Abstract
Objective
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first detected during pregnancy. It can result in pregnancy complications such as birth injury, stillbirth. Fatty acid-binding protein 4 (FABP4), found in adipose tissue, is associated with insulin resistance, and type 2 diabetes. The aim of this study was to investigate whether FABP4 in the placenta and decidua of pregnant women with GDM is higher than that in normal pregnant women, and whether serum from pregnant women with GDM may cause adipocytes to secrete more FABP4 than does serum from a normal pregnant group.
Methods
We obtained placentas, deciduas, and serum from 12 pregnant women with GDM and 12 normal pregnant women and performed enzyme-linked immunosorbent assay, real time quantitative-polymerase chain reaction. We cultured human pre-adipocytes for 17 days with GDM and non-GDM serum and performed western blot, real time quantitative-polymerase chain reaction, and oil red O staining.
Results
Expression of FABP4 in serum, placenta and decidua of pregnant women with GDM was significantly higher than that in normal pregnant women. Serum from pregnant women with GDM increased the expression of FABP4 mRNA and decreased the expression of adiponectin mRNA in human pre-adipocytes significantly. Adipocyte cultured in GDM serum showed significantly greater lipid accumulation than those cultured in normal serum.
Conclusion
Our results suggest that FABP4 is higher in placenta and decidua from pregnant women with GDM. Increased circulating FABP4 in maternal serum from pregnant women with GDM may originate from adipocytes and the placenta. Circulating FABP4 can induce increased insulin resistance and decreased insulin sensitivity.
Keywords: Adiponectin, Fatty acid-binding protein 4, Pregnancy in diabetics
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first detected during pregnancy. Uncontrolled GDM may result in pregnancy complications such as macrosomia, birth injury, stillbirth, and iatrogenic preterm birth [1]. Although the incidence of GDM is increasing worldwide, the precise pathogenesis of GDM is unknown.
In normal pregnancy, insulin sensitivity decreases and insulin resistance increases with advancing gestation. The progressive increase in insulin resistance after the middle trimester of gestation is associated with an increased maternal body fat mass and placenta hormonal effects [2], and decreased insulin sensitivity is associated with inflammatory factors such as tumor necrosis factor-α and interleukin-6 [3].
The disappearance of increased insulin resistance and decreased insulin sensitivity following delivery indicates that placental hormones may have major effects in maternal glucose metabolism and result in glucose intolerance.
Several studies reported that placental hormones such as human placenta lactogen (hPL) [4], estrogen [5], and progesterone [6] are associated with the pathogenesis of GDM. However, those studies did not explain the interaction between substances originating in the placenta and effects on maternal organs.
Fatty acid-binding protein 4 (FABP4), also known as adipocyte P2, was first found in adipose tissue and mature adipocytes. Expression of FABP4 is induced during adipocyte differentiation and is controlled by insulin and peroxisome proliferator-activated receptor γ agonists [7]. FABP4 activates hormone-sensitive lipase in mature adipocytes, regulating lipolysis [8]. Increases in circulating FABP4 levels are associated with obesity, insulin resistance, and type 2 diabetes [9]. In studies of the relationship between FABP4 and the pathogenesis of GDM, only one study reported that FABP4 levels in the serum of pregnant women with GDM were higher than those in serum of normal pregnant women [10].
Adiponectin is primarily secreted by adipose tissue and is negatively correlated with adiposity [11]. Also, concentrations of adiponectin are decreased in diabetes and cardiovascular disease [12]. A low adiponectin concentration in serum is associated with low insulin sensitivity [13].
We hypothesized that because FABP4 can affect insulin resistance in type 2 diabetes mellitus, FABP4 is higher in the placenta and decidua of mothers with GDM. Placental hormones may induce upregulation of FABP4 in the placenta and decidua in GDM. Those substances can cross into the maternal circulation and activate adipocytes that may secrete FABP4 and inhibit adiponectin in pregnant women with GDM more than in normal pregnant women. The synergistic effects of FABP4 from the placenta and adipocytes may induce insulin resistance and GDM.
This study explored whether FABP4 levels are higher in the placenta and decidua of pregnant women with GDM than in those of normal pregnant women. We also investigated whether serum from pregnant women with GDM may induce adipocytes to secrete FABP4 more than serum from normal pregnant women does.
Materials and methods
1. Subjects
We extracted the serum, placenta and decidua from 12 patients diagnosed with GDM who underwent elective repeat cesarean sections, and 12 gestational week-matched controls who also underwent elective repeat cesarean sections. All sera were collected at one day prior to the operation. The baseline characteristics of the patients are summarized in Table 1. Exclusion criteria were fetal structural anomaly, multiple gestations, preeclampsia, and other maternal medical diseases.
Table 1. Characteristics between gestational diabetes mellitus and normal group.
| GDM (n=12) | Control (n=12) | P-value | |
|---|---|---|---|
| Maternal age (yr) | 35.42±5.18 | 32.08±5.21 | 0.13 |
| Gestational age at delivery (wk) | 37.70±2.69 | 38.08±1.01 | 0.52 |
| Body mass index (kg/m2) | 29.66±4.14 | 27.79±5.47 | 0.36 |
| Birth weight (g) | 3,085±577 | 3,171±392 | 0.68 |
Values are presented as mean±standard deviation.
GDM, gestational diabetes mellitus.
All pregnant women were screened at 24 to 28 weeks of gestation for GDM using a 50-g glucose challenge test. When the result was positive, a 100-g oral glucose tolerance test was performed. The diagnostic criteria of Carpenter and Coustan were used, which define GDM as two values exceeding the threshold for positivity [14].
The current study was approved by the institutional review board of Kangwon National University Hospital. We obtained written informed consent from all patients before collection of any tissue. We collected about 1×1-cm-sized placental tissue on the center of placenta and 0.5×0.5-cm-sized decidua in the placental bed. All samples were collected directly into liquid nitrogen and stored in sealed containers at −70℃ until analysis.
2. Tissue preparation and enzyme-linked immunosorbent assay measurements for FABP4
Tissues were washed with cold phosphate buffered saline (PBS) and homogenized with 100 µL of tissue protein extraction reagent (Thermo Scientific, Rockford, IL, USA) containing protease inhibitor cocktail (Roche, Hamburg, Germany). The FABP4 was measured using enzyme-linked immunosorbent assay kit according to the manufacturer's instructions (R&D System, Minneapolis, MN, USA) and normalized to total tissue protein content. The total tissue protein concentration was determined using the Pierce BCA protein assay reagent (Thermo Scientific). All experimental samples were assayed in triplicate.
3. Cell culture and differentiation
Primary subcutaneous human pre-adipocytes, a fibroblast growth kit, and an adipocyte differentiation toolkit were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Culture and differentiation of primary subcutaneous human pre-adipocytes were performed following the ATCC protocol.
The cells were cultured with a fibroblast growth kit (ATCC, PCS-210-041) containing 2% fetal bovine serum at 37℃ in a humidified 5% CO2 incubator. The cells were seeded and maintained in 6-well plates until they reached more than 90% confluence. After the cells reached confluence, the medium was removed, and the cells were washed with PBS and then cultured using the adipocyte differentiation toolkit (ATCC, PCS-500-050). Briefly, the cells were washed gently with PBS on day 0 (confluence) and incubated with 2 mL adipocyte differentiation initiation medium (ATCC) for 48 hours. On day 2, the medium was replaced with adipocyte differentiation maintenance medium (ATCC) for 48 hours. The adipocyte differentiation maintenance medium was changed every 48 hours.
To investigate the effects of sera from pregnant women with GDM and normal pregnant women on primary human subcutaneous pre-adipocytes, the adipocytes were treated with 5% serum from normal pregnant women or from pregnant women with GDM at day 7 post-induction. We used three serum samples randomly selected from pregnant women with GDM and normal pregnant women, respectively. To observe the effect of the sera in the treatment and control groups, cells from both groups were handled identically. The next day, cells were harvested for gene expression or stained with oil red O. All experiments were performed in triplicate.
4. Gene expression analysis (RNA isolation and real-time polymerase chain reaction)
Total RNA was extracted from placenta and decidua tissue and adipocytes with TRIzol reagent (Invitrogen, Fostercity, CA, USA), and the quality and concentration of RNA were estimated with a NanoDrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). cDNA was synthesized using QuantiTect reverse transcription kits (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Gene expression levels were determined with QuanStudio Real-Time PCR Software (Applied Biosystems, Foster City, CA, USA) using the Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) and normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The amplification conditions were 95℃ for 10 minutes, followed by 40 cycles at 95℃ for 15 seconds and 60℃ for 1 minute. The expression values were calculated using the 2−ΔΔCt method according to the manufacturer's instructions.
The primers used were as follows: FABP4 forward primer, 5′-ggcatggccaaacctaacat-3′, and reverse primer, 5′-ttccatcccatttctgcacat-3′; adiponectin forward primer, 5′-ccctctcttacaagcccatca-3′, and reverse primer 5′-gagccagtctggtagtacatca-3′; and GAPDH forward primer, 5′-cgccacagtttcccggaggg-3′, and reverse primer, 5′-ccctccaaaatcaagtgggg-3′.
5. Western blot analysis
The extracted proteins from cells were separated in 12% sodium dodecyl sulphate-polyacrylamide gels, transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA), blocked with 5% (w/v) nonfat skim milk in Tris-buffered saline, 0.1% Tween 20 for 1 hour, and incubated overnight at 4℃ with the rabbit ant-FABP4 polyclonal antibody (1:500; Abcam, Cambridge, MA, USA) or mouse anti-adiponectin monoclonal antibody (1:500, Abcam). Immunoreactive proteins were detected using a horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence assay kits (Millipore, Billerica, MA, USA).
6. Observation of lipid accumulation by oil red O staining
Cells differentiated in the 6-well plate were washed with PBS and fixed with 4% paraformaldehyde for 1 hour at room temperature. After removing the 4% paraformaldehyde, cells were washed with 60% isopropanol solution and dried completely in a hood. The fixed cells were stained with diluted oil red O solution (Sigma-Aldrich, St. Louis, MO, USA; oil red O:distilled water=6:4) for 2 hours. The stained lipid droplets were observed, and images were obtained from randomly selected fields under a microscope (Olympus, Tokyo, Japan). In addition to determine the accumulation of lipid content, the stain was eluted with 100% isopropanol and measured at 510 nm using a spectrophotometer (Spectra MAX 190, Molecular Device, Sunnyvale, CA, USA).
7. Statistical analysis
All data are expressed as mean±standard deviation or mean±standard error. Differences between 2 independent groups in Table 1 and Fig. 1 were analyzed using Mann-Whitney U-test, whereas the differences between 3 groups in Figs. 2 and 3 were performed with Kruskal-Wallis test using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
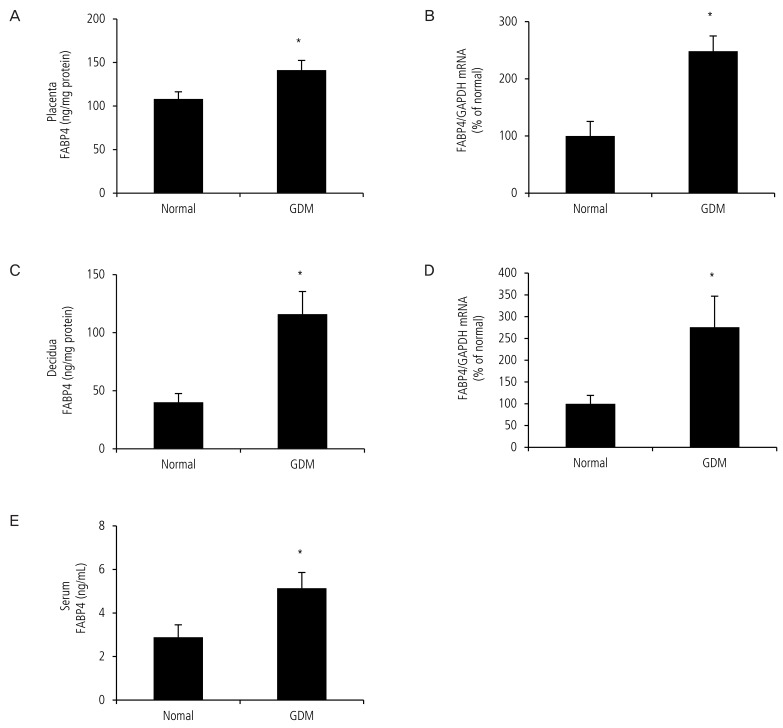
Fig. 1. Expression of fatty acid-binding protein 4 (FABP4) in the placenta and decidua of pregnant women with gestational diabetes mellitus (GDM) was higher than that in normal pregnant women. The placenta and decidua of pregnant women with GDM and normal pregnant women were submitted to real-time polymerase chain reaction and enzyme-linked immunosorbent assay. (A) FABP4 protein in the placenta of women with GDM was higher than that in the normal pregnant group. (B) Expression of FABP4 mRNA was upregulated in the placenta of women with GDM. (C) FABP4 protein in the decidua from women with GDM was higher than that in the normal pregnant group. (D) Expression of FABP4 mRNA was upregulated in the decidua of women with GDM. (E) FABP4 protein in the serum from women with GDM was higher than that in the normal pregnant group. Data are expressed as mean±standard error. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. *P<0.05: normal vs. GDM.
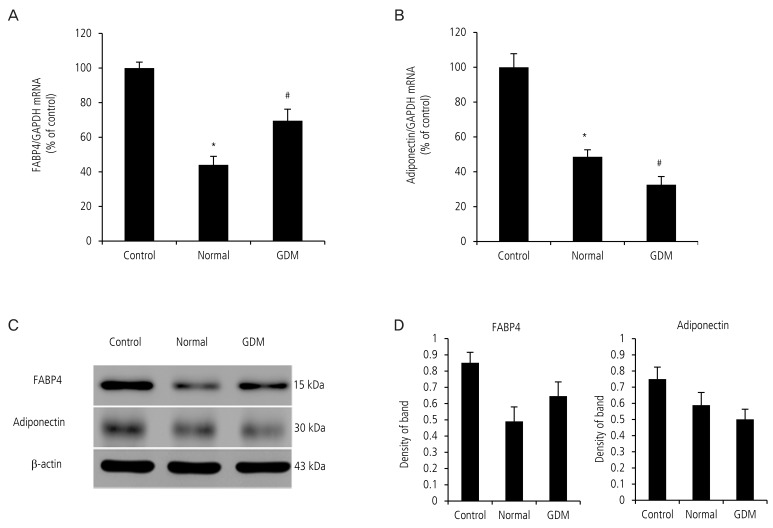
Fig. 2. Serum from pregnant women with gestational diabetes mellitus (GDM) increased the expression of fatty acid-binding protein 4 (FABP4) mRNA and decreased the expression of adiponectin mRNA in primary subcutaneous human pre-adipocytes. After primary human pre-adipocytes were cultured using serum from pregnant women with GDM and normal pregnant women for 24 hours, real-time polymerase chain reaction and Western blot was performed. (A) Expression of FABP4 mRNA in adipocytes cultured in serum from pregnant women with GDM was higher than that from the normal group. (B) Expression of adiponectin mRNA in adipocytes cultured in serum from mothers with GDM was lower than that from the normal group. (C) FABP4 and adiponectin protein level in adipocyte were determined by Western blot (representative blots are shown). (D) Band densities in the Western blot were quantified using ImageJ software. Trends toward increased FABP4 protein expression and decreased adiponectin protein expression in GDM group were shown. Data are expressed as mean±standard deviation. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. *P<0.05: control vs. normal, #P<0.05: normal vs. GDM.
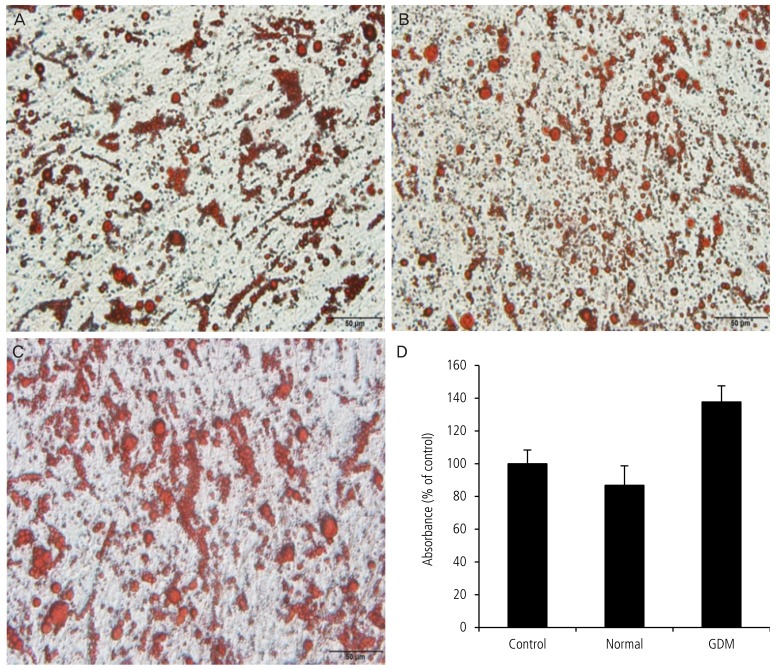
Fig. 3. Lipid accumulation in adipocytes exposed to serum from mothers with gestational diabetes mellitus (GDM) was greater than that in cells cultured in serum from the normal group. After primary human pre-adipocytes were cultured using serum from pregnant women with GDM and normal pregnant women for 24 hours, oil red O staining was performed. A representative experiment is shown. (A) Control group using fetal bovine serum culture medium. (B) Normal group using 5% serum from a normal pregnant woman. (C) GDM group using 5% serum from pregnant women with GDM. (D) Absorbance rate using spectrophotometer. Data are expressed as mean±standard deviation. *P<0.05: normal vs. GDM.
Results
1. Expression of FABP4 in the serum, placenta and decidua of pregnant women with GDM was higher than that in normal pregnant women
We first confirmed expression of FABP4 mRNA by real-time polymerase chain reaction in the placenta and decidua and found that FABP4 mRNA was significantly upregulated in placenta (100.0±25.6 vs. 248.0±26.6, P<0.05, Fig. 1B) and decidua (100.0±19.2 vs. 275.9±71.2,P<0.05, Fig. 1D) from pregnant women with GDM. We then measured the total FABP4 levels in the placenta, decidua and serum by enzyme-linked immunosorbent assay in 24 normal pregnant women and with GDM. The average protein level of FABP4 was significantly higher in the placenta (108.1±9.0 vs. 141.3±11.0, P<0.05, Fig. 1A) decidua (40.1±8.4 vs. 116.0±15.3, P<0.05, Fig. 1C) and serum (2.9±0.6 vs. 5.1±0.7, P<0.05, Fig. 1E) from pregnant women with GDM.
We suggest that excessive production of FABP4 in GDM may result from placental hormones, which may enter the maternal circulation more than they would during a normal pregnancy.
2. Serum from pregnant women with GDM increased FABP4 mRNA and decreased adiponectin mRNA in primary subcutaneous human pre-adipocytes
To address our hypothesis that circulating placental hormones in GDM upregulate FABP4 and down-regulate adiponectin in adipocytes, we exposed adipocytes to serum from pregnant women with GDM and normal pregnant women in culture media for 24 hours. The serum from pregnant women with GDM increased the expression of FABP4 mRNA in the adipocytes significantly (100.0±3.4 vs. 44.06±4.89 vs. 69.6±6.6, P<0.05, Fig. 2A). However, the serum from pregnant women with GDM decreased the expression of adiponectin mRNA in the adipocytes significantly (100.0±7.8 vs. 48.6±4.0 vs. 32.6±4.7, P<0.05, Fig. 2B).
In contrast, serum from pregnant women with GDM did not alter FABP4 protein and adiponectin protein expression in the adipocyte (Fig. 2C). However, trends toward increased FABP4 protein expression and decreased adiponectin protein expression in GDM group were demonstrated (Fig. 2C, D).
3. Exposure to serum from pregnant women with GDM induced greater lipid accumulation than did exposure to serum from the normal group
To investigate whether serum from normal pregnant women and with GDM affects lipid accumulation in adipocytes, we performed oil red O staining after adipocyte culture for 24 hours (Fig. 3). Lipid accumulation in the group receiving serum from pregnant women with GDM was significantly greater than that receiving serum from the normal pregnant women (100.0±8.4 vs. 86.8±11.9 vs. 137.7±9.9, P<0.05, Fig. 3).
Discussion
Several conclusions may be drawn from our results. First, serum from pregnant women with GDM affects adipocyte differentiation and proliferation to increase the level of FABP4 and decrease that of adiponectin. Second, serum from pregnant women with GDM induces lipid accumulation in the adipocytes. Third, expression of FABP4 mRNA in the placenta and decidua of pregnant women with GDM is greater than that in normal tissues.
Our findings indicated that increased FABP4 originating from the placenta and adipocytes in pregnant women with GDM circulates in maternal blood. Our results can explain previous reports that FABP4 in maternal serum of pregnant women with GDM was higher than that in a normal group [10]. Circulating FABP4 is associated with lipolysis and may aggravate insulin resistance compared to normal physiological insulin resistance [15].
Candidates for placental hormones to induce FABP4 overexpression in the placenta and deciduas in GDM include hPL and progesterone. Both increase continuously until term and may be associated with increased insulin resistance with advancing gestation [2,16,17].
hPL is first detected in the maternal blood at 6 weeks of gestation. This placenta hormone increases continuously based on placenta size at term. Also, hPL stimulates beta cell replication in the pancreas and plays important roles in pancreatic adaptation to maternal insulin resistance [18].
Placental progesterone can reduce insulin sensitivity and increase the insulin resistance that causes the progesterone-mediated decrease in glucose transporter type 4 expression in skeletal muscle and adipose tissue. Pregnant women receiving regular injections of progesterone more often have abnormal glucose tests and GDM compared to control women [5].
Elevated placental hormones originating from serum in GDM may increase the expression of FABP4 mRNA in adipocytes. The synergistic effects of FABP4 from the placenta and adipocytes can act on the metabolic and inflammatory pathways via adipocytes. These activities may play crucial roles in the development of insulin resistance and type 2 diabetes mellitus.
FABP4 increases accumulation of foam cells via inhibition of the peroxisome proliferator-activated receptor γ–liver X receptor α–adenosine triphosphate-binding cassette A1 pathway [19,20].
In our study, administration of serum from pregnant women with GDM induced lipid accumulation more than administration of serum from normal pregnant women did. This finding is similar to previous results. Because few studies reported the interaction between hPL and FABP4 or between progesterone and FABP4, additional experiments on the relationship between specific hormones and FABPs are needed.
Adiponectin concentrations in the third trimester were lower than in non-pregnant than in pregnant women and were strongly related to insulin sensitivity. Pregnancy results in adiponectin changes in lean women. Adiponectin may play important roles in the pathogenesis of GDM in lean women [21,22].
In our study, serum originating from pregnant women with GDM suppressed expression of adiponectin mRNA in adipocytes. Decreased adiponectin may be negatively correlated with insulin sensitivity.
In conclusion, our results suggest that increased circulating FABP4 in the serum of pregnant women with GDM may originate from adipocytes and the placenta. Circulating FABPs can induce an increase in insulin resistance and a decrease in insulin sensitivity.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2014R1A1A1037863); the Research Grant from Kangwon National University (C1011736-01-01); Kangwon National University Hospital.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus: the insulin and cytokine network. Diabetes Care. 2007;30(Suppl 2):S120–S126. doi: 10.2337/dc07-s203. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes. 2008;57:606–613. doi: 10.2337/db07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalkhoff RK, Kissebah AH, Kim HJ. Carbohydrate and lipid metabolism during normal pregnancy: relationship to gestational hormone action. Semin Perinatol. 1978;2:291–307. [PubMed] [Google Scholar]
- 5.Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67:341–347. doi: 10.1210/jcem-67-2-341. [DOI] [PubMed] [Google Scholar]
- 6.Waters TP, Schultz BA, Mercer BM, Catalano PM. Effect of 17alpha-hydroxyprogesterone caproate on glucose intolerance in pregnancy. Obstet Gynecol. 2009;114:45–49. doi: 10.1097/AOG.0b013e3181a9454b. [DOI] [PubMed] [Google Scholar]
- 7.Hunt CR, Ro JH, Dobson DE, Min HY, Spiegelman BM. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986;83:3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuhashi M, Saitoh S, Shimamoto K, Miura T. Fatty acid-binding protein 4 (FABP4): pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin Med Insights Cardiol. 2015;8(Suppl 3):23–33. doi: 10.4137/CMC.S17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toruner F, Altinova AE, Akturk M, Kaya M, Arslan E, Bukan N, et al. The relationship between adipocyte fatty acid binding protein-4, retinol binding protein-4 levels and early diabetic nephropathy in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;91:203–207. doi: 10.1016/j.diabres.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Li YY, Xiao R, Li CP, Huangfu J, Mao JF. Increased plasma levels of FABP4 and PTEN is associated with more severe insulin resistance in women with gestational diabetes mellitus. Med Sci Monit. 2015;21:426–431. doi: 10.12659/MSM.892431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 12.Hauguel-de Mouzon S, Catalano P. Adiponectin: are measurements clinically useful in pregnancy? Diabetes Care. 2013;36:1434–1436. doi: 10.2337/dc12-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mather KJ, Goldberg RB. Clinical use of adiponectin as a marker of metabolic dysregulation. Best Pract Res Clin Endocrinol Metab. 2014;28:107–117. doi: 10.1016/j.beem.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 15.Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162:1008–1014. doi: 10.1016/0002-9378(90)91306-w. [DOI] [PubMed] [Google Scholar]
- 17.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286:2516–2518. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]
- 18.Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011;18:409–416. doi: 10.1097/MED.0b013e32834c800d. [DOI] [PubMed] [Google Scholar]
- 19.Baz B, Riveline JP, Gautier JF. Endocrinology of pregnancy: gestational diabetes mellitus. Definition, aetiological and clinical aspects. Eur J Endocrinol. 2016;174:R43–R51. doi: 10.1530/EJE-15-0378. [DOI] [PubMed] [Google Scholar]
- 20.Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86:568–573. doi: 10.1210/jcem.86.2.7137. [DOI] [PubMed] [Google Scholar]
- 21.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27:799–800. doi: 10.2337/diacare.27.3.799. [DOI] [PubMed] [Google Scholar]
- 22.Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:2306–2311. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]