Abstract
The intestinal epithelium can be easily disrupted during gut inflammation as seen in inflammatory bowel disease (IBD), such as ulcerative colitis or Crohn’s disease. For a long time, research into the pathophysiology of IBD has been focused on immune cell–mediated mechanisms. Recent evidence, however, suggests that the intestinal epithelium might play a major role in the development and perpetuation of IBD. It is now clear that IBD can be triggered by disturbances in epithelial barrier integrity via dysfunctions in intestinal epithelial cell–intrinsic molecular circuits that control the homeostasis, renewal, and repair of intestinal epithelial cells. The intestinal epithelium in the healthy individual represents a semi-permeable physical barrier shielding the interior of the body from invasions of pathogens on the one hand and allowing selective passage of nutrients on the other hand. However, the intestinal epithelium must be considered much more than a simple physical barrier. Instead, the epithelium is a highly dynamic tissue that responds to a plenitude of signals including the intestinal microbiota and signals from the immune system. This epithelial response to these signals regulates barrier function, the composition of the microbiota, and mucosal immune homeostasis within the lamina propria. The epithelium can thus be regarded as a translator between the microbiota and the immune system and aberrant signal transduction between the epithelium and adjacent immune cells might promote immune dysregulation in IBD. This review summarizes the important cellular and molecular barrier components of the intestinal epithelium and emphasizes the mechanisms leading to barrier dysfunction during intestinal inflammation.
Keywords: Intestinal Epithelial Barrier, Intestinal Inflammation, Immune-Epithelial Crosstalk
Abbreviations used in this paper: BMP, bone morphogenic protein; CD, Crohn's disease; Fz, frizzled; HD, humans α-defensin; IBD, inflammatory bowel disease; IECs, intestinal epithelial cells; IL, interleukin; JAMs, junctional adhesion molecules; Lgr5, leucine rich repeat containing G-protein coupled receptor 5; MARVEL, myelin and lymphocyte and related proteins for vesicle trafficking and membrane link; MLCK, myosin light chain kinase; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NOD-2, nucleotide-binding oligomerization domain-containing protein 2; STAT, signal transducer and activator of transcription; TAMP, tight junction–associated MARVEL protein; TJ, tight junction; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin; UC, ulcerative colitis
Summary.
Intestinal epithelial barrier integrity defects are frequently seen during intestinal inflammation. This article highlights the composition of the intestinal epithelium and summarizes mechanisms that lead to the disruption of barrier integrity promoting the development of inflammatory bowel diseases.
The intestine has to meet a lifelong service of proper food digestion and nutrient absorption; however, this responsibility for import from the outside to the inside of the body is not without challenges. The intestinal tract is exposed to a plethora of food-borne antigens and bacterial antigens in the microbiota. At the same time the gut harbors a large part of the immune cells of the body whose task is to identify and fight off foreign antigens and microbial threats. The epithelial cell layer prevents excessive contact of these antigens with the immune cells and thereby also protects the gut from unwanted immune reactions. This is achieved by the sophisticated organization of the intestinal epithelium, which establishes a tightly regulated barrier.1 The intestinal epithelium is built of monolayered columnar epithelial cells that are tightly connected by tight junctions (TJs).2 Although TJs can be considered as a part of the physical barrier, specialized intestinal epithelial cells (IECs), such as goblet cells and Paneth cells, take over miscellaneous functions of antimicrobial defense, which make them crucial parts of the innate immune system. Goblet cells secrete a variety of antimicrobial molecules, such as trefoil factors and mucins.3 Mucin secretion constitutes a thick mucus layer to prevent excessive direct contact of bacteria to the epithelial cell surface and thereby to protect against invasive pathogens. Paneth cells are professional producers of antimicrobial peptides, which are secreted within the crypts of the small intestine.4 However, controlled antigen delivery to immune cells plays an important role in the education of the gut immune system. For instance, specialized epithelial cells, M (microfold)-cells, which are abundant within the follicle-associated epithelium but also appear along the crypt-villous axis, take up intestinal microbes and their antigens and forward them to resident immune cells in the gut-associated lymphoid tissue, supporting the maturation of the immune system.5 Thus the intestinal epithelium, rather than a strict barrier, constitutes a highly regulated gate controlling the admission of antigens to serve the host’s health. Epithelial barrier integrity is challenged by the high rate of cell turnover. The epithelium is completely renewed within only 4–5 days with cells shedding into the gut lumen at the surface and proliferation of stem cells within the intestinal crypt replacing the constant cell loss.6 A failure of coordinated replenishment can cause severe barrier defects leading to excessive invasion of luminal antigens and intestinal inflammation, such as seen in patients with ulcerative colitis (UC) or Crohn's disease (CD).
Recent experimental evidence indeed implicates a crucial function of barrier dysfunction in the onset of inflammatory bowel disease (IBD). This article focuses on the role of the intestinal epithelium during maintenance of homeostasis and highlights the mechanisms of epithelial barrier dysfunction during intestinal inflammation.
In the steady state epithelial homeostasis is maintained by proliferation and cell shedding. The intestinal epithelium constitutes a physical barrier by its structural organization, by specialized innate immune cell functions, and by a tight regulation of the ratio between proliferation and cell death. The 3-dimensional structure of the small intestinal epithelium is characterized by invaginations denoted crypts of Lieberkühn merging with protrusions termed villi. In the large intestine villi are lacking resulting in a rather flat mucosal surface. Stem cells at the crypt base give rise to fast cycling progenitor cells, which migrate up toward the villus tip while they differentiate into the mature epithelial cell types. Finally, once reaching the villus tip of the small intestine or the epithelial surface of the colon, IECs are shed into the lumen and replaced by neighboring cells.
Because IECs have a limited lifespan of about 4–5 days, the epithelium has to be constantly replenished to prevent disruption of the intestinal epithelial barrier. This is enabled by multipotent intestinal stem cells, which reside at the crypt base. Stem cells divide, giving rise to epithelial transit-amplifying cells, progenitor cells with very high proliferative capacity. Intestinal stem cells were first identified by several groups in the 1970s after using H3-thymidine to label and track proliferating cell populations.7, 8 During the last decade especially the group of Hans Clever’s elegantly refined stem cell fate mapping by creating a mouse-model to lineage trace a candidate marker for actively dividing crypt base columnar cells, which where intermingled with Paneth cells at the crypt base. These studies ultimately led to the identification of a specific marker for crypt base columnar stem cells, the leucine rich repeat containing G-protein coupled receptor-5 (Lgr5).9 Since then, several other potential stem cell markers have been identified by in vivo linage tracing or by transgenic fluorescent labelling of cells, which are residing either at the crypt base columnar region or at the +4 position (fourth cell position from the crypt base center) of the crypt. Stem cells at the +4 position are located directly above the Paneth cells and have been described as quiescent, reserve stem cell populations, which are reactivated into a stem cell fate under specific physiological conditions, such as injury.10
The constant proliferation at the crypt base is thought to provide the steric force that moves cells up the crypt villus axis. While migrating upward cells start to differentiate into the various mature IECs of the secretory or absorptive cell type.11 Intestinal epithelial homeostasis not only depends on the ratio between proliferation and cell death, but also the balance between proliferating progenitor cells and differentiating IECs. This is warranted by the specialized microenvironment in which stem and progenitor cells reside, which is defined as stem cell niche. Within this niche, IECs, such as Paneth cells, and neighboring mesenchymal cells at the crypt base secrete signaling molecules that determine proliferation and differentiation. This special expression gives rise to a concentration gradient that regulates the preservation of the stem cell niche, proliferation of progenitor cells, and differentiation of IECs.
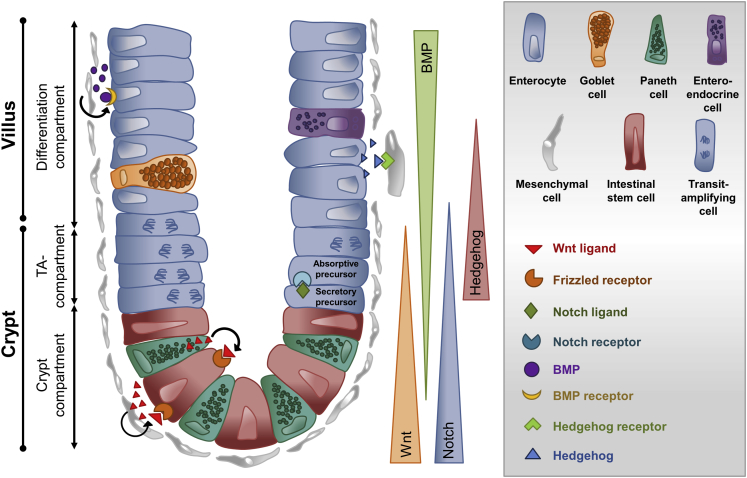
The 4 most important signaling molecules regulating the composition and proliferation within the niche are Wnt, Notch, bone morphogenic proteins (BMPs), and hedgehog (Figure 1).12 Wnt ligands are produced by epithelial cells, Paneth cells, or mesenchymal cells and signaling into target cells leads to the activation of either the canonical and noncanonical Wnt pathway.13 Canonical Wnt signaling drives proliferation and is mainly concentrated at the lower crypt, where stem cells are located.14 Wnt signaling extends up to the transit amplifying area where the rapidly proliferating progenies of stem cells reside. In sharp contrast, noncanonical Wnt signaling is predominantly observed in the upper crypt area where proliferation ceases and differentiation becomes important. The expression of Wnt ligands and frizzled (Fz)-receptors was precisely studied by Gregorieff et al,15 who revealed a strong expression of Wnt3 and Wnt9b by Paneth cells and the Wnt receptors Fz5 and Fz7 on stem cells in the small intestinal crypt, activating the canonical Wnt pathway. Wnt activation leads to the accumulation of β-catenin and subsequently to the transcription of several target genes that govern proliferation of intestinal stem cells. Wnt proteins can also be produced by mesenchymal cells neighboring the crypt, such as myofibroblasts.16 Myofibroblasts can express Wnt2b, Wnt4, and Wnt5, which lead to the activation of the noncanonical pathway, driving cellular polarity and motility and therefore supporting the differentiation of IECs.17 Although Wnt ligands and Fz receptors activate Wnt signaling, the pathway can be negatively regulated by antagonistic mechanisms, such as the secreted Fz-related proteins.18, 19 Secreted Fz-related proteins are secreted predominantly by mesenchymal cells in close proximity to Wnt-producing intestinal stem cells to regulate Wnt signalling.15
Figure 1.
Schematic depiction of the small intestinal crypt compartment and the 4 main driving signaling pathways for maintenance of the stem cell niche. All relevant intestinal cell types and signaling molecules are represented in the grey box. In summary, Paneth cells and mesenchymal cells are producers of Wnt ligands, which directly bind to frizzled receptors expressed by intestinal stem cells. Secretory precursors of the transit-amplifying compartment secrete Notch ligands, which bind to Notch receptors expressed by absorptive precursors. Hedgehog signals are produced by epithelial cells and act directly on Hedgehog receptor-expressing mesenchymal cells, preserving them to produce BMPs, which bind to their receptors expressed by epithelial cells counteracting the Wnt pathway. TA, transit-amplifying.
Another important protein family regulating epithelial proliferation and differentiation are BMPs, which belong to the transforming growth factor-β superfamily.20 BMPs are produced by IECs and by mesenchymal cells throughout the crypt-villus axis. BMP antagonists, such as Noggin, were found to be expressed by myofibroblasts in the intestinal crypt.21 In contrast to canonical Wnt signaling and the gradient of Wnt ligands, expression of BMPs increases along the crypt villus axis.22 Conditional deletion of the type I BMP receptor Bmpr1a in mice resulted in disturbed epithelial homeostasis and regeneration with an increase in stem and progenitor cell populations and has led to the conclusion that BMP signaling is essential to control Wnt signaling and to warrant proper stem cell self-renewal.22 One of the main decisions that have to be made during intestinal epithelial differentiation is the commitment to either the secretory or absorptive cell lineage. The most important signaling pathway regulating this decision seems to be the Notch signaling pathway. Notch receptors are expressed predominantly in the crypt compartment and their ligands (members of the Delta and Serrate/Jagged subfamilies) are expressed by the neighboring cells.23 Several studies on Notch signaling in the gut have given rise to a model of how differentiation into the 2 main lineages is organized: if 1 progenitor cell expresses the notch receptor and its neighboring cell expresses the Notch ligand Delta1, Notch signaling in this progenitor cell leads to the expression of Notch-responsive genes, such as Hes1 and Hes5. These can be considered as master regulators of IEC differentiation and their expression drives the cell toward the absorptive cell fate. Interestingly, the Delta1 is especially expressed in cells of the secretory linage, thereby inhibiting the neighboring cell to become also secretory, a model that is known as lateral inhibition.24 Thus lateral inhibition warrants coordinated differentiation into both major IEC lineages. Interestingly, recent evidence indicated that Notch signaling and thereby the balance of absorptive and secretory IEC can be regulated by inflammatory cytokines.25 For example, interleukin (IL) 33 was shown to down-regulate Notch signaling in epithelial progenitor cells thereby promoting differentiation of these to Paneth and goblet cells. Because these cells are important contributors of antimicrobial defense, it indicates that immune-epithelial crosstalk is a central mechanism of host defence.26
Mesenchymal cells in the vicinity of the intestinal crypt are considered critical for the maintenance of the niche. The niche-promoting functions of mesenchymal cells are dependent on hedgehog signaling. Hedgehog ligands, such as Indian hedgehog and sonic hedgehog, are expressed by epithelial cells and bind to the Patched 1 and Patched 2 receptors expressed on mesenchymal cells.6 Paracrine hedgehog signaling was shown not only to be important for the expansion of the mesenchymal cells but also for overall intestinal homeostasis, because mesenchymal cells support the maintenance of the crypt-villus axis.27 Collectively, epithelial proliferation and differentiation is maintained by several signaling pathways creating a defined milieu of niche factors controlling IEC fate. Disturbances in 1 of these signaling pathways might lead to the breakdown of the epithelial barrier and finally to the development of inflammation or to an undesired proliferative activity, resulting in the formation of tumors.
Maintenance of intestinal homeostasis requires structural integrity of the epithelium. The constant proliferation within the crypt thus requires a disposal of cells at more or less the same rate. Of note, the rate of apoptosis or necrosis in the steady-state gut is low and activation of caspases can be seen mainly in cells at the villous tip, especially in cells that are already in the process of cell shedding. The connection of cell death and cell shedding is still not fully understood, but experimental evidences rather implicate that epithelial cell shedding does not require cell death and that caspase activation is rather a consequence than a cause of the shedding process. Recently, significant progress has been made in understanding the molecular mechanisms of controlled IEC shedding. Watson et al28, 29 used multiphoton microscopy to demonstrate that IECs are expulsed from the villus tip leaving a gap in the epithelium. Interestingly, it was shown that in the steady state, these gaps do not compromise intestinal barrier integrity because they were sealed with the TJ protein occludin and the TJ-associated scaffolding protein zonula occludens-1. Further investigations showed that the expulsion of IECs is carried out through a regulated pattern of molecular mechanisms: in a first step rearrangement of tight and adherens junctions and cytoskeletal proteins around the basolateral membranes is initiated. Rho-associated kinase, myosin light chain kinase (MLCK) and dynamin II activation build tension around the shedding cell and force them to be pushed into the lumen.30 Meanwhile the gap is sealed with occludin, adjacent cells capture the space, and new junctions are made to tightly connect the cells.
Barrier Dysfunction as a Potential Cause of Intestinal Inflammation in Inflammatory Bowel Disease
Considering the endoscopic view in a patient suffering an acute flare, it is obvious that there must be a barrier dysfunction in patients with IBD. However, this observation leads to the question whether or not the barrier dysfunction is cause or consequence of IBD. As discussed in the following, several lines of evidence underline that a barrier defect alone suffices to develop IBD.
Besides the endoscopic view even the microscopic view strongly supports this impression by revealing a decrease in goblet cells31; defective defensin production32 and a reduced thickness of mucus layer; and an altered composition with regard to mucins, phosphatidylcholine, and glycosylation.33 In addition, IBD has been associated with a dysbiosis that by itself can result in increased intestinal permeability.34 Indirect data suggest that the barrier defect might precede the onset of disease. This includes the fact that even patients with quiescent IBD have been characterized by an increased paracellular permeability.35 Furthermore, up to 40% of first-degree relatives of patients with CD show an altered small intestinal permeability.36, 37, 38, 39, 40, 41 Although these relatives showed no overt symptoms of IBD, because studies with a long-term follow-up are currently not available, it is difficult to determine the impact of the altered small intestinal permeability. There is only 1 study of a first-degree relative that showed impaired permeability without symptoms during the time of evaluation but developed ileocolonic CD years after.42 Of note, relatives of patients with IBD often show subclinical intestinal inflammation. For example, in studies measuring the fecal calprotectin concentrations, which represent the neutrophil flux in the intestine, patients with CD and their relatives showed significantly increased calprotectin levels compared with control subjects.43, 44 Additionally, asymptomatic first-degree relatives of patients with CD show higher levels of basal intestinal mucosal cytokines, such as IL2, IL6, and IL8, in their intestinal mucosa than control subjects.45
IBD can also be initiated by virtue of environmental triggers. Such substances as sodium caprate (an ingredient of milk fat) or acetylsalicylic acid can act on TJ proteins in noninflamed intestinal mucosa in patients with CD, leading to increased intestinal permeability.46, 47, 48 This provides evidence that patients with IBD show a hyperresponsiveness toward damaging agents, such as nonsteroidal anti-inflammatory drugs like aspirin, providing a potential first-step to the onset of IBD. Vice versa a dysregulated barrier function can predict the risk of a relapse for small intestinal CD.36, 42, 49 Of note, therapeutic strategies might affect barrier functions: in particular TNF-α antibodies have been proven to revert intestinal barrier defects in patients responsive to this strategy.50
In line with the previously mentioned findings in humans, animal studies show that a barrier defect can precede intestinal inflammation in vivo. For example, IL10-/- mice develop spontaneous colitis while aging.51 Strikingly, IL10-/- mice, at 2 weeks of age, showed an increase in ileal and colonic permeability before the onset of intestinal inflammation. This primary permeability defect was associated with increased secretion of interferon-γ and tumor necrosis factor (TNF)-α as inflammation developed. When raised under pathogen-free conditions these mice showed neither inflammation nor increased intestinal permeability, suggesting a dysregulated epithelial barrier response toward normal enteric microflora in these mice. Samp/YitFc mice show spontaneous ileal inflammation, which resembles many features of human CD.52 In an approach to analyze if the inflammation was caused by a primary immune cell defect or a dysregulated epithelial barrier response, Olson et al52 studied bone marrow chimeras. Indeed, similar to IL10-/- mice, the authors could show that barrier permeability preceded the development of ileal inflammation. Animal study in mice deficient for the xenobiotic transporter mdr1a also supported that alterations in the intestinal barrier alone can be sufficient to drive colitis.53 The authors of that study showed impaired phosphorylation of the TJ proteins occludin and zonula occludens-1 in mdr1a-deficient mice, which was associated with spontaneous development of colitis.
The complex signaling environment of the intestinal epithelium and the sensitive balance between proliferation and cell shedding provides great potential of disturbances. Physiologic epithelial cell shedding is a process that involves rearrangement of TJ proteins to extrude the cell from the epithelium. MLCK is an important regulator of cell shedding. Mice with experimental colitis had increased expression of MLCK, which resulted in dysregulation of TJs and a severe loss of epithelial barrier function.54 Interestingly MLCK was also shown to be up-regulated in active IBD,55 implicating its involvement in altered epithelial integrity.
Another important molecule of epithelial integrity is Rho-A. The importance of Rho-A with regard to inflammation was shown by López-Posadas et al,56 who showed that Rho-A signaling was impaired in patients with IBD, because of reduced expression of the Rho-A prenylation enzyme geranylgeranyltransferase-I. Mice lacking either Rho-A or geranylgeranyltransferase-I in IECs suffered from chronic intestinal inflammation, cytoskeleton rearrangement, and aberrant cell shedding. Another important molecule for regulated cell shedding and epithelial integrity is Rho-associated kinase, a downstream effector of Rho-A and important for signal transduction pathways controlling adhesion, transmigration, phagocytosis, and proliferation.57, 58 Rho-associated kinase was found to be highly activated in the inflamed intestinal mucosa of patients with CD,59 suggesting impaired cytoskeletal rearrangements. Another major cause of barrier dysfunction is excessive cell death, such as apoptosis or necroptosis, which has been demonstrated to drive severe gut inflammation and bacterial translocation in mouse models.60 Surprisingly, mice with a deficiency in junctional adhesion molecule (JAM)-A showed enhanced intestinal epithelial permeability and translocation of bacteria leading to lymphocyte infiltration but not to the development of colitis.61 Similar results were obtained from Claudin-2 transgenic mice, which displayed increased mucosal permeability, but did not show signs of mucosal inflammation. Colonic macrophages from these mice exhibited immune anergy resulting in immune compensation.62 This could be an analogous situation to that observed in asymptomatic first-degree relatives of patients with CD. They show a leaky intestinal barrier but need a further trigger to develop disease.
Although only the minority of diseases can be explained by the presence of mutations in risk loci, still several genes that are involved in the regulation of the intestinal barrier have been associated with IBD susceptibility including genes involved in bacterial antigen recognition, such as the nucleotide-binding oligomerization domain-containing protein (NOD)2/CARD15 variants63, 64 or ATGl16L1 and IRGM.65, 66, 67 This is of particular importance for rather monogenetic diseases mostly presenting as an early onset of IBD because in the case of a primary epithelial defect bone marrow transplantation is not an option.68
In summary, the previously mentioned studies indicated that a primary defect in barrier function can induce chronic intestinal inflammation. However, one has to recognize that inflammation in the lamina propria can equally induce barrier dysfunction69 and it remains to be determined in which cases barrier dysfunction is cause or consequence of inflammation.
Impact of Barrier and Channel Properties of Tight Junction Proteins in Inflammatory Bowel Disease
Barrier function is not only dependent on coordinated proliferation and cell death. The paracellular barrier function of the intestinal epithelium is determined TJs, a multiprotein complex, which tightens the paracellular cleft, but also specifically regulates its permeability. The barrier and passage function is controlled by 2 families of 4-fold-membrane-spanning TJ proteins: the TAMP (TJ-associated MARVEL [myelin and lymphocyte and related proteins for vesicle trafficking and membrane link] protein) family,70 comprised of occludin, tricellulin, and marvelD3, and the large family of claudins (for review see71, 72). Currently, 27 claudins are known in mammalia.73 Of note, although many claudins possess barrier-establishing properties, several others in contrast mediate specific permeabilities (ie, by forming paracellular channels selective for small cations, anions, or water). For example, claudin-2 forms a channel for cations74, 75 and water76 through a common pore.77
TJ proteins with 1 transmembrane domain are JAMs78 and angulins.79 They are not directly committed to barrier or channel functions. Instead JAM contributes to cohesion of the TJ and the angulins act as regulators (eg, of tricellulin). Along the intestine, TJ proteins are differently expressed in the proximal and distal segments80, 81 with increasing expression of typical barrier-forming claudins, such as claudin-1, -3, -4, -5, and -8. In consequence, the tightness of cell junctions along the gut axis increases from small to large intestine. Conversely, the expression of claudin-12, which together with claudin-2 has been described as important for vitamin D–regulated Ca2+-uptake,82 is high in the surface epithelium of the jejunum and decreases toward the colon. The channel-forming protein claudin-2 is typical for leaky epithelia characterized by high ion and water permeability. In the gut it is abundant in the surface epithelia of duodenum, jejunum, and ileum. However, claudin-2 is present to a certain amount also in the (healthy) colon, but here it is localized within the crypts.81 Partly in parallel, claudin-15 also forms paracellular cation channels83, 84 and is predominantly expressed in the small intestine, and is also present in colon segments.85 However, in the intestine of young mice claudin-2 and claudin-15 abundance was found to develop in a reciprocal way, suggesting a teamwork behavior in maintenance of intestinal cation permeability. Importantly, a claudin-15-mediated sodium back-leak proved to be essential for sugar absorption via the sodium glucose transporter 1.83 The TAMP occludin is expressed all over the intestine with increasing abundance toward the colon, which would be in concordance with a tightening role. Another TAMP, tricellulin, is predominantly located at the contacts of 3 cells86 and tightens the tricellular TJ against macromolecule passage.87 Loss of occludin from the bicellular TJ leads to a shift of tricellulin from tricellular to bicellular TJs.88 Collectively, TJs are mandatory for barrier regulation. Their composition differs in a temporal and spatial manner to regulate the requirements of paracellular transport.
During inflammation several effects on TJs have been observed. Ultrastructurally, changes in the continuity and number of TJ strands point to a disturbed paracellular barrier. The continuous, linear strand pattern of TJs typical for tight epithelia change in IBD to a particle-type appearance with strand breaks and loss of continuity, and the number of horizontally oriented strands is reduced in CD and in UC.89, 90
When analyzing effects of inflammation on the molecular level, altered expression and localization of several TJ proteins can be detected. First and foremost, claudin-2 abundance increases in various inflammatory diseases, such as CD,90 UC,89 and celiac disease.91 Functionally, this leads to a flux of cations and water via the paracellular pathway into the gut lumen, which gives rise to leak flux diarrhea.92 Also for claudin-15 an increased expression has been reported in celiac disease,91 which may add to the claudin-2-induced effects. Proinflammatory cytokines, such as TNF-α90 and IL13,89 have been linked to the observed claudin-2 increase. In addition, TNF-α causes a dislocalization of the barrier formers claudin-5 and claudin-8 from the TJ to sub-TJ membrane compartments and into endosomes.90 In Caco-2 cells, the stimulation with IL1β caused an increase in intestinal TJ permeability by redistribution of occludin and elevated expression of MLCK.93 IL6 produced mainly by epithelial cells and immune cells of the lamina propria increased the paracellular permeability to cations and led to an increase of the pore-forming claudin-2 in IEC.94
Expression of other barrier-forming claudins is also often found to be down-regulated in several inflammatory or immune-mediated diseases (eg, claudin-4 and -7 in UC95; claudin-4 in collagenous colitis96; claudin-5 and -8 in CD90; or claudin-3, -5, and -7 in celiac disease91). Occludin down-regulation has been reported for CD,90 UC,89 and collagenous colitis.96 The lowered expression depends on the cytokines interferon-γ and TNF-α.97 In intestinal cell lines occludin knockdown increases macromolecule permeability.98, 99 As for JAM-A, a complex role in intestinal homeostasis by regulating epithelial permeability and proliferation is assumed during inflammatory processes.100
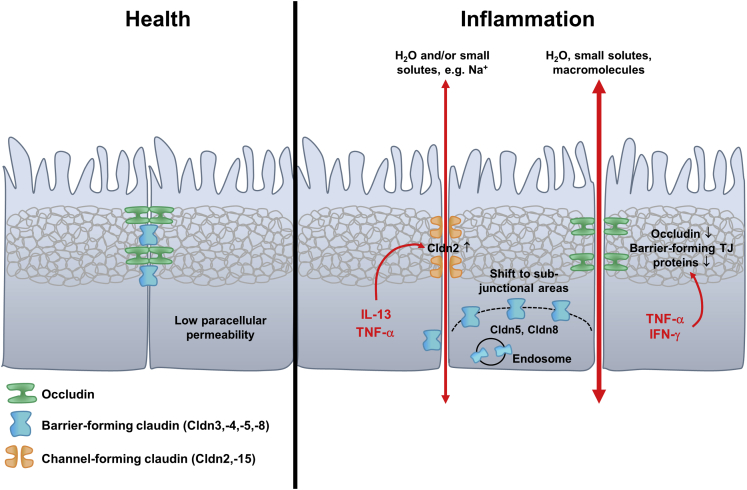
In summary, the segmental heterogeneity of TJ protein expression patterns determines distinct functions of the small and large segments of the healthy intestine. In IBD, expression changes cause segment-specific alterations in paracellular barrier and channel functions (Figure 2). These changes may lead to 2 general effects.101 First is increased paracellular transport of solutes and water, typically mediated by up-regulated claudin-2 and down-regulated barrier-forming claudins. As a consequence, ions and water diffuse from blood to lumen, causing leak-flux diarrhea.92 Second is increased permeability to large molecules including luminal pathogens (eg, food antigens and bacterial lipopolysaccharides), which may initiate an immune response and cause or maintain inflammatory processes.
Figure 2.
Paracellular intestinal barrier in health and inflammation. The paracellular barrier in healthy distal intestinal tissue is characterized by high expression levels of barrier-forming TJ proteins, which leads to a low paracellular permeability. In inflammation, the TJ barrier is disturbed. Channel-forming claudins are up-regulated in their expression (eg, by TNF-α or IL13), leading to an increased permeability for ions and water (pore pathway). Barrier-forming TJ proteins are down-regulated and can also be shifted into subjunctional regions or into endosomes, which further destabilizes the TJ barrier. Occludin, which is, besides tricellulin, involved in regulation of permeability for macromolecules, is also down-regulated by inflammatory processes (eg, by TNF-α or interferon-γ), leading to increased paracellular permeability for macromolecules (leak-pathway). IFN, interferon.
In summary, there are many specific and complex effects in different inflammatory processes and diseases making TJ proteins substantial factors for epithelial barrier impairment, but also for intervention.
Specialized Epithelial Cells Have Innate Immune Cell Functions
The intestinal epithelium is not only a physical barrier, it also has innate immune cell functions to actively combat pathogens through antimicrobial peptides and a protective mucus layer. The secretion of mucus and antimicrobial proteins by secretory cells, such as goblet cells and Paneth cells, provides a shield against bacteria, fungi, and other antigens in the intestinal lumen.102 The mucus layer varies in its thickness between small and large intestine.103 In the small intestine a loosely attached mucus layer can be observed that increases in thickness toward the colon. Although the mucus of the small intestine composes only 1 layer, the colon mucus is 2-layered. Although the outer layer is loose and can be accessed by bacteria, the inner layer is attached to the epithelial surface and is considered to have protective functions because it is impermeable to the luminal bacteria. The main framework of the mucus barrier is built by gel forming mucins, such as Mucin-2 and Mucin-6 secreted by goblet cells.104, 105 Mucins are glycoproteins building a network that is largely impermeable for pathogens. Furthermore, goblet cells produce trefoil factor 3, which has been shown to be important for mucosal restitution together with mucins.106, 107, 108 Another goblet cell product, Resistin-like molecule-β, was shown to act as an immune-effector molecule. On nematode infection, Th2-derived cytokines induced Resistin-like molecule-β expression in goblet cells and impaired the chemosensory function of the nematode.109
Another important epithelial cell type that secretes host defense molecules is the Paneth cell of the small intestinal crypt. Intermingled between intestinal stem cells, Paneth cells secrete a plenty of antimicrobial peptides, the most abundant ones of which are defensins. In humans α-defensin (HD) 5 acts against gram-positive and gram-negative bacteria, by permeabilizing the plasma membrane,110 whereas HD6 showed a specialized way of inactivating pathogens. Accordingly, Chu et al111 provided proof of evidence that HD6 was able to fight off Salmonella typhimurium by attaching to the bacterium and performing a self-assembly, thereby creating nanonets that enclose bacteria. Of note, HD5 and HD6 are down-regulated in patients suffering from CD.112 Lysozyme is another important molecule released by Paneth cells and has been shown to be an efficient glycosidase that hydrolyses peptidoglycan of the bacterial cell wall.113 The lectin-regenerating islet-derived protein III-γ is also expressed by Paneth cells (but also from other IEC types).114, 115 Similar to lysozyme, it acts against the bacterial cell wall. It has been shown that mice deficient for regenerating islet-derived protein III-γ showed signs of constitutive inflammatory responses.116 Given the special location of Paneth cells intermingled with stem cells in the intestinal crypts, one might suggest a gradient of antimicrobial peptides with high expression levels at the crypt base where the stem cells reside. Therefore, Paneth cells might be considered antimicrobial guardians of the stem-cell niche.
Influence of the Gut Microbiota on Intestinal Barrier Integrity
Over the past years, several studies documented changes in the commensal gut microflora of patients with IBD (reviewed in117), including a reduced complexity of commensals or a shift toward a specific phylum. It is currently not clear whether these disturbances are cause or consequence of the manifestation of IBD.
The intestinal microbiota represents the entirety of microorganisms in the human intestine and includes not only bacteria but also fungi and viruses.118 The most frequent microorganisms are commensal bacteria that are beneficial for the host. They help to digest nutrients and also compete with pathogens for the same ecological niches.119 To distinguish between harmful pathogens and beneficial commensals, IECs and innate immune cells, such as dendritic cells and macrophages, are equipped with a variety of innate immune receptors, known as pathogen recognition receptors,120 which recognize conserved microbe-associated molecular patterns, such as bacterial DNA, bacterial components like flagellin, or components of the bacterial cell wall like lipoteichoic acid, muramyl dipeptide, peptidoglycan, or lipopolysaccharide.121, 122 Pathogen recognition receptors include Toll-like receptors, NOD-like receptors, and Rig-I like receptors. Microbe-associated molecular patterns are constantly stimulating IECs to produce antimicrobial peptides, such as REGIII-γ and angiogenin-4 (reviewed in123) or cytokines, such as IL33124 and IL25.125 These mediators are important to form so-called tolerogenic macrophages and dendritic cells to preserve a symbiotic situation and balance the microbial composition.126 Importantly, this interplay of microbes with the intestinal epithelium and the immune system regulates homeostasis of the intestine.
It is known that patients suffering from IBD show disturbances in the recognition of pathogens because of alterations in the expression of pathogen recognition receptors. Frameshift mutations of the NOD-2 gene were identified in patients with CD.127, 128 NOD-2 has been shown to be required for the regulation of commensals in the intestine129, 130 because it is a member of the NOD-like receptor family and expressed in the cytosol of IECs recognizing bacterial muramyl dipeptide.131, 132 Interestingly, mice deficient for NOD-2 showed impaired goblet cell function and increased numbers of interferon-γ producing intraepithelial lymphocytes.130 Furthermore it was shown that NOD-2-deficient mice were intensely enriched in Bacteroides vulgatus, usually a known commensal microbe, potentially causing higher susceptibility to mucosal inflammation. Another example for the interaction of the microbiota with the intestinal epithelium is the production of the IL2 cytokine member, thymic stromal lymphopoietin (TSLP), which is produced by IECs in response to commensals, such as gram-negative Escherichia coli or gram-positive Lactobacillus rhamnosous.133 TSLP signals via signal transducer and activator of transcription (STAT) activation and leads to up-regulation of CD40, CD80, and CD86 on dendritic cells mediating a noninflammatory TH2134 or Treg135 induction.136 These findings were confirmed by Taylor et al,137 who could show that mice with genetic ablation of the TSLP receptor displayed increased IL12/23p40 and interferon-γ production during acute intestinal inflammation induced by dextran sodium sulphate. Hence, the overexpression of IL12 from dendritic cells is suggested to counterregulate the development of TH2 cells. Interestingly, IECs from patients with CD failed to produce TSLP, which correlated with the inability to control IL12 release, disturbing intestinal homeostasis.138
These findings support a disturbed microbiota in patients with IBD, which can be initiated because of a dysregulated epithelial-immune cell communication. The appreciation of intestinal microbes as being beneficial for the host, especially in shaping the intestinal barrier, has been the justification of using probiotics as a dietary supplement. Extensive studies analyzed the beneficial effect of probiotics, such as L rhamnosus, Enterococcus faecalis, and Bifidobaceterium brevis, on the intestinal epithelial barrier function. By using mouse models investigators could show a beneficial effect of probiotics countering intestinal inflammation. This might be caused by barrier-supportive effects because intestinal permeability was reduced when mice or rats were treated with a probiotic cocktail.139, 140, 141, 142, 143, 144
Taken together, the intestinal microbiota shows to be an important regulator of epithelial–immune cell communication and patients with IBD often show a dysbiosis. Shaping the gut microflora with specific probiotics is now seen as a supportive therapeutic approach in the treatment of IBD.
Inflammatory Mediators Are Major Regulators of Epithelial Cell Function and Barrier Establishment
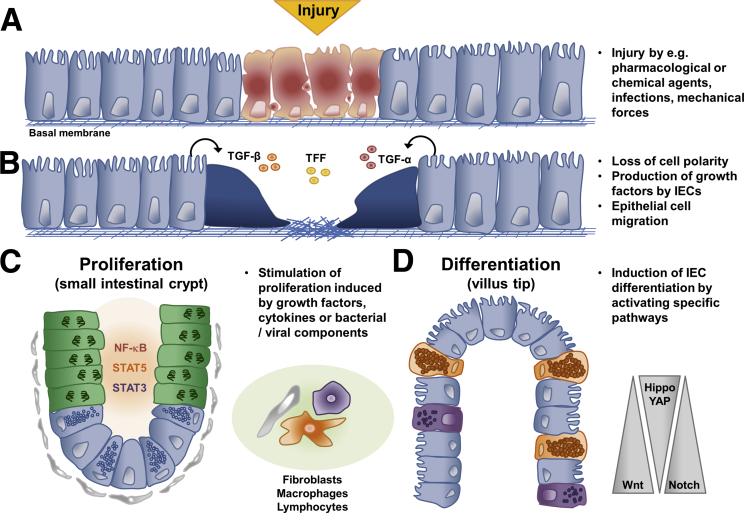
The inflammatory process leads to a strong burst of environmental factors that are processed by the intestinal epithelium to rebuild the epithelial barrier. The mechanisms of epithelial repair can be divided into 3 specific processes. First, in an initial phase, which is termed epithelial restitution, epithelial cells lose their columnar polarity and migrate to the site of the injury to rapidly seal the defective barrier (Figure 3).145 Factors that induce this first step of healing are predominantly produced by epithelial cells, to warrant this fast response after injury. Bioactive transforming growth factor-α and transforming growth factor-β have been shown to be important for the restitution process.146, 147 Moreover, goblet cell products, such as trefoil factors and galectin-2 and -4, have been shown to be involved in the first step of epithelial wound closure.107, 148 Subsequently these factors lead to changes in the cytoskeleton and finally to epithelial cell migration.149, 150, 151
Figure 3.
Representative model of mechanisms involved in mucosal healing. A few hours after epithelial injury (A), epithelial cells lose their polarity induced by the secretion of factors, such as TGF-α, TGF-β, or TFF (B). This induces epithelial cell migration to close the wound. After several hours, growth factors, cytokines, or bacterial/viral components induce the proliferation of IECs in the intestinal crypt (C). In a later phase, IECs differentiate into specialized IECs (D). For cell type nomenclature see Figure 1. TFF, Trefoil factor; TGF, transforming growth factor.
In a second step, depending on the cause of injury, epithelial cells receive signals, such as cytokines, growth factors, and bacterial products. The receipt of these signals induces signaling pathways resulting in the activation of transcription factors, such as STAT-3, and -5 and nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB).152 Many of these signaling events seem to be host protective. Mice with a specific deletion of STAT3 in IECs are highly susceptible to dextran sodium sulphate and showed impaired wound healing.153, 154 A major inducer of STAT3 activation in the intestinal epithelium is IL22, produced by group 3 innate lymphoid cells, which is highly up-regulated during intestinal inflammation.155 By in vitro studies Lindemans et al156 provided evidence that intestinal organoids increase their growth after stimulation with IL22 by activating STAT3 signaling. Interestingly, genome-wide association studies indicate that IL22 is located at a UC risk locus.157 Davidson et al158 reported alterations of the colonic stem cell gene signature during dextran sodium sulphate–induced colitis in mice. Lgr5+ stem cells were diminished in the distal colon and alterations in the crypt structure could be observed. Furthermore, they could detect decreased expression levels of Lgr5, achaete-scute homolog, and homeodomain-only protein mRNA. During the recovery phase Lgr5+ stem cells reappeared. Genetic ablation of STAT5 in IECs and also specifically in Lgr5+ stem cells led to destruction of the intestinal crypt compartment with diminished proliferation and severe inflammation.159
NF-κB up-regulation is often seen during intestinal inflammation but NF-κB has been shown to have proinflammatory and anti-inflammatory roles (for review see160). During inflammatory processes NF-κB mediates wound healing in IECs.161, 162 In contrast, abolition of canonical NF-κB activity in IECs, shown by deletion of NF-κ-B essential modulator or by combined deficiency of IκB kinase 1 together with IκB kinase 2, caused dramatic inflammation of the colon. This demonstrates the necessity of functional NF-κB activity during mucosal healing in the colon.163
In a third step, the activated replenishment of new IECs acquires also a well-defined differentiation process of the epithelial progenitors into mature IECs of either the absorptive or secretory linage. Inflammatory cytokines, such as IL6, induce epithelial cell proliferation through the IL6 coreceptor gp130, which was shown to induce YAP/Notch signaling.164 Notch activation induced expression of Hes-1, inhibiting the differentiation of Paneth and goblet cells. Of note, patients suffering from IBD often manifest elevated levels of IL6 and diminished Paneth and goblet cells.
Increasing evidence indicates that secreted factors during inflammation are able to shape the stem cell compartment. The Wnt pathway is another major pathway in the intestinal crypt, and is important for preservation of the stem cell niche. During intestinal injury it was revealed that Wnt signaling is up-regulated by cytokines inducing STAT6 activation produced by M2 macrophages165 and that the inhibition of the Wnt pathway (eg, by accelerated expression of the Wnt antagonist DKK1) leads to a reduced proliferation rate during inflammation in the large intestine.166
Taken together, it is evident that several cytokines released during intestinal inflammation not only activate immune cells to eradicate microbial challenges but at the same time act on epithelial cells to protect the barrier from the consequences thereof. More research has been undertaken to better understand the complex interplay of immune and epithelial cells and the meaning of this trans-communication for the development of IBD.
Conclusions
The intestinal epithelium can be seen as a translator between the intestinal microbiota and the immune system. The epithelium receives signals from the microbiota via pathogen recognition receptors and translates these into signals forwarded to mucosal immune cells. Vice versa, IECs receive signals from immune cells and translate them into signals shaping barrier function and even the composition of the gut microflora. Previous concepts trying to explain the development of IBD primarily focused on either immune-intrinsic or barrier intrinsic dysfunction. However, none of these concepts alone has been successful to explain all features of IBD. At the same time, data in the literature do not fully support a causative defect in the intestinal epithelial barrier as a primary etiologic factor leading to the manifestation of IBD. An emerging and attractive hypothesis integrating aspects from immune and epithelial explanation approaches is that IBDs are caused by a dysregulated communication between IECs and the immune system. Identifying important molecular interactions of the intestinal epithelium with the microbiota and the immune system influencing intestinal permeability and communication with the immune system will provide important insight into the pathogenesis of IBD and has potential to yield novel therapeutic approaches.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work received funding from Deutsche Forschungsgemeinschaft projects within FOR2438 (TP05), SFB1181 (C05), and the clinical research unit KFO257. Further support was given by the projects SPP1656, SFB796 (B09), BE3686/2, SI749/8-1, the Interdisciplinary Centre for Clinical Research of the University Erlangen-Nürnberg, and the European Community's 7th Framework Program.
References
- 1.Mandel L.J., Bacallao R., Zampighi G. Uncoupling of the molecular “fence” and paracellular “gate” functions in epithelial tight junctions. Nature. 1993;361:552–555. doi: 10.1038/361552a0. [DOI] [PubMed] [Google Scholar]
- 2.Ivanov A.I. Structure and regulation of intestinal epithelial tight junctions: current concepts and unanswered questions. Adv Exp Med Biol. 2012;763:132–148. doi: 10.1007/978-1-4614-4711-5_6. [DOI] [PubMed] [Google Scholar]
- 3.McCauley H.A., Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med. 2015;21:492–503. doi: 10.1016/j.molmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Kopp Z.A., Jain U., Van Limbergen J. Do antimicrobial peptides and complement collaborate in the intestinal mucosa? Front Immunol. 2015;6:17. doi: 10.3389/fimmu.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohno H. Intestinal M cells. J Biochem. 2016;159:151–160. doi: 10.1093/jb/mvv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H., Leblond C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 8.Potten C.S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 9.Barker N., van Es J.H., Kuipers J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 10.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 11.Noah T.K., Donahue B., Shroyer N.F. Intestinal development and differentiation. Exp Cell Res. 2011;317:2702–2710. doi: 10.1016/j.yexcr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanuytsel T., Senger S., Fasano A. Major signaling pathways in intestinal stem cells. Biochim Biophys Acta - Gen Subj. 2013;1830:2410–2426. doi: 10.1016/j.bbagen.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krausova M., Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26:570–579. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregorieff A., Pinto D., Begthel H. Expression Pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Kabiri Z., Greicius G., Madan B. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 17.Sugimura R., Li L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res C Embryo Today. 2010;90:243–256. doi: 10.1002/bdrc.20195. [DOI] [PubMed] [Google Scholar]
- 18.Bovolenta P., Esteve P., Ruiz J.M. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson C.T., Mamo T., Maran A. Molecular strategies for modulating Wnt signaling. Front Biosci (Landmark Ed) 2017;22:137–156. doi: 10.2741/4477. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J., Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Batts L.E., Polk D.B., Dubois R.N. Bmp signaling is required for intestinal growth and morphogenesis. Dev Dyn. 2006;235:1563–1570. doi: 10.1002/dvdy.20741. [DOI] [PubMed] [Google Scholar]
- 22.He X.C., Zhang J., Tong W.G. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–β-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 23.VanDussen K.L., Carulli A.J., Keeley T.M. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crosnier C., Stamataki D., Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 25.Shang Y., Smith S., Hu X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell. 2016;7:159–174. doi: 10.1007/s13238-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahapatro M., Foersch S., Hefele M. Programming of intestinal epithelial differentiation by IL-33 derived from pericryptal fibroblasts in response to systemic infection. Cell Rep. 2016;15:1743–1756. doi: 10.1016/j.celrep.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 27.Büller N.V.J.A., Rosekrans S.L., Westerlund J. Hedgehog signaling and maintenance of homeostasis in the intestinal epithelium. Physiology. 2012;27:148–155. doi: 10.1152/physiol.00003.2012. [DOI] [PubMed] [Google Scholar]
- 28.Kiesslich R., Goetz M., Angus E.M. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. 2007;133:1769–1778. doi: 10.1053/j.gastro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Watson A.J.M., Chu S., Sieck L. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–912. doi: 10.1053/j.gastro.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Marchiando A.M., Shen L., Graham W.V. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208–1218. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pullan R.D., Thomas G.A., Rhodes M. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courth L.F., Ostaff M.J., Mailänder-Sánchez D. Crohn’s disease-derived monocytes fail to induce Paneth cell defensins. Proc Natl Acad Sci. 2015;112:14000–14005. doi: 10.1073/pnas.1510084112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wehkamp J., Koslowski M., Wang G. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn’s disease. Mucosal Immunol. 2008;1:S67–S74. doi: 10.1038/mi.2008.48. [DOI] [PubMed] [Google Scholar]
- 34.Fava F. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J Gastroenterol. 2011;17:557. doi: 10.3748/wjg.v17.i5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vivinus-Nébot M., Frin-Mathy G., Bzioueche H. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744–752. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 36.Hollander D., Vadheim C.M., Brettholz E. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 37.May G.R., Sutherland L.R., Meddings J.B. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology. 1993;104:1627–1632. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- 38.Munkholm P., Langholz E., Hollander D. Intestinal permeability in patients with Crohn’s disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68–72. doi: 10.1136/gut.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeters M., Geypens B., Claus D. Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 40.Russell R., Satsangi J. IBD: a family affair. Best Pract Res Clin Gastroenterol. 2004;18:525–539. doi: 10.1016/j.bpg.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Fries W., Renda M.C., Lo Presti M.A. Intestinal permeability and genetic determinants in patients, first-degree relatives, and controls in a high-incidence area of Crohn’s disease in Southern Italy. Am J Gastroenterol. 2005;100:2730–2736. doi: 10.1111/j.1572-0241.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 42.Irvine E.J., Marshall J.K. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology. 2000;119:1740–1744. doi: 10.1053/gast.2000.20231. [DOI] [PubMed] [Google Scholar]
- 43.Thjodleifsson B., Sigthorsson G., Cariglia N. Subclinical intestinal inflammation: an inherited abnormality in Crohn’s disease relatives? Gastroenterology. 2003;124:1728–1737. doi: 10.1016/s0016-5085(03)00383-4. [DOI] [PubMed] [Google Scholar]
- 44.Montalto M., Curigliano V., Santoro L. Fecal calprotectin in first-degree relatives of patients with ulcerative colitis. Am J Gastroenterol. 2007;102:132–136. doi: 10.1111/j.1572-0241.2006.00884.x. [DOI] [PubMed] [Google Scholar]
- 45.Indaram A.V., Nandi S., Weissman S. Elevated basal intestinal mucosal cytokine levels in asymptomatic first-degree relatives of patients with Crohn’s disease. World J Gastroenterol. 2000;6:49–52. doi: 10.3748/wjg.v6.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Söderholm J.D., Olaison G., Peterson K.H. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn’s disease. Gut. 2002;50:307–313. doi: 10.1136/gut.50.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamora S.A., Hilsden R.J., Meddings J.B. Intestinal permeability before and after ibuprofen in families of children with Crohn’s disease. Can J Gastroenterol. 1999;13:31–36. doi: 10.1155/1999/457315. [DOI] [PubMed] [Google Scholar]
- 48.Hilsden R.J., Meddings J.B., Sutherland L.R. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn’s disease. Gastroenterology. 1996;110:1395–1403. doi: 10.1053/gast.1996.v110.pm8613043. [DOI] [PubMed] [Google Scholar]
- 49.Wyatt J., Vogelsang H., Hübl W. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet (London, England) 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 50.Zeissig S. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madsen K.L., Malfair D., Gray D. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Olson T.S., Reuter B.K., Scott K.G.-E. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resta-Lenert S., Smitham J., Barrett K.E. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/- mice. AJP Gastrointest Liver Physiol. 2005;289:G153–G162. doi: 10.1152/ajpgi.00395.2004. [DOI] [PubMed] [Google Scholar]
- 54.Su L., Nalle S.C., Shen L. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blair S.A., Kane S.V., Clayburgh D.R. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Investig. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 56.López-Posadas R., Becker C., Günther C. Rho-A prenylation and signaling link epithelial homeostasis to intestinal inflammation. J Clin Invest. 2016;126:611–626. doi: 10.1172/JCI80997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benoit Y.D., Lussier C., Ducharme P.-A. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol Cell. 2009;101:695–708. doi: 10.1042/BC20090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kümper S., Mardakheh F.K., McCarthy A. Rho-associated kinase (ROCK) function is essential for cell cycle progression, senescence and tumorigenesis. Elife. 2016;5:e12994. doi: 10.7554/eLife.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segain J.–P., Raingeard de la Blétière D., Sauzeau V. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology. 2003;124:1180–1187. doi: 10.1016/s0016-5085(03)00283-x. [DOI] [PubMed] [Google Scholar]
- 60.Günther C., Buchen B., Neurath M.F. Regulation and pathophysiological role of epithelial turnover in the gut. Semin Cell Dev Biol. 2014;35:40–50. doi: 10.1016/j.semcdb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Khounlotham M., Kim W., Peatman E. Compromised intestinal epithelial barrier induces adaptive immune compensation that protects from colitis. Immunity. 2012;37:563–573. doi: 10.1016/j.immuni.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmad R., Chaturvedi R., Olivares-Villagómez D. Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunol. 2014;7:1340–1353. doi: 10.1038/mi.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D'Inca R., Annese V., Di Leo V. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1455–1461. doi: 10.1111/j.1365-2036.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- 64.Buhner S. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hampe J., Franke A., Rosenstiel P. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 66.Parkes M., Barrett J.C., Prescott N.J. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCarroll S.A., Huett A., Kuballa P. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn’s disease. Nat Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uhlig H.H., Schwerd T., Koletzko S. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007. doi: 10.1053/j.gastro.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lissner D., Schumann M., Batra A. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297–1305. doi: 10.1097/MIB.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raleigh D.R., Marchiando A.M., Zhang Y. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–1213. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gunzel D., Yu A.S.L. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Günzel D., Fromm M. Claudins and other tight junction proteins. Compr Physiol. 2012;2:1819–1852. doi: 10.1002/cphy.c110045. [DOI] [PubMed] [Google Scholar]
- 73.Mineta K., Yamamoto Y., Yamazaki Y. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–612. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 74.Furuse M., Furuse K., Sasaki H. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amasheh S., Meiri N., Gitter A.H. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115(Pt 24):4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 76.Rosenthal R., Milatz S., Krug S.M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 77.Rosenthal R., Günzel D., Krug S.M. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2016;219:521–536. doi: 10.1111/apha.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ebnet K. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 79.Higashi T., Tokuda S., Kitajiri S.I. Analysis of the “angulin” proteins LSR, ILDR1 and ILDR2-tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci. 2013;126:966–977. doi: 10.1242/jcs.116442. [DOI] [PubMed] [Google Scholar]
- 80.Lameris A.L., Huybers S., Kaukinen K. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48:58–69. doi: 10.3109/00365521.2012.741616. [DOI] [PubMed] [Google Scholar]
- 81.Markov A.G., Veshnyakova A., Fromm M. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B. 2010;180:591–598. doi: 10.1007/s00360-009-0440-7. [DOI] [PubMed] [Google Scholar]
- 82.Fujita H., Sugimoto K., Inatomi S. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–1921. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tamura A., Hayashi H., Imasato M. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 84.Van Itallie C.M., Fanning A.S., Anderson J.M. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol - Ren Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 85.Fujita H. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem. 2006;54:933–944. doi: 10.1369/jhc.6A6944.2006. [DOI] [PubMed] [Google Scholar]
- 86.Ikenouchi J., Furuse M., Furuse K. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krug S.M., Amasheh S., Richter J.F. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ikenouchi J., Sasaki H., Tsukita S. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell. 2008;19:4687–4693. doi: 10.1091/mbc.E08-05-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heller F., Florian P., Bojarski C. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Zeissig S., Burgel N., Gunzel D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schumann M., Günzel D., Buergel N. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220–228. doi: 10.1136/gutjnl-2011-300123. [DOI] [PubMed] [Google Scholar]
- 92.Sandle G.I. Pathogenesis of diarrhea in ulcerative colitis: new views on an old problem. J Clin Gastroenterol. 2005;39(Suppl 2):S49–S52. doi: 10.1097/01.mcg.0000155520.04253.37. [DOI] [PubMed] [Google Scholar]
- 93.Al-Sadi R., Ye D., Dokladny K. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suzuki T., Yoshinaga N., Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oshima T., Miwa H., Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008;23(Suppl 2):S146–S150. doi: 10.1111/j.1440-1746.2008.05405.x. [DOI] [PubMed] [Google Scholar]
- 96.Bürgel N., Bojarski C., Mankertz J. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433–443. doi: 10.1053/gast.2002.34784. [DOI] [PubMed] [Google Scholar]
- 97.Amasheh M., Grotjohann I., Amasheh S. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: a novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol. 2009;44:1226–1235. doi: 10.1080/00365520903131973. [DOI] [PubMed] [Google Scholar]
- 98.Al-Sadi R., Khatib K., Guo S. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1054–G1064. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buschmann M.M., Shen L., Rajapakse H. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell. 2013;24:3056–3068. doi: 10.1091/mbc.E12-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laukoetter M.G., Nava P., Lee W.Y. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krug S.M., Schulzke J.D., Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi: 10.1016/j.semcdb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 102.Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2015;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johansson M.E.V., Sjövall H., Hansson G.C. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lievin-Le Moal V., Servin A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin N., Xu L., Sun M. The protective effect of trefoil factor 3 on the intestinal tight junction barrier is mediated by toll-like receptor 2 via a PI3K/Akt dependent mechanism. Biochem Biophys Res Commun. 2013;440:143–149. doi: 10.1016/j.bbrc.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 107.Dignass A., Lynch-Devaney K., Kindon H. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wright N.A., Poulsom R., Stamp G. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104:12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 109.Artis D., Wang M.L., Keilbaugh S.A. RELM /FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 111.Chu H., Pazgier M., Jung G. Human-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science. 2012;337:477–481. doi: 10.1126/science.1218831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wehkamp J., Schmid M., Stange E.F. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr Opin Gastroenterol. 2007;23:370–378. doi: 10.1097/MOG.0b013e328136c580. [DOI] [PubMed] [Google Scholar]
- 113.Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 114.Cash H.L., Whitham C.V., Behrendt C.L. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mukherjee S., Partch C.L., Lehotzky R.E. Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J Biol Chem. 2009;284:4881–4888. doi: 10.1074/jbc.M808077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Loonen L.M., Stolte E.H., Jaklofsky M.T. REG3γ-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol. 2014;7:939–947. doi: 10.1038/mi.2013.109. [DOI] [PubMed] [Google Scholar]
- 117.Matsuoka K., Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Plottel C.S., Blaser M.J. Microbiome and malignancy. Cell Host Microbe. 2011;10:324–335. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kelly D., Conway S., Aminov R. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 2005;26:326–333. doi: 10.1016/j.it.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 120.Fukata M., Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6:451–463. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schnare M., Barton G.M., Holt A.C. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 122.Kaisho T., Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 123.Duerkop B.A., Vaishnava S., Hooper L.V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31:368–376. doi: 10.1016/j.immuni.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 124.Schiering C., Krausgruber T., Chomka A. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zaph C., Du Y., Saenz S.A. Commensal-dependent expression of IL-25 regulates the IL-23–IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rossi O., van Baarlen P., Wells J.M. Springer; Berlin: 2011. Host-recognition of pathogens and commensals in the mammalian intestine; pp. 291–321. [DOI] [PubMed] [Google Scholar]
- 127.Hugot J.-P., Chamaillard M., Zouali H. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 128.Ogura Y., Bonen D.K., Inohara N. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 129.Petnicki-Ocwieja T., Hrncir T., Liu Y.-J. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramanan D., Tang M.S., Bowcutt R. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41:311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Inohara N., Ogura Y., Fontalba A. Host recognition of bacterial muramyl dipeptide mediated through NOD2: implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 132.Girardin S.E., Boneca I.G., Viala J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 133.Zeuthen L.H., Fink L.N., Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2007;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kido M., Tanaka J., Aoki N. Helicobacter pylori promotes the production of thymic stromal lymphopoietin by gastric epithelial cells and induces dendritic cell-mediated inflammatory Th2 responses. Infect Immunol. 2010;78:108–114. doi: 10.1128/IAI.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iliev I.D., Spadoni I., Mileti E. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 136.Roan F., Bell B.D., Stoklasek T.A. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J Leukoc Biol. 2012;91:877–886. doi: 10.1189/jlb.1211622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taylor B.C., Zaph C., Troy A.E. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rimoldi M., Chieppa M., Salucci V. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 139.Kim M.S., Byun J.S., Yoon Y.S. A probiotic combination attenuates experimental colitis through inhibition of innate cytokine production. Benef Microbes. December 23, 2016:1–12. doi: 10.3920/BM2016.0031. [DOI] [PubMed] [Google Scholar]
- 140.Darbaky Y., Evrard B., Patrier S. Oral probiotic treatment of Lactobacillus rhamnosus Lcr35 ® prevents visceral hypersensitivity to a colonic inflammation and an acute psychological stress. J Appl Microbiol. 2017;122:188–200. doi: 10.1111/jam.13320. [DOI] [PubMed] [Google Scholar]
- 141.Miyauchi E., Morita H., Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci. 2009;92:2400–2408. doi: 10.3168/jds.2008-1698. [DOI] [PubMed] [Google Scholar]
- 142.Zareie M., Johnson-Henry K., Jury J. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Laudanno O.M., Cesolari J.A., Godoy A. Bioflora probiotic in immunomodulation and prophylaxis of intestinal bacterial translocation in rats. Dig Dis Sci. 2008;53:2667–2670. doi: 10.1007/s10620-007-0179-5. [DOI] [PubMed] [Google Scholar]
- 144.Mennigen R., Nolte K., Rijcken E. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. AJP Gastrointest Liver Physiol. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 145.Sturm A., Dignass A.U. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348–353. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hoffmann P., Zeeh J.M., Lakshmanan J. Increased expression of transforming growth factor alpha precursors in acute experimental colitis in rats. Gut. 1997;41:195–202. doi: 10.1136/gut.41.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beck P.L., Rosenberg I.M., Xavier R.J. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. doi: 10.1016/s0002-9440(10)63853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Paclik D., Lohse K., Wiedenmann B. Galectin-2 and -4, but not Galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm Bowel Dis. 2008;14:1366–1372. doi: 10.1002/ibd.20499. [DOI] [PubMed] [Google Scholar]
- 149.Lotz M.M., Rabinovitz I., Mercurio A.M. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol. 2000;156:985–996. doi: 10.1016/S0002-9440(10)64966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lotz M.M., Nusrat A., Madara J.L. Intestinal epithelial restitution involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am J Pathol. 1997;150:747–760. [PMC free article] [PubMed] [Google Scholar]
- 151.Turner J.R., Basson M.D., Basson T. Roles of extracellular matrix and cytoskeleton in intestinal epithelial restitution. Front Gastrointest Res Basel. 2002;25:1–13. [Google Scholar]
- 152.Waldner M.J., Neurath M.F. Mechanisms of immune signaling in colitis-associated cancer. Cell Mol Gastroenterol Hepatol. 2015;1:6–16. doi: 10.1016/j.jcmgh.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pickert G., Neufert C., Leppkes M. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Neufert C., Pickert G., Zheng Y. Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle. 2010;9:652–655. doi: 10.4161/cc.9.4.10615. [DOI] [PubMed] [Google Scholar]
- 155.Hanash A.M., Dudakov J.A., Hua G. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lindemans C.A., Calafiore M., Mertelsmann A.M. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Silverberg M.S., Cho J.H., Rioux J.D. Ulcerative colitis–risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216–220. doi: 10.1038/ng.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Davidson L.A., Goldsby J.S., Callaway E.S. Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta - Mol Basis Dis. 2012;1822:1600–1607. doi: 10.1016/j.bbadis.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gilbert S., Nivarthi H., Mayhew C.N. Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Reports. 2015;4:209–225. doi: 10.1016/j.stemcr.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Egan L.J., de Lecea A., Lehrman E.D. Nuclear factor- B activation promotes restitution of wounded intestinal epithelial monolayers. AJP Cell Physiol. 2003;285:C1028–C1035. doi: 10.1152/ajpcell.00167.2003. [DOI] [PubMed] [Google Scholar]
- 162.Strauch E.D., Bass B.L., Rao J.N. NF-κB regulates intestinal epithelial cell and bile salt-induced migration after injury. Ann Surg. 2003;237:494–501. doi: 10.1097/01.SLA.0000060459.03270.E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nenci A., Becker C., Wullaert A. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 164.Taniguchi K., Wu L.-W., Grivennikov S.I. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cosín-Roger J., Ortiz-Masiá D., Calatayud S. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 2016;9:986–998. doi: 10.1038/mi.2015.123. [DOI] [PubMed] [Google Scholar]
- 166.Koch S., Nava P., Addis C. The Wnt Antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology. 2011;141:259–268. doi: 10.1053/j.gastro.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]