Abstract
Background
Population-based national data on the trends in expenditures related to heart failure (HF) is scarce. Assessing the time trends in health care expenditures for HF in the United States can help to better define the burden of this condition.
Methods
Using 10-year data (2002–2011) from the national Medical Expenditure Panel Survey (weighted sample of 188,708,194 U.S adults aged ≥18 years) and a two-part model (adjusting for demographics, comorbidities and time); we estimated adjusted mean and incremental medical expenditures by HF status. The costs were direct total health care expenditures (out-of-pocket payments and payments by private insurance, Medicaid, Medicare, and other sources) from various sources (office-based visits, hospital outpatient, emergency room, inpatient hospital, pharmacy, home health care, and other medical expenditures).
Results
Compared to expenditures for individuals without HF ($5,511 [95% confidence interval (CI): 5,405–5,617]), individuals with HF had a four-fold higher mean expenditures of ($23,854 [95%CI: 21,733–25,975]). Individuals with HF had $3,446 (95%CI: 2,592–4,299) higher direct incremental expenditures compared with those without HF, after adjusting for demographics and comorbidities. Among those with HF, costs continuously increased by $5836 (28% relative increase), from $21,316 (95%CI: 18,359–24,272) in 2002/2003 to $27,152 (95%CI: 20,066–34,237) in 2010/2011; and inpatient costs ($11,318 over the whole period) were the single largest component of total medical expenditure. The estimated unadjusted total direct medical expenditures for US adults with HF were $30 billion/year and the adjusted total incremental expenditure $5.8 billion/year.
Conclusions
Heart failure is costly and over a recent 10-year period, direct expenditure related to HF increased markedly, mainly driven by inpatient costs.
Keywords: heart failure, costs, trends, outcomes
Introduction
Heart failure (HF) is associated with a substantial burden of morbidity and mortality in the US. 1 In 2012, an estimated 5.7 million Americans ≥20 years of age had HF, and this number is projected to increase by 46% by the year 2030.1 Over time, the hospitalizations for HF have remained high, and are a significant concern for the US healthcare system, especially in terms of costs, which are heighted by the development and implementation of life-prolonging therapies, as well as aging of the population, which will lead to more people at risk for developing HF.1 The HF costs are presumably driven by hospitalizations, home nursing or hospice service, and medical devices such as cardiac resynchronization therapy and ventricular assist devices, as well as transplantation. In the US, total cost for HF was estimated to be $30.7 billion in 2012, with a 68% was attributable to direct medical costs.2 It is purported that the costs of HF have been rising or will rise over time in the US.2 However, extant studies on HF costs have been limited to short time period,3,4 focused on a single aspect of expenses (mainly in-hospital costs) 5–8 or have not had a national reach.7,8 These have either focused on limited period of time (a few years 3 or the last few months of life 4 and/or have mainly predated the widespread use of novel devices like cardiac resynchronization and defibrillator 9 or left ventricular assist devices 10. Changes in the use of and spending on hospitalizations, outpatient visits, and other medical services related to HF care remain unclear. Few studies have comprehensively, quantified the change in the use of all these medical components in the U.S or used nationally representative data 7,8. Overall, there is a lack of nationwide data over a prolonged time period to reliability assess the trends in resource use among HF patients in the US.
Using the framework of the Medical Expenditure Panel Survey Household Component (MEPS-HC) 11, the largest nationally representative survey of medical costs the United States, we examined the changes over time in direct health care expenditures among U.S. adults with HF from 2002 to 2011, with the aim of assess how the changing demographics or quality of HF care the US has impacted the cost of HF care for different US populations.
Methods
Data source and Study Population
We used data from the 2002–2011 Medical Expenditure Panel Survey (MEPS). We identified 1,764 (weighted sample of 1,675,414) US adults (aged ≥18 years) with heart failure using an ICD-9 code from the Medical Expenditure Panel Survey Household Component (MEPS-HC). The MEPS includes several waves of national surveys of families and individuals, their medical providers, and employers in the U.S. It samples data on an average of 39,000 individuals per year to estimate the use of medical resources in the U.S. population. The MEPS sample is drawn from reporting units in the previous year’s National Health Interview Survey, a nationally representative sample (with oversampling for Blacks and Hispanics) of the US civilian non-institutionalized population. The MEPS has a complex design consisting of clustering, stratification, and multistage and disproportionate sampling with oversampling of minorities. We included MEPS rounds of interviews covering two full calendar years from 2002 to 2011. The included participants were individuals aged 18 years and above, with heart failure (HF) enrolled in the Medical Expenditure Panel Survey Household Component (MEPS-HC), during the 2002 – 2011 period. We merged data from HC survey of the medical condition files and full-year consolidated files using the unique person identifier (DUPERSID) on a one-to-one match.12 We pooled 10-year data to ensure sufficient sample size and increase precision of our estimates. The medical conditions and procedures reported by the MEPS-HC related to heart failure was recorded by an interviewer as verbatim text and then converted by professional coders to ICD-9-CM codes. The error rate for any coder did not exceed 2.5% on verification. To protect the confidentiality of respondents, fully specified ICD-9-CM codes were collapsed to three digits.12 The MEPS collects information on health care use, expenditures, sources of payment, health status, the status of health insurance coverage, and demographic and socioeconomic characteristics of the civilian, non-institutionalized population of the U.S. Medical use and expenditures were collected from both household respondents and their medical providers.
Heart Failure definition
People with HF were defined on the basis of self-report that led to medical visits or treatment within the interview year. The self-reported HF condition was transcribed and classified with International Classification of Diseases, Ninth Revision, Clinical Modification codes (ICD-9) using the ICD-9-CM diagnostic code 428 for heart failure.12,13 In the MEPS-HC, diagnoses codes are derived by professional coders based on survey interviews; and only the first three digits of these codes are reported in MEPS. Information on each respondent is annualized, in which a calendar year is the duration of time for which information is reported in MEPS. Respondents were included in the study based on the availability of a HF diagnosis at any time during the year; with no requirement for hospital admission to be included in the study.
Outcomes
Our focus was on direct medical costs, as these constitute the vast majority of HF related costs. These costs include the total direct health care expenditures for the calendar year for each individual. The direct medical costs of HF were estimated by point of service, with the following point-of-service categories used hospital (inpatient, outpatient, and emergency department), physician (office-based visits), prescription, home health, and other (including nursing home, rehabilitation, vision, medical supplies, dental). The costs include out-of-pocket payments and payments by private insurance, Medicaid, Medicare, and other sources; medical expenditures include (including office-based medical provider, hospital outpatient, emergency room (ER), inpatient hospital (including zero night stays), pharmacy, dental, home health care, and other medical expenditures reported during the calendar year. The cost over the 2002–2011 period were be adjusted to the 2014 dollar value using the consumer price index obtained from the Bureau of Labor Statistics.14 We used the 10-year pooled cross-sectional data and adjusted the analytic sampling weight variable by dividing it by the number of years being pooled. The sum of these adjusted weights represents the average annual population size for the pooled period. We combined 10 years of data (2002–2011), as over each year these have a common variance structure necessary to ensure compatibility of our variables within the complex sample design.
Covariates
The covariates defined on the basis of self-report included demographic and clinical variables. Recent studies showed that socio demographic and binary indicators of disease are important covariates that affect medical expenditures 15 and that binary indicators of disease are more effective in accounting for disease burden.16–18 Covariates are age, sex, race/ethnicity, marital status, educational level, health insurance, metropolitan statistical area (MSA), region, poverty/income ratio (income level), calendar year, and comorbidities – diabetes, hypertension, cardiovascular diseases (CVD), emphysema, joint pain, arthritis and asthma. Binary indicators of comorbidities were based on self- report of a positive response to the question, “Have you ever been diagnosed with diabetes, hypertension, stroke, emphysema, joint pain, arthritis, or asthma?” Cardiovascular disease (CVD) was defined by a positive response to a question, “Have you ever been diagnosed with coronary heart disease, angina, myocardial infarction, or other heart diseases?”
Age was categorized into 18–44, 45–64 and 65–85 years. Sex was dichotomized as male vs. female, race/ethnicity as non-Hispanic White (NHW), non-Hispanic Black (NHB), and Hispanic and Others; marital status as married, widow/divorced/single (non married) and never married; education as less than high school (grade ≤ 12 years), high school, and college or more (grade ≥13 years); insurance as private, public only, and uninsured at all time in the year; region as Northeast, Midwest, South and West. Income level was defined as a percentage of poverty level and grouped in to four categories: poor (< 125% federal poverty level), low income (≥125% and <200% federal poverty level), middle income (≥200% and <400% federal poverty level) and high income (greater than equal to 400% poverty level). The MSA was coded as yes versus no at end of the year -31 December, and categorized as MSA (urban) vs. non-MSA (rural). Calendar year was coded in relevant categories (e.g. 2002/2003, 2004/2005, 2006/2007, 2008/2009, and 2010/2011) to pool data.
Statistical Analyses
The baseline characteristics of patients are presented by HF status, as percentages for categorical variables, with differences tested for using χ2 tests. We estimated the unadjusted mean direct medical expenditures for individuals by HF status and then compared heart failure vs. non-heart failure using test post-estimation command with survey data. We then used a two-part model to estimate the adjusted direct medical expenditures by HF status after controlling for confounding factors. The ‘margins’ function in STATA is used to extrapolate the incremental effects and their standard errors from the combined first and second parts of the final model. The two-part model was done using a two part generalized linear model allowing for mixed discrete-continuous variables.19,20 A probit model was used to estimate the probability of observing a zero versus positive medical expenditure, and a generalized linear model then estimated conditional on having a positive medical expenditure.20–22 The use of GLM in the second part of the model has an advantage over log Ordinary Least Squares (OLS) since it relaxes the normality and homoscedasticity assumptions and avoids bias associated with retransforming to the raw scale.14 The model addresses the zero concentration as well as the positive skewness of expenditures 23 and allows users to calculate incremental effects and standard errors from the two parts of the model. 20 We adjusted for socio-demographic factors (age, sex, race, marital status, education, health insurance, metropolitan statistical area, region, and income level) and comorbidities (diabetes, hypertension, CVD, stroke, emphysema, joint pain, arthritis, and asthma). To determine the family distribution for the generalized linear model, we used the modified Park test, 19,20 taking into account the complex survey design. The Park test verifies the use of a gamma distribution with a log link as the best–fitting GLM for consistent estimation of coefficients and marginal effects of medical expenditures. 20,24 The variance inflation factor for all predictors used in the two-part model was estimated to rule out multicollinearity problems. We hypothesized that HF costs have been increasing over time, and these vary by age, presence of CVD (excluding HF), and race. The change in cost over time represents the mean cost per person per year over a 10-year period. We used standard pairwise comparison methods of Sidak, Scheffe, and Bonferroni to compare the pooled total mean healthcare expenditure among HF if the changes over 10 years were statistically significant. We compared total mean expenditures between ten year groups (2002/03 vs. 2004/05, 2002/03 vs. 2006/07, 2002/03 vs. 2008/09, 2002/03 vs. 2010/11, 2004/05 vs. 2006/07; 2004/05 vs. 2008/09, 2004/05 vs. 2010/11, 2006/07 vs. 2008/09, 2006/07 vs. 2010/11, 2008/09 vs. 2010/11).
For all the analyses, we accounted for the complex sampling design of MEPS dataset by using sampling weight, variance estimation stratum and primary sampling unit (clustering). A p value < 0.05 was considered statistically significant. All analyses were performed using STATA 14 (Stata Corp, College Station, TX).25
Results
Population Characteristics
The characteristics of US adults with and without HF in the U.S. during the 2002–2011 period are shown in Table 1. Of the weighted population representing 188,708,194 U.S. adults aged ≥18 years, 0.9% had HF (with a prevalence of 0.1%, 0.76% and 3.0%, among the 18–44 years, 44–64 years, and 65 years or more age groups, respectively). HF was more frequent among older patients (65 years and above), non-Hispanic Whites or non-Hispanic Black (but less frequent among Hispanic or Others), non-married, less than High school and High school graduates, publicly insured, rural and southern dwellers, and poor, and low income earners. People with HF had a higher frequency of hypertension, diabetes, CVD, stroke and emphysema. The HF prevalence increased in 2004/2005, 2006/2007 and 2008/2009, as compared to 2002/2003.
Table 1.
Sample demographics among adults with heart failure
| Variables | All (%) | Heart failure (%) | No-heart failure (%) | p-value |
|---|---|---|---|---|
| N(n) | 188,708,194 (187,341) | 1,675,414 (1,764) | 187,032,780 (185,577) | |
| Age category | ||||
| Age 18–44 | 45.7 | 5.3 | 46.1 | <0.001 |
| Age 45–64 | 35.4 | 30.2 | 35.4 | |
| Age 65–85 | 18.9 | 64.5 | 18.5 | |
| Sex | ||||
| Male | 45.6 | 46.2 | 45.5 | 0.731 |
| Female | 54.4 | 53.8 | 54.5 | |
| Race/ethnicity | ||||
| Non-Hispanic White | 72.1 | 78.5 | 72.0 | <0.001 |
| Non-Hispanic Black | 10.5 | 14.3 | 10.5 | |
| Hispanic | 11.3 | 4.3 | 11.4 | |
| Others | 6.1 | 2.9 | 6.1 | |
| Marital status | ||||
| Married | 55.5 | 43.8 | 55.6 | <0.001 |
| Non-married a | 21.3 | 49.1 | 21.0 | |
| Never married | 23.2 | 7.1 | 23.4 | |
| Education category | ||||
| <High School | 17.4 | 32.3 | 17.3 | <0.001 |
| High School | 30.5 | 34.6 | 30.4 | |
| College or more | 52.1 | 33.1 | 52.3 | |
| Insurance | ||||
| Private | 72.1 | 49.0 | 72.3 | <0.001 |
| Public | 16.4 | 46.4 | 16.1 | |
| Uninsured | 11.5 | 4.6 | 11.6 | |
| Metropolitan statistical status | ||||
| Urban | 82.9 | 75.8 | 82.9 | <0.001 |
| Rural | 17.1 | 24.2 | 17.1 | |
| Census region | ||||
| Northeast | 18.7 | 16.6 | 18.7 | <0.001 |
| Midwest | 22.9 | 27.2 | 22.8 | |
| South | 35.9 | 40.4 | 35.9 | |
| West | 22.5 | 15.8 | 22.6 | |
| Income category | ||||
| Poor income | 15.1 | 23.5 | 15.0 | <0.001 |
| Low income | 12.9 | 22.4 | 12.8 | |
| Middle income | 30.2 | 28.9 | 30.2 | |
| High income | 41.8 | 25.2 | 42.0 | |
| Chronic conditions | ||||
| Diabetes | 9.5 | 40.8 | 9.2 | <0.001 |
| Hypertension | 32.9 | 80.7 | 32.4 | <0.001 |
| Cardiovascular disease | 13.6 | 91.9 | 12.9 | <0.001 |
| Stroke | 3.5 | 22.7 | 3.3 | <0.001 |
| Emphysema | 2.1 | 15.8 | 2.0 | <0.001 |
| Joint pain | 37.9 | 67.5 | 37.6 | <0.001 |
| Arthritis | 26.1 | 65.0 | 25.7 | <0.001 |
| Asthma | 10.5 | 22.1 | 10.4 | <0.001 |
| Year category | ||||
| Year 2002/03 | 19.2 | 18.5 | 19.2 | 0.057 |
| Year 2004/05 | 19.6 | 22.1 | 19.6 | |
| Year 2006/07 | 19.9 | 20.3 | 19.9 | |
| Year 2008/09 | 20.5 | 21.6 | 20.5 | |
| Year 2010/11 | 20.8 | 17.5 | 20.8 |
N - weighted sample size; n - unweighted sample size; %, weighted percentage.
Non-married stands for widowed/divorced and separated.
Unadjusted Cost Differences between Individuals with and without Heart Failure
The total mean unadjusted direct expenditures for individuals with HF increased from $21,316 (95%CI: 18,359–24,272) in 2002/2003 to $24,582 (95%CI: 20,392–28,772) in 2004/2005, and then declined to $23,153 (95%CI: 18,960–27,346) in 2008/2009, to then increased again to 27,152 (95%CI: 20,066–34,237) in 2010/2011 (Table 2). Total mean unadjusted medical expenditures for individuals without HF increased continuously from $4,987 (95%CI: 4,792–5,181) in 2002/2003 to $5,952 (95%CI: 5,738–6,166) in 2010/2011. The overall change in direct costs over the 10-year period was $5836, corresponding to a 28% increase. The multiple comparison test of Sidak, Scheffe and Bonferroni showed that the total mean expenditures among individuals with HF were not statistically significant over the 10-year period at 95% CI. Compared to individuals without HF ($5,511 [95%CI: 5,405–5,617]), the unadjusted pooled mean expenditures over the 10-year study period for individuals with HF was more than fourfold ($23,854 ([95%CI: 21,733–25,975]) (Table 2).
Table 2.
Unadjusted mean of total healthcare expenditures by heart failure (HF) status among adults
| Costs | No-heart failure Mean ($) | 95% CI | Heart Failure Mean ($) | 95% CI | P value * |
|---|---|---|---|---|---|
| Total costs | |||||
| 2002/03 | 4,987 | 4,792–5,181 | 21,316 | 18,359–24,272 | <0.001 |
| 2004/05 | 5,440 | 5,195–5,685 | 24,582 | 20,392–28,772 | <0.001 |
| 2006/07 | 5,502 | 5,311–5,693 | 23,273 | 19,489–27,057 | <0.001 |
| 2008/09 | 5,632 | 5,448–5,815 | 23,153 | 18,960–27,346 | <0.001 |
| 2010/11 | 5,952 | 5,738–6,166 | 27,152 | 20,066–34,237 | <0.001 |
| Pooled sample | 5,511 | 5,405–5,617 | 23,854 | 21,733–25,975 | <0.001 |
| Inpatient | |||||
| 2002/03 | 1,499 | 1,361–1,638 | 9,972 | 7,302–12,642 | <0.001 |
| 2004/05 | 1,535 | 1,415–1,654 | 12,981 | 9,310–16,652 | <0.001 |
| 2006/07 | 1,575 | 1,452–1,698 | 10,064 | 7,262–12,865 | <0.001 |
| 2008/09 | 1,481 | 1,364–1,598 | 9,835 | 7,154–12,516 | <0.001 |
| 2010/11 | 1,681 | 1,542–1,821 | 13,922 | 7,232–20,611 | <0.001 |
| Pooled sample | 1,556 | 1,492–1,619 | 11,318 | 9,577–13,059 | <0.001 |
| Office based | |||||
| 2002/03 | 1,111 | 1,076–1,145 | 2,739 | 2,208–3,271 | <0.001 |
| 2004/05 | 1,282 | 1,219–1,345 | 3,082 | 2,510–3,653 | <0.001 |
| 2006/07 | 1,359 | 1,296–1,422 | 3,559 | 2,844–4,275 | <0.001 |
| 2008/09 | 1,422 | 1,369–1,476 | 4,043 | 2,738–5,349 | <0.001 |
| 2010/11 | 1,452 | 1,390–1,513 | 3,347 | 2,680–4,014 | <0.001 |
| Pooled sample | 1,329 | 1,301–1,356 | 3,370 | 2,981–3,759 | <0.001 |
| Medications | |||||
| 2002/03 | 1,065 | 1,027–1,104 | 4,490 | 3,989–4,992 | <0.001 |
| 2004/05 | 1,219 | 1,169–1,268 | 4,449 | 4,027–4,870 | <0.001 |
| 2006/07 | 1,265 | 1,212–1,317 | 4,894 | 4,005–5,784 | <0.001 |
| 2008/09 | 1,298 | 1,246–1,349 | 4,807 | 3,986–5,629 | <0.001 |
| 2010/11 | 1,406 | 1,313–1,499 | 4,720 | 3,944–5,496 | <0.001 |
| Pooled sample | 1,254 | 1,222–1,285 | 4,672 | 4,325–5,019 | <0.001 |
| Outpatient | |||||
| 2002/03 | 546 | 507–585 | 1,067 | 684–1,449 | 0.008 |
| 2004/05 | 540 | 503–577 | 1,330 | 731–1,930 | 0.010 |
| 2006/07 | 508 | 473–542 | 1,433 | 723–2,143 | 0.010 |
| 2008/09 | 551 | 494–609 | 1,240 | 726–1,775 | 0.009 |
| 2010/11 | 567 | 522–611 | 1,232 | 426–2,037 | 0.108 |
| Pooled sample | 543 | 521–564 | 1,266 | 995–1,536 | <0.001 |
| Emergency Room | |||||
| 2002/03 | 161 | 152–171 | 492 | 356–628 | <0.001 |
| 2004/05 | 187 | 174–200 | 504 | 326–681 | <0.001 |
| 2006/07 | 189 | 177–201 | 479 | 344–614 | <0.001 |
| 2008/09 | 224 | 207–241 | 681 | 417–944 | <0.001 |
| 2010/11 | 227 | 212–242 | 709 | 476–942 | <0.001 |
| Pooled sample | 198 | 192–205 | 571 | 478–663 | <0.001 |
| Home Health | |||||
| 2002/03 | 153 | 125–181 | 1,902 | 1,267–2,538 | <0.001 |
| 2004/05 | 225 | 76–373 | 1,425 | 915–1,934 | <0.001 |
| 2006/07 | 149 | 128–171 | 2,107 | 899–3,315 | 0.001 |
| 2008/09 | 205 | 154–256 | 1,862 | 1,121–2,603 | <0.001 |
| 2010/11 | 182 | 147–217 | 2,563 | 1,246–3,879 | <0.001 |
| Pooled sample | 183 | 149–217 | 1,945 | 1,502–2,388 | <0.001 |
| Others | |||||
| 2002/03 | 448 | 430–465 | 650 | 471–829 | 0.026 |
| 2004/05 | 450 | 434–466 | 809 | 610–1,008 | <0.001 |
| 2006/07 | 454 | 437–471 | 734 | 474–994 | 0.036 |
| 2008/09 | 447 | 429–466 | 681 | 446–917 | 0.050 |
| 2010/11 | 434 | 415–453 | 657 | 439–875 | 0.045 |
| Pooled sample | 446 | 437–456 | 710 | 614–807 | <0.001 |
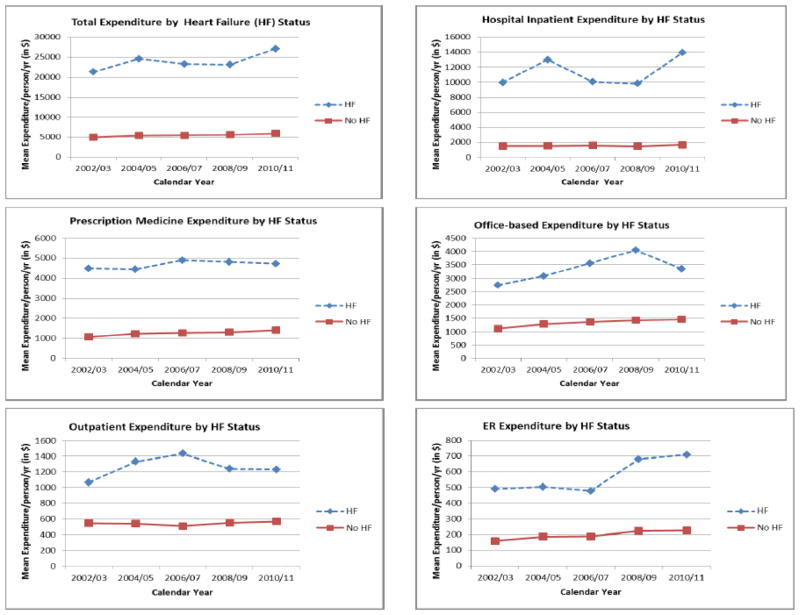
The unadjusted inpatient expenditure for people with heart failure rose initially from ($9,972 ([95%CI: 7,302–12,642]) in 2002/03 to $12,981 (95%CI: 9,310–16,652) in 2004/05 and then decreased continuously to $9,835 (95%CI: 7,154–12,516) in 2008/09 and then increased in 2010/11 ($13,922 ([95%CI: 7,232–20,611]) (Figure 1 and Table 2).
Figure 1.
Trends in total direct healthcare expenditures and healthcare services by heart failure status
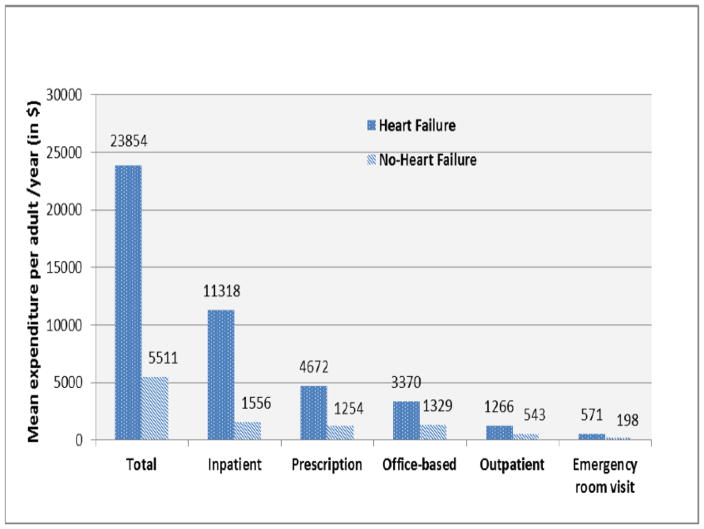
Table 2 and Figure 1 show the annual expenditure per person by health care services for those with HF and without HF, over the 2002–2011 period. The overall change in each component of HF costs over the 10-year period were $3950 (39.6% relative increase) for inpatient costs, $608 (22.2% relative increase) for office visits, $230 (5.1% relative increase) for medications, $165 (15.5% relative increase) for outpatient care, $217 (44.1% relative increase) for ER visits, $661 (34.7% relative increase) for home health, and $7 (1.16% relative increase) for other costs Figure 2 shows the annual mean expenditure per person by health care services for those with HF and without HF over the 2002–2011 period. Inpatient cost ($11,318) of HF was the largest single component of the total medical expenditure. Prescription medication ($4,672), office-based visit ($3,370), outpatient visit ($1,266) and Emergency room visit ($571) accounted a large proportion of expenditures for HF in descending order.
Figure 2.
Mean total medical expenditure by heart failure status for the 2002–2011 US population
Adjusted Comparison of Individuals With and Without Heart Failure
Accounting for demographics, comorbidities and the effect of time, individuals with HF had $3,446 (95% CI 2,592–4,299) significantly higher expenditures than those without HF (Table 3). Individuals aged > 45 years or 65–85 years had significantly higher expenditures relative to those aged 18–44 years. Being female, high school or more, urban resident and publicly insured were significantly associated with higher total health care expenditures compared to their reference groups. Non-Hispanic Black or Hispanic/Other race, non married or never married, uninsured, residence in the South, as well as low, middle or high income were significantly associated with lower total health care expenditures as compared to their counterparts. Comorbidities were associated with significantly higher expenditures, namely diabetes $2,823 (95% CI: 2,506 – 3,139), hypertension $ 1,334 (95% CI 1,138 – 1,530), CVD $ 3,333 (95% CI: 3,018 – 3,649), stroke $ 2,982 (95% CI: 2,502 – 3,462). Compared with 2002/2003, adjusted mean expenditures were significantly higher by $505 (95% CI 194–816) in 2004/2005, $470 (174–765) in 2006/2007, and $279 (13– 545) in 2008/2009, and $ 684 (370–998) in 2010/2011 (Table 3).
Table 3.
Two-part regression model: Incremental effects of healthcare expenditures among adults with heart failure
| Variables | Incremental Cost | 95% CI | P-value |
|---|---|---|---|
| Primary independent variable | |||
| No-Heart failure (Ref.) | – | – | |
| Heart failure | 3,446*** | 2,592–4,299 | <0.001 |
| Covariates | |||
| Age | |||
| 18–44 (Ref.) | – | – | |
| 45–64 | 1,419*** | 1,170–1,668 | <0.001 |
| 65–85 | 1,851*** | 1,585–2,116 | <0.001 |
| Sex | |||
| Male (Ref.) | – | – | |
| Female | 1,066*** | 846–1,287 | <0.001 |
| Race | |||
| Non-Hispanic White (Ref.) | – | – | |
| Non-Hispanic Black | −436** | −717– −155 | 0.002 |
| Hispanic | −983*** | −1,243– −722 | <0.001 |
| Others | −995*** | −1,532– −458 | <0.001 |
| Marital Status | |||
| Married (Ref.) | – | – | |
| Non-married a | −349** | −555– −143 | 0.001 |
| Never married | −538*** | −807– −268 | <0.001 |
| Education | |||
| <High School (Ref.) | – | – | |
| High school | 494** | 207–782 | 0.001 |
| College or more | 777*** | 516–1,039 | <0.001 |
| Insurance status | |||
| Private (Ref.) | – | – | |
| Public insured | 996*** | 665–1,327 | <0.001 |
| Uninsured | −3,093*** | −3,281– −2,905 | <0.001 |
| Setting | |||
| Rural (Ref.) | – | – | – |
| Urban | 338** | 104–572 | 0.005 |
| Region | |||
| Northeast (Ref.) | – | – | – |
| Midwest | 54 | −335–445 | 0.783 |
| South | −371* | −735 −8.0 | 0.045 |
| West | 16 | −432–465 | 0.943 |
| Income | |||
| Poor income (Ref.) | – | – | |
| Low income | −807*** | −1,162– −453 | <0.001 |
| Middle income | −1,107*** | −1,439– −775 | <0.001 |
| High income | −901*** | −1,248– −554 | <0.001 |
| Diabetes | |||
| No Diabetes (Ref.) | – | – | |
| Diabetes | 2,823*** | 2,506–3,139 | <0.001 |
| Hypertension | |||
| No Hypertension (Ref.) | – | – | |
| Hypertension | 1,334*** | 1,138–1,530 | <0.001 |
| Cardiovascular disease | |||
| No cardiovascular disease (Ref.) | – | – | |
| Cardiovascular disease | 3,333*** | 3,018–3,649 | <0.001 |
| Stroke | |||
| No Stroke (Ref.) | – | – | |
| Stroke | 2,982*** | 2,502–3,462 | <0.001 |
| Emphysema | |||
| No Emphysema (Ref.) | – | – | |
| Emphysema | 2,016*** | 1,508–2,523 | <0.001 |
| Joint pain | |||
| No Joint Pain (Ref.) | – | – | |
| Joint pain | 1,182*** | 991–1,373 | <0.001 |
| Arthritis | |||
| No Arthritis (Ref.) | – | – | |
| Arthritis | 1,714*** | 1,492–1,936 | <0.001 |
| Asthma | |||
| No Asthma (Ref.) | – | – | |
| Asthma | 1,459*** | 978–1,941 | <0.001 |
| Year category | |||
| Year 2002/03 (Ref.) | – | – | |
| Year 2004/05 | 505** | 194–816 | 0.001 |
| Year 2006/07 | 470** | 174–765 | 0.002 |
| Year 2008/09 | 279* | 13–545 | 0.039 |
| Year 2010/11 | 684*** | 370–998 | <0.001 |
Non-married stands for widowed/divorced and separated.; Primary outcome variable in this model is total health care expenditures
Economic Burden of Heart Failure in the US
We extrapolated the individual costs estimates, to the entire US population. Based on the unadjusted mean, the annual aggregate cost during the 2002–2011 period among adults with HF was estimated at $40 billion for the entire US population. At the population level, the adjusted total incremental cost for HF was $5.8 billion per year, when comparing those with HF to those without HF.
Discussion
We demonstrated that direct health care expenditures among adults with HF increased from 2002/2003 through 2010/2011 (by approximately 30%). Individuals with HF had more than four times higher total direct health care expenditures compared with those without HFs during the 2002–2011 time frame and nearly half of the HF-related expenditures came from hospital inpatient care. The temporal trend for increased total medical expenditures was driven largely by inpatient hospitalization costs, with a relatively modest contribution for other types of expenditures. The observed decrease in HF costs in 2008–2009 may simply be consistent with the economic recession during this period. The increase in HF costs was influenced by age (>65 years), a higher level of education, public insurance status, urban dwellers, and the presence of comorbidities. Our estimates of the population-level cost of HF are somewhat consistent with the global estimates. In 2012, the global economic cost of HF was estimated at $108 billion per annum, with direct costs accounted for ~ 60% ($65 billion) and indirect costs accounted for ~ 40% ($43 billion) of the overall spend.26
The prevalence of HF increased over the study period. This may reflect more patients surviving with heart disease going on to developing heart failure and well as increased survival in those diagnosed with heart failure during the study period. Better HF management over time, with implementation of quality of care standards (including the more widespread use of potentially costly devices (implantable cardiac defibrillators, cardiac resynchronization and mechanical assist devices) and transplant has improved survival and consequently costs. The upwards trends in HF costs may also be partly explained by the improvement in detection of HF with preserved ejection fraction (HFpEF), as evidenced by studies indicating a temporal increase in the proportion of HFpEF cases detected in the community 27 and well as in hospitalizations for HFpEF.28
There were a number of demographic and clinical characteristics associated with health care expenditures in patients with HF. Older age is associated with high costs, which is consistent with the increase in HF incidence with ageing. HF is the most common diagnosis in hospitalized elderly patients aged >65 years.1 That the incremental costs in the US South are less than that in rest of the country contrast with the higher burden of HF in this region. It is well known that the South-Eastern region of the US for example (spanning from Georgia in the east to Oklahoma in the west - commonly referred to as the ‘stroke belt’ owing to elevated rates of cerebrovascular events in this region) has an excess frequency of HF and its risk factors such as hypertension, diabetes mellitus, compared with the rest of the country, but with 69% higher age-adjusted mortality from HF than the national average.29 Compared to the rest of the country, the South may be more rural and have less teaching hospitals, and thus less of an ability to address the burden of HF. Indeed, previous studies have shown that highest-cost discharges for HF were more likely to be observed in urban and teaching hospitals.30 Comorbidities that are also risk factors for HF such as obesity, sleep apnea, and diabetes mellitus, also played role in the rising burden of costs.
Our study provides important insights into factors associated with HF expenditures, thus has important implications for providing value-driven care to HF patients. The observed trends can be used to evaluate the effectiveness of the control and prevention programs or policies to stem the tide of HF, and point to the potential needs for a shift in the HF care delivery to a preventive approach, given the importance of preclinical HF. The study period predates most of the Patient Protection and Affordable Care Act based reforms and expenditures trends evident in this study establish a baseline in which subsequent expenditure trends can be compared. The disproportionately elevated costs of HF points to the need for efforts aimed at preventing the progression from asymptomatic stages of HF to more symptomatic, especially as projections of future HF costs (based on the MEPS data) indicate a doubling of costs from 2012 to 2030, if the HF incidence trends remain the same.31
Comparisons with other studies
Our study is the first of its kind to comprehensively examine US national trends in HF expenditures over a substantially long period of time (a decade). To our knowledge, no study has used national level data to examine the trend of the financial burden of HF from the patients’ or payers’ perspectives including a quantification of all components of direct HF expenditures. We assembled data on inpatient, outpatient, and emergency room (ER) visits, and prescription medication use. This differs from previous studies on HF costs that have either focused on shorter periods of time (a few years 3,32 or the last few months of life 4), on in-patient care/hospital costs only;5–8,30 or have mainly predated the widespread use of novel devices like cardiac resynchronization and defibrillator 9 or left ventricular assist device,10 as well as heart transplant, which have costs that can potentially outweighs all the other HF costs. Of note, some aspects of the other studies that used the MSEP data differed significantly from ours. Heindenreich et al conducted a simulation exercise focusing on costs projections over the 2010–2030 period.33 Voigt et al. examined costs during the 2007–2012 period, but did not examine trends in costs.32
Our findings of increasing HF costs are in agreement with other reports that have examined the medical cost of HFs over time. Indeed, in the prospective Cardiovascular Health study (CHS) the mean 10-year medical costs were significantly higher for the prevalent HF cohort (54,704 dollars vs. 41,780 dollars) compared those without HF.34 Also, a study of the lifetime costs of HF, showed that comorbidities such as diabetes mellitus significantly influence the expenditures 35.
Strengths and limitations
The strengths of our study include the examination of trends in costs over a decade using a nationally representative sample, including multiple cost categories (inpatient, outpatient, prescription medications, dental, ER, and home health expenditures), and the use of a robust cost estimation method to assess incremental costs accounting for a variety of comorbidities and thus evaluating the independent effect of HF.
Our study had some limitations. First, comorbidities was based on self-report, thus a potential for bias. However, self-reported comorbidities have been shown to be reliable.36 Second, our estimates may be lower than the actual HF costs, as people with early stages of HF such as stage B, which is generally asymptomatic and account for an important fraction of those with HF (up to 50% of those with left ventricular systolic dysfunction are asymptomatic in the early phase of the condition),37 may not have been accounted for. Third, institutionalized individuals who tend to be sicker but with a lower survival, and potential higher expenditures were not included in MEPS. Fourth, the HF costs were derived using survey data, which are subject to sampling error; thus there is a certain degree of uncertainty in our point estimates that is difficult to quantify. Fifth, we did not include costs from the use of over-the-counter medications or investigate the indirect costs of lost productivity from morbidity premature mortality; the latter costs can be very substantial as indicated by a simulation that projected an increase in these from US$9.8 billion in 2012 to $16.6 billion by 2030..31 The data on costs related to transitions of care in HF (hospice, short-term and long-term care facilities were not included in the estimates as these are not available in the MSEP surveys. We did not have data on the etiology of heart failure; we could not tell whether this was consecutive to ischemic heart disease or not. Our analysis did not also examine types of HF (HF with reduced ejection fraction [HFrEF] vs. HFpEF) as the relative contribution of each type to costs has changed over time, given the continuous refinement in the capacity to diagnose HFpEF over recent years. While some studies, have suggested that preserved ejection fraction (≥50%) is associated with a 23.6% higher lifetime costs,35 other have postulated that that the costs may be similar between those with HFrEF and HFpEF, 38 but the latter data was published at a time when detection of HFpEF was much less than currently and many of the new devices or late-stage therapies indicated for HFrEF were not in use. Finally, we also did not specifically have information on the contribution of costs related to the use of implantable cardiac defibrillators, cardiac resynchronization, left ventricular assist devices and heart transplant. We also could not make a distinction between initial and repeat hospitalization, as well as whether the HF diagnosis was primary from secondary, given that these may have an impact on costs.
Conclusion
This study provides insights into high burden of HF-related costs in the United States over time, as well as the key determinants of high expenditures. Further research is needed to better characterize how the HF costs vary with health resource utilization overtime. HF greatly contributes to the increase in health costs in the U.S. population, indicating the potential savings from interventions to improve prevention and management of HF in the U.S. population. Specifically, policy interventions directed towards reducing inpatient hospitalization use could have a significant impact on the trajectory of the overall HF related costs. Improved cardiology and primary care access, systems of care, awareness on diet and physical activity, shift in care modalities (outpatient care/home healthcare) and reducing risk factor of HF by treating comorbidities are ways to minimize the substantial burden. The disproportionally high costs of HF points to the need for a shift of HF care towards a preventive approach and comprehensive disease management.
Acknowledgments
Sources of Funding:
This study was supported by Grant K24DK093699 from The National Institute of Diabetes and Digestive and Kidney Disease (PI: Leonard Egede).
Grants, contracts, and other forms of financial support:
Dr. Gregg C. Fonarow reports receiving research funding from the National Institutes of Health and consulting for Amgen, Janssen, Novartis, Medtronic, and St Jude.
Footnotes
Disclaimer:
This article represents the views of the authors and not those of NIH, VHA or HSR&D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–54. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greiner MA, Hammill BG, Fonarow GC, Whellan DJ, Eapen ZJ, Hernandez AF, Curtis LH. Predicting costs among medicare beneficiaries with heart failure. Am J Cardiol. 2012;109:705–11. doi: 10.1016/j.amjcard.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unroe KT, Greiner MA, Hernandez AF, Whellan DJ, Kaul P, Schulman KA, Peterson ED, Curtis LH. Resource use in the last 6 months of life among medicare beneficiaries with heart failure, 2000–2007. Arch Intern Med. 2011;171:196–203. doi: 10.1001/archinternmed.2010.371. [DOI] [PubMed] [Google Scholar]
- 5.Whellan DJ, Greiner MA, Schulman KA, Curtis LH. Costs of inpatient care among Medicare beneficiaries with heart failure, 2001 to 2004. Circ Cardiovasc Qual Outcomes. 2010;3:33–40. doi: 10.1161/CIRCOUTCOMES.109.854760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauptman PJ, Swindle J, Burroughs TE, Schnitzler MA. Resource utilization in patients hospitalized with heart failure: insights from a contemporary national hospital database. Am Heart J. 2008;155:978–985. e1. doi: 10.1016/j.ahj.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Wexler DJ, Chen J, Smith GL, Radford MJ, Yaari S, Bradford WD, Krumholz HM. Predictors of costs of caring for elderly patients discharged with heart failure. Am Heart J. 2001;142:350–7. doi: 10.1067/mhj.2001.116476. [DOI] [PubMed] [Google Scholar]
- 8.Ong MK, Mangione CM, Romano PS, Zhou Q, Auerbach AD, Chun A, Davidson B, Ganiats TG, Greenfield S, Gropper MA, Malik S, Rosenthal JT, Escarce JJ. Looking forward, looking back: assessing variations in hospital resource use and outcomes for elderly patients with heart failure. Circ Cardiovasc Qual Outcomes. 2009;2:548–57. doi: 10.1161/CIRCOUTCOMES.108.825612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen AY-J, Wang X, Doris J, Moore N. Proportion of patients in a congestive heart failure care management program meeting criteria for cardiac resynchronization therapy. Am J Cardiol. 2004;94:673–6. doi: 10.1016/j.amjcard.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez AF, Shea AM, Milano CA, Rogers JG, Hammill BG, O’Connor CM, Schulman KA, Peterson ED, Curtis LH. Long-term outcomes and costs of ventricular assist devices among Medicare beneficiaries. JAMA. 2008;300:2398–406. doi: 10.1001/jama.2008.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen JW, Cohen SB, Banthin JS. The medical expenditure panel survey: a national information resource to support healthcare cost research and inform policy and practice. Med Care. 2009;47:S44–50. doi: 10.1097/MLR.0b013e3181a23e3a. [DOI] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality (AHRQb) [Accessed June 20, 2016];Medical Expenditure Panel Survey, 2011 Medical conditions 2013b. Available from: http://meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h146/h146doc.pdf.
- 13.Trogdon JG, Murphy LB, Khavjou OA, Li R, Maylahn CM, Tangka FK, Nurmagambetov TA, Ekwueme DU, Nwaise I, Chapman DP, Orenstein D. Costs of Chronic Diseases at the State Level: The Chronic Disease Cost Calculator. Prev Chronic Dis. 2015;12:E140. doi: 10.5888/pcd12.150131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CPI Inflation Calculator. Washington, DC: U.S. Bureau of Labor Statistics; [Accessed 15 June 2016.15]. Available from: http://data.bls.gov/cgi-bin/cpicalc.pl. [Google Scholar]
- 15.Ozieh MN, Bishu KG, Dismuke CE, Egede LE. Trends in health care expenditure in U.S. adults with diabetes: 2002–2011. Diabetes Care. 2015;38:1844–51. doi: 10.2337/dc15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egede LE, Lynch CP, Gebregziabher M, Hunt KJ, Echols C, Gilbert GE, Mauldin PD. Differential impact of longitudinal medication non-adherence on mortality by race/ethnicity among veterans with diabetes. J Gen Intern Med. 2013;28:208–15. doi: 10.1007/s11606-012-2200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egede LE, Gebregziabher M, Echols C, Lynch CP. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother. 2014;48:562–70. doi: 10.1177/1060028014526362. [DOI] [PubMed] [Google Scholar]
- 18.Bishu KG, Gebregziabher M, Dismuke CE, Egede LE. Quantifying the Incremental and Aggregate Cost of Missed Workdays in Adults with Diabetes. J Gen Intern Med. 2015;30:1773–9. doi: 10.1007/s11606-015-3338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–94. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 20.Belotti F, Deb P, Manning WG, Norton EC. twopm: Two-part models. Stata J. 2015;15:3–20. [Google Scholar]
- 21.Ku L. Health insurance coverage and medical expenditures of immigrants and native-born citizens in the United States. Am J Public Health. 2009;99:1322–8. doi: 10.2105/AJPH.2008.144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnett SBL, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Duan N, Manning WG, Morris CN, Newhouse JP. A Comparison of Alternative Models for the Demand for Medical Care. J Bus Econ Stat. 1983;1:115–126. [Google Scholar]
- 24.Desai PR, Lawson KA, Barner JC, Rascati KL. Identifying patient characteristics associated with high schizophrenia-related direct medical costs in community-dwelling patients. J Manag Care Pharm. 2013;19:468–77. doi: 10.18553/jmcp.2013.19.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp. Statistical Software. College Station, TX: StataCorp LP; 2015. Stata: Release 14. [Google Scholar]
- 26.Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–76. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC Get With the Guidelines Scientific Advisory Committee and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 29.Mujib M, Zhang Y, Feller MA, Ahmed A. Evidence of a “Heart Failure Belt” in the Southeastern United States. Am J Cardiol. 2011;107:935–937. doi: 10.1016/j.amjcard.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziaeian B, Sharma PP, Yu T-C, Johnson KW, Fonarow GC. Factors associated with variations in hospital expenditures for acute heart failure in the United States. Am Heart J. 2015;169:282–289.e15. doi: 10.1016/j.ahj.2014.11.007. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pi??a IL, Trogdon JG. Forecasting the impact of heart failure in the united states a policy statement from the american heart association. Circ Hear Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voigt J, Sasha John M, Taylor A, Krucoff M, Reynolds MR, Michael Gibson C. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the united states. Clin Cardiol. 2014;37:312–321. doi: 10.1002/clc.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG American Heart Association Advocacy Coordinating Committee, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao L, Anstrom KJ, Gottdiener JS, Pappas PA, Whellan DJ, Kitzman DW, Aurigemma GP, Mark DB, Schulman KA, Jollis JG. Long-term costs and resource use in elderly participants with congestive heart failure in the Cardiovascular Health Study. Am Heart J. 2007;153:245–52. doi: 10.1016/j.ahj.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, Roger VL. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Echouffo-Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the Risk of Progression From Asymptomatic Left Ventricular Dysfunction to Overt Heart Failure: A Systematic Overview and Meta-Analysis. JACC Heart Fail. 2016;4:237–48. doi: 10.1016/j.jchf.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Liao L. Costs for Heart Failure With Normal vs Reduced Ejection Fraction. Arch Intern Med. 2006;166:112. doi: 10.1001/archinte.166.1.112. [DOI] [PubMed] [Google Scholar]