Abstract
Gastric cancer is the third most common cause of cancer-related death. Although the incidence of gastric cancer in the United States is relatively low, it remains significantly higher in some countries, including Japan and Korea. Interactions between cancer stem cells and the tumor microenvironment can have a substantial impact on tumor characteristics and contribute to heterogeneity. The mechanisms responsible for maintaining malignant cancer stem cells within the tumor microenvironment in human gastric cancer are largely unknown. Tumor cell and genetic heterogeneity contribute to either de novo intrinsic or the therapy-induced emergence of drug-resistant clones and eventual tumor recurrence. Although chemotherapy often is capable of inducing cell death in tumors, many cancer patients experience recurrence because of failure to effectively target the cancer stem cells, which are believed to be key tumor-initiating cells. Among the population of stem cells within the stomach that may be targeted during chronic Helicobacter pylori infection and altered into tumor-initiating cells are those cells marked by the cluster-of-differentiation (CD)44 cell surface receptor. CD44 variable isoforms (CD44v) have been implicated as key players in malignant transformation whereby their expression is highly restricted and specific, unlike the canonical CD44 standard isoform. Overall, CD44v, in particular CD44v9, are believed to mark the gastric cancer cells that contribute to increased resistance for chemotherapy- or radiation-induced cell death. This review focuses on the following: the alteration of the gastric stem cell during bacterial infection, and the role of CD44v in the initiation, maintenance, and growth of tumors associated with gastric cancer.
Keywords: Helicobacter pylori, CD44v9, CD44v6, Inflammation
Abbreviations used in this paper: Cag, cytotoxin-associated gene; CD, cluster-of-differentiation; CD44v9, CD44 variant isoform containing exon v9; CSC, cancer stem cell; Lgr5, leucine-rich, repeat-containing, G-protein–coupled receptor 5; MDSC, myeloid-derived suppressor cell; PDL1, programmed cell death 1 ligand; PDTX, patient-derived tumor xenograft; ROS, reactive oxygen species; SPEM, spasmolytic polypeptide expressing metaplasia; xCT, SLC7A11
Summary.
Cell surface adhesion molecule CD44 has been identified as a gastric cancer stem cell marker. CD44 variant isoforms are expressed abundantly in epithelial-type carcinomas and are associated with initiation and progression of gastric cancer.
Gastric cancer is the fifth most common cancer worldwide and the third most common cause of cancer-related death.1 The incidence of gastric cancer in the United States is relatively low as a result of the diagnosis and treatment of the major risk factor Helicobacter pylori.2 However, the 5-year survival rate for patients diagnosed with this malignancy is only 29%.3 Importantly, the incidence of gastric cancer varies throughout the world. For example, it is 4 times more common in Japan than in the United Kingdom and occurs at a younger age.4 A variety of etiologic factors contribute to this higher incidence in countries such as Japan, including H pylori prevalence and virulence,5, 6 as well as dietary7 and genetic variations8, 9 among these populations. Given the poor response of gastric cancer to various existing treatment modalities, there is an unmet need for approaches to predict individual therapy responses. Solid tumors consist of not only malignant cells but also various types of stromal cells, fibroblasts, endothelial cells, and hematopoietic cells such as macrophages and lymphocytes (reviewed by Quante and Wang10 and Quante et al11). Interactions between cancer stem cells and the tumor microenvironment can have a substantial impact on tumor characteristics and contribute to heterogeneity. Heterogeneity contributes to tumor recurrence.12 Although chemotherapy often is capable of inducing cell death in tumors, many cancer patients experience recurrence because of failure to effectively target the cancer stem cells, which are believed to be key tumor-initiating cells.13, 14 These cancer stem cells are responsible for the formation, maintenance, and continued growth of the tumor,14, 15 and thus highlights the need to target cancer stem cells during treatment. The mechanisms responsible for maintaining malignant cancer stem cells within the tumor microenvironment in human gastric cancer are largely unknown.
The Correa et al16 model reported that gastric atrophy (parietal cell loss) was one of several significant changes that occurred after chronic inflammation. We now understand that the major cause of chronic inflammation in the normal, acid-secreting stomach is H pylori bacterial colonization.17 It is widely accepted that inflammation that is caused by H pylori infection is a trigger for the development of gastric cancer. An explanation for the causal role of H pylori infection in the pathogenesis of gastric cancer has been described by disruption of differentiation of epithelia as a consequence of altered gastric stem cell phenotype.18, 19 The chronic nature of H pylori gastritis is critical to the carcinogenic potential of this infection. The long-term interaction of the bacteria and inflammatory mediators with gastric epithelial, progenitor, and stem cells, results in the accumulation of mutations, epigenetic modifications, and deregulation of cell function that ultimately may lead to cancer.19, 20, 21 Therefore, H pylori infection plays a critical role during the initiating steps of gastric cancer.
Initiation of Gastric Cancer
Alterations in Epithelial Gastric Stem Cells
Abnormal differentiation (metaplasia) is associated with cancer and may reflect the permanent alteration in the behavior of the stem cells, thus making the gastric stem cell a candidate H pylori target. It is hypothesized that tumors develop because of a rare subpopulation of cells (known as cancer stem cells [CSCs]).10 Although the origin of gastric cancer stem cells remains uncertain, there are a number of key studies that show the expansion of gastric stem cells during bacterial infection that may lead to their alteration and transformation into tumor-initiating cells.19, 20, 21 Among the populations of stem cells within the stomach that may be targeted during bacterial infection, that may lead to metaplasia or aberrant epithelial cell proliferation and differentiation, are cells expressing the leucine-rich, repeat-containing, G-protein–coupled receptor 5 (Lgr5) and the cluster-of-differentiation (CD)44 cell surface receptor.19, 20, 21, 22 Troy marks a specific subset of chief cells that are capable of replenishing entire gastric units in response to injury.23 In addition, the Sox2+ stem cell compartment has been shown to be critical for normal tissue regeneration,24 and villin+ is a quiescent stem cell population that becomes apparent upon cytokine stimulation.25 The expansion of Troy+, Sox2+, and villin+ cell populations in response to H pylori infection has not been investigated thoroughly.
Lgr5, located in adult stem cells at the base on the antral glands of the stomach, are capable of long-term renewal of the epithelium.26 By using lineage tracing to mark cells derived from Lgr5+ stem cells, Sigal et al19 analyzed the response of these gastric stem cells to H pylori infection. The investigators showed that the bacteria formed distinct microcolonies deep in the stomach glands where infection accelerated Lgr5+ stem cell proliferation.19 The findings show that H pylori can colonize and manipulate the gastric stem cell compartment and this has significant implications for H pylori–induced gastric disease. These studies also suggest that alterations to stem cells may be responsible for the development of gastric cancer. In support of this idea, human studies have shown that there is enhanced Lgr5 expression in patients with progressive dedifferentiation and metastasis of gastric cancer.27, 28
In another study, the investigators deleted Smad4 and PTEN in murine gastric Lgr5+ stem cells by the inducible Cre–LoxP system. In mice with altered/mutant Lgr5+ stem cells there was a rapid onset and progression from adenoma to invasive intestinal-type gastric cancer in the antrum.29 Moreover, it has been shown in an organoid culture system that Lgr5+ gastric stem cell homeostasis is regulated by the Notch signaling pathway.30 In this study, it also was shown that chronic activation of Notch within gastric Lgr5+ stem cells induced the development of antral polyps in mice, implicating this pathway in gastric tumorigenesis.30 In addition, GLI2A is an activator form of the Hedgehog transcription factor GLI2, and its expression within Lgr5+ gastric stem cells drives the rapid development of gastric adenocarcinoma.20 Thus, alterations in Lgr5+ gastric stem cells, which potentially are induced by H pylori infection, may be an initiating event for the development of gastric cancer, indicating the potential of Lgr5 as an early diagnostic and prognostic biomarker.
Another host molecule that may influence carcinogenesis in conjunction with H pylori is CD44. CD44 is a cell surface adhesion molecule that is expressed on a variety of cells, including gastric epithelial cells, that recently was identified as a gastric cancer stem cell marker, whereby cells expressing CD44 have been shown to possess the properties of cancer stem cells.31 Defined as a unique subpopulation in tumors that possess the ability to initiate tumor growth and sustain tumor self-renewal, a subpopulation of CD44-positive cells showed spheroid colony formation in serum-free media in vitro as well as tumorigenic ability when injected orthotopically into stomachs of immunodeficient mice in vivo. In addition, the CD44-positive gastric cancer cells showed the stem cell properties of self-renewal, and CD44 knockdown by short hairpin RNA resulted in reduced spheroid colony formation and tumors in immunodeficient mice.31 In the normal stomach, CD44 labels a population of undifferentiated cells in the isthmus region where stem cells are known to reside.22 Atrophy of parietal cells that is induced by Helicobacter infection or tamoxifen treatment results in the expansion of CD44-positive cells into the base of the gastric glands.22
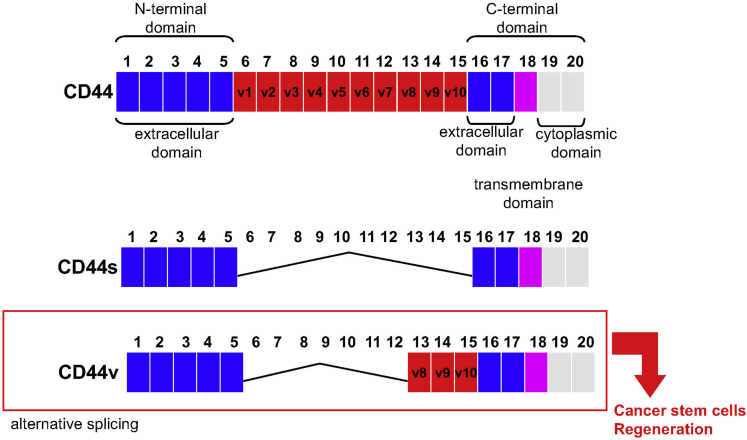
Alternative messenger RNA splicing produces CD44 variant isoforms that are expressed abundantly in epithelial-type carcinomas, although the standard CD44 isoform is expressed predominantly in hematopoietic cells and normal epithelial cell subsets (Figure 1).32, 33 The involvement of CD44 variant isoforms in gastric cancer has not been well studied. What is known, however, is that early studies by Heider et al34 showed that the normal epithelium expresses 2 of 12 CD44 variant RNAs containing exons V5 and V6. Intestinal-type tumors express a more complex pattern of amplification products that hybridized to exons V5 and V6. In the sample of a diffuse-type tumor, expression of exon V5, but not V6, could be detected.34 However, CD44 variant isoform containing exox v9 (CD44v9) also has been detected as a potential predictive marker for recurrence in multiple early gastric cancers.35
Figure 1.
CD44 variant isoforms contain insertions close to the transmembrane region that are generated by messenger RNA splicing. Although the standard CD44 isoform (CD44s) is expressed predominantly in hematopoietic cells and normal epithelial cell subsets, CD44v8–10 is identified as a cancer stem cell marker. Recently, CD44v9 was shown to emerge during gastric regeneration.
CD44 variant isoforms, in particular the isoform containing exon v6 (CD44v6), was identified as a marker for invasive intramucosal carcinoma and premalignant lesions.36 Moreover, in cases of sporadic and hereditary diffuse gastric cancer, CD44v6 expression correlated inversely with the expression of E-cadherin.36 Another variant isoform, CD44v8–10, has been shown to be a cancer stem cell marker in primary gastric cancer.37 We have observed an increase in CD44v9 expression in the stomachs of patients infected with H pylori (unpublished data). In addition, the higher expression for CD44v9 was observed in gastric cancer tissues, with greater expression rates for CD44v9 in the intestinal type or well-differentiated gastric cancer than in the diffuse type or poorly differentiated gastric cancer.38, 39 Within cancer cells, CD44v9 interacts with the glutamate-cysteine transporter SLC7A11 (xCT), stabilizes the protein, and thereby potentiates defense against reactive oxygen species that subsequently promotes tumor growth40 (Figure 2). Thus, CD44 and its splice variants are associated positively with the initiation and progression of gastric cancer and may play important roles in the diagnosis, therapy, and prognosis of this disease.
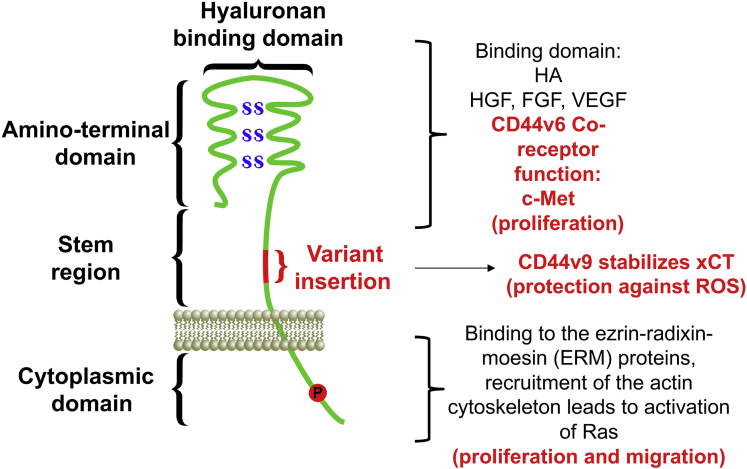
Figure 2.
Structure, binding sites, and interactions of CD44. The CD44 protein is composed of an extracellular N-terminal domain, a stem region, and the carboxyl terminal cytoplasmic domain/tail. The stem region, close to the transmembrane region, is the site of variant exon product insertion. The N-terminal domain contains highly conserved disulfide bonds (SS) that are essential for hyaluronan (HA) binding. The C-terminal cytoplasmic tail contains several phosphorylation (P) sites that regulate the interaction of CD44 with the cytoskeletal linker proteins for the regulation of cell proliferation and importantly migration. Note that CD44v6 acts as a co-receptor for c-Met signaling and c-Met ligands including hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) to regulate proliferation. CD44v9 stabilizes the cysteine–glutamate transporter xCT for the protection against ROS and subsequent cell survival and proliferation.
Notably, CD44v6 acts as the co-receptor for c-Met.41, 42 The extracellular domain of CD44v6 is necessary for c-Met activation, which is dependent on hepatocyte growth factor binding41 (Figure 2). The co-receptor function of CD44v6 for c-Met is of particular interest given that studies pinpoint CD44v6 as a marker of early invasive intramucosal gastric carcinoma.36 The cytotoxin-associated gene (Cag) pathogenicity island is a strain-specific constituent of H pylori that augments cancer risk.43 The Cag pathogenicity island encodes a type IV secretion system that is a multimolecular complex that mediates the translocation of bacterial factors into the host cell.43, 44 Evidence in the literature shows that H pylori–expressed CagA accumulates in gastric cancer cells specifically expressing CD44 and showing suppression of autophagy by their resistance to reactive oxygen species (ROS), and thus suggesting that CD44+ cells are resistant to oxidative stress.45 In cells lacking CD44 expression, CagA is degraded by autophagy induced by the accumulation of ROS.45 Upon delivery into the host cells by the type IV Cag secretion system, CagA translocates into the host cell cytoplasm where it can stimulate cell signaling through interaction with several host proteins,43, 46, 47 including the tyrosine kinase c-Met receptor.48, 49, 50 CagA exerts effects within host cells by inducing hyperproliferation and disrupting apical-junctional complexes and cellular polarity.51, 52, 53 Suzuki et al54 showed that CagA CM motifs interact with Met, leading to sustained PI3K-AKT signaling in response to H pylori, leading to β-catenin activation and cellular proliferation. We have published that CD44v6 acts as a co-receptor for the function of c-Met in response to H pylori infection and bacterial-induced epithelial proliferation.21 Collectively, these studies suggest that CD44 and its variant isoforms may not simply be markers of the gastric cancer stem cell, but also actively involved in the initiation and progression of disease.
Unregulated Spasmolytic Polypeptide/Trefoil Factor 2–Expressing Metaplasia in the Initiation of Gastric Cancer
It is accepted that loss of acid-secreting parietal cells is a prerequisite for the development of metaplasia and a mucosal lineage change associated with increased risk for gastric cancer.55, 56, 57 Loss of parietal cells results in the transdifferentiation of the chief cell lineage into a mucous cell metaplasia identified as spasmolytic polypeptide expressing metaplasia (SPEM).58 Although Helicobacter infection, tamoxifen treatment, and parietal cell–specific protonophores (DMP-777 and L635) are known inducers of SPEM,58, 59 parietal cell loss alone is not sufficient to induce metaplasia.60 In a study using a mouse model expressing the diphtheria toxin receptor specifically in parietal cells to induce their death, metaplastic reprogramming of chief cells was not observed, suggesting mechanisms beyond parietal cell injury and apoptosis.60 Metaplastic mucous cells arising for the loss of parietal cells express trefoil factor 2, also known as spasmolytic polypeptide, thus leading to the designation of this lineage as SPEM.56, 61, 62 SPEM also is associated with increased expression of cell surface protein CD44, in particular CD44v9.63 SPEM also has been identified in the mucosa surrounding intestinal-type gastric cancers in human beings.62 Importantly, mice infected with Helicobacter felis for more than 12 months showed progression of the metaplasia to dysplasia.56, 57, 64 These reports indicated that metaplastic glands induced by parietal cell loss and chronic inflammation progress toward dysplasia. Interestingly, we reported the induction of SPEM after gastric injury.65 SPEM was identified in the ulcer margin in the regenerating gastric glands and disappeared when the mucosa returned to its normal compendium of cell lineages, suggesting a possible role for SPEM in ulcer repair.65 Collectively, these studies suggest that in response to gastric ulceration, SPEM is a regulated mechanism that may contribute to repair. SPEM in the setting of parietal cell atrophy and chronic inflammation is an unregulated precursor lineage for the development of dysplasia associated with gastric cancer development. The stem cell marker CD44v9 also marks SPEM and may contribute to the production of metaplasia.
Maintenance of Gastric Cancer: The Role of the Cancer Stem Cells
The gastrointestinal tumor microenvironment is required for tumor initiation, progression, and metastasis. Solid tumors are heterogeneous and consist of cancer cells, cancer stem cells, and various types of stromal cells, fibroblasts, endothelial cells, and hematopoietic cells, mainly macrophages and lymphocytes.10, 11, 40, 66 Poor response of gastric cancer to various existing treatment modalities may be accounted for by the cellular heterogeneity of the tumor microenvironment. In particular, chemotherapy is one of the standard methods of treatment in many cancers including gastric cancer.67 Although chemotherapy often is capable of inducing cell death in tumors, many cancer patients experience recurrence because of failure to effectively target the cancer stem cells, which are believed to be key tumor-initiating cells. One CSC model proposes that the growth of the tumor is driven by a population of self-sustaining cells with stem cell properties of proliferation and an ability to differentiate into the entire heterogeneous population of the tumor.13, 14 These CSC are responsible for the formation, maintenance, and continued growth of the tumor.14, 15 This model highlights the need to target CSCs with chemotherapy. The targeted agent eliminates the chemoresistant CSC population, preventing recurrence of the tumor, while the chemotherapy targets the differentiated cells. As discussed earlier, variant isoforms of CD44 including CD44v6 and CD44v9 have been reported to have prognostic value in gastric cancer. For example, CD44v9 expression in primary early gastric cancer is a predictive marker for recurrence,35 and the presence of CD44v9-positive circulating cancer cells is associated strongly with recurrence and poor survival rates in colorectal cancer.68 In our laboratory, gastric organoids derived from the tumor tissue of a patient with diffuse-type gastric cancer expressing CD44v9 were resistant to cisplatin. Interestingly, inhibition of xCT by sulfasalazine sensitized the organoids to cisplatin-induced atrophy (unpublished data). Thus, this is an example by which therapy targeted to the CD44v9–xCT system may impair the ROS defense by cancer stem cells and thereby sensitize them to currently available treatments.
Immune suppression and adaptive immune resistance are other mechanisms that contribute to the maintenance and growth of tumors. The immune system typically detects and eliminates cancer development via a process termed immune surveillance.69, 70, 71 However, during immune evasion, the tumor cells escape the immune system. For example, tumor-derived cytokines can induce the differentiation of immune effectors to a suppressive phenotype such as tumor-associated macrophages, myeloid-derived suppressor cells (MDSCs), and regulatory CD4+ T cells.72 Tumor-associated macrophages within the tumor microenvironment have been described as protumorigenic, supporting cancer initiation and progression.71, 73, 74, 75 Another example is the suppressive function of MDSCs. MDSCs are a heterogeneous myeloid cell population that infiltrates tissue in response to infection, injury, autoimmune disease, and cancer.76 A study by the Merchant laboratory identified Schlafen 4 (Slfn4) as a GLI1 target gene and myeloid differentiation factor that correlated with the development of SPEM in mice.77 A recent study by the same group then showed that migration of Slfn4-expressing cells from the bone marrow to the stomach in response to Helicobacter infection showed MDSC markers, and acquired the ability to inhibit T-cell proliferation.78 This supports MDSC suppression of T-cell function and subsequent dampening of the immune response to create a microenvironment favoring tumor growth.79
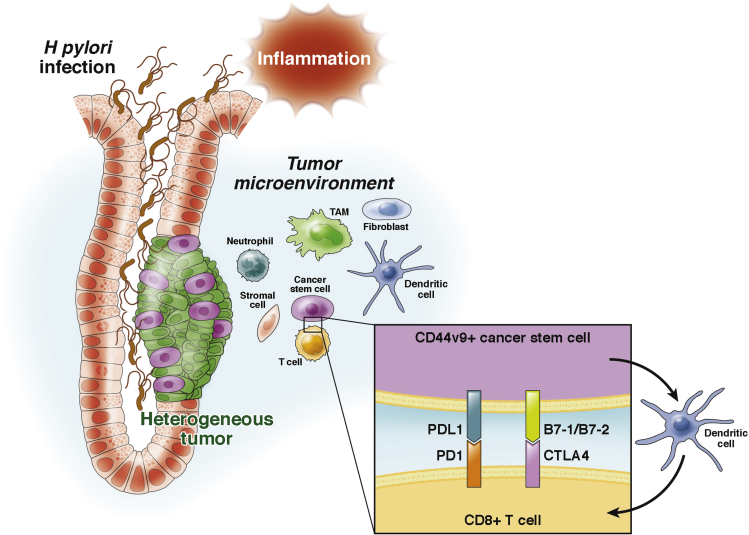
Tumor cells may evade the immune response by inducing T-cell inactivation. Dendritic cells within the tumor microenvironment present tumor-specific antigens on major histocompatibility complex class I to CD8+ T cells that then can prime an antigen-specific T-cell response.80 In colorectal tumors, high densities of cytotoxic and memory T cells in the tumor microenvironment are associated with reduced recurrence of disease.81 Tumors can evade immune surveillance by expressing molecules such as programmed cell death 1 ligand (PDL1), which interacts with PD1 and subsequently inhibits CD8+ cytotoxic T-lymphocyte proliferation, survival, and effector function82, 83, 84 (Figure 3). In addition, CTLA-4, which is expressed minimally on the surface of resting T lymphocytes, is highly expressed on activated T lymphocytes.85, 86 Importantly, ligands B7-1/B7-2, expressed on tumor cells, bind to CTLA-4 and inhibit effector T-cell function85, 86 (Figure 3). Although anti-PD1 antibodies are already in clinical trials for gastric cancer treatment,87 whether PD1-PDL1 or CTLA-4/B7 interactions inhibit CD8+ cytotoxic T-cell effector function within the gastric tumor microenvironment has not been well studied. Importantly, a preclinical model that predicts the efficacy of such targeted therapies in individual patients with gastric cancer does not exist. In support of this notion, however, PDL1+ (B7-H1+) gastric cancer stem cells show an increased proliferative capacity.88 PDL1 also has been identified as an independent prognostic factor for patients with stage II/III gastric cancer, suggesting that patients with stage II/III gastric cancer might be appropriate for PD1/PDL1-targeted therapy.89 Furthermore, selective expression of the PDL1 was observed on CD44(+) cells in squamous cell carcinoma of the head and neck90 and breast cancer cells.91 In addition, PDL1 expression was associated with worse overall survival in patients with stage II/III gastric cancer, suggesting that these patients might be appropriate for PD1/PDL1-targeted therapy.89
Figure 3.
Illustration for the proposed development of gastric cancer initiated by chronic inflammation. During H pylori infection, CD44v9 expression emerges, a marker of the gastric cancer stem cell. Despite loss of H pylori infection over time, CD44v9 expression is maintained in cancer stem cells within the tumor. We may predict that tumor antigen secreted by the CD44v9+ cells activate dendritic cells that subsequently activate CD8+ cytotoxic T cells to express PD1 and CTLA-4, a mechanism by which cancer stem cells can evade the immune response via inactivation of T-cell effector function. TAM, tumor-associated macrophages.
Future Directions
Given the poor response of gastric cancer to various existing treatment modalities, there is an unmet need for approaches to predict individual therapy responses. Although cancer cell lines and patient-derived tumor xenografts (PDTXs) have proven very valuable in fundamental cancer research, both models have disadvantages. Cell lines lack the tumor heterogeneity found in tumor tissues, and although PDTXs bear promise as preclinical models for human cancer, there are several limitations. For example, similarities between PDTX and parental tumors cannot be assumed until rigorously tested, tumor–host interactions are not always conserved across species and tumor immunity is entirely absent. As mentioned earlier, a preclinical model that predicts the efficacy of such targeted therapies in individual patients with gastric cancer does not exist. We and others have shown that gastric organoids not only have the tissue architecture and physiological function of the human stomach, but are also an experimentally tractable system allowing for cell and genetic manipulation.21, 30, 65, 92, 93 Moreover, patient-derived gastric tumor organoids can be transplanted orthotopically into NOD.Cg-Rag1[tmMom]/L2rg[tmWjl]Tg(CMV-IL3, CSF2, KITLG)/Eav/J (NRGS) (NRG-SGM3) mice expressing human-derived immune cells so that the role of the immune response within the tumor microenvironment may be identified in vivo (unpublished data). Understanding the immune response within the tumor microenvironment is crucial not only to addressing important biological questions, but also for the success of current and future immunotherapy. Potential studies that use organoids include the following: (1) in vitro and in vivo organoid-based approaches for the study of the interaction between cancer stem cells and the immune microenvironment using organoid/immune cell co-cultures, (2) a preclinical organoid-based platform for anticancer drug evaluation, and (3) in vitro and in vivo preclinical models that will allow us to effectively evaluate novel cancer therapeutics as well as to identify predictive biomarkers for gastric cancer. Although organoids have been widely used for biological and clinical studies, this system does show key challenges, as follows: (1) the lack of the native microenvironment, (2) organoids within the same culture may be phenotypically heterogeneous, and (3) maintenance of epithelial heterogeneity within the organoid culture over time is unclear. Despite these challenges, the development of cutting edge in vitro and in vivo organoid-based approaches from a common patient sample is a critical first step for personalized medicine providing a preclinical approach to prevent, diagnose, or treat gastric cancer.
Footnotes
Conflicts of interest The author discloses no conflicts.
Funding This work was supported by National Institutes of Health grants R01 DK083402-06A1 and 1U19AI116491-01, and the College of Medicine Bridge Funding Program.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Piazuelo M.B., Epplein M., Correa P. Gastric cancer: an infectious disease. Infect Dis Clin North Am. 2010;24:853–869. doi: 10.1016/j.idc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Neugut A.I., Hayek M., Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996;23:281–291. [PubMed] [Google Scholar]
- 5.Covacci A., Telford J.L., Del Giudice G. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 6.Parsonnet J., Friedman G.D., Vandersteen D.P. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 7.Joossens J.V., Hill M.J., Elliott P. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol. 1996;25:494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]
- 8.El-Omar E. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743–747. doi: 10.1136/gut.48.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Omar E.M., Chow W.H., Rabkin C.S. Gastric cancer and H. pylori: host genetics open the way. Gastroenterology. 2001;121:1002–1005. [PubMed] [Google Scholar]
- 10.Quante M., Wang T.C. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology. 2008;23:350–359. doi: 10.1152/physiol.00031.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quante M., Varga J., Wang T.C. The gastrointestinal tumor microenvironment. Gastroenterology. 2013;145:63–78. doi: 10.1053/j.gastro.2013.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S., Cong Y., Wang D. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2014;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor M.L., Xiang D., Shigdar S. Cancer stem cells: a contentious hypothesis now moving forward. Cancer Lett. 2014;344:180–187. doi: 10.1016/j.canlet.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Brungs D., Aghmesheh M., Vine K.L. Gastric cancer stem cells: evidence, potential markers, and clinical implications. J Gastroenterol. 2016;51:313–326. doi: 10.1007/s00535-015-1125-5. [DOI] [PubMed] [Google Scholar]
- 15.Dewi D.L., Ishii H., Kano Y. Cancer stem cell theory in gastrointestinal malignancies: recent progress and upcoming challenges. J Gastroenterol. 2011;46:1145–1157. doi: 10.1007/s00535-011-0442-6. [DOI] [PubMed] [Google Scholar]
- 16.Correa P., Haenszel W., Cuello C. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 17.Marshall B.J., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 18.Bessède E., Staedel C., Acuña Amador L. Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene. 2014;33:4123–4131. doi: 10.1038/onc.2013.380. [DOI] [PubMed] [Google Scholar]
- 19.Sigal M., Rothenberg M.E., Logan C.Y. Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology. 2015;148:1392–1404 e21. doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 20.Syu L.J., Zhao X., Zhang Y. Invasive mouse gastric adenocarcinomas arising from Lgr5+ stem cells are dependent on crosstalk between the Hedgehog/GLI2 and mTOR pathways. Oncotarget. 2016;7:10255–10270. doi: 10.18632/oncotarget.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertaux-Skeirik N., Feng R., Schumacher M.A. CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLoS Pathog. 2015;11:e1004663. doi: 10.1371/journal.ppat.1004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khurana S.S., Riehl T.E., Moore B.D. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stange D.E., Koo B.K., Huch M. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold K., Sarkar A., Yram M.A. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao X.T., Ziel J.W., McKimpson W. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker N., Huch M., Kujala P. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Z.X., Sun Y., Bu Z.D. Intestinal stem cell marker LGR5 expression during gastric carcinogenesis. World J Gastroenterol. 2013;19:8714–8721. doi: 10.3748/wjg.v19.i46.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bu Z., Zheng Z., Zhang L. LGR5 is a promising biomarker for patients with stage I and II gastric cancer. Chin J Cancer Res. 2013;25:79–89. doi: 10.3978/j.issn.1000-9604.2013.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X.B., Yang G., Zhu L. Gastric Lgr5(+) stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res. 2016;26:838–849. doi: 10.1038/cr.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demitrack E.S., Gifford G.B., Keeley T.M. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522–2536. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takaishi S., Okumura T., Tu S. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orian-Rousseau V. CD44 Acts as a signaling platform controlling tumor progression and metastasis. Front Immunol. 2015;6:154. doi: 10.3389/fimmu.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orian-Rousseau V., Ponta H. Perspectives of CD44 targeting therapies. Arch Toxicol. 2015;89:3–14. doi: 10.1007/s00204-014-1424-2. [DOI] [PubMed] [Google Scholar]
- 34.Heider K.H., Dammrich J., Skroch-Angel P. Differential expression of CD44 splice variants in intestinal- and diffuse-type human gastric carcinomas and normal gastric mucosa. Cancer Res. 1993;53:4197–4203. [PubMed] [Google Scholar]
- 35.Hirata K., Suzuki H., Imaeda H. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109:379–386. doi: 10.1038/bjc.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Cunha C.B., Oliveira C., Wen X. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab Invest. 2010;90:1604–1614. doi: 10.1038/labinvest.2010.155. [DOI] [PubMed] [Google Scholar]
- 37.Lau W.M., Teng E., Chong H.S. CD44v8-10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 2014;74:2630–2641. doi: 10.1158/0008-5472.CAN-13-2309. [DOI] [PubMed] [Google Scholar]
- 38.Jang B.I., Li Y., Graham D.Y. The role of CD44 in the pathogenesis, diagnosis, and therapy of gastric cancer. Gut Liver. 2011;5:397–405. doi: 10.5009/gnl.2011.5.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Go S.I., Ko G.H., Lee W.S. CD44 variant 9 serves as a poor prognostic marker in early gastric cancer, but not in advanced gastric cancer. Cancer Res Treat. 2016;48:142–152. doi: 10.4143/crt.2014.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishimoto T., Nagano O., Yae T. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 41.Orian-Rousseau V., Chen L., Sleeman J.P. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orian-Rousseau V., Morrison H., Matzke A. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell. 2007;18:76–83. doi: 10.1091/mbc.E06-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odenbreit S., Puls J., Sedlmaier B. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 44.Segal E.D., Falkow S., Tompkins L.S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci U S A. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsugawa H., Suzuki H., Saya H. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Higashi H., Tsutsumi R., Muto S. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 47.Selbach The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics. 2004;4:2961–2968. doi: 10.1002/pmic.200400915. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira M.J., Costa A.C., Costa A.M. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J Biol Chem. 2006;281:34888–34896. doi: 10.1074/jbc.M607067200. [DOI] [PubMed] [Google Scholar]
- 49.Oliveira M.J., Costa A.M., Costa A.C. CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. J Infect Dis. 2009;200:745–755. doi: 10.1086/604727. [DOI] [PubMed] [Google Scholar]
- 50.Churin Y., Al-Ghoul L., Kepp O. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003;161:249–255. doi: 10.1083/jcb.200208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amieva M.R., Vogelmann R., Covacci A. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franco A.T., Israel D.A., Washington M.K. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saadat I., Higashi H., Obuse C. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki M., Mimuro H., Kiga K. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 55.El-Zimaity H.M., Ota H., Graham D.Y. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428–1436. doi: 10.1002/cncr.10375. [DOI] [PubMed] [Google Scholar]
- 56.Wang T.C., Goldenring J.R., Dangler C. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 57.Fox J.G., Li X., Cahill R.J. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 58.Nam K.T., Lee H.J., Sousa J.F. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weis V.G., Sousa J.F., LaFleur B.J. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013;62:1270–1279. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burclaff J., Osaki L.H., Liu D. Targeted apoptosis of parietal cells is insufficient to induce metaplasia in stomach. Gastroenterology. 2017;152:762–766. doi: 10.1053/j.gastro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang T.C., Dangler C.A., Chen D. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt P.H., Lee J.R., Joshi V. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 63.Wada T., Ishimoto T., Seishima R. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104:1323–1329. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nomura S., Baxter T., Yamaguchi H. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 65.Engevik A.C., Feng R., Choi E. The development of spasmolytic polypeptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell Mol Gastroenterol Hepatol. 2016;2:605–624. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quante M., Tu S.P., Tomita H. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmad S.A., Xia B.T., Bailey C.E. An update on gastric cancer. Curr Probl Surg. 2016;53:449–490. doi: 10.1067/j.cpsurg.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Katoh S., Goi T., Naruse T. Cancer stem cell marker in circulating tumor cells: expression of CD44 variant exon 9 is strongly correlated to treatment refractoriness, recurrence and prognosis of human colorectal cancer. Anticancer Res. 2015;35:239–244. [PubMed] [Google Scholar]
- 69.Matsueda S., Graham D.Y. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20:1657–1666. doi: 10.3748/wjg.v20.i7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burnet F.M. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 71.Wang M., Busuttil R.A., Pattison S. Immunological battlefield in gastric cancer and role of immunotherapies. World J Gastroenterol. 2016;22:6373–6384. doi: 10.3748/wjg.v22.i28.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee K., Hwang H., Nam K.T. Immune response and the tumor microenvironment: how they communicate to regulate gastric cancer. Gut Liver. 2014;8:131–139. doi: 10.5009/gnl.2014.8.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murray P.J., Allen J.E., Biswas S.K. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan Y., Zhang J., Li J.H. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial-mesenchymal transition in gastric cancer. Onco Targets Ther. 2016;9:3975–3983. doi: 10.2147/OTT.S103112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Zaatari M., Kao J.Y., Tessier A. Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets. PLoS One. 2013;8:e58935. doi: 10.1371/journal.pone.0058935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding L., Hayes M.M., Photenhauer A. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest. 2016;126:2867–2880. doi: 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 81.Galon J., Costes A., Sanchez-Cabo F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 82.Ahmadzadeh M., Johnson L.A., Heemskerk B. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen X., Fosco D., Kline D.E. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol. 2014;44:2603–2616. doi: 10.1002/eji.201344423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reissfelder C., Stamova S., Gossmann C. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739–751. doi: 10.1172/JCI74894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCoy K.D., Hermans I.F., Fraser J.H. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) can regulate dendritic cell-induced activation and cytotoxicity of CD8(+) T cells independently of CD4(+) T cell help. J Exp Med. 1999;189:1157–1162. doi: 10.1084/jem.189.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCoy K.D., Le Gros G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell Biol. 1999;77:1–10. doi: 10.1046/j.1440-1711.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 87.Muro K., Chung H.C., Shankaran V. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y., Wu K.E., Zhao E. B7-H1 enhances proliferation ability of gastric cancer stem-like cells as a receptor. Oncol Lett. 2015;9:1833–1838. doi: 10.3892/ol.2015.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tamura T., Ohira M., Tanaka H. Programmed death-1 ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric cancer. Anticancer Res. 2015;35:5369–5376. [PubMed] [Google Scholar]
- 90.Lee Y., Shin J.H., Longmire M. CD44+ cells in head and neck squamous cell carcinoma suppress T-cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin Cancer Res. 2016;22:3571–3581. doi: 10.1158/1078-0432.CCR-15-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alsuliman A., Colak D., Al-Harazi O. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer. 2015;14:149. doi: 10.1186/s12943-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCracken K.W., Catá E.M., Crawford C.M. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCracken K.W., Aihara E., Martin B. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–187. doi: 10.1038/nature21021. [DOI] [PMC free article] [PubMed] [Google Scholar]