malik B, Stillman M. Chemotherapy-induced peripheral neuropathy. Curr Neurol Neurosci Rep. 2008;8:56–65. doi: 10.1007/s11910-008-0010-5. [DOI] [PubMed] [Google Scholar]
- 102.Mantyh P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain. 2013;154(Suppl 1):S54–S62. doi: 10.1016/j.pain.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 103.Mantyh PW. Bone cancer pain: From mechanism to therapy. Curr Opin Support Palliat Care. 2014;8:83–90. doi: 10.1097/SPC.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Massey RL, Kim HK, Abdi S. Brief review: Chemotherapy-induced painful peripheral neuropathy (CIPPN): Current status and future directions. Can J Anesth. 2014;61:754–762. doi: 10.1007/s12630-014-0171-4. [DOI] [PubMed] [Google Scholar]
- 105.McCaffrey G, Thompson ML, Majuta L, Fealk MN, Chartier S, Longo G, Mantyh PW. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res. 2014;74:7014–7023. doi: 10.1158/0008-5472.CAN-14-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mendoza TR, Koyyalagunta D, Burton AW, Thomas SK, Phan MH, Giralt SA, Shah JJ, Cleeland CS. Changes in pain and other symptoms in patients with painful multiple myeloma-related vertebral fracture treated with kypho-plasty or vertebroplasty. J Pain. 2012;13:564–570. doi: 10.1016/j.jpain.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mercadante S, Catala E, Arcuri E, Casuccio A. Celiac plexus block for pancreatic cancer pain: Factors influencing pain, symptoms and quality of life. J Pain Symptom Manage. 2003;26:1140–1147. doi: 10.1016/j.jpainsymman.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 108.Miaskowski C, Cooper BA, Melisko M, Chen LM, Mastick J, West C, Paul SM, Dunn LB, Schmidt BL, Hammer M, Cartwright F, Wright F, Langford DJ, Lee K, Aouizerat BE. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer. 2014;120:2371–2378. doi: 10.1002/cncr.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mick G, Baron R, Correa-Illanes G, Hans G, Mayoral V, Frias X, Sintes D, Keller T. Is an easy and reliable diagnosis of localized neuropathic pain (LNP) possible in general practice? Development of a screening tool based on IASP criteria. Curr Med Res Opin. 2014;30:1357–1366. doi: 10.1185/03007995.2014.907562. [DOI] [PubMed] [Google Scholar]
- 110.Mieog JS, Morden JP, Bliss JM, Coombes RC, van de Velde CJ, IES Steering Committee Carpal tunnel syndrome and musculoskeletal symptoms in postmenopausal women with early breast cancer treated with exemestane or tamoxifen after 2–3 years of tamoxifen: A retrospective analysis of the Intergroup Exemestane Study. Lancet Oncol. 2012;13:420–432. doi: 10.1016/S1470-2045(11)70328-X. [DOI] [PubMed] [Google Scholar]
- 111.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40:872–882. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 112.Mitera G, Zeiadin N, Kirou-Mauro A, DeAngelis C, Wong J, Sanjeevan T, Sinclair E, Danjoux C, Barnes E, Tsao M, Sahgal A, Chow E. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39:259–267. doi: 10.1016/j.jpainsymman.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 113.Montgomery K, Laird B, Lesley C, Fallon M. A characterization study of breakthrough pain in cancer induced bone pain. Palliat Medicine. 2012;26:565–566. [Google Scholar]
- 114.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine DL, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau JL. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 115.Mulvey MR, Rolke R, Klepstad P, Caraceni A, Fallon M, Colvin L, Laird B, Bennett MI, IASP Cancer Pain SIG and the EAPC Research Network Confirming neuropathic pain in cancer patients: Applying the NeuPSIG grading system in clinical practice and clinical research. Pain. 2014;155:859–863. doi: 10.1016/j.pain.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 116.Nahman-Averbuch H, Yarnitsky D, Granovsky Y, Sprecher E, Steiner M, Tzuk-Shina T, Pud D. Pronociceptive pain modulation in patients with painful chemotherapy-induced polyneuropathy. J Pain Symptom Manage. 2011;42:229–238. doi: 10.1016/j.jpainsymman.2010.10.268. [DOI] [PubMed] [Google Scholar]
- 117.Niu L, Wang Y, Yao F, Wei C, Chen Y, Zhang L, Chen J, Li J, Zuo J, Xu K. Alleviating visceral cancer pain in patients with pancreatic cancer using cryoablation and celiac plexus block. Cryobiology. 2013;66:105–111. doi: 10.1016/j.cryobiol.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 118.Nomiya T, Teruyama K, Wada H, Nemoto K. Time course of pain relief in patients treated with radiotherapy for cancer pain: A prospective study. Clin J Pain. 2010;26:38–42. doi: 10.1097/AJP.0b013e3181b0c82c. [DOI] [PubMed] [Google Scholar]
- 119.Nudelman KN, McDonald BC, Wang Y, Smith DJ, West JD, O’Neill DP, Zanville NR, Champion VL, Schneider BP, Saykin AJ. Cerebral perfusion and gray matter changes associated with chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2016;34:677–683. doi: 10.1200/JCO.2015.62.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pachman DR, Watson JC, Lustberg MB, Wagner-Johnston ND, Chan A, Broadfield L, Cheung YT, Steer C, Storey DJ, Chandwani KD, Paice J, Jean-Pierre P, Oh J, Kamath J, Fallon M, Strik H, Koeppen S, Loprinzi CL. Management options for established chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2014;22:2281–2295. doi: 10.1007/s00520-014-2289-x. [DOI] [PubMed] [Google Scholar]
- 121.Paice JA. Chronic treatment-related pain in cancer survivors. Pain. 2011;152:S84–S89. doi: 10.1016/j.pain.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 122.Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. Ca Cancer J Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 123.Phillips LL. Managing the pain of bone metastases in the home environment. Am J Hosp Palliat Med. 1998;15:32–42. doi: 10.1177/104990919801500108. [DOI] [PubMed] [Google Scholar]
- 124.Pignataro RM, Swisher AK. Chemotherapy induced peripheral neuropathy: Risk factors, pathophysiology, assessment, and potential physical therapy interventions. Rehabil Oncol. 2010;28:10–18. [Google Scholar]
- 125.Pollen JJ, Schmidt JD. Bone pain in metastatic cancer of prostate. Urology. 1979;13:129–134. doi: 10.1016/0090-4295(79)90280-2. [DOI] [PubMed] [Google Scholar]
- 126.Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236–2247. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 127.Portenoy RK, Hagen NA. Breakthrough pain: Definition, prevalence and characteristics. Pain. 1990;41:273–281. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- 128.Reddy SM, Vergo MT, Paice JA, Kwon N, Helenowski IB, Benson AB, Mulcahy MF, Nimeiri HS, Harden RN. Quantitative sensory testing at baseline and during cycle 1 oxaliplatin infusion detects subclinical peripheral neuropathy and predicts clinically overt chronic neuropathy in gastrointestinal malignancies. Clin Colorectal Cancer. 2016;15:37–46. doi: 10.1016/j.clcc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 129.Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS, Hanna EY. Survival patterns in squamous cell carcinoma of the head and neck: Pain as an independent prognostic factor for survival. J Pain. 2014;15:1015–1022. doi: 10.1016/j.jpain.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riccio AI, Wodajo FM, Malawer M. Metastatic carcinoma of the long bones. Am Fam Physician. 2007;76:1489–1494. [PubMed] [Google Scholar]
- 131.Richardson PG, Laubach JP, Schlossman RL, Mitsiades C, Anderson K. Complications of multiple myeloma therapy, part 1: Risk reduction and management of peripheral neu-ropathy and asthenia. J Natl Compr Canc Netw. 2010;8:S4–S12. doi: 10.6004/jnccn.2010.0115. [DOI] [PubMed] [Google Scholar]
- 132.Rizzoli R, Body JJ, Brandi ML, Cannata-Andia J, Chappard D, El Maghraoui A, Gluer CC, Kendler D, Napoli N, Papaioannou A, Pierroz DD, Rahme M, Van Poznak CH, De Villiers TJ, El Hajj Fuleihan G. Cancer-associated bone disease. Osteoporos Int. 2013;24:2929–2953. doi: 10.1007/s00198-013-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodionov V, Bogomolova O, Rodionova M. Clinical symptoms of bone marrow metastases in breast cancer patients. Breast. 2011;20:S41. [Google Scholar]
- 134.Rustoen T, Moum T, Padilla G, Paul S, Miaskowski C. Predictors of quality of life in oncology outpatients with pain from bone metastasis. J Pain Symptom Manage. 2005;30:234–242. doi: 10.1016/j.jpainsymman.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 135.Rykowski JJ, Hilgier M. Efficacy of neurolytic celiac plexus block in varying locations of pancreatic cancer: Influence on pain relief. Anesthesiology. 2000;92:347–354. doi: 10.1097/00000542-200002000-00014. [DOI] [PubMed] [Google Scholar]
- 136.Ryo H, Sakai H, Yoneda S, Noguchi Y. Bone metastasis of primary lung cancer [in Japanese] Nihon Kokyuki Gakkai Zasshi. 1998;36:317–322. [PubMed] [Google Scholar]
- 137.Sansur CA, Pouratian N, Dumont AS, Schiff D, Shaffrey CI, Shaffrey ME. Part II: Spinal-cord neoplasms–primary tumours of the bony spine and adjacent soft tissues. Lancet Oncol. 2007;8:137–147. doi: 10.1016/S1470-2045(07)70033-5. [DOI] [PubMed] [Google Scholar]
- 138.Schmidt BL. The neurobiology of cancer pain. J Oral Maxillofac Surg. 2015;73:S132–S135. doi: 10.1016/j.joms.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schmidt BL. What pain tells us about cancer. Pain. 2015;156(Suppl 1):S32–S34. doi: 10.1097/j.pain.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scott AC, McConnell S, Laird B, Colvin L, Fallon M. Quantitative sensory testing to assess the sensory characteristics of cancer-induced bone pain after radiotherapy and potential clinical biomarkers of response. Eur J Pain. 2012;16:123–133. doi: 10.1016/j.ejpain.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 141.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 142.Sestak I, Sapunar F, Cuzick J. Aromatase inhibitor-induced carpal tunnel syndrome: Results from the ATAC trial. J Clin Oncol. 2009;27:4961–4965. doi: 10.1200/JCO.2009.22.0236. [DOI] [PubMed] [Google Scholar]
- 143.Sharma M, Simpson KH, Bennett MI, Gupta S. Practical Management of Complex Cancer Pain. Oxford: Oxford University Press; 2014. [Google Scholar]
- 144.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Ca Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 145.Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, Schwartz AG, Brown LM, Greenberg RS, Schoenberg JB, Pottern LM, Hoover RN, Fraumeni JF., Jr Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80:1830–1837. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Slosky LM, Largent-Milnes TM, Vanderah TW. Use of animal models in understanding cancer-induced bone pain. Cancer Growth Metastasis. 2015;8:47–62. doi: 10.4137/CGM.S21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: Chemotherapy-induced peripheral neuropathy. Cancer. 2012;118:2250–2260. doi: 10.1002/cncr.27463. [DOI] [PubMed] [Google Scholar]
- 148.Taverner T. Neuropathic pain in people with cancer (part 1): Incidence, manifestation, and assessment. Int J Palliat Nurs. 2014;20:442–447. doi: 10.12968/ijpn.2014.20.9.442. [DOI] [PubMed] [Google Scholar]
- 149.Tersmette AC, Petersen GM, Offerhaus GJ, Falatko FC, Brune KA, Goggins M, Rozenblum E, Wilentz RE, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738–744. [PubMed] [Google Scholar]
- 150.Themistocleous AC. The pain in neuropathy study (PiNS): A cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157:1132–1145. doi: 10.1097/j.pain.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tofthagen C, Visovsky CM, Hopgood R. Chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2013;17:138–144. doi: 10.1188/13.CJON.138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toyama S, Shimoyama N, Ishida Y, Koyasu T, Szeto HH, Shimoyama M. Characterization of acute and chronic neuropathies induced by oxaliplatin in mice and differential effects of a novel mitochondria-targeted antioxidant on the neuropathies. Anesthesiology. 2014;120:459–473. doi: 10.1097/01.anes.0000435634.34709.65. [DOI] [PubMed] [Google Scholar]
- 153.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JW, Wang SJ. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trinkaus M, Simmons C, Myers J, Dranatisaris G, Clemons M. Skeletal-related events (SREs) in breast cancer patients with bone metastases treated in the nontrial setting. Support Care Cancer. 2010;18:197–203. doi: 10.1007/s00520-009-0645-z. [DOI] [PubMed] [Google Scholar]
- 155.Turk DC, Fillingim RB, Ohrbach R, Patel KV. Assessment of psychosocial and functional impact of chronic pain. J Pain. 2016;17:T21–T49. doi: 10.1016/j.jpain.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 156.Vardeh D, Mannion RJ, Woolf CJ. Towards a mechanism based pain diagnosis. J Pain. 2016;17:T50–T69. doi: 10.1016/j.jpain.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ventzel L, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Chemotherapy-induced pain and neuropathy: A prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain. 2016;157:560–568. doi: 10.1097/j.pain.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 158.Vichaya EG, Wang XS, Boyette-Davis JA, Mendoza TR, He Z, Thomas SK, Shah N, Williams LA, Cleeland CS, Dougherty PM. Subclinical pretreatment sensory deficits appear to predict the development of pain and numbness in patients with multiple myeloma undergoing chemotherapy. Cancer Chemother Pharmacol. 2013;71:1531–1540. doi: 10.1007/s00280-013-2152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wilson KG, Chochinov HM, Allard P, Chary S, Gagnon PR, Macmillan K, De Luca M, O’Shea F, Kuhl D, Fainsinger RL. Prevalence and correlates of pain in the Canadian National Palliative Care Survey. Pain Res Manag. 2009;14:365–370. doi: 10.1155/2009/251239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wong K, Zeng L, Zhang L, Bedard G, Wong E, Tsao M, Barnes E, Danjoux C, Sahgal A, Holden L, Lauzon N, Chow E. Minimal clinically important differences in the brief pain inventory in patients with bone metastases. Support Care Cancer. 2013;21:1893–1899. doi: 10.1007/s00520-013-1731-9. [DOI] [PubMed] [Google Scholar]
- 161.Yang Y, Zhang YG, Lin GA, Xie HQ, Pan HT, Huang BQ, Liu JD, Liu H, Zhang N, Li L, Chen JH. Spinal changes of a newly isolated neuropeptide endomorphin-2 concomitant with vincristine-induced allodynia. PLoS One. 2014;9:e89583. doi: 10.1371/journal.pone.0089583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zedan AH, Vilholm OJ. Chemotherapy-induced polyneuropathy: Major agents and assessment by questionnaires. Basic Clin Pharmacol Toxicol. 2014;115:193–200. doi: 10.1111/bcpt.12262. [DOI] [PubMed] [Google Scholar]


