Summary
Memory skills strongly differ across the general population, however little is known about the brain characteristics supporting superior memory performance. Here, we assess functional brain network organization of 23 of the world’s most successful memory athletes and matched controls by fMRI during both task-free resting state baseline and active memory encoding. We demonstrate that in a group of naïve controls, functional connectivity changes induced by six weeks of mnemonic training were correlated with the network organization that distinguishes athletes from controls. During rest, this effect was mainly driven by connections between rather than within the visual, medial temporal lobe and default mode networks, whereas during task it was driven by connectivity within these networks. Similarity with memory athlete connectivity patterns predicted memory improvements up to 4 months after training. In conclusion, mnemonic training drives distributed rather than regional changes, reorganizing the brain’s functional network organization to enable superior memory performance.
Keywords: resting state, brain networks, dynamics, memory, mnemonic, cognitive training, method of loci
Introduction
Memory is one of the core components of human cognition. Memory is critical for learning new information and allows one to plan for the future (Schacter et al., 2007). The sense of self is defined, in part, by one’s ability to remember past events. It is understandable, therefore, that few brain disorders are feared more than Alzheimer’s disease, the quintessential disorder of memory loss. The medial temporal lobes have been linked to memory since the seminal early reports on patient H.M (Scoville and Milner, 1957). Increasingly, however, the field has moved from a region-based understanding of memory function to a network-based approach. The network approach maintains the importance of MTL structures while highlighting the relevance of their interactions with cortical structures like the angular gyrus and posterior cingulate cortex, among others (Greicius et al., 2003, 2006; Vincent et al., 2006). The network approach has begun to inform our understanding of Alzheimer’s disease and how it might spread progressively to other brain regions (Seeley et al., 2009).
In order to better understand the network structure supporting memory, we focus here not on memory loss but on memory gain. The top participants of the annual World Memory Championships regularly demonstrate the ability to memorize hundreds of words, digits or other abstract information units within minutes (Foer, 2011). Surprisingly, such memory skills do not seem to be associated with extraordinary brain anatomy or general cognitive superiority, but are acquired through deliberate training in mnemonic strategies (Maguire et al., 2003; Dresler and Konrad, 2013). The most prominent mnemonic technique is the method of loci, an ancient technique used extensively by Greek and Roman orators (Yates, 1966)). It utilizes well-established memories of visuospatial routes: During encoding, to-be-remembered information is visualized at salient points along such a route, which in turn is mentally retraced during retrieval. While numerous behavioral studies have demonstrated the efficacy of mnemonic strategies such as the method of loci (Worthen and Hunt, 2011), data on the brain changes underlying mnemonics are sparse. Previous fMRI studies have demonstrated transient activation of visuospatial brain regions during use of the method of loci in both expert and novice users (Maguire et al., 2003; Nyberg et al., 2003). More long-lasting changes in baseline brain function or anatomy, however, have not been observed in mnemonic experts, possibly because distributed effects or distinctive brain network connectivity patterns are difficult to detect on the basis of very small sample sizes. To elucidate changes in baseline brain function due to extensive training in mnemonic strategies, here we investigate brain networks that are associated with memory and visuospatial processing. We compare fMRI functional connectivity patterns of a comparably large sample of the world’s leading memory athletes with mnemonics-naïve subjects before and after an intense training in the method of loci.
Results
Memory assessment and training
We investigated 23 memory athletes (aged 28±8.6 years, 9 female) out of the Top-50 of the memory sports world ranking list with magnetic resonance imaging assessing both brain anatomy and function during task-free rest before engaging in memory tasks. All of these participants attribute their superior memory skills to deliberate training in mnemonic strategies. The memory athletes were compared with a control group closely matched for age, sex, intelligence, and handedness. 17 of the 23 athletes participated in a word learning task under fMRI conditions where they demonstrated their superior memory abilities compared to controls (70.8±0.6 vs. 39.9±3.6 of 72 words correctly recalled 20 minutes after encoding; Median: 72 vs. 41; Wilcoxon signed ranks test p<0.001, r=0.62).
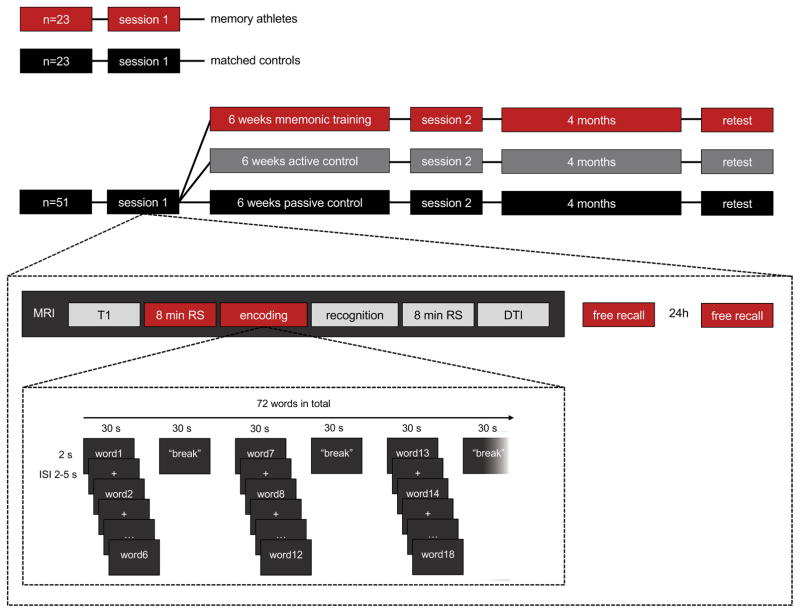
To whether naïve controls can improve their memory with mnemonic training similar to that of memory athletes, 51 participants (aged 24±3.0 years, all male) without any prior experience in mnemonic strategies completed two fMRI sessions over a six-week interval (Fig. 1). In each session, all participants performed a memory test in which they memorized 72 words. Memory was tested with free recall after 20 minutes and again after 24 hours. After the 24 h retest of the first session, subjects were pseudo-randomly assigned to either six weeks (40 x 30 minutes) of mnemonic training in the method of loci, or an active (n-back working memory training) or passive (no training) control condition (Fig. 1). At the conclusion of the six-week training period, participants returned for a post-training assessment that again included a resting-state fMRI scan and a further encoding session of 72 new words, followed by free recall after 20 minute and 24 hour delays. Four months after training completion, participants of all three groups were invited again for a memory test of the 72 words used in the first session to assess potential long-term benefits of mnemonic training.
Figure 1.
Top: study schema. All participants underwent at least one experimental session; participants of the training arm underwent a second experimental session after six weeks, plus a retest after four months. Bottom: Sequence of MRI scans and memory tasks performed in pre- and post-training sessions.
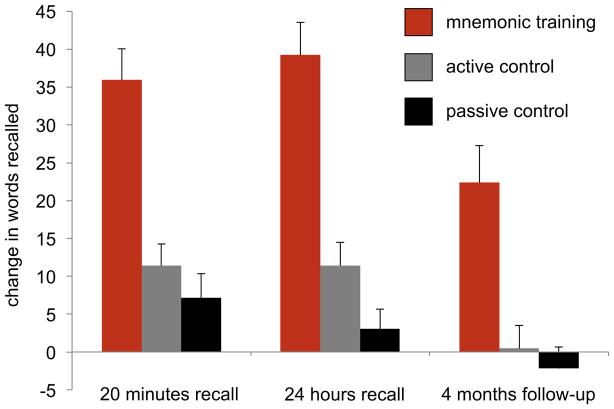
We observed significantly improved memory performance in the participants of the mnemonic training condition in the second experimental session, and this improvement was significantly greater than observed in participants of the active and passive control groups (F2,48>20, p<.001, η2>.4 each). These effects persisted at the four-month follow-up (F2,43=13.4, p<.001, η2=.39; Fig. 2 and supplemental table S2).
Figure 2.
Mnemonic training has potent and enduring effects on memory capacity. Participants in the mnemonic condition showed significantly greater improvement in memory performance after training than participants of the active and passive control groups (p<.001, η2=.3 each, no significant difference between control groups). Mean changes from pre- to post-training sessions in free recall of 72 learned words ± standard error of the mean are shown. During a four month follow-up, subjects re-encoded the list of words from their baseline visit and were asked to recall the list after a 15 minute delay.
Resting state brain network connectivity
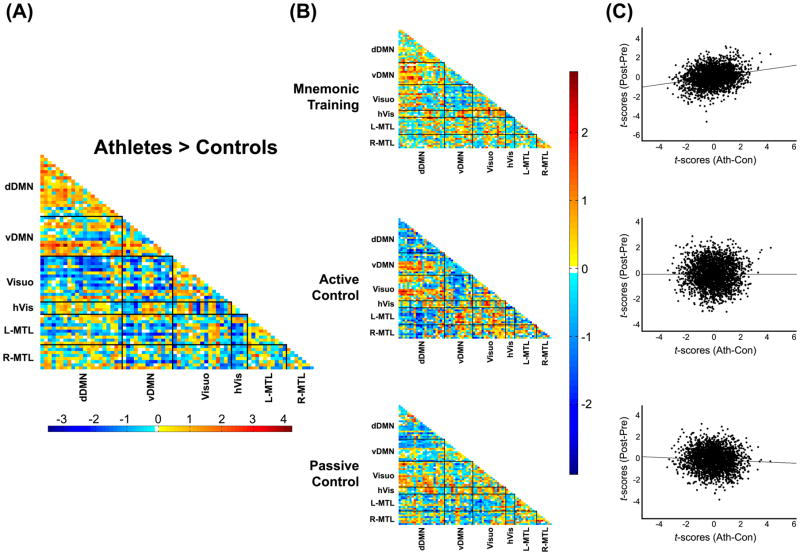
We were interested in the functional organization of brain networks underlying mnemonic expertise in memory athletes in comparison to brain network reorganization as a result of an intense mnemonic training in naïve subjects. All participants underwent a T1-weighted anatomical scan and an 8-minute resting-state fMRI (rs-fMRI) scan with a 3.0T scanner. Scans were completed before engaging in any memory-related activity, ensuring the assessment of pure baseline brain network organization. After fMRI data preprocessing, functional connectivity (FC) was calculated between 71 regions of interest (ROIs) distributed across 6 brain networks related to memory and visuospatial processing (Fig. 3). FC was compared between athletes and controls with a two-sample t-test, producing a 71 x 71 connectivity matrix cataloguing differences in pairwise FC (athletes-controls connectivity matrix, Fig. 4). This difference matrix was then used as a starting point to test whether this network organization was innate to the athletes or could be instilled by 6 weeks of mnemonic training in naïve subjects. In the training groups we therefore calculated pre- and post-training connectivity matrices in the same manner as above. Using paired t-tests, we produced three 71 x 71 connectivity difference matrices documenting training changes in connectivity for each training condition. We then compared these FC changes for each training group with the FC pattern that distinguished athletes from controls by correlating the two T-score matrices. We found that mnemonic training elicited changes in brain network organization that significantly resembled the network connectivity patterns that distinguish memory athletes from controls (Fig. 4, r=.22, p<.005). Neither the active nor passive control group experienced similar changes in neural network organization (r<.02, p>.6 each). In contrast to this multivariate effect of global connectivity similarity, none of the univariate differences between any of the groups were significant after correction for multiple comparisons via false discovery rate. In other words, without comparison to the athlete/control connectivity difference pattern, no connectivity changes through mnemonic training would have been observed in our sample.
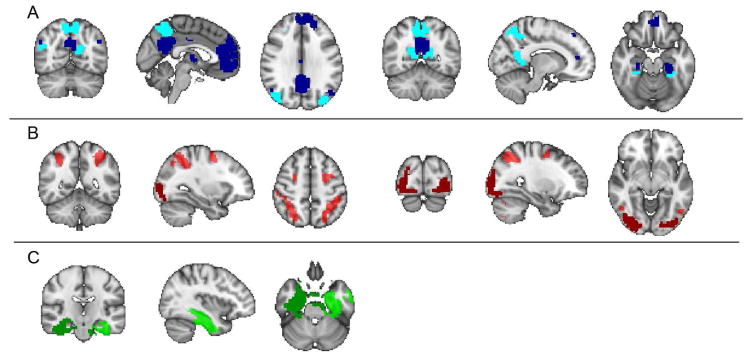
Figure 3.
Brain networks examined with resting-state fMRI analyses: Six networks based on Shirer et al. 2012 were selected due to their hypothesized recruitment by the memory task: (A) ventral (dark blue) and dorsal (light blue) default mode network, (B) higher visual (dark red) and visuospatial (light red) network, (C) left (dark green) and right (light green) medial temporal lobe.
Figure 4.
Similarity of training-induced connectivity changes with athlete-control connectivity differences. (A) Brain network connectivity differences between memory athletes and controls. (B) Connectivity changes from pre- to post-training assessment for each training condition. (C) Scatterplots and correlations between the memory athlete vs. control connectivity difference matrix and the pre- vs. post-training connectivity difference matrices. The pattern of connectivity differences between memory athletes and controls correlates significantly with the pattern of connectivity changes in the mnemonic training condition (r=.222, p=.005), but does not correlate significantly with the connectivity pattern changes in the active (r=.011, p=.943) or passive (r=−.061, p=.632) control groups.
Association with behavioral measures
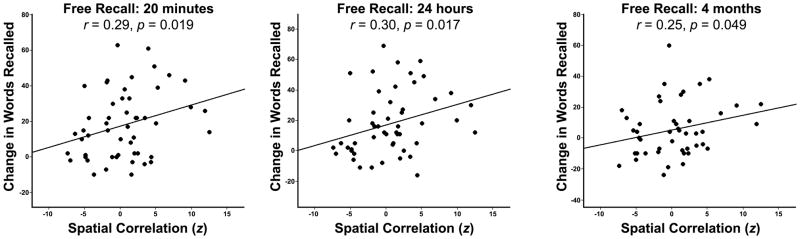
We next examined whether brain network re-organization was related to improved memory performance. We calculated the correlation of each individual subject’s connectivity-change matrix (post- minus pre-training FC matrix), to the athletes-controls matrix, producing 51 different similarity values, one for each participant across the three training arms. These values were regressed against the participants’ change-in-free-recall scores (post-training minus pre-training free-recall performance). We found that the correlation of individual-change matrices to the athletes-controls matrix was significantly related to the participants’ changes in free recall performance. This was true for 20 minute delayed recall, 24 hour delayed recall, and in a follow-up memory test four months after the end of training (Fig. 5, Z=2.07, p=.019; Z=2.12, p=.017; Z=1.65, p=.049, respectively).
Figure 5.
Memory performance is correlated with functional connectivity changes. The spatial correlation strength of change-in-FC matrices to the athletes-controls matrix was significantly related to the participants’ performance on the free-recall tasks at 20 minutes, 24 hours, and in an additional learning session at 15 minutes for the baseline list of words re-encoded at the 4 month follow-up visit.
Given that both memory athletes and participants of the mnemonic condition after training showed strong ceiling effects in the memory task, no meaningful correlations were possible within these groups. Further emphasizing the multivariate nature of our findings, for all other comparisons simple within-group univariate correlations with behavior were not significant after correction for multiple comparisons. We also did not find significant associations with training speed within the mnemonic training group.
Identification of pivotal connections and hubs
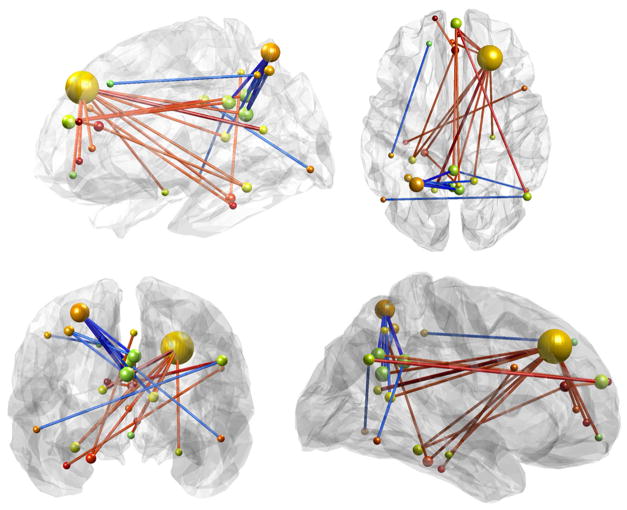
To understand the nature of the multivariate finding in more detail, we tested whether the effect is distributed across all connections between our selected ROIs or driven by more discriminative connections. We focused on those 25 connections in the athletes-controls matrix whose T-score absolute (i.e. both positive and negative) values were among the top 1% of largest differences. We tested across participants if similarity between the individual pre-post training connectivity difference matrices with the athlete-control difference matrix differed between this restricted set of 25 connections and the whole set of 2485 connections. We found a significant increase in similarity in the mnemonic training condition (t=2.61, p=.019), but not for the active (t=0.59, p=.57) or passive (t=−1.65, p=.12) control group. This suggests that the top 1% of connections carried a disproportional amount of information, thus allowing a more specific interpretation of the observed multivariate effect: Connectivity between two major hubs (MPFC and right-DLPFC) and a number of regions important for memory processes, including the left parahippocampal gyrus, bilateral retrosplenial cortex, posterior cingulate cortex and right angular gyrus were pivotal for the observed similarity between training effects and memory athlete connectivity patterns (Fig. 6).
Figure 6.
The top 1% of differential connections between memory athletes and matched controls are shown. Red connections depict stronger and blue connections weaker functional connectivity in memory athletes as compared to controls.
Resting state network dynamics
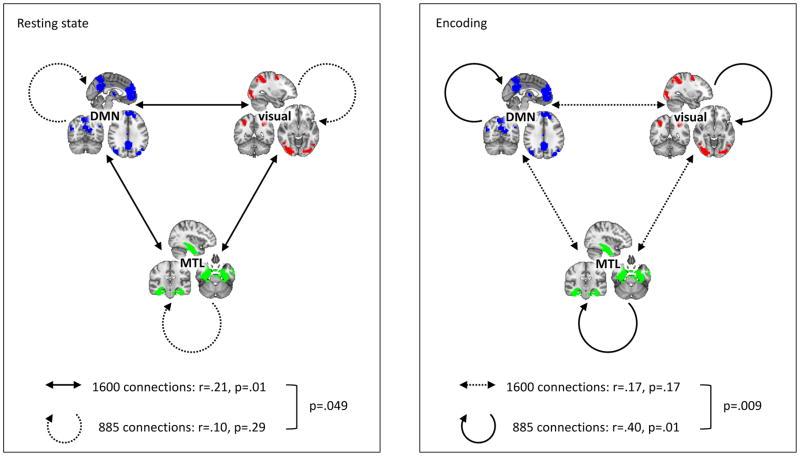
To gain additional insight into network dynamics, we investigated if the effect was more prominent within or between brain networks. We repeated the correlational similarity analyses for 885 connections lying entirely within either the default mode network (ventral and dorsal combined), or the visual network (visuospatial and higher visual combined), or the MTL (left and right combined); and separately for the 1600 connections between either of the default mode, visual, and MTL networks (Fig. 7). We found for neither condition significant resemblance of the pre vs. post connectivity differences with athlete vs. control connectivity differences within the networks (mnemonic training: r=0.10, p=0.29; active control: r=−0.05, p=0.64; passive control: r=−0.17, p=0.13). In contrast, we did find a significant correlation for pre vs. post connectivity differences with athlete vs. control connectivity differences between the networks in the mnemonic training condition (r=0.21, p=0.01), whereas the respective correlations for the active or passive control conditions were not significant (active control: r=0.02, p=0.82; passive control: r=0.00, p=0.96). Importantly, for the mnemonic training condition similarity with athlete-control connectivity patterns was significantly larger for between vs. within network connectivity (t=2.17, p=.049). Hence, the observed effect was mainly driven by between rather than within network connectivity patterns during task-free baseline rest.
Figure 7.
During resting state, similarity between mnemonic training-induced connectivity changes and athlete/control connectivity differences is mainly driven by between brain network connectivity. During encoding, in contrast, similarity between mnemonic training-induced connectivity changes and athlete/control connectivity differences is mainly driven by within brain network connectivity.
Brain network connectivity during encoding
To replicate our findings and to test whether the observed multivariate similarity between brain network connectivity patterns of memory athletes and after mnemonic training was restricted to baseline rest or is also present during active memory encoding, we repeated the described analyses also for connectivity as seen in the fMRI encoding task data. We were able to replicate the main finding of a correlational similarity between athlete/control and pre/post training connectivity difference patterns for the mnemonic condition (r=0.26, p=0.02), but not for the active (r=0.03, p=0.74) or passive (r=−0.03, p=0.70) control groups.
Strikingly, in the within vs. between network analyses for the task recordings we found the opposite effect than for task-free resting state data: we observed a significant correlation for pre-post with athlete-control connectivity patterns within the networks specifically in the mnemonic training condition (mnemonic training: r=0.40, p=0.01; active control: r=0.00, p=0.97; passive control: r=0.04, p=0.70), however no significant similarity for between network connectivity in any of the training groups (mnemonic training: r=0.17, p=0.17; active control: r=0.05, p=0.65; passive control: r=−0.07, p=0.50). For the mnemonic training condition, similarity with athlete-control connectivity patterns was significantly larger for within vs. between network connectivity (t=3.0, p=.01). Hence, in contrast to task-free resting state, the similarity effect was driven by within rather than between network connectivity patterns during task.
Discussion
Our results demonstrate that superior memory is supported by a multivariate resting-state functional connectivity profile distributed throughout the default mode network, visual networks and the medial temporal lobe. This superior-memory connectivity profile can be instilled in naïve controls by a 6-week period of mnemonic training in the method of loci: The greater the degree to which an individual’s functional connectivity profile after training resembled the memory athletes’ connectivity pattern, the more that individual profited on measures of short- and long-delay memory through training. The improved memory observed after mnemonic training persists for as long as four months after training concludes. Of note, the training-induced similarity with the superior memory connectivity profile can be observed both during task-free baseline resting state and for background brain connectivity during active encoding. During rest, similarity between training-induced changes and the specific connectivity pattern of memory athletes is mainly driven by connectivity between brain networks, whereas during encoding it is driven by within network connectivity.
One hypothesis for the efficacy of mnemonic strategies invokes their use of naturally evolved skills, such as visuospatial memory and navigation (Maguire et al., 2003). In the method of loci, abstract and unrelated information units are transformed into concrete and related information patterns that can more easily be processed by memory-related brain structures, such as the hippocampus. The method of loci has been associated with hippocampal place and grid cells (Becchetti, 2010), which are also active during mental navigation (Bellmund et al., 2016), and involved in episodic memory encoding and retrieval (Miller et al., 2013; Monaco et al., 2014). Brain regions critical for visuospatial memory and navigation such as retrosplenial and hippocampal areas are engaged during mnemonic encoding in memory athletes (Maguire et al., 2003). Acquisition of the method of loci in novices is related to activation increases in the left hippocampal region, its use during encoding with increased activation in the left occipito-parietal cortex, retrosplenial cortex and dorsolateral prefrontal cortex (Nyberg et al., 2003), and its use during recall with increased activation in the left parahippocampal gyrus and retrosplenial cortex (Kondo et al., 2005). These studies converge with our data in that the left parahippocampal gyrus and bilateral retrosplenial cortex both showed significant changes in network connectivity between memory athletes and controls.
We identified the right dorsolateral prefrontal cortex (DLPFC) as a hub for a number of connections that contributed most strongly to the transfer effect. The DLPFC is more strongly activated when information is encoded in a more structured way, e.g. by chunking (Bor et al., 2003). In particular, the right DLPFC has been linked to the use of memory strategies: patients with right DLPFC lesions are specifically impaired when using strategies during memory tasks (Chase et al., 2008), and transcranial magnetic stimulation of the right DLPFC interferes with retrieval only in users of encoding strategies (Manenti et al., 2010). The right DLPFC shows activation increases mainly for the encoding of visual material (Kelley et al., 1998; Epstein et al., 2002), particularly during encoding via visuospatial mnemonics such as the method of loci (Kondo et al., 2005). The prominent role of right DLPFC we found in the brain connectivity profile of experts in the method of loci is therefore convergent with previous work linking this brain region to visuospatial processing and encoding strategies.
Our results suggest participation of the medial prefrontal cortex (MPFC) in the functional connectivity profile supporting superior memory. Separate research on mental schemas has highlighted the role of the MPFC in memory processes: Mental schemas enhance learning by allowing efficient encoding of newly acquired information through incorporation in pre-existing knowledge structures (Tse et al., 2007). Schema utilization improves learning, and is associated with increased activity in, and connectivity between, the MPFC and information-related cortices (van Kesteren et al., 2010a). Furthermore, the manipulation of prior schema knowledge was shown to influence MPFC-hippocampal connectivity during encoding and post-encoding rest (van Kesteren et al., 2010b). Mnemonics, such as the method of loci, can be conceptualized as utilizing schemas, providing pre-learned knowledge structures into which new information can be rapidly encoded.
Analyzing network dynamics, we observed that the similarity of mnemonic training-induced brain reorganization with superior memory connectivity patterns was mainly driven by between network connectivity during task-free baseline resting state, and by within network connectivity during actual encoding. While task-related brain processes are known to be intrinsically related to task-independent measurements collected at rest (Hampson et al., 2006; Tavor et al., 2016; Shine et al., 2016), the specific association between task-free and task-related brain function is not well understood yet. Segregated processing modes coexist with a more global and integrated coordination of brain networks (Tognoli & Kelso, 2014), and the pattern of segregated vs. integrated brain network processing dynamically changes depending on cognitive task demands (Shine et al., 2016). Our data suggest that during rest, global between network measurements are more informative than regional within network measurements for detecting superior memory capacity, whereas the opposite is true during task engagement.
In conclusion, we demonstrate that superior memory capacity is supported by distributed changes in functional connectivity rather than by focal changes in single brain regions. The brain network organization associated with superior memory can be achieved by mnemonic training. Among the distributed differences across memory and visuospatial brain regions, we found most robustly increased functional connectivity among the right DLPFC, the MPFC and structures of the medial temporal lobe in expert users of mnemonics and in naïve subjects after mnemonic training. On the level of network dynamics, effects were driven between brain networks during rest and within networks during active encoding, corroborating differential neural processing during these two states also for the phenomenon of memory expertise. Collectively, these results demonstrate the role of mnemonic strategies in altering functional networks and improving memory performance, and support the use of fMRI brain connectivity measures as a powerful tool in the study of brain plasticity.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact Martin Dresler (martin.dresler@donders.ru.nl). Restrictions apply to the raw data of memory athletes, as these allow personal identification of the participants.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Memory athletes of the Top-50 of the memory sports world rankings were recruited via email, phone calls or personally. Control participants were matched for age, sex, handedness, smoking status, and IQ. Where relevant, to ensure matching with the generally high intellectual level of the memory athletes, control participants were recruited among gifted students of academic foundations and members of the high-IQ society Mensa via mailing lists. Seven control participants were selected among the participants of the training arm according to their high cognitive performance shown in the screening session, evenly distributed among the three training conditions. All control participants were tested with a standardized memory test (Bäumler, 1974). Exclusion criterion was a performance of more than two standard deviations above the mean according to the norms provided with the memory test to avoid including ‘natural’ superior memorizers in the control group, however none of the participants reached this criterion. None of the cognitive tests were performed immediately before the fMRI session, in order to prevent the resting state network activity being influenced by previous learning. Participants for the training arm of the study were recruited via mailing lists and public announcements among students of the universities of Munich. In a screening session, exclusion criteria (experience in mnemonic strategies, psychiatric or neurological history, more than 5 cigarettes per day, other drug consumption) were checked. In addition, fluid reasoning (Weiß and Weiß, 2006) and memory abilities (Bäumler, 1974) were tested, and performance was used to pseudo-randomly assign participants to the three training conditions to ensure similar cognitive baseline levels between groups. To minimize motivational or compliance effects of the condition assignment, all participants of the training arm were offered to participate in an additional mnemonic strategy or working memory training after conclusion of the study. One participant dropped out of the active control condition after one week of n-back training due to 33 lack of commitment. One further participant had to be excluded before condition assignment due to a pathological finding in brain anatomy. Both participants were replaced by newly recruited subjects. All participants were paid and provided written informed consent to the study in line with the approval by the ethics committee of the medical faculty of the University of Munich. For a detailed overview over participants see Supplemental Table S1.
METHOD DETAILS
Cognitive training
Immediately after the 24 h free recall of session 1, all participants of the training arm were pseudo-randomly assigned to one of three training conditions. Participants of the mnemonic training condition started within one week after condition assignment with a 2 hours introduction course in mnemonic strategies at the Max Planck Institute of Psychiatry. They were introduced into the method of loci, were taught their first loci route within and outside of the institute, applied this route in a first memorization task under supervision, were familiarized with the home-based training platform (http://memocamp.com), instructed how to build new routes, and provided with a training plan for the upcoming week. Training plans gave instructions on which set of locations to use to ensure equal training of all routes and reduce interference of word list memorized on preceding days. The training consisted of 30 minutes of training per day for 40 days at home via a web-based training platform. During the first two weeks of the training, participants built and memorized three further loci routes, with which they trained to memorize lists of random words. During the next four weeks, training was restricted to memorizing lists of random words or images with the four loci routes. The task demand (number of words to be memorized) changed dynamically according to the individual performance of the participant: 5 to be memorized words were presented on the first trial; the number of presented words increased in subsequent training runs by 5 as soon as a subject managed to perfectly remember all words in a given run. Speed of training success was defined as the average number of training runs a participant needed per level increase until he successfully reached level 8 (i.e. 40 words presented), as this level was reached by most mnemonic training participants (16 out of 17), but can hardly be achieved by mnemonics-naïve individuals. Logfiles of the training sessions were checked each day to monitor compliance. In case of a missed or too short training session, participants were contacted on the following morning and instructed to expand the following training session to make up for the missed training time. Once a week, subjects came to the laboratory, were interviewed regarding problems with the training regime, trained under direct supervision, and were provided with a training plan for the next week.
Participants of the active control condition started within one week after condition assignment with an introduction into the home-based n-back working memory training program. We used a very demanding version of the dual n-back task, in which participants had to monitor and update series of both visually presented spatial locations and auditorily presented letters (Jaeggi et al., 2008). The value of n varied between blocks of trials, with adjustments made continuously based on performance. The task demand thus changed adaptively according to the individual performance of the participant. Participants trained 30 minutes each day for 40 days. Logfiles of the training sessions were checked each day to control for compliance. In case of a missed or too short training session, participants were contacted on the following morning and instructed to expand the following training session to make up for the missed training time. Once a week, subjects came to the laboratory, were interviewed regarding problems with the training regime, and trained under direct supervision.
The passive control group did not receive any training between the two experimental sessions.
Behavioral data acquisition
All participants of the training arm of the study and performed a word-encoding task in the scanner during pre- and post-training sessions. In the post-training session, participants of the mnemonic training condition were asked to apply the method of loci to the task. We used two lists of 72 concrete nouns, with one list being presented per session. Words in both lists were counterbalanced for word length and frequency, and were presented in a random order within each list. To prevent order effects across sessions, word lists were presented in a crossover-designed manner. Words were presented individually for 2 s each, with a jittered inter-stimulus interval of 2–5 s. After six words, a fixation cross was presented for 30 s, which was followed by the next 6 words etc. Participants were instructed not to rehearse during the fixation cross periods, and to think of nothing in particular, comparable to the resting state scan before, however with eyes open.
After the encoding task, a word order recognition task of all 72 words followed. Triplets of words from the word lists encoded before were presented for 10 seconds, after which participants had to indicate within 3 seconds if the order of words was exactly as presented before or in a changed order. Presentation and response to each triplet of target words was followed by a control condition, in which participants had to indicate if triplets of new words were shown in ascending order according to their number of syllables. Recognition data have not been analyzed yet and will be presented elsewhere.
Immediately after leaving the scanner, participants had to indicate on a 4-point scale if they had been continuously alert, partly tired, partly drowsy, or partly asleep during the rs-fMRI scan, and if they had their eyes closed during the resting state and open during the encoding session. Analysis of this data indicated that all participants adhered to the eyes closed instructions and no participant reported having been drowsy or asleep during rs-fMRI. Participants were then brought to the behavioral laboratory, where had to freely recall all 72 words presented during the encoding session. Subjects wrote down all remembered words; after 5 min they were asked if they would need more time; after another 5 min recall was terminated. After 24 hours, another free recall of 5+5 min was performed via telephone. Recall score was defined as number of words correctly recalled ignoring order and spelling mistakes. On average, participants forgot 10.3 ±7.0 words in the 24 hour recall compared to 20 min recall in the pre-training assessment, and 10.7 ±8.5 words in the 24 hour recall compared to 20 min recall in the post-training assessment (paired t-test: t>8.9, p<0.01 each).
During the final retest after four months, participants performed the encoding task once more, this time outside the scanner. The word list of their first session was used for re-test, and long-term effects were calculated as difference between first session and re-test session performance. Participants of the mnemonic training condition were asked to use the method of loci for encoding, and all confirmed use of the strategy after the task. Encoding was followed by a delay period of 15 minutes, filled with a reasoning task, after which participants had to freely recall all memorized words. Of the 51 study participants, 2 participants each of the mnemonic training and passive control conditions and 1 participant of the passive control group were not available for the follow-up test session.
MRI data acquisition
All imaging data were collected at the Max Planck Institute of Psychiatry using a 3 T (GE Discovery MR750) scanner with a 12-channel head coil. A standard localizer, coil calibration and a 3D T1-weighted anatomical scan (TR 7.1 ms, TE 2.2 ms, slice thickness 1.3 mm, in-plane FOV 240 mm, 320×320x128 matrix, 12° flip angle) preceded fMRI data collection. Eight minutes of rs-fMRI with eyes closed were collected (EPI sequence, TR 2.5 s, TE 30 ms), covering the whole brain with 34 slices, using a 64×64 matrix with 3 mm slice thickness and 1 mm slice spacing, and a field of view of 240 x 240 mm2. The images were AC–PC aligned and acquired using an interleaved slice acquisition scheme.
After rs-fMRI data collection, participants performed a word encoding task (see “behavioral data acquisition” section above). We obtained 292 T2*-weighted blood oxygenation level-dependent (BOLD) images for each encoding phase of the experiment, using the following EPI sequence: repetition time (TR), 2.5 s; echo time (TE), 30 ms; flip angle, 90°; 42 ascending axial slices; field of view (FOV), 240 × 240 mm; 64 × 64 matrix; slice thickness, 2 mm.
Participants further performed a word order recognition task in the scanner, and underwent a second rs-fMRI and a DTI scan (Fig. 1). Data of these additional scans have not been analyzed yet and will be presented elsewhere.
ROI selection
Functional connectivity (FC) for all participants was calculated across 71 ROIs modified from Shirer et. al 2012. To generate the modified ROIs, we first divided the brain into 91 regions: 90 of which covered 14 major networks described by Shirer et al. 2012, and the rest of the gray matter voxels were treated as a single region. We then divided each region into round(nN/p) parcels using Ward clustering (Michel et al., 2012), where n is the number of gray matter voxels in the given region, p is the total number of gray matter voxels in the brain, and N is a user-defined number of parcels, set to 500 in accordance with the literature (Van Essen and Ugurbil, 2012). To constrain the parcels to be spatially-contiguous, only Pearson’s correlations between fMRI time courses of spatially-adjacent voxels were considered during Ward clustering. Whereas the Shirer et. al 2012 atlas did not cover a large portion of cortex and subcortical regions, this processing produced an atlas covering all brain regions (Altmann et al., 2015; Richiardi et al., 2015). From among these 500 ROIs, we selected 71 ROIs that covered six brain networks chosen a priori as being related to memory or visuospatial processing and so potentially relevant to mnemonic training, namely the dorsal and ventral default mode networks, the visuospatial and higher visual networks, and the left and right medial temporal lobes (Fig. 3).
QUANTIFICATION AND STATISTICAL ANALYSIS
Behavioral data analysis
For statistical analysis of training-related change in 20 min free recall (defined as the difference between pre- and post-training scores in 20 min free recall) and in 24 h free recall (defined likewise), we performed ANOVAs, each with the three levels mnemonic training, active control, and passive control. For training-related change in 20 min recall, we found a significant effect (F2,48=21.5, p<.001, η2=.47), with Bonferroni-corrected post hoc tests indicating a significant difference between mnemonic training and both active and passive control (p<.001 each), but not between the latter two (p>.9). Also for training-related change in 24 h recall, we found a significant effect (F2,48=33.2, p<.001, η2=.58), with Bonferroni-corrected post hoc tests indicating a significant difference between mnemonic training and both active and passive control (p<.001 each), but not between the latter two (p=.29).
To test for long-term effects, we performed another ANOVA for the change from pre-training 20 min recall to the 4 months retest, with the three levels mnemonic training, active control, and passive control. We found a significant effect (F2,43=13.3, p<.001, η2=.38), with Bonferroni-corrected post hoc tests indicating a significant difference between mnemonic training and both active and passive control (p<.001 each), but not between the latter two (p>.9).
17 of the 23 athletes and their respective controls also underwent encoding and retrieval of 72 words as described above, however were assessed only with short-term free recall, i.e. without the 24 hours or 4 months retest. Due to massive ceiling effects in the athletes group (70.8±0.6 vs. 39.9±3.6 of 72 words correctly recalled 20 minutes after encoding; Median: 72 vs. 41), we used a Wilcoxon signed ranks test to analyze the difference between athletes and controls.
For detailed memory data see Supplemental Table S2.
Resting state fMRI analysis
rs-fMRI data were processed and analyzed using the FMRIB Software Library (FSL: version 4.1). We applied motion correction (for motion parameters see Table S3), removed nonbrain structures, and performed spatial smoothing with a 6mm FWHM Gaussian Kernel. The data were aligned to the MNI152 standard space image with affine linear registration. This was followed by noise regression of movement, cerebral spinal fluid, white matter, and global signal. The data were additionally filtered with a bandpass filter of 0.01–0.1 Hz, restricting analysis to low frequency BOLD fluctuations.
Functional connectivity (FC) for all participants was calculated across 71 ROIs that covered six brain networks chosen a priori as being related to memory or visuospatial processing and so potentially relevant to mnemonic training: dorsal and ventral default mode network, visuospatial and higher visual network, and left and right medial temporal lobe (see Fig. 3). We extracted the mean time series for each ROI, and calculated the Pearson correlation coefficient between the time series of all ROIs, producing a 71 x 71 matrix of correlation coefficients. This was done separately for memory athletes and matched controls. FC was compared between athletes and controls with a two-sample t-test, producing a 71 x 71 matrix cataloguing differences in pairwise FC. We then generated the same connectivity difference matrices for all the training groups: The pre- and post-training FC matrices were compared with a paired-samples t-test. This was done separately for the three training conditions, producing corresponding 71 x 71 matrices of t-values for the mnemonic, and active and passive control conditions.
The FC changes that occurred in each training group were then compared with the differences in FC that distinguish memory athletes from matched controls by calculating the spatial correlation of the t-score matrices. To do this, we correlated the matrices of t-values for each training group separately with the matrix of t-values from the t-test of athletes versus controls. To test the resulting correlations for significance we constructed the permutation distribution for each of the correlations: By randomly permuting the athlete and control pairs (while keeping the matching intact) we generated in a first step 10.000 matrices containing t-values of the permuted athlete vs. control samples. In a second step, each of these matrices was correlated, analog to the procedure above, with each of the training group matrices, resulting in a permutation distribution for each of the three correlations. From these we constructed the p-values by assessing the proportion of correlations from the permutation tests that had a higher absolute value than the absolute value of the correlations of the non-permuted data.
Association with behavior
We next examined the relationship between network reorganization and improved performance on the free-recall task. We calculated the spatial correlation of each subject’s change-matrix to the athletes-controls matrix, and regressed the subjects’ spatial correlations with their changes in performance on the free-recall task. This was done separately for the 20 minute delay free-recall, the 24 hour delay free-recall, and the 4 month follow-up. In addition, we analyzed the amount of forgetting that occurred from 20 min recall to 24 hour recall in both the pre- and post-training assessment. For the mnemonic training group, we also associated the training speed (average number of training runs needed to reach 40 presented words as described above) with network reorganization. Correlations were converted to Z-scores with the Fisher transformation, and these Z-scores were used to assess the significance of the spatial correlation.
Identification of pivotal connections and hubs
We selected the top 1% (i.e. 25) connections with the highest t-scores in the athlete-control connectivity difference and visualized them using the Flexible Brain Graph Visualizer (Richiardi et al., 2012; http://sourceforge.net/projects/flexbgv). To test if the observed similarity between mnemonic training effects and athlete/control connectivity differences holds also for this restricted set of 25 connections, we selected the same connections in each training group and repeated the similarity analysis as described above (with 1000 permutations) on these connections.
We then tested whether this restriction to this set of top connectivity differences significantly increased similarity of pre-post training changes with athlete-control connectivity differences. Instead of the group pre-post training difference matrices, we correlated the individual pre-post training difference matrices with the athlete-control difference matrix, thus obtaining one correlation per participant. We did this for the full set of 2485 connections and for the top 1% of connections. Via paired t-test we then compared how the selection of regions influenced the previously observed connectivity similarity between training effects and athlete-control connectivity differences.
Within vs. between network analysis
The 71 ROIs were in total part of 3 larger networks: visual (visuospatial + higher visual combined, 19 ROIs), medial temporal lobe (left + right combined, 18 ROIs), and default mode network (dorsal + ventral combined, 34 ROIs). To investigate whether the training effect we observed was driven by within or between network connectivity changes, we sorted our whole set of 2485 unique connections into 885 connections lying entirely within either the DMN, visual, and MTL network; and in 1600 connections from a given ROI to a ROI outside of its own network. Then we repeated the correlational similarity analysis on the individual level as described above for both of these sets separately.
Task-based fMRI analyses
Following rs-fMRI, participants completed an encoding task within the fMRI scanner (see description of behavioral data acquisition above). All fMRI data acquired during encoding were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). The first five volumes were discarded to allow for T1-equilibration. The remaining volumes were realigned to the mean image of each session (for memory athletes and matched controls), or across sessions (for participants in the training arm of the study). The structural scan was co-registered to the mean functional scan and segmented into grey matter, white matter, and cerebrospinal fluid using the “New Segmentation” algorithm. All images (functional and structural) were spatially normalized to the Montreal Neurological Institute (MNI) EPI template using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL; Ashburner 2007), and functional images were further smoothed with a 3D Gaussian kernel (8 mm full-width at half maximum, FWHM). Task data of one participant (active control group) had to be excluded because of technical difficulties.
Next, we assessed functional connectivity during the encoding task. We used a voxel-wise general linear model (GLM) to remove nuisance-related effects. Nuisance regressors comprised the six realignment parameters, as well as additional regressors that captured scan-to-scan motion (Power et al. 2012). Specifically, we calculated the framewise displacement (FD) for every scan at time t by FD(t) = |Δdx(t)| + |Δdy(t)| + |Δdz(t)| + r|α(t)| + r|β(t)| + r|γ(t)|, where (dx, dy, dz) is the translational-, and (α, β, γ) the rotational movement. Scans that exceeded a head motion limit of FD(t) > 0.3 mm were removed, indicated in one additional regressor per removed scan. For one participant (matched control group) more than 50% of the scans during encoding exceeded the FD-limit, and we therefore excluded this data set from all task-based functional connectivity analysis. Within the remaining sample, the % of excluded scans was relatively low, and neither the amount of excluded scans nor average FD differed between groups (all p>.38, see Table S4). Finally, the data was high-pass filtered at a cut-off of 128 s. For training groups, both sessions (initial, delayed) were modeled in one GLM. The residuals of this model were used for all following task-based connectivity analysis.
To capture encoding effects rather than fixation periods that might contain rs-fMRI fluctuations, we only used volumes from the 12 encoding periods for our task-based functional connectivity analysis (30 s each; 144 volumes in total). Encoding volumes were concatenated and the average residual time course was extracted based on all voxels within each of the ROIs. Since the parts of the cerebellum were not covered during task data acquisition, we restricted the analysis to 70 ROIs fully covered. Time courses were correlated (Pearson’s r), yielding a 70 × 70 correlation matrix per participant and encoding session. Correlations were Fisher’s z transformed and remaining analyses steps were identical to the analysis of rs-fMRI data (see above).
As a control we repeated the whole analysis and modeled task-related events in addition to nuisance regressors. This so-called “background connectivity” has been demonstrated to be unrelated to task-evoked responses, and is thus thought to provide an index of sustained processing during cognitive operations (Al-Aidroos et al. 2012; Duncan et al. 2014; Tompary et al. 2015). We modeled the BOLD response for all encoding trials as a single task regressor, time-locked to the onset of each trial. Instructions were binned within a separate regressor of no interest. All events were estimated as a boxcar function with a duration of 3 s (encoding trials), or 5 s (instructions), and were convolved with a canonical hemodynamic response function. None of the results changed essentially in this background connectivity analysis, in particular none of the significant results became insignificant or vice versa.
Control analyses
As a control analysis for our choice of brain parcellation scheme, we repeated the correlational similarity analysis of connectivity differences with those ROIs from the Brainnetome parcellation (Fan et al., 2016) that had at least 100 Voxels overlap with the preselected ROIs from our parcellation (82 of the Brainnetome 273 parcels remained). We were able to replicate our main results (mnemonic training: r= 0.23, p<0.003; active control: r=−0.06, p=0.59; passive control: r=−0.07, p=0.59). An exploratory whole brain analysis using the Brainnetome parcellation yielded results in the same direction as our main analysis with pre-selected brain networks, which however were not significant (mnemonic training: r= 0.08, p=0.35; active control: r=0.02, p=0.78; passive control: r=−0.06, p=0.51).
To check whether the observed effects were based on the actual mnemonic training or might have been induced already by the exposure to the visuospatial imagery strategy in the introductory course in the method of loci, we performed two control analyses. First, we compared baseline performance on training day 1 with the weekly means of the individual top scores in the training discipline “memorizing random words in five minutes”, which most closely resembles the task conditions during the fMRI sessions. We observed a continuous increase in memory performance over the six weeks of training from 16.6±1.2 to 42.3±3.85 words memorized in 5 minutes (see supplemental figure S1). Second, we analyzed data from an independent study on mnemonic strategies, where participants underwent an fMRI RS scan (same scanner, sequences, procedures as in our main study) immediately before and after a 2-day introductory course into visuospatial mnemonic strategies including the method of loci. Hence, participants (n=18, age 23.5±3.4 years, all male) were as familiar with the general principles of the method of loci as participants in our main study, however lacked the intense training phase. In this control analysis, we did not find the similarity with athlete/control connectivity differences that we observed in our main study (r=−0.02, p=0.85). Both control analyses combined therefore confirm the interpretation that the observed behavioral, brain network reorganization and similarity effects are related to the intense training in the method of loci and not just on the mere exposure to the visuospatial principles of the strategy.
As a control analysis for the restricted set of top 1% connectivity differences between athletes and controls, we also tested the opposite direction, i.e. selected the top 1% connectivity changes in the mnemonic training group and correlated these with the athlete/control connectivity matrices. We observed a marginally significant similarity (r=0.68, p=0.055).
Crucially, to check whether our general findings rely on the comparison with the athlete/control connectivity differences or could be observed in analyses restricted to the training sample, we performed simple univariate analyses that compared connectivity changes directly with behavioral measures as described above.
Gray matter analysis
T1-weighted data were analyzed with FMRIB Software Library (FSL)-VBM, a voxel-based morphometry style analysis (Ashburner and Friston, 2000; Good et al., 2001) performed with FSL tools (Smith et al., 2004). First, anatomical images were brain extracted using the Brain Extraction Tool (Smith et al., 2002). Next, tissue-type segmentation was performed using FAST4 (Zhang et al., 2001). The resultant gray matter partial volume images were then aligned to MNI152 standard space using the affine registration tool FLIRT (Jenkinson and Smith, 2001), followed by nonlinear registration using FNIRT (Anderson et al., 2007), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). For the use of gray matter volume as a voxelwise regressor in the fMRI data analysis, a four-dimensional (4D) image was created by concatenating every participant’s standard space gray matter image.
For direct comparison of gray matter volume, the individual standard space gray matter images were averaged to create a study-specific template, to which the native gray matter images were then nonlinearly reregistered. The registered partial volume images were then modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated segmented images were smoothed with an isotropic Gaussian kernel with σ = 3 mm. Finally, to test for significant differences between memory athletes and matched controls, a voxelwise general linear model was applied using permutation-based nonparametric testing, with Threshold-Free Cluster Enhancement (TFCE) as implemented in FSL (Smith and Nichols, 2009) and p < 0.05 familywise error corrected. We used the same preprocessing, analysis, and thresholding to examine pre/post changes within the three training conditions.
We also analyzed gray matter volume within the functional regions of interest (fROIs) used in the functional connectivity analyses. We masked each subject’s processed T1-weighted gray matter segmentation image with a fROI and calculated the gray matter volume within the masked area. Gray matter volume was defined as the average volume within the area of the segmentation image masked by a fROI; this was calculated separately for each of the 71 fROIs. We compared gray matter volume in memory athletes with matched controls across all 71 fROIs using a two-sample t-test. Results were thresholded with an FDR correction to account for multiple comparisons (q < 0.05). The same processing and thresholding was used to examine pre/post changes in the three training conditions; however, in this analysis we used a paired-samples t-test instead of a two-sample t-test.
DATA AND SOFTWARE AVAILABILITY
Data resources
ROIs are available via http://findlab.stanford.edu/functional_ROIs.html.
ADDITIONAL RESOURCES
None.
Supplementary Material
Acknowledgments
We thank S. Weisig and P. Schuster for their help with data collection. MD is supported by grants from the Netherlands Organisation for Scientific Research (NWO), the Volkswagen Foundation, and the German Academic Exchange Service (DAAD). NCJM and GF are supported by grants from the Netherlands Organisation for Scientific Research (NWO). MG is supported by grants from the Feldman Family Foundation and the National Institutes of Health (RO1NS073498).
Footnotes
Author Contributions: MD and BNK designed the study and collected the data. MD, WS, NCJM, and IW analyzed the data. MD, WS, NCJM, and MG wrote the manuscript. All authors discussed the results and the revision and commented on the manuscript. 23
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Aidroos N, Said CP, Turk-Browne NB. Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proc Natl Acad Sci U S A. 2012;109:14675–14680. doi: 10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A, Ng B, Landau SM, Jagust WJ, Greicius MD. Regional brain hypometabolism is unrelated to regional amyloid plaque burden. Brain. 2015;138:3734–3746. doi: 10.1093/brain/awv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JLR, Jenkinson M, Smith S. FMRIB technical reports TR07JA1 and TR07JA2. 2007 available at www.fmrib.ox.ac.uk/analysis/techrep.
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bäumler G. Lern- und Gedächtnistest LGT-3. Göttingen: Hogrefe; 1974. [Google Scholar]
- Becchetti A. Hippocampal formation and the classical art of memory. Proc Natl Acad Sci USA. 2010;107:E104. doi: 10.1073/pnas.1004950107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmund JL, Deuker L, Navarro Schröder T, Doeller CF. Grid-cell representations in mental simulation. Elife. 2016;30:5. doi: 10.7554/eLife.17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361–367. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Chase HW, Clark L, Sahakian BJ, Bullmore ET, Robbins TW. Dissociable roles of prefrontal subregions in self-ordered working memory performance. Neuropsychologia. 2008;46:2650–2661. doi: 10.1016/j.neuropsychologia.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Dresler M, Konrad BN. Mnemonic expertise during wakefulness and sleep. Behav Brain Sci. 2013;36:616–617. doi: 10.1017/S0140525X13001301. [DOI] [PubMed] [Google Scholar]
- Duncan K, Tompary A, Davachi L. Associative encoding and retrieval are predicted by functional connectivity in distinct hippocampal area CA1 pathways. J Neurosci. 2014;34:11188–11198. doi: 10.1523/JNEUROSCI.0521-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein CM, Sekino M, Yamaguchi K, Kamiya S, Ueno S. Asymmetries of prefrontal cortex in human episodic memory: effects of transcranial magnetic stimulation on learning abstract patterns. Neurosci Lett. 2002;320:5–8. doi: 10.1016/s0304-3940(01)02573-3. [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, et al. The Human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foer J. Moonwalking with Einstein. London: Penguin Books; 2011. [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Suzuki M, Mugikura S, Abe N, Takahashi S, Iijima T, Fujii T. Changes in brain activation associated with use of a memory strategy: a functional MRI study. Neuroimage. 2005;24:1154–1163. doi: 10.1016/j.neuroimage.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Valentine ER, Wilding JM, Kapur N. Routes to remembering: the brains behind superior memory. Nat Neurosci. 2003;6:90–95. doi: 10.1038/nn988. [DOI] [PubMed] [Google Scholar]
- Manenti R, Cotelli M, Calabria M, Maioli C, Miniussi C. The role of the dorsolateral prefrontal cortex in retrieval from long-term memory depends on strategies: a repetitive transcranial magnetic stimulation study. Neuroscience. 2010;166:501–507. doi: 10.1016/j.neuroscience.2009.12.037. [DOI] [PubMed] [Google Scholar]
- Michel V, Gramfort A, Varoquaux G, Eger E, Keribin C, Thirion B. A supervised clustering approach for fMRI-based inference of brain states. Patt Recog. 2012;45:2041–731. [Google Scholar]
- Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, Hefft S, Merkow M, Polyn SM, Jacobs J, et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 2013;342:1111–1114. doi: 10.1126/science.1244056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco JD, Rao G, Roth ED, Knierim JJ. Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat Neurosci. 2014;17:725–731. doi: 10.1038/nn.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Neely AS, Petersson KM, Ingvar M, Bäckman L. Neural correlates of training-related memory improvement in adulthood and aging. Proc Natl Acad Sci USA. 2003;100:13728–13733. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richiardi J, Gschwind M, Simioni S, Annoni JM, Greco B, Hagmann P, Schluep M, Vuilleumier P, Van De Ville D. Classifying minimally disabled multiple sclerosis patients from resting state functional connectivity. Neuroimage. 2012;62:2021–2033. doi: 10.1016/j.neuroimage.2012.05.078. [DOI] [PubMed] [Google Scholar]
- Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Büchel C, et al. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:1241–1244. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner BJ. Loss of recent memory after bilateral hippocampal lesions. Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, Moodie CA, Poldrack RA. The Dynamics of Functional Brain Networks: Integrated Network States during Cognitive Task Performance. Neuron. 2016;92:544–554. doi: 10.1016/j.neuron.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Tavor I, Parker Jones O, Mars RB, Smith SM, Behrens TE, Jbabdi S. Task-free MRI predicts individual differences in brain activity during task performance. Science. 2016;352:216–220. doi: 10.1126/science.aad8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoli E, Kelso JA. The metastable brain. Neuron. 2014;81:35–48. doi: 10.1016/j.neuron.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompary A, Duncan K, Davachi L. Consolidation of Associative and Item Memory Is Related to Post-Encoding Functional Connectivity between the Ventral Tegmental Area and Different Medial Temporal Lobe Subregions during an Unrelated Task. J Neurosci. 2015;35:7326–7331. doi: 10.1523/JNEUROSCI.4816-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K. The future of the human connectome. Neuroimage. 2012;62:1299–1310. doi: 10.1016/j.neuroimage.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MT, Rijpkema M, Ruiter DJ, Fernández G. Retrieval of associative information congruent with prior knowledge is related to increased medial prefrontal activity and connectivity. J Neurosci. 2010a;30:15888–15894. doi: 10.1523/JNEUROSCI.2674-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MT, Fernández G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci USA. 2010b;107:7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Weiß HR, Weiß B. Grundintelligenztest Skala 2 – Revision. Göttingen: Hogrefe; 2006. CFT 20-R. [Google Scholar]
- Worthen JB, Hunt RR. Mnemonology. New York: Psychology Press; 2011. [Google Scholar]
- Yates FA. The Art of Memory. London: Routledge & Kegan Paul; 1966. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.