Abstract
mRNA vaccines elicit a potent immune response including antibodies and cytotoxic T cells. mRNA vaccines are currently evaluated in clinical trials for cancer immunotherapy applications, but also have great potential as prophylactic vaccines. Efficient delivery of mRNA vaccines will be key for their success and translation to the clinic. Among potential nonviral vectors, lipid nanoparticles are particularly promising. Indeed, lipid nanoparticles can be synthesized with relative ease in a scalable manner, protect the mRNA against degradation, facilitate endosomal escape, can be targeted to the desired cell type by surface decoration with ligands, and as needed, can be codelivered with adjuvants.
Keywords: : adjuvant, cancer immunotherapy, cationic lipid, drug delivery, lipid nanoparticle, mRNA, oligonucleotide, therapeutic vaccine, vaccine
Recently, mRNA vaccines have generated significant interest to complement or even replace traditional vaccines due to a number of important attributes that they possess. Although subunit vaccines have been used successfully to elicit humoral immunity against a wide variety of pathogens, they fail to induce cellular immunity which is required to eradicate the intracellular pathogen reservoir of many chronic diseases, including viral infections such as HIV or hepatitis C. Live-attenuated vaccines are the most potent in activating both arms of the adaptive immune system – cellular and humoral immunity. However, these vaccines exhibit considerable safety drawbacks. Indeed, attenuated pathogens have the very rare potential to revert to a pathogenic form and cause disease. This is of special concern in immune deficient individuals, or in immunosuppressed patients, where guidelines generally recommend that no live-attenuated vaccines should be administered [1]. Subunit vaccines have been developed as a safer alternative, while recognizing that they are less efficient and often require adjuvants.
With the vaccine limitations outlined above in mind, mRNA vaccines combine the advantages of subunit vaccines and live-attenuated vaccines without the risks associated with live-attenuated or DNA vaccines. Successful cytosolic delivery of mRNA, encoding for an antigen, results in vaccine epitope synthesis of the transfected cells. The presence of clearly defined antigens in the cytosol can enable presentation of both endogenous and exogenous antigens, and provide T-cell activation while being safe [2–4].
The promise of activating the humoral and the cellular arms of the immune system has driven the development of DNA vaccines over the last decades. In fact, DNA and mRNA vaccines share many similarities, where the main difference between the two vaccines is the target location for the delivery of the oligonucleotides. DNA therapeutics have to reach the nucleus, while for mRNA therapeutics, the cytosol is the target. As a result, mRNA therapeutics are easier to deliver because they do not require crossing the nuclear membrane. In addition, even if mRNA reaches the nucleus, it does not integrate itself or alters the genome [5]. Although recombination among single-stranded RNA is rarely possible, cytosolic mRNA has no interaction with the genome [6]. Moreover, mRNA essentially represents the minimal genetic information, and is only transiently expressed until the mRNA has been degraded. mRNA can encode multiple proteins possessing very different chemical and physical properties, while leaving its physiochemical properties largely unaffected. Accordingly, mRNA provides the technological basis to deliver a wide variety of antigens, modulators and cell-signaling factors in a single molecule. Simultaneously, mRNA exhibits self-adjuvating properties in that it binds to pattern-recognition receptors like TLR7 that promote cellular immunity [7,8]. Finally, mRNA synthesis and purification are fast, easy and low cost when compared with other vaccines.
The main challenge faced by mRNA vaccines for clinical approval is their intracellular delivery. Because of its sensitivity toward catalytic hydrolysis by omnipresent ribonucleases [9], mRNA is highly unstable under physiological conditions. Therefore, unprotected mRNA delivered by itself is unsuitable for broad therapeutic applications, and was therefore ignored by the pharmaceutical industry for a long time. It was the development of RNA interference and its tremendous therapeutic potential that triggered intense efforts toward stabilization of RNA in vivo. Several strategies have been developed for RNA delivery, including RNA-conjugates, modified RNA, viral vectors and microparticles and nanoparticles [10–12]. While linking RNA to molecules offers some level of protection against degradation, it can promote binding to serum proteins and subsequent aggregation that can lead to vascular blockage [13]. Viral vectors were the obvious choice for delivery, because viruses have naturally evolved to become highly efficient at nucleic-acid delivery. However, several limitations are generally associated with these vectors, including immunogenicity [14], carcinogenesis [15], broad tropism [16], packaging capacity [17] and production difficulties [18]. In contrast to viral analogues, nonviral vectors exhibit significantly reduced transfection efficiency but tend to have lower immunogenicity than viruses and patients do not have pre-existing immunity against the nonviral vector. Furthermore, nonviral vectors, whose sizes are larger than those of viruses, have the potential to carry larger genetic payloads, while at the same time being simple to synthesize. With the development of new materials and preparation techniques, as well as a better understanding of the mechanisms involved, nonviral vectors are becoming the preferred vehicle to deliver mRNA [19–22]. The most common technologies use lipids [23], polymers [24], followed by peptides [25] and inorganic nanoparticles [26].
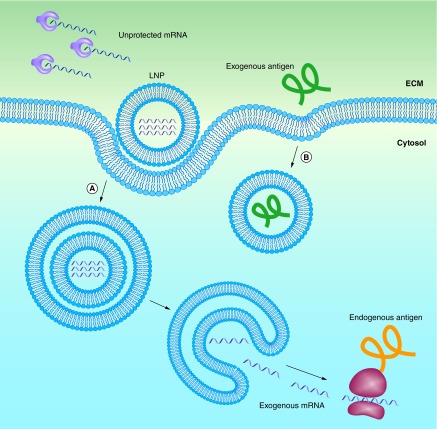
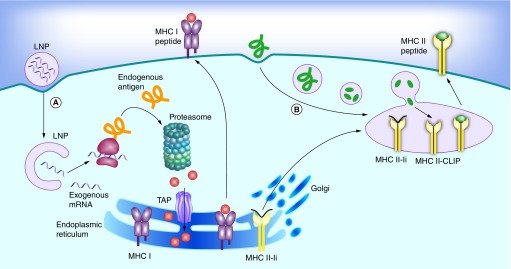
Independent of the materials or technologies used, ‘good’ nonviral vectors should: efficiently bind and condense RNA, protect against degradation in the extracellular space and localize the payload at the membrane of the desired target cell, followed by cellular uptake and endosomal escape into the cytosol. This process, along with the barriers that need to be overcome, is outlined schematically in Figure 1A. Note that this is much more than what is needed for the delivery of protein or peptide antigens, where endocytosis is sufficient (Figure 1B). Currently, lipid nanoparticles (LNPs) are among the most frequently used vectors for in vivo RNA delivery [27]. Although most of the work on LNPs is aimed at treating genetic conditions in a number of different tissues, a considerable amount of work aims to target the immune system (Table 1). The most important targets for mRNA vaccines are professional antigen presenting cells (APCs), with dendritic cells (DCs) likely being the most relevant cell type. Indeed, DCs play a critical role in antigen processing and presentation to elicit an immune response against specific antigens. The transfected DCs express the mRNA-encoded antigen in the native form. The antigens are subsequently processed by the proteasome, and the generated peptide epitopes enter the endoplasmic reticulum where they are loaded onto major histocompatibility complex (MHC) class I molecules. The MHC class I molecules are transported to the surface of the cell where the epitopes are presented to CD8 T cells along with costimulatory signals (Figure 2A). Presentation of antigen fragments on MHC II induces antigen-specific antibodies. The MHC class II pathway may be further enhanced with mRNA coding for both the antigen and the lysosomal sorting signal LAMP1. This entire process is depicted schematically in Figure 2A, and has been thoroughly reviewed by Heath and Carbone [28]. Note that this is different from protein or peptide antigens which are degraded in the late endosome and loaded on MHCII for presentation to CD 4 T cells (Figure 2B). There is a pathway for the presentation of protein antigens on MHCI termed cross-presentation. However, this process is not yet fully understood, and is often too weak to elicit a potent cytotoxic immune response [29].
Figure 1. . Lipid nanoparticles protect mRNA from degradation, and facilitate endocytosis and endosomal escape.
(A) mRNA can be encapsulated in lipid nanoparticles (LNPs) for protection from enzymatic degradation. A positively charged LNP favors localization of mRNA at the negatively charged cell membrane, including subsequent endocytosis into the cytosol. In order to be transcribed, the mRNA must escape both the LNP and the endosome. (B) Extracellular proteins based vaccines are endocytosed in a similar manner, but do not need to escape from the endosome to be presented on MHCII.
Table 1. . Overview of published lipid nanoparticle mRNA vaccines used in vivo.
| Composition of lipids used | Size (nm) | Zeta-potential (mV) | Antigen | Species | mRNA dose | Successful administration routes | Ref. |
|---|---|---|---|---|---|---|---|
| PC, PS, cholesterol |
<200 |
|
Influenza virus nucleoprotein |
Mice |
N/A |
IV, SC |
[30] |
| DOTAP |
|
|
OVA |
Mice |
2 × 5 µg |
IV, ID |
[31] |
| DOTAP, DOPE |
|
|
OVA |
Mice |
2 × 3 µg |
IV, ID |
[31] |
| HVJ-liposome made from: PS, PC, cholesterol |
|
|
gp100 |
Mice |
2 × 8 µg |
Intraspleenic |
[32] |
| DOTAP, DOPE |
|
|
HIV gag |
Mice |
2 × 20 µg |
SC |
[33] |
| Unifectin, protamine |
|
|
B-Gal |
Mice |
1 × 30 µg |
IV, SC, ID |
[34] |
| Histidylated lipoplex |
60–100 |
|
MART-1 |
Mice |
2 × 12.5 µg |
IV |
[35] |
| Man11-LPR100 |
140–170 |
+17 to +25 |
MART-1 |
Mice |
2 × 25 µg |
IV |
[36] |
| Stemfect transfection kit (Stemgent) |
180/300† |
+40/-12† |
OVA |
Mice |
3 × 9 µg |
IN |
[37] |
| DSPC, cholesterol, PEG DMG 2000, DLinDMA |
130–165 |
|
RSV-F rep. HIV gp |
Mice |
2 × 0.01 µg |
IM |
[38] |
| Squalene, Span 85, DOTAP |
129 |
+30.1 |
RSV-F rep. |
Mice |
2 × 0.15 µg |
IM |
[39] |
| Squalene, Span 85, DOTAP |
129 |
+30.1 |
HIV gp140 rep. |
Rabbits |
2 × 25 µg |
IM |
[39] |
| Squalene, Span 85, DOTAP | 129 | +30.1 | IE-1 hCMV rep. | Macaques | 2 × 75 µg | IM | [39] |
DLinDMA: 1,2-dilinoleyloxy-3-dimethylaminopropane; DOPE: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP: 1,2-Dioleyl-3-trimethylammonium-propane chloride salt; DSPC: 1,2-Diastearoyl-sn-glycero-3-phosphocholine; Histidylated lipoplex: PEGylated derivative of histidylated polylysine and L-histidine-(N,N-di-n-hexadecylamine)ethylamide liposomes; HVJ-liposome: liposome with fusion proteins derived from the hemagglutinating virus of Japan (HVJ); ID: Intradermal; IM: Intramuscular; IN: Intranodal; IV: Intravenous; Man11-LPR100: Mannosylated and histidylated lipopolyplexes (Man11-LPR100) obtained by adding mannosylated and histidylated liposomes to mRNA-PEGylated histidylated polylysine polyplexes; PC: Dipalmitoylphosphatidylcholine; PEG DMG 2000: 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]; PS: Phosphatidylserine; SC: Subcutaneous; Span 85: sorbitane trioleate.
†In water/in 10% FBS buffer.
Figure 2. . Antigen presentation on MHC I and II pathways in dendritic cells.
(A) Endogenous proteins with pathogen or self origin are primarily displayed on the MHC I pathway. These proteins are degraded into smaller peptides by the proteasome. The peptides are transported into the endoplasmic reticulum for loading onto the MHC class I molecules. This MHC I–peptide complex is then displayed at the cell surface to CD8 T-cells. (B) On the other hand, proteins that enter the cell on the endocytic route are displayed on the MHC II pathway. For this purpose, the MHC class II molecules are protected with the invariant chain (Ii) from binding to endogenous peptides in the endoplasmic reticulum. The MHC II-Ii complex is then exported through the Golgi to the MIIC/CIIV compartment, where the invariant chain is replaced with antigens. The MHC II–peptide complex is then displayed at the cell surface to CD4 T-cells.
LNPs generally consist of an aqueous core surrounded by a lipid bilayer shell that is made of a combination of different lipids, each serving distinct functions [40]. However, other structures have been reported [41,42]. Most LNP formulations rely on cationic lipids to efficiently complex the negatively charged RNA, although some anionic and neutral formulations have been used in the past [23]. Because several studies have shown that cationic lipids bearing a permanent positive charge are more toxic and less efficient [43], the potency of LNPs has been advanced significantly with the development of new, ionizable lipids and lipid-like materials [44]. This new generation of lipids and lipidoids contains amine groups which maintain a neutral or mildly cationic surface charge at physiological pH, thereby reducing nonspecific lipid–protein interactions and facilitating oligonucleotide release in the cytosol. In the endosome, the amine groups are thought to be ionized upon acidification and help to induce hexagonal phase structures, which disrupt the membrane of the late endosomes. This, in turn, facilitates cellular uptake and endosomal escape of mRNA into the cytoplasm [45,46]. Some of these ionizable lipids were identified by systematically modifying the polar head and nonpolar tail structures of the lipids [47–49] and others were discovered by combining large structural libraries into lipid-like lipidoids [50–52].
In addition to ionizable cationic lipids, phospholipids, cholesterol and lipid-anchored polyethylene glycol (PEG) are the most commonly used components for LNP formulations. Generally, phospholipids play a structural role in LNPs. They help with the formation and disruption of the lipid bilayer to facilitate endosomal escape. Furthermore, some phospholipids possess polymorphic features and promote a transition from a lamellar to a hexagonal phase in the endosome [53,54]. In addition, the negatively charged phosphate group appears to be involved in cationic charge neutralization, which is important for phase changes and endosomal escape [55–57]. Cholesterol serves as a stabilizing element in LNPs and plays a crucial role in the transfection of cells [58,59]. Increasing the cholesterol content in LNPs is associated with a lower transition temperature, which aids in the transition from lamellar to hexagonal phases [60]. The transition to the hexagonal phase is important for the release of the mRNA from the LNP and its translocation across the endosomal membrane [61]. Lipid-anchored PEGs preferentially deposit on the LNP surface, where they act as a barrier which sterically stabilizes the LNP and reduces nonspecific binding to proteins [62]. The PEG coating strongly influences the properties of the LNPs and has to be tailored carefully. A higher PEG content usually increases the blood circulation time of LNPs, while reducing cellular uptake and interaction with the endosomal membrane [63–65]. LNPs are incredibly versatile. Indeed, water-soluble molecules, such as proteins and carbohydrates, can be entrapped within the LNP aqueous core, whereas lipophilic compounds can be incorporated into the LNP lipid bilayer. This, in turn, can facilitate the codelivery of immunopotentiators, also known as adjuvants, which is important to enhance vaccine efficacy [66,67]. The surface of an LNP may be decorated with specific targeting sequences which help with homing and subsequent uptake. LNPs could even be simultaneously formulated with multiple antigens, signaling factors and adjuvants for tailored applications. Some of these LNP synthesis strategies are well established and will be reviewed in the following section.
Synthesis of lipid nanoparticles
The method via which LNPs are synthesized is critical, because it directly affects both the LNP size and encapsulation efficiency. In general, LNPs are formed by condensing lipids from an ethanol solution in water. Depending on the LNP synthesis method, mRNA is dissolved in the aqueous phase and encapsulated in the condensation process, or is complexed to the finished LNPs in a second step. The theory of vesicle formation assumes that LNP formation is based on disk-like bilayered fragments whose edges are stabilized by ethanol [68]. When diluting ethanol in water, these planar fragments grow and fuse to even bigger rafts. At low ethanol concentrations, the destabilized structures bend to form closed LNPs. The faster the increase in the polarity of the ethanol solution, the smaller the fragments will be before closing into vesicles, resulting in overall smaller LNPs. Two important factors that directly influence the rate at which the polarity of the ethanol solution changes are the rate of mixing and the volumetric ratio between the aqueous and lipid phases [69,70]. The mixing rate, for example, influences both the size and the homogeneity of the LNPs. The properties of individual LNPs strongly depend on local, microscopic mixing rates, where diffusive transport effects can lead to LNPs with variable compositions. Therefore, rapid mixing of the ethanol–lipid phase with excess water is key for the synthesis of small, uniform LNPs.
Early synthesis methods relied on the formation of micrometer-sized vesicles by suspending lipids in water, followed by sonication to produce submicrometer-sized particles [71]. This top–down approach has many limitations, including molecular degradation, contamination and lack of scalability. Other synthesis methods include the condensation of a lipid ethanol solution by rapid injection into a vigorously stirred aqueous buffer [72]. The preformed vesicles are then complexed with RNA in slightly acidic ethanol–water solutions [73,74]. However, this synthesis method lacks reproducibility due to variable injection and mixing rates. Extrusion of a lipid film through a small filter has also been a very popular synthesis method, and has often been used at the laboratory scale using syringe miniextruders [75]. Newer synthesis methods directly mix the lipid–ethanol phase with an aqueous solution of mRNA in a small T-piece [76]. Here, the flow, and hence, the mixing rates, can be controlled with pumps. In this way, LNPs with diameters of 70 nm or larger and high encapsulation efficiencies can be generated [47]. The macroscopic mixing techniques mentioned above enable a wide range of local mixing rates, leading to LNPs with high polydispersity and often poor reproducibility. Microfluidic mixing, such as, hydrodynamic flow focusing, was developed to generate more uniform particles [77]. However, with hydrodynamic flow focusing, small particles are only generated with ethanol–water flow ratios of 30 or higher, which leads to substantial dilution [78]
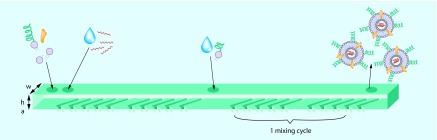
Higher mixing rates, with minimal mass transport effects, are achieved with staggered herringbone micro mixers, as depicted in Figure 3 [79]. A series of herringbone structures induce a rotational chaotic flow, essentially wrapping the fluids into one another. This phenomenon is also termed turbulent flow. In this way, the microfluidic device enables extremely rapid mixing of two fluids, with an associated fast increase in the polarity of the lipid solution. The time required for mixing in the staggered herringbone micro mixer, tmix, decreases with the flow velocity, U, as follows: tmix ˜ λ/[U ln(Ul/D)], where λ and l are parameters determined by the geometry of the microfluidic device and D is the diffusion coefficient [79]. At low flow rates, mixing rates are also low, leading to larger LNPs as previously described. Belliveau et al. further investigated the effect of flow rate on the size and polydispersity of LNPs generated with the staggered herringbone micro mixer. It was determined that increasing the total flow rate from 0.02 to 4 ml/min results in a continuous decrease in the polydispersity of the LNPs. The size of the LNPs remained constant at flow rates above 2 ml/min [63]. Zhigaltsev et al. varied the aqueous/ethanol flow rate ratios, and found that limit-sized particles can be generated with a flow rate ratio of 3/1. Limit-size systems are defined in this context as the smallest achievable aggregates compatible with the packing of the molecular constituents in a defined and energetically stable structure [70]. These finding suggests that with an aqueous flow rate of 1.5 ml/min and an ethanol flow rate of 0.5 ml/min, monodisperse limit-sized particles can be generated. Leung et al. used the staggered herringbone micromixer to encapsulate plasmid DNA and negatively charged gold nanoparticles into LNPs containing cationic lipids [80].
Figure 3. . Staggered herringbone mixer for lipid nanoparticle synthesis.
Lipids dissolved in ethanol and an aqueous buffer of mRNA are pumped into the two primary inlets of the microfluidic mixer using syringe pumps. The herringbone structures induce chaotic advection in the laminar flow that enables rapid mixing of ethanol and the aqueous phase. Although the mixing time depends on the flow rate, approximately 15 cycles are needed for complete mixing. The optional secondary inlet can be used to prevent lipid nanoparticle fusion by further dilution with buffer, or to add water-soluble lipid derivatives to the lipid nanoparticles. Approximate dimension are w = 200 μm, h = 77 μm, a = 18 μm.
The staggered herringbone micromixer offers a number of advantages over other synthesis methods. High encapsulation efficiency and the ability to generate small particles are among the most obvious advantages. Minimal material loss due to small dead volumes and low flow rate ratios are also important attributes for the synthesis of mRNA–LNPs. Furthermore, massive parallelization of the microfluidic device in a variety of materials is possible, enabling large-scale, pharmaceutical-grade synthesis. For these reasons, we expect that microfluidic mixing using the staggered herringbone structure will be one of the synthesis methods of choice going forward, for both small- and large-scale synthesis of LNPs and possibly other nanoparticle systems.
Targeting antigen-presenting cells
Decorating the LNPs with immune cell receptors may facilitate the uptake by the desired type of immune cells. For the immune system to be activated, or for an immune response to be elicited, professional APCs need to encounter an antigen and a danger signal. APCs are concentrated at high density in lymph nodes (LNs). For LN targeting, mRNA can be injected directly in the LNs, or LNPs can be designed to accumulate in the LNs. We will discuss direct LN injection in the next section, and focus here on the tailoring of LNPs for LN accumulation. The two most important parameters for LN accumulation are LNP size and surface composition. Generally, reports indicate decreasing lymphatic uptake with increasing LNP size. Only small LNPs with a diameter smaller than about 150 nm appear to enter the lymphatic capillaries, and are subsequently drained to the peripheral lymphatics [81–83]. On the other hand, larger LNPs are retained at the injection site [84,85]. Larger LNPs are believed to be recognized and cleared more rapidly by the complement system because they present a larger number of recognition sites on their surface [86].
Coating the particles with a PEG-containing lipid can reduce complement activation. The right amount of PEG coating on the LNPs is critical. A recent study by Carstens et al. showed that PEG coating clearly improves lymphatic drainage. A similar study by Kaur et al. came to the same conclusion for the LNPs that they considered [87,88]. However, improved lymphatic drainage does not automatically translate in a more potent immune response. The observed enhancement in lymphatic drainage is possibly due to a higher shielding of the LNPs’ cationic charges against unspecific interactions with proteins [89]. Interestingly, a higher PEG content in the LNPs is also known to adversely affect cellular LNP uptake via endocytosis and endosomal escape [90,91]. It is well known that enhanced PEGylation of LNPs leads to longer blood circulation times. However, anti-PEG antibody response following repeated intravenous (IV) administration of PEGylated LNPs has been reported to dramatically accelerate blood clearance of the LNPs and to lead to acute hypersensitivity [92,93]. This finding is very concerning for immunotherapy applications, where multiple dosing may be required for long-lasting protection. A possible solution may be found by modifying the PEG molecule into a less immunogenic variant, or by using different administration routes.
Active targeting of DCs has been studied extensively in recent years. The term active targeting is somewhat misleading in that the LNPs are not actually actively guided toward DCs. Instead, by decorating the LNP surfaces with suitable molecules, uptake by DCs is enhanced. DCs are studded with different receptors, including lectins that recognize carbohydrate moieties present on many pathogens, and are involved in antigen capture and presentation. A wide variety of different DC receptors have been identified, including the mannose receptor [94], DC-SIGN [95], DEC-205 [96] and Langerin [97]. In recent years, these receptors have been characterized and used for targeted protein and protein–LNP vaccines. Initial experiments used mannose monosaccharides or disaccharides to target vaccines to DCs, often with little success [98]. The binding affinity of such monosaccharaides is very weak, typically in the mM range. The apparent affinity can be enhanced by orders of magnitude by coupling the monosaccharaides to a scaffold that forms a multivalent cluster or by using multibranched saccharides [99,100]. Pharmacokinetics and biodistribution of such LNPs is altered significantly when varying the density of the sugar moieties [100]. A high DC specificity was observed for LNPs containing 11% mannosylated lipids, while no specificity was observed for LNPs containing 3% mannosylated lipids [101]. Mannosylated mRNA–LNPs coding for MART-1 also showed higher vaccination rates compared with their nonmannosylated analogs [36]. It would be interesting to investigate if decorating LNPs with ligands for different DC subsets also increases the potency of mRNA vaccines, as has been shown in the case of protein-based vaccines [102].
Adjuvanting lipid nanoparticles
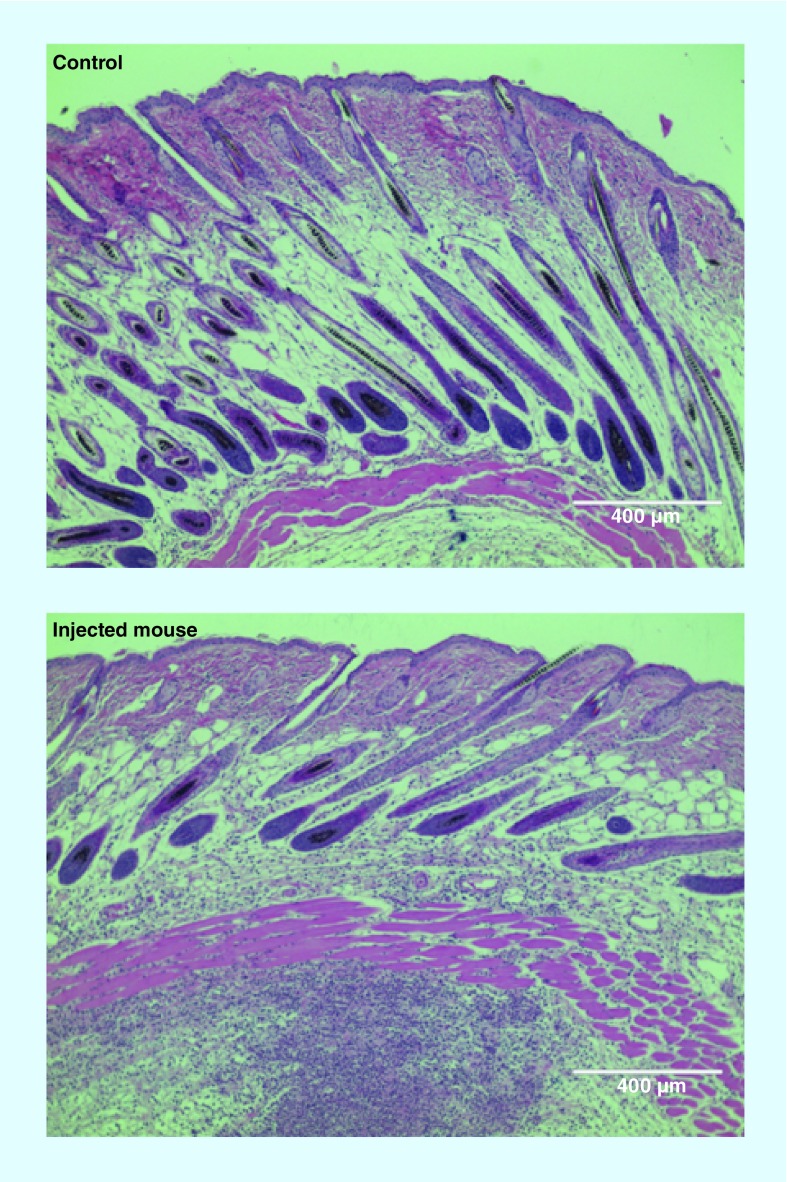
Adjuvants can be added readily to LNPs to increase the immune response. To this end, ongoing research needs to identify the best adjuvant candidates and effective doses. Aluminum salts were first used to enhance the immune response of traditional vaccines [103]. The role of such adjuvants was initially related to the depot effect that prolonged antigen exposure, but is still not understood in detail. The LNP vector can have an adjuvant effect by itself [104]. Some of the lipids can activate the immune system and are able to induce inflammation. Activation of the immune system is a problem for gene therapy delivery in protein replacement therapy, but is a desirable advantage for vaccination. In particular, LNPs containing cationic lipids, such as 1,2-dioleyl-3-trimethylammonium-propane chloride salt, have been shown to activate Toll-like receptor 4 (TLR4) and induce a strong proinflammatory response with Th1 type cytokines, including IL-2, IFN-γ and TNF-α [105]. Indeed, the proinflammatory effect of LNPs is something that we have observed after injection of LNPs containing ionizable cationic lipid (Figure 4). A strong monocyte infiltration is observed 24 h after the injection of LNPs containing mRNA coding for GFP.
Figure 4. . Injection of a cationic lipid nanoparticle formulation into the SC induces inflammation.
The control figure is a mouse skin section 24 h after a saline injection. The injected mouse figure corresponds to a mouse skin section 24 h after lipid nanoparticle injection, coding for green fluorescent protein. An infiltration of monocytes, characterized by a higher density of blue dots, is visible below the cutaneous muscle layer. The lipid nanoparticle consisted of C12-200, DOPE, cholesterol, and a PEGylated lipid.
TLRs are a class of receptors expressed on APCs that recognize structurally conserved molecular motifs from pathogens. TLRs have become the target of adjuvant development because following their activation, cytokines are produced, which trigger inflammation [106]. Including adjuvants with the LNPs provides a way to further increase the potency of the vaccine and guide the immune response in the desired direction. Currently, a wide variety of different adjuvants have been tested, primarily with protein–LNP vaccines. For example, Yanasarn et al. have evaluated the adjuvant effect of neutral, cationic and anionic protein-carrying LNPs [107]. Others have incorporated the bacteria derived monophosphoryl lipid A into their LNPs. This resulted in more potent vaccines than those obtained using nonlipid A formulations [108–110]. Other adjuvants include hydrophilic oligonucleotides, such as, the unmethylated dinucleotides CpG, which are similar to bacterial DNA and trigger TLR9 receptors [111]. Protein vaccines coencapsulated with CpGs in liposomes showed an improved cellular immune response and different antibody response compared with the protein alone [112,113]. An exciting approach was reported by Wu et al. who used the medicinal chemistry potential of the pharma company Novartis to develop TLR agonists small-molecule immune potentiators to tune the immune activation and to limit side effects [114]. We would expect that small molecule TLR agonists could be tailored for formulation in LNPs, and produced at a much lower cost than many of the ligands used today.
However, there is growing evidence that the addition of non-mRNA adjuvants may not be necessary. Mammalian cells can sense foreign mRNA with so-called pattern-recognition receptors. These include the innate immune receptors TLR3, TLR7 and TLR8 that are located in the endosomes and sampling its content [115]. The cytosol is sampled for nonself mRNA by cytoplasmic innate immune receptors, the retinoic acid-inducible gene I (RIG-I), the protein kinase RNA-activated (PKR), 2′–5′-oligoadenylate synthase (OAS) and the melanoma differentiation-associated antigen 5 (MDA5) [116]. Activation of these receptors results in upregulation of transcription of genes coding for type I interferons, proinflammatory cytokines: IL-6, Il-12, TNF and chemokines. Furthermore, via phosphorylation of eukaryotic translation initiation factor 2α (eiF2α), the protein translation will be slowed down and ultimately inhibited [117]. activation of OAS leads to overexpression of RNase L that degrades foreign and cellular RNA [118]. These receptor-mediated responses have evolved to protect cells from viral RNA and help mediate an antiviral immune response. For the purpose of mRNA-mediated protein replacement therapy, this is a major problem that can be overcome by the use of naturally occurring modified nucleotides to suppress activation of these innate immune receptors [119,120]. However, for vaccine applications, it remains to be determined weather modified mRNA, omitting stalled translation and enhanced protein degradation, or unmodified mRNA activating the innate immune system will perform better.
A particularly innovative approach has been developed by the German biotech company CureVac, who tailored both mRNA stability and immunogenicity by optimizing the nucleotide sequence, and hence the codon sequence, while relying exclusively on unmodified nucleotides that translate into the same amino acid sequence. Their RNA adjuvant consists of a single-stranded, noncoding, noncapped RNA sequence containing several poly U-repeats that is complexed with a polymeric carrier to increase stability against degradation [121]. This general adjuvant not only increases the immunogenicity of mRNA vaccines, but also works for peptide and protein vaccines [121,122]. An issue that has to be analyzed in detail is the fact that, through codon optimization, we do obtain the same full-length protein but a different set of cryptic peptides. Translation of alternative out of frame open reading frames or from alternative starts sites, including noncanonical triplets such as CUG, ACG and GUG, lead to shorter so called cryptic peptides [123]. These shorter peptides are presented on MHC complexes and hence are alternative antigens for immune recognition [124]. These naturally occurring cryptic peptides may contribute to a therapeutic immune response, and may be lost upon codon optimization, as a different nucleotides sequence leads to a different set of cryptic peptides [125]. Although these optimized sequences are sufficiently stable to work without any vector, it remains to be seen if they would improve in efficiency if they are delivered in an LNP vector that helps with endosomal escape.
Routes of administration
In order to mount a strong adaptive immune response, a vaccine needs to reach the LNs, where T-cell activation and proliferation occurs. Furthermore, affinity maturation and isotype switching of antibodies takes place in germinal centers in the LNs. In order to target these sites, LNPs need to be tailored carefully. Properties like LNP composition, charge, size and size distribution directly affect the pharmacokinetic characteristics and potency of the vector system [126,127]. The route of administration likely influences both the immune response and side effects, and is therefore an important factor. Nevertheless, reports on the impact of the administration route on the quality and strength of the immune response are few, especially for mRNA–particle vaccines and even for protein-particle vaccines.
Intramuscular injection (IM) of vaccines is the most often practiced route of administration in patients. Indeed, this route of vaccination is simple to carry out and does not require much training for its implementation. The second most practiced route of vaccine administration for routine vaccinations is subcutaneous (SC) injection. The human SC tissue is tightly connected with its underlying bone and muscle tissues, making SC less practical for humans than for rodents. It is straightforward for LNPs administered by either route to reach the LNs. Factors that determine lymphatic trafficking include particle size, charge and colloidal stability [128,129]. LNPs smaller than 150 nm are efficiently drained via afferent lymphatic vessels to the draining LNs. Also, larger LNPs are readily phagocytosed by immune cells and then trafficked to the LNs.
Intradermal (ID) injection delivers LNPs directly into the skin, an organ which is densely populated with Langerhans cells in the epidermis and with multiple DC subtypes in the dermis. The ID route of administration has been shown to effectively induce a Th1 type immune response and cytotoxic T-cell induction for mRNA–LNP vaccines [31]. Moreover, several studies with traditional vaccines have revealed that ID administration may require as little as one-fifth of a standard IM dose to elicit a comparable immune responses [130,131]. Together with recently developed transdermal drug delivery technologies like microneedles, ID applications may have great potential for dose sparing.
IV injections of LNP–mRNA vaccines are less common because of the potential of systemic side effects. Indeed, injecting immunogenic material in the blood stream may lead to massive cytokine production, also known as cytokine storm, that can lead to shock and death [125]. Additionally, vital organs, including the liver and lungs, are transfected by mRNA vaccine delivery using LNPs. Expression of the antigen by these organs could recruit T cells that induce tissue damage and inflammation. Nevertheless, Perche et al. showed that 24 h after IV administration of their LNPs, 3% of splenic DCs were expressing the antigen [36]. This value was further enhanced to 13% using mannosylated lipids, with no toxic side effects observed in mice. Surprisingly, the vaccine potency correlated with the number of transfected DCs, suggesting that DCs are primarily responsible for the observed result.
Mucosal delivery of a vaccine can have the additional benefit of mucosal immunity, including the secretion of IgA antibodies. Intranasal (IN) administration of LNPs coding for the chicken protein ovalbumin (OVA) has been shown to elicit an OVA-specific cytotoxic T-cell response against E.G7-OVA lymphoma [37]. From the nasal epithelium, M cells transport the LNPs to the underlying nasal-associated lymphoid tissue where high numbers of B cells, T cells and DCs reside. IN vaccine delivery is a convenient, noninvasive way of vaccine administration that allows harvesting the potential of mucosal immunity, despite some reported cases of Bells palsy after IN administration of inactivated influenza vaccine [132].
Injection of the vaccine into the LNs is the most direct way of delivering vaccines to the LNs. Currently, no intranodal (IN) immunizations with LNP–mRNA vaccines have been reported. However, for IN administration of mRNA vaccines, vectors may not be necessary. The IN injection of naked mRNA encoding antigens has been reported to induce a potent T-cell response [133]. The challenge of IN immunization is the injection into the LN, which can be achieved using ultrasound guidance [134]. In spite of the obvious benefit, the additional equipment and need for specially trained personnel will likely prevent direct LN injection from becoming widely adopted. Finally, reported intraperitoneal injection of an mRNA–LNP coding for beta-galactosidase did not result in any significant immunization [34].
A recent study using LNPs with mRNA coding for luciferase compared different routes of administration [135]. The total amount of protein produced was largest for IV administration, while duration of luciferase expression was the longest for ID injection followed by IM administration. How the route of administration of mRNA–LNPs influences both the total amount of protein produced, as well as the duration of expression, are two important parameters that have implications when determining a route of administration for a particular vaccine. A study investigating the different routes of administration in the context of both antibody titers and cytotoxic T cells would be very interesting.
Self-amplifying mRNA
Self-amplifying mRNA has been used to prolong protein expression and to increase the immunogenicity of mRNA vaccines, which leads to a dramatic decrease in the effective dose compared with nonreplicating mRNA [136,137]. Self-amplifying mRNAs, also termed replicons, are based on RNA viruses where the structural viral proteins are replaced with suitable mRNA encoding antigens, as well as with RNA polymerases for RNA replication. The most studied replicons are derived from alphavirus and from flaviviruses. When introduced into the cytosol of cells, the mRNA will express the heterologous genes and replicate. Through the mRNA amplification, large amounts of desired antigens can be synthesized, accounting for up to 20% of total cell protein [138]. Self-amplifying mRNAs not only code for the antigens of interest, but also for the viral, RNA-dependent polymerase to amplify the replicon. As a result, self-amplifying mRNA is much larger than nonamplifying mRNA. The size of self-amplifying mRNA, including the 5′ un-translated region, the poly-A tail and the gene of interest, can be as large as 10 kb. Accordingly, delivery of self-amplifying mRNA requires a vector capable of transporting such a large payload. In this respect, LNPs have been used to successfully deliver self-amplifying mRNA [38,139]. Geall et al. showed that self-amplifying mRNA encapsulated in LNPs exhibits overall higher immunogenicity than the nonencapsulated variant [38]. However, it was not reported that SAM is more immunogenic than transient mRNA. For future applications in humans, the extent of immune response against the polymerase will need to be determined. Especially for repeated applications, an immune response against the polymerase could reduce the efficiency and be a safety issue.
Prophylactic & therapeutic vaccines
mRNA vaccines can be used for both prophylactic and therapeutic vaccination. The many advantages over protein or DNA vaccines enable the application of mRNA as a prophylactic against diseases where conventional vaccines have not shown sufficient efficacy. This is due to the nature of the immune activation and number of antigens that can be delivered. Because of the short production times, mRNA vaccines can also be used to respond rapidly to emerging threats or seasonal strains of pathogens [139]. Currently, no mRNA therapeutic is approved for use in humans, and a beneficial safety profile in patients still has to be demonstrated. A first clinical application will likely not be a prophylactic vaccine, because the tolerance for side effects is very low for a drug that is injected into healthy individuals. Establishing the safety profile in a therapeutic application, such as cancer immunotherapy, will be followed by prophylactic applications. Cancer immunotherapy appears to be an ideal application, because a strong CD8 T-cell response is likely required to cure cancer, which is precisely the strength of mRNA vaccines. The feasibility of both prophylactic and therapeutic mRNA vaccines has been demonstrated in many preclinical studies. While there have not been any clinical trials delivering mRNA vaccines with LNPs, the results from two clinical trials have been reported. A Phase 1/2 trial of protamine-complexed mRNA, coding for six different cancer-associated antigens, delivered intradermally to metastatic melanoma patients, reported encouraging results [140]. In a Phase 1/2a study, advanced prostate cancer patients treated with full-length mRNA vaccine encoding for several tumor associated antigens, experience prolonged survival [121]. The vaccine was also administered intradermally and consisted of free modified mRNA and mRNA complexed with protamine.
Conclusion & future perspective
The field of mRNA therapeutics has entered a very exciting phase with multiple clinical studies ongoing using mRNA for cancer immunotherapy. Although no study yet employs LNP–mRNA formulations, LNPs offer a number of advantages over other vectors, including protection of nonstabilized mRNA, the large payload that can be delivered, adjuvants that can be codelivered, the possibility to decorate them with targeting ligands and the ease of simple synthesis.
We believe that valuable lessons can be learned from the clinical translation of siRNA–LNPs. Many of the components used in LNPs to deliver mRNA have also been used to deliver siRNA. Several clinical studies delivering siRNA in LNP carries have been conducted in recent years [141]. While the exact composition of formulations used to deliver the much larger mRNA molecules will likely be different from the ones used for siRNA, many of the challenges involved are the same [142]. Among the most problematic are the potential toxicity of LNP components, including cationic lipids, phospholipids or combinations thereof. The immunogenicity of PEG and the decreased interaction of the LNPs with the endosomal membranes that hinders endosomal escape are also important issues for both siRNA and mRNA delivery.
The remaining challenges for LNP–mRNA vaccines involve the complexity associated with identifying the best formulation. In this respect, a major challenge of all vaccine research is that antibody titers and T-cell counts are ‘second-order’ effects, indicating that they are not a direct result of immune cell transfection, but instead, a result of how well these cells promote the immune response. Consequently, lymphatic drainage and transfection potency are not the only features that need to be considered. Two different LNPs, for example, may be able to drain to the lymphatics and transfect DCs exceptionally well. However, the measurable outcome may be completely different if one LNP fails to activate the appropriate signaling pathways that result in a complete immune response. Hence, there is currently no high-throughput assay to efficiently evaluate different formulations and to predict in vivo immune responses, as well as to address dosage and side effects.
Another challenge is that detailed mechanistic knowledge, for example, of how LNPs assist in endocytosis and endosomal escape, is still lacking, thereby making the rational improvement or design of LNPs very difficult. For most formulations, the bottleneck has not been identified, whether it is endocytosis, endosomal escape, stability of the mRNA, DC activation or something different. Findings from protein or protein–LNP vaccines are only helpful to a certain extent, because antigen processing and presentation is completely different for mRNA–LNP vaccines. Even when optimizing the transfection efficiency of the LNPs, there are differences that need to be considered. Another challenge is the definition of a standard for how the potency of LNP formulations should be determined. For this purpose, the community uses different administration routes and different antigens at variable time points. Much like the expression of luciferase is a standard for mRNA-based protein replacement therapeutics, the mRNA vaccine community needs to establish its own standards.
Besides improvements to the vector, the community should not forget the payload itself. The true power of mRNA–LNPs over naked mRNA is the coadministration of various different signals to the same cell as well as the decoration of the LNPs with targeting ligands. LNPs need to be developed further to potentiate this advantage and live up to their full potential. Furthermore, it is still unclear how the different administration routes behave for LNP-based mRNA vaccines. A comparative evaluation of LNPs is required for several administration routes to determine the optimal parameters for the desired vaccination.
Finally, mRNA vaccines will have to demonstrate that they are superior to DNA vaccines, and that there is not a significant reduction in potency upon translation from small animal models to humans. The first DNA vaccine entered clinical trials almost 20 years ago without a single product licensed for use in humans [143]. This is in part because the potency of DNA vaccines in humans has been lower than that suggested by preclinical studies in small animals [144]. A major advantage of mRNA vaccines over DNA vaccines is regulatory in nature. Both the regulatory agencies in USA and Germany, namely, the US FDA, and the Paul Ehrlich Institute, respectively, do not classify nonreplicating mRNA as gene therapy. This, in turn, eases the requirements for preclinical and toxicological studies [145]. mRNA vaccines represent a very exciting application with multiple clinical-stage applications. Currently, no LNP–mRNA vaccine has been tested in patients, because there are still a number of unanswered questions. Nevertheless, we believe that addressing these questions, including LNP composition, codelivered adjuvants and decoration with targeting ligands, will uncover the true potential of LNP formulations over other delivery vectors.
Executive summary.
Differences between DNA & mRNA vaccines
mRNA vaccines, like DNA vaccines, induce synthesis of antigens in transfected cells, and hence, activate a broad immune response, including antibodies, Th1 helper CD4 T cells and cytotoxic CD8 T cells.
mRNA, with the cytosol as its target, is easier to deliver and much safer than DNA, because the mRNA in the cytosol does not interact with the genome in the nucleus and is only transiently expressed.
In contrast to DNA vaccines, the FDA does not consider nonreplicating mRNA vaccines gene therapies.
Advantages & challenges of lipid nanoparticles
Lipid nanoparticles (LNPs) protect the mRNA against degradation and assist in endocytosis and endosomal escape.
Adjuvants can be incorporated in LNPs and assist in immune activation and potentially tailoring of the immune response.
LNPs can be targeted to specific cell types by decorating their surfaces with specific ligands.
LNP synthesis, using methods such as microfluidic devices, is robust and upscalable.
Some cationic lipidoids exhibit toxicity and repeated application can induce an immune response against polyethylene glycol.
Future perspectives of LNP mRNA vaccines
mRNA vaccines are currently evaluated in clinical trials, albeit not formulated in LNPs.
LNP delivery of siRNA is already in clinical trials, and lessons from these experiences can be helpful for the translation of mRNA vaccines.
mRNA vaccines are currently evaluated for applications in cancer immunotherapy, with prophylactic vaccines applications to follow.
Acknowledgements
The authors thank K Kauffman for his thorough review of the manuscript, including providing critical feedback.
Footnotes
Financial & competing interests disclosure
This work was funded by the National Institutes of Health (Grant# EB 000244). Robert Langer is a co-founder and member of the board of directors of Moderna therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Crawford NW, Bines JE, Royle J, Buttery JP. Optimizing immunization in pediatric special risk groups. Expert Rev. Vaccines. 2011;10(2):175–186. doi: 10.1586/erv.10.157. [DOI] [PubMed] [Google Scholar]
- 2.Liu MA. Immunologic basis of vaccine vectors. Immunity. 2010;33(4):504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Hilleman MR. Recombinant vector vaccines in vaccinology. Dev. Biol. Stand. 1994;82:3–20. [PubMed] [Google Scholar]
- 4.Deering RP, Kommareddy S, Ulmer JB, Brito LA, Geall AJ. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines. Expert Opin. Drug Deliv. 2014;11(6):885–899. doi: 10.1517/17425247.2014.901308. [DOI] [PubMed] [Google Scholar]
- 5.Pascolo S. Vaccination with messenger RNA (mRNA) In: Bauer PDS, Hartmann PDG, editors. Toll-Like Receptors (TLRs) and Innate Immunity. Springer Berlin Heidelberg; Germany: 2008. pp. 221–235. [Google Scholar]
- 6.Jäschke A, Helm M. RNA sex. Chem. Biol. 2003;10(12):1148–1150. doi: 10.1016/j.chembiol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Fotin-Mleczek M, Duchardt KM, Lorenz C, et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 2011;34(1):1–15. doi: 10.1097/CJI.0b013e3181f7dbe8. [DOI] [PubMed] [Google Scholar]
- 8.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29(17):3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorrentino S. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell. Mol. Life Sci. CMLS. 1998;54(8):785–794. doi: 10.1007/s000180050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chira S, Jackson CS, Oprea I, et al. Progresses towards safe and efficient gene therapy vectors. Oncotarget. 2015;6(31):30675–30703. doi: 10.18632/oncotarget.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ku SH, Jo SD, Lee YK, Kim K, Kim SH. Chemical and structural modifications of RNAi therapeutics. Adv. Drug Deliv. Rev. 2015 doi: 10.1016/j.addr.2015.10.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Lundstrom K. Alphaviruses in gene therapy. Viruses. 2009;1(1):13–25. doi: 10.3390/v1010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogris M, Brunner S, Schüller S, Kircheis R, Wagner E. PEGylated DNA/transferring–PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6(4):595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 14.Bessis N, GarciaCozar FJ, Boissier M-C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(S1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 15.Baum C, Kustikova O, Modlich U, Li Z, Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum. Gene Ther. 2006;17(3):253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 16.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007;8(8):573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 18.Bouard D, Alazard-Dany N, Cosset F-L. Viral vectors: from virology to transgene expression. Br. J. Pharmacol. 2009;157(2):153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez H, Hwang SJ, Davis ME. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem. 1999;10(6):1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 20.Davis ME. The First Targeted Delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 21.Monopoli MP, Åberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012;7(12):779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Lytton-Jean AKR, Chen Y, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol. 2012;7(6):389–393. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem. Rev. 2009;109(2):259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 24.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 25.Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J. 2007;9(1):E18–E29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokolova V, Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew. Chem. Int. Ed. 2008;47(8):1382–1395. doi: 10.1002/anie.200703039. [DOI] [PubMed] [Google Scholar]
- 27.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Review on the problems and recent solutions in RNA delivery with a focus on RNAi.
- 28.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 2001;1(2):126–134. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]; • Review of the mechanisms of antigen processing and T-cell activation.
- 29.Kasturi SP, Pulendran B. Cross-presentation: avoiding trafficking chaos? Nat. Immunol. 2008;9(5):461–463. doi: 10.1038/ni0508-461. [DOI] [PubMed] [Google Scholar]
- 30.Martinon F, Krishnan S, Lenzen G, et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993;23(7):1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 31.Hess PR, Boczkowski D, Nair SK, Snyder D, Gilboa E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol. Immunother. 2005;55(6):672–683. doi: 10.1007/s00262-005-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou W-Z, Hoon DSB, Huang SKS, et al. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum. Gene Ther. 1999;10(16):2719–2724. doi: 10.1089/10430349950016762. [DOI] [PubMed] [Google Scholar]
- 33.Pollard C, Rejman J, De Haes W, et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 2013;21(1):251–259. doi: 10.1038/mt.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur. J. Immunol. 2000;30(1):1–7. doi: 10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Mockey M, Bourseau E, Chandrashekhar V, et al. mRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes. Cancer Gene Ther. 2007;14(9):802–814. doi: 10.1038/sj.cgt.7701072. [DOI] [PubMed] [Google Scholar]
- 36.Perche F, Benvegnu T, Berchel M, et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomed. Nanotechnol. Biol. Med. 2011;7(4):445–453. doi: 10.1016/j.nano.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Phua KKL, Staats HF, Leong KW, Nair SK. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci. Rep. 2014;4:5128. doi: 10.1038/srep05128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geall AJ, Verma A, Otten GR, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl Acad. Sci. USA. 2012;109(36):14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brito LA, Chan M, Shaw CA, et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014;22(12):2118–2129. doi: 10.1038/mt.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Jr FCS. Lipid-based nanoparticles for nucleic acid delivery. Pharm. Res. 2007;24(3):438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 41.Kuntsche J, Horst JC, Bunjes H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011;417(1):120–137. doi: 10.1016/j.ijpharm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Zhigaltsev IV, Maurer N, Edwards K, Karlsson G, Cullis PR. Formation of drug–arylsulfonate complexes inside liposomes: a novel approach to improve drug retention. J. Control. Rel. 2006;110(2):378–386. doi: 10.1016/j.jconrel.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Sato Y, Hatakeyama H, Sakurai Y, Hyodo M, Akita H, Harashima H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J. Control. Rel. 2012;163(3):267–276. doi: 10.1016/j.jconrel.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 45.Basha G, Novobrantseva TI, Rosin N, et al. Influence of cationic lipid composition on gene silencing properties of lipid nanoparticle formulations of sirna in antigen-presenting cells. Mol. Ther. 2011;19(12):2186–2200. doi: 10.1038/mt.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahay G, Querbes W, Alabi C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 2013;31(7):653–658. doi: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semple SC, Akinc A, Chen J, et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28(2):172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]; • Methodical synthesis of cationic lipids which result in an important advancement in synthetic transfection technology.
- 48.Maier MA, Jayaraman M, Matsuda S, et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013;21(8):1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayaraman M, Ansell SM, Mui BL, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo . Angew. Chem. Int. Ed. Engl. 2012;51(34):8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Love KT, Mahon KP, Levins CG, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA. 2010;107(5):1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitehead KA, Dorkin JR, Vegas AJ, et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 2014;5:4277. doi: 10.1038/ncomms5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong Y, Love KT, Dorkin JR, et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl Acad. Sci. USA. 2014;111(11):3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui L, Chen D, Zhu L. Conformation transformation determined by different self-assembled phases in a DNA complex with cationic polyhedral oligomeric silsesquioxane lipid. ACS Nano. 2008;2(5):921–927. doi: 10.1021/nn800177v. [DOI] [PubMed] [Google Scholar]
- 54.Dobbs W, Heinrich B, Bourgogne C, et al. Mesomorphic imidazolium salts: new vectors for efficient siRNA transfection. J. Am. Chem. Soc. 2009;131(37):13338–13346. doi: 10.1021/ja903028f. [DOI] [PubMed] [Google Scholar]
- 55.Smisterová J, Wagenaar A, Stuart MCA, et al. Molecular shape of the cationic lipid controls the structure of cationic lipid/dioleylphosphatidylethanolamine–DNA complexes and the efficiency of gene delivery. J. Biol. Chem. 2001;276(50):47615–47622. doi: 10.1074/jbc.M106199200. [DOI] [PubMed] [Google Scholar]
- 56.Hirsch-Lerner D, Zhang M, Eliyahu H, Ferrari ME, Wheeler CJ, Barenholz Y. Effect of ‘helper lipid’ on lipoplex electrostatics. Biochim. Biophys. Acta BBA - Biomembr. 2005;1714(2):71–84. doi: 10.1016/j.bbamem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Koiwai K, Tokuhisa K, Karinaga R, et al. Transition from a normal to inverted cylinder for an amidine-bearing lipid/pDNA complex and its excellent transfection. Bioconjug. Chem. 2005;16(6):1349–1351. doi: 10.1021/bc050164a. [DOI] [PubMed] [Google Scholar]
- 58.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 59.Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol. Pharm. 2009;6(3):763–771. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi H, Sinoda K, Hatta I. Effects of cholesterol on the lamellar and the inverted hexagonal phases of dielaidoylphosphatidylethanolamine. Biochim. Biophys. Acta. 1996;1289(2):209–216. doi: 10.1016/0304-4165(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 61.Zuhorn IS, Bakowsky U, Polushkin E, et al. Nonbilayer phase of lipoplex–membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol. Ther. 2005;11(5):801–810. doi: 10.1016/j.ymthe.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 62.Woodle MC. Sterically stabilized liposome therapeutics. Adv. Drug Deliv. Rev. 1995;16(2):249–265. [Google Scholar]
- 63.Belliveau NM, Huft J, Lin PJ, et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids. 2012;1(8):e37. doi: 10.1038/mtna.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Study on the impact of mixing parameters in the microfluidic mixer on the formation of lipid nanoparticles.
- 64.Li S-D, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J. Control. Rel. Off. J. Control Rel. Soc. 2010;145(3):178–181. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buyens K, De Smedt SC, Braeckmans K, et al. Liposome based systems for systemic siRNA delivery: stability in blood sets the requirements for optimal carrier design. J. Control Rel. 2012;158(3):362–370. doi: 10.1016/j.jconrel.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 67.Schlosser E, Mueller M, Fischer S, et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine. 2008;26(13):1626–1637. doi: 10.1016/j.vaccine.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 68.Lasic DD. The mechanism of vesicle formation. Biochem. J. 1988;256(1):1–11. doi: 10.1042/bj2560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv. Pharm. Bull. 2015;5(3):305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhigaltsev IV, Belliveau N, Hafez I, et al. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir. 2012;28(7):3633–3640. doi: 10.1021/la204833h. [DOI] [PubMed] [Google Scholar]
- 71.Huang C-H. Phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry (Mosc.) 1969;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- 72.Batzri S, Korn ED. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta BBA – Biomembr. 1973;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- 73.Semple SC, Klimuk SK, Harasym TO, et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta BBA – Biomembr. 2001;1510(1):152–166. doi: 10.1016/s0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 74.Maurer N, Wong KF, Stark H, et al. Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys. J. 2001;80(5):2310–2326. doi: 10.1016/S0006-3495(01)76202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berger N, Sachse A, Bender J, Schubert R, Brandl M. Filter extrusion of liposomes using different devices: comparison of liposome size, encapsulation efficiency, and process characteristics. Int. J. Pharm. 2001;223(1):55–68. doi: 10.1016/s0378-5173(01)00721-9. [DOI] [PubMed] [Google Scholar]
- 76.Jeffs LB, Palmer LR, Ambegia EG, Giesbrecht C, Ewanick S, MacLachlan I. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm. Res. 2005;22(3):362–372. doi: 10.1007/s11095-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 77.Karnik R, Gu F, Basto P, et al. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008;8(9):2906–2912. doi: 10.1021/nl801736q. [DOI] [PubMed] [Google Scholar]
- 78.Jahn A, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir. 2007;23(11):6289–6293. doi: 10.1021/la070051a. [DOI] [PubMed] [Google Scholar]
- 79.Stroock AD, Dertinger SKW, Ajdari A, Mezić I, Stone HA, Whitesides GM. Chaotic mixer for microchannels. Science. 2002;295(5555):647–651. doi: 10.1126/science.1066238. [DOI] [PubMed] [Google Scholar]; •• Fundamental paper on the mechanisms of mixing in microchannels at low Reynolds numbers.
- 80.Leung AKK, Tam YYC, Chen S, Hafez IM, Cullis PR. Microfluidic mixing: a general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B. 2015;119(28):8698–8706. doi: 10.1021/acs.jpcb.5b02891. [DOI] [PubMed] [Google Scholar]
- 81.Swartz MA. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001;50(1):3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 82.Allen TM, Hansen CB, Guo LSS. Subcutaneous administration of liposomes: a comparison with the intravenous and intraperitoneal routes of injection. Biochim. Biophys. Acta BBA - Biomembr. 1993;1150(1):9–16. doi: 10.1016/0005-2736(93)90115-g. [DOI] [PubMed] [Google Scholar]
- 83.Oussoren C, Velinova M, Scherphof G, van der Want JJ, van Rooijen N, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: IV. Fate of liposomes in regional lymph nodes. Biochim. Biophys. Acta BBA - Biomembr. 1998;1370(2):259–272. doi: 10.1016/s0005-2736(97)00275-7. [DOI] [PubMed] [Google Scholar]
- 84.Henriksen-Lacey M, Bramwell VW, Christensen D, Agger E-M, Andersen P, Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J. Control. Rel. 2010;142(2):180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 85.Henriksen-Lacey M, Christensen D, Bramwell VW, et al. Comparison of the depot effect and immunogenicity of liposomes based on dimethyldioctadecylammonium (DDA), 3β-[n-(n′,n′-dimethylaminoethane)carbomyl], cholesterol (DC-Chol), and 1,2-dioleoyl-3-trimethylammonium propane (dotap): prolonged liposome retention mediates stronger Th1 responses. Mol. Pharm. 2011;8(1):153–161. doi: 10.1021/mp100208f. [DOI] [PubMed] [Google Scholar]
- 86.Ishida T, Harashima H, Kiwada H. Liposome clearance. Biosci. Rep. 2002;22(2):197–224. doi: 10.1023/a:1020134521778. [DOI] [PubMed] [Google Scholar]
- 87.Carstens MG, Camps MGM, Henriksen-Lacey M, et al. Effect of vesicle size on tissue localization and immunogenicity of liposomal DNA vaccines. Vaccine. 2011;29(29):4761–4770. doi: 10.1016/j.vaccine.2011.04.081. [DOI] [PubMed] [Google Scholar]
- 88.Kaur R, Bramwell VW, Kirby DJ, Perrie Y. Pegylation of DDA:TDB liposomal adjuvants reduces the vaccine depot effect and alters the Th1/Th2 immune responses. J. Control Rel. 2012;158(1):72–77. doi: 10.1016/j.jconrel.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 89.van den Berg JH, Oosterhuis K, Hennink WE, et al. Shielding the cationic charge of nanoparticle-formulated dermal DNA vaccines is essential for antigen expression and immunogenicity. J. Control Rel. 2010;141(2):234–240. doi: 10.1016/j.jconrel.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 2004;83(3):97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 91.Remaut K, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Pegylation of liposomes favours the endosomal degradation of the delivered phosphodiester oligonucleotides. J. Control Rel. 2007;117(2):256–266. doi: 10.1016/j.jconrel.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 92.Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015;7(5):655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Judge A, McClintock K, Phelps JR, MacLachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol. Ther. 2006;13(2):328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Martinez-Pomares L. The mannose receptor. J. Leukoc. Biol. 2012;92(6):1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 95.Geijtenbeek TBH, Torensma R, van Vliet SJ, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100(5):575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 96.Jiang W, Swiggard WJ, Heufler C, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375(6527):151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 97.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-Type lectin specific to langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12(1):71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 98.Karanikas V, Hwang LA, Pearson J, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan–MUC1 fusion protein. J. Clin. Invest. 1997;100(11):2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Espuelas S, Thumann C, Heurtault B, Schuber F, Frisch B. Influence of ligand valency on the targeting of immature human dendritic cells by mannosylated liposomes. Bioconjug. Chem. 2008;19(12):2385–2393. doi: 10.1021/bc8002524. [DOI] [PubMed] [Google Scholar]
- 100.Reina JJ, Rojo J. Glycodendritic structures: tools to interact with DC-SIGN. Braz. J. Pharm. Sci. 2013;49(SPE):109–124. [Google Scholar]
- 101.Perche F, Gosset D, Mével M, et al. Selective gene delivery in dendritic cells with mannosylated and histidylated lipopolyplexes. J. Drug Target. 2011;19(5):315–325. doi: 10.3109/1061186X.2010.504262. [DOI] [PubMed] [Google Scholar]
- 102.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo . Science. 2007;315(5808):107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 103.Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28(Suppl. 3):C25–C36. doi: 10.1016/j.vaccine.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 104.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007;59(6):478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 105.Kedmi R, Ben-Arie N, Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31(26):6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 106.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 107.Yanasarn N, Sloat BR, Cui Z. Negatively charged liposomes show potent adjuvant activity when simply admixed with protein antigens. Mol. Pharm. 2011;8(4):1174–1185. doi: 10.1021/mp200016d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adler-Moore J, Munoz M, Kim H, et al. Characterization of the murine Th2 response to immunization with liposomal M2e influenza vaccine. Vaccine. 2011;29(27):4460–4468. doi: 10.1016/j.vaccine.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 109.Ravindran R, Maji M, Ali N. Vaccination with liposomal leishmanial antigens adjuvanted with monophosphoryl lipid–trehalose dicorynomycolate (MPL-TDM) confers long-term protection against visceral leishmaniasis through a human administrable route. Mol. Pharm. 2012;9(1):59–70. doi: 10.1021/mp2002494. [DOI] [PubMed] [Google Scholar]
- 110.Rizwan SB, McBurney WT, Young K, et al. Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid A stimulate robust cellular and humoral immune responses. J. Control Rel. 2013;165(1):16–21. doi: 10.1016/j.jconrel.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 111.Shirota H, Klinman DM. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Exp. Rev. Vaccines. 2014;13(2):299–312. doi: 10.1586/14760584.2014.863715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Erikçi E, Gursel M, Gürsel İ. Differential immune activation following encapsulation of immunostimulatory CpG oligodeoxynucleotide in nanoliposomes. Biomaterials. 2011;32(6):1715–1723. doi: 10.1016/j.biomaterials.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 113.Bal SM, Hortensius S, Ding Z, Jiskoot W, Bouwstra JA. Co-encapsulation of antigen and Toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine. 2011;29(5):1045–1052. doi: 10.1016/j.vaccine.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 114.Wu TY-H, Singh M, Miller AT, et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 2014;6(263):263ra160–263ra160. doi: 10.1126/scitranslmed.3009980. [DOI] [PubMed] [Google Scholar]
- 115.Jensen S, Thomsen AR. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J. Virol. 2012;86(6):2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics – developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 117.Balachandran S, Roberts PC, Brown LE, et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13(1):129–141. doi: 10.1016/s1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- 118.Banerjee S, Chakrabarti A, Jha BK, et al. Cell-type-specific effects of RNase L on viral induction of beta interferon. mBio. 2014;5(2):e00856–e00814. doi: 10.1128/mBio.00856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]; •• Fundamental paper on the effect of nucleoside modification in RNA recognition.
- 120.Karikó K, Muramatsu H, Welsh FA, et al. Incorporation of pseudouridine into mrna yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. J. Am. Soc. Gene Ther. 2008;16(11):1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kübler H, Scheel B, Gnad-Vogt U, et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man Phase I/IIa study. J. Immunother. Cancer. 2015;3(1):26. doi: 10.1186/s40425-015-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Riedmann EM. Human vaccines and immunotherapeutics. Hum. Vaccines Immunother. 2013;9(10):2034–2037. doi: 10.4161/hv.26556. [DOI] [PubMed] [Google Scholar]
- 123.Peabody DS. Translation initiation at non-AUG triplets in mammalian cells. J. Biol. Chem. 1989;264(9):5031–5035. [PubMed] [Google Scholar]
- 124.Malarkannan S, Horng T, Shih PP, Schwab S, Shastri N. Presentation of out-of-frame peptide/MHC Class I complexes by a novel translation initiation mechanism. Immunity. 1999;10(6):681–690. doi: 10.1016/s1074-7613(00)80067-9. [DOI] [PubMed] [Google Scholar]
- 125.Mauro VP, Chappell SA. A critical analysis of codon optimization in human therapeutics. Trends Mol. Med. 2014;20(11):604–613. doi: 10.1016/j.molmed.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Litzinger DC, Buiting AMJ, van Rooijen N, Huang L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochim. Biophys. Acta BBA - Biomembr. 1994;1190(1):99–107. doi: 10.1016/0005-2736(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 127.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10(11):787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]; • Review on the problems and advances in vaccinology with a focus on the delivery of antigens and enhancement of efficacy.
- 128.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control Rel. 2006;112(1):26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 129.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 130.Midoux P, Pichon C. Lipid-based mRNA vaccine delivery systems. Expert Rev. Vaccines. 2015;14(2):221–234. doi: 10.1586/14760584.2015.986104. [DOI] [PubMed] [Google Scholar]
- 131.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 2004;351(22):2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 132.Mutsch M, Zhou W, Rhodes P, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 133.Kreiter S, Selmi A, Diken M, et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70(22):9031–9040. doi: 10.1158/0008-5472.CAN-10-0699. [DOI] [PubMed] [Google Scholar]
- 134.Tagawa ST, Lee P, Snively J, et al. Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with stage IV melanoma. Cancer. 2003;98(1):144–154. doi: 10.1002/cncr.11462. [DOI] [PubMed] [Google Scholar]
- 135.Pardi N, Tuyishime S, Muramatsu H, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control Rel. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McCullough KC, Milona P, Thomann-Harwood L, et al. Self-amplifying replicon RNA vaccine delivery to dendritic cells by synthetic nanoparticles. Vaccines. 2014;2(4):735–754. doi: 10.3390/vaccines2040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ulmer JB, Mason PW, Geall A, Mandl CW. RNA-based vaccines. Vaccine. 2012;30(30):4414–4418. doi: 10.1016/j.vaccine.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 138.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo . Virology. 1997;239(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 139.Hekele A, Bertholet S, Archer J, et al. Rapidly produced SAM® vaccine against H7N9 influenza is immunogenic in mice. Emerg. Microbes Infect. 2013;2(8):e52. doi: 10.1038/emi.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weide B, Pascolo S, Scheel B, et al. Direct injection of protamine-protected mRNA: results of a Phase 1/2 vaccination trial in metastatic melanoma patients. J. Immunother. 2009;32(5):498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- 141.Xue HY, Guo P, Wen W-C, Wong HL. Lipid-based nanocarriers for RNA delivery. Curr. Pharm. Des. 2015;21(22):3140–3147. doi: 10.2174/1381612821666150531164540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kauffman KJ, Dorkin JR, Yang JH, et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15(11):7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 143.MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 1998;178(1):92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 144.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011;53(3):296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weide B, Garbe C, Rammensee H-G, Pascolo S. Plasmid DNA- and messenger RNA-based anti-cancer vaccination. Immunol. Lett. 2008;115(1):33–42. doi: 10.1016/j.imlet.2007.09.012. [DOI] [PubMed] [Google Scholar]