Abstract
Rationale
Pneumonia remains the most common major infection after cardiac surgery despite numerous preventive measures.
Objectives
To prospectively examine the timing, pathogens, and risk factors, including modifiable management practices, for post-operative pneumonia and estimate its impact on clinical outcomes.
Methods
5,158 adult cardiac surgery patients were prospectively enrolled in a cohort study across 10 centers. All infections were adjudicated by an independent committee. Competing risk models were used to assess the association of patient characteristics and management practices with pneumonia within 65 days of surgery. Mortality was assessed by Cox proportional hazards model, and length of stay using a multi-state model.
Measurements and Main Results
The cumulative incidence of p neumonia was 2.4% ,33% of which occurred after discharge. Older age, lower hemoglobin level, chronic obstructive pulmonary disease, steroid use, operative time and left ventricular assist device/heart transplant were risk factors. Ventilation time (24–48 vs ≤24 hours;HR,2·83; 95% CI,1·72–4·66; >48 hours HR,4·67; 95% CI,2·70–8·08), nasogastric tubes (HR,1·80; 95% CI,1·10–2·94), and each unit of blood cells transfused (HR,1·16; 95% CI,1·08–1·26) increased pneumonia risk. Prophylactic use of second-generation cephalosporins (HR,0·66; 95% CI, 0·45–0·97) and platelet transfusions (HR, 0·49, 95% CI, 0·30–0·79) were protective. Pneumonia was associated with a marked increase in mortality (HR,8·89; 95% CI,5·02–15·75), and longer LOS of 13·55 ± 1·95 days (bootstrap 95% CI,10·31–16·58).
Conclusions
Pneumonia continues to impose a major impact on the health of patients after cardiac surgery. Adjusting for baseline risk, several specific management practices were associated with pneumonia, which offer targets for quality improvement and further research.
INTRODUCTION
Healthcare-associated infections (HAIs) represent the leading cause of non-cardiac morbidity after cardiac surgery,1 and pneumonia is particularly common and responsible for adverse patient outcomes.2–4 Moreover, pneumonia is frequently cited as among the most costly and resource-intensive of all HAIs.5–7
Patients undergoing cardiac surgery are highly susceptible to pneumonia. Many patients present with cardiac symptoms requiring hospitalization prior to urgent surgery, predisposing them to pathogens. Common risk factors among cardiac surgery patients, such as COPD, heart failure, and advanced age, result in a higher risk profile for pneumonia. Moreover, cardiopulmonary bypass, with its effect on systemic inflammatory mediators and its potential for lung injury, may further contribute to the risk of developing pneumonia.8 Significant fluid shifts in the perioperative setting often leading to pulmonary edema, combined with the frequent need for transfusion of blood products may affect pneumonia risk.9 Any prolonged use of mechanical ventilation places patients at increased risk for pneumonia.10,11 Finally, postoperative pain due to sternotomy or thoracotomy can affect pulmonary mechanics. Variations in management practices, such as fluid administration and ventilator management, are likely to affect the incidence of pneumonia,12,13 but few insights exist about the relationship between these “process of care” variables and pneumonia.
The purpose of this study was to examine the time course, pathogens, and risk factors, including modifiable management practices, for post-operative pneumonia in patients undergoing cardiac surgery. This study is a sub-study of a larger prospective cohort study conducted within centers participating in the Cardiothoracic Surgical Trials Network (CTSN), looking at all serious post-operative infections.14
PATIENTS AND METHODS
Study Design and Patients
The primary objective of this CTSN observational study was to identify management practices associated with risk of infection after cardiac surgery. This study was conducted in 10 centers from the Cardiothoracic Surgical Trials Network. The patient population included all adults receiving cardiac surgical interventions, excluding those with active systemic infections, including endocarditis.
We targeted a minimum sample size of 5,000 patients to obtain at least 200 patients with major infections. This sample size was based not on explicit statistical criteria, but on acquiring an adequate number of events (at least 10 per variable) to ensure stability of coefficient estimates in our models.15,16 Patients were followed for up to 60 ± 5 days after surgery with two planned post-discharge assessments at 30 and 60 days after surgery; the last date of follow-up was 11/29/2010. We collected data on patient characteristics (demographics, baseline laboratory values, co-morbidities), surgery-related factors (such as prior IABP, surgery time, operative procedure), and management practices (such as antimicrobial prophylaxis, glycemic control, line management) (see Appendix E1). Data were transmitted from sites electronically to a secure server administered by the Data Coordinating Center (DCC). Participating institutional review boards approved the protocol (see Appendix E2 for participating sites) , and the study was overseen by a National Heart, Lung, and Blood Institute-appointed Data Safety and Monitoring Board. All patients provided written informed consent to participate and release their medical information.
Endpoints
The primary endpoint for this analysis was pneumonia within 65 days of the index cardiac surgery. Pneumonia was classified according to definitions from the Centers for Disease Control and the National Healthcare Safety Network surveillance (see Appendix E1). Other secondary endpoints included all-cause mortality, reoperation, and hospital readmissions. All pneumonias were reviewed by an independent event adjudication committee. The date of final event adjudication was June 2011.
Data Analysis
We used the proportional sub-distribution hazards model,17 a variant of the Cox proportional hazards model, to account for death as a competing event when assessing the effect of baseline characteristics and management practices on pneumonia. Model building proceeded in two stages. We first identified patient- and procedure-related risk factors that were individually associated (at p < 0.10) with pneumonia and remained significant (at p < 0.05) in the multivariable model using a backward selection procedure. We then evaluated the additional contribution of management practices utilized prior to the first pneumonia (see Appendix E1 for details), except for post-operative transfusions, where the exact timing could not always be ascertained. Management practices that were individually associated with pneumonia (at p < 0.10) were placed in a multivariable model that contained all the patient- and procedure-specific variables in the first stage. Using a backward selection approach, the final competing risk model for pneumonia consisted of only management practices with a p < 0.05, adjusting for all the first stage baseline risk factors.
The standard Cox proportional hazards model was applied to the mortality analysis, with pneumonia treated as a time-dependent covariate. Patient characteristics that are known risk factors for mortality in the elderly cardiac surgery population and that were significant in our model were controlled for in the analysis.
To estimate the incremental length of stay (LOS) due to pneumonia, we used a multi-state approach that treats pneumonia as a time-dependent exposure and mortality as a competing risk.18 LOS was defined as the time from index procedure to hospital discharge or death. The model assumed a time-inhomogeneous Markov process with one initial state (index surgery), one intermediate state (pneumonia), and two absorbing states (death and discharged alive). All patients started in the initial state and could transition to the other states. Patients who developed pneumonia could have had a prior or subsequent infection and patients who did not develop pneumonia could have had other infections. A bootstrap standard error and 95% CI was computed for the additional LOS due to pneumonia based on 1000 bootstrap samples. All analyses were done in SAS statistical software (SAS® v9.4; Cary, NC), and R 3.1.1.19,20
RESULTS
Patients Characteristics
Among the patients enrolled (n= 5,158), the mean age was 64.4±13.2 years, the median body mass index (BMI) was 28.2 kg/m2, and the proportion of women was 33% (n=1,708; Table 1). Diabetes was present in 23% (n=1169) of patients, 29% (n=1,505) had heart failure, and 14% (n=746) had COPD of mild or greater severity. Nineteen percent (n=958) of patients had undergone prior cardiac surgery. The most common procedures were isolated coronary artery bypass grafting (CABG) (33%, n=1,677) and isolated valve surgery (36%, n=1,878).
Table 1.
Patient and Operative Characteristics*
| Baseline and Operative Characteristics | Overall (N=5158) |
|---|---|
| Demographics | |
| Age, mean (SD) | 64.4 (13.2) |
| Male | 3450 (66.9) |
| White | 4322 (83.9) |
| BMI | 28.2 (25.1, 32.3) |
| Insurance | |
| Medicaid | 233 (4.5) |
| Medicare | 1928 (37.5) |
| Government (Other) | 626 (12.2) |
| Private | 2099 (40.8) |
| None/Self | 260 (5.1) |
| Baseline Laboratories | |
| WBC, ×103/ml | 7.0 (5.7, 8.4) |
| Creatinine, mg/dL | 1.0 (0.8, 1.2) |
| Hemoglobin, g/dL | 13.4 (12.0, 14.5) |
| Cardiac morbidity | |
| Heart failure | 1505 (29.2) |
| Ejection fraction | 55.0 (48.0, 60.0) |
| Previous cardiac surgery | 958 (18.6) |
| Noncardiac morbidity | |
| Corticosteroids | 176 (3.4) |
| Diabetes‡ | 1169 (22.7) |
| COPD | |
| None | 4412 (85.5) |
| Mild or moderate | 644 (12.5) |
| Severe | 102 (2.0) |
| Operative | |
| Surgery time, hours | 4.2 (3.3, 5.2) |
| Bypass time, minutes§ | 105.0 (78.0, 140.0) |
| Sternotomy | 4669 (90.5) |
| Surgery Type | |
| Elective | 3806 (73.8) |
| Urgent | 1214 (23.5) |
| Emergent | 138 (2.7) |
| Procedure | |
| Isolated CABG | 1677 (32.5) |
| Isolated valve | 1878 (36.4) |
| CABG + valve | 692 (13.4) |
| LVAD/Tx | 122 (2.4) |
| Thoracic aortic | 428 (8.3) |
| Otherll | 361 (7.0) |
Abbreviations: CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LVAD/Tx, left ventricular assist device or heart transplant surgery; SD, standard deviation.
Continuous variables are expressed as median (IQR) and categorical variables as count (%).
Insulin or oral medications.
91.1% of patients had on-pump surgical procedures.
Other: ventricular septal defect repairs, atrial septal defect repairs, aneurysmectomies, PFO closures, ablations, septal myectomies, excision of cardiac tumors, pericardiectomies, and limited other procedures.
Frequency and Timing of Pneumonia and Microorganisms
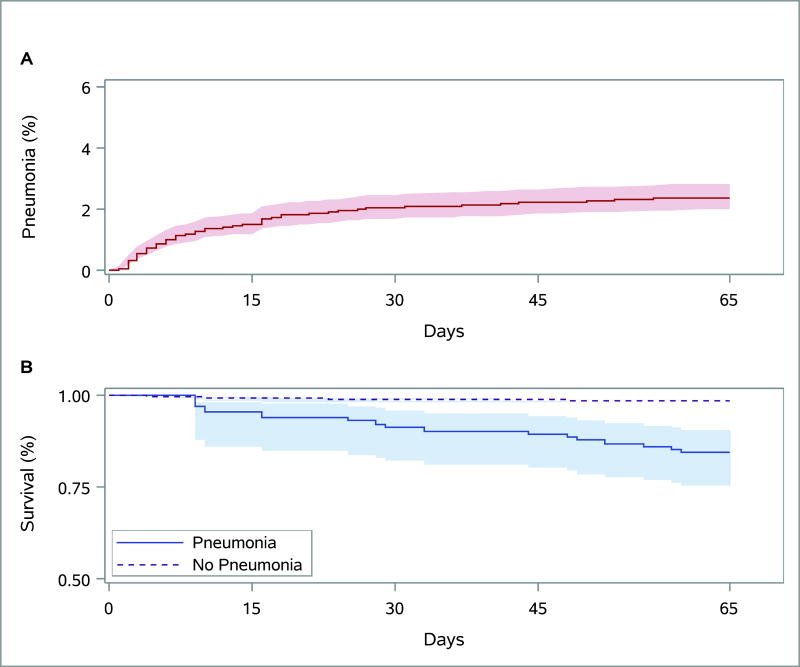
A total of 123 patients experienced 125 pneumonia events (crude cumulative incidence 2.4%, Figure 1A), accounting for 41.5% of all major infections (n=301) in this cohort (Figure 1A). The initial diagnosis was made during the index hospitalization in 67% (n=82) of patients who developed pneumonia, nearly half of which (n=58) occurred during the first week after surgery. The mean time to the first episode of pneumonia was 14.4 days, with a median time of 8 days (range 1–62). Of the 123 patients, who experienced pneumonia, 106 (86%) contracted it within 30 days following cardiac surgery.
Figure 1.
Cumulative incidence function with 95% confidence intervals for pneumonia with death as a competing risk.(A) Extended Kaplan-Meier curve with 95% confidence intervals for mortality based on method of Snappin and colleagues.(B).
Central Picture with legend: Cumulative incidence of pneumonia over 65 days
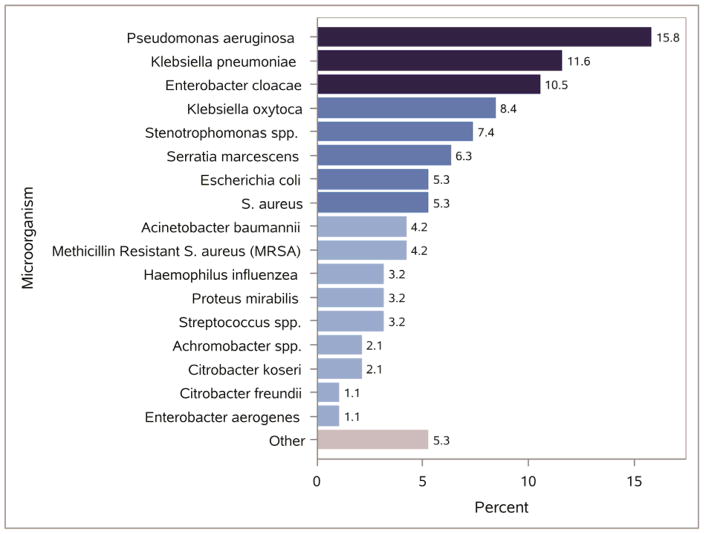
The distribution of microorganisms isolated from patients who developed pneumonia is shown in Figure 2. The three most common isolates were Pseudomonas Aeruginosa (15.8%, n=15), Klebsiella pneumonia (11.6%, n=11), and Enterobacter cloacae (10.5%, n=10).
Figure 2. Distribution of Identified Microorganisms among Patients Developing Pneumonia.
Organism data were available for 62% of pneumonia cases. Up to different three organisms was recorded for each event. Other category: Neisseria sicca, Alcaligenes xylosoxidans, Corynebacterium pseudodiptheriticum, Morganella morganii, Serratia plymuthica
Baseline Risk Factors Related to Pneumonia
Preoperative patient characteristics that were associated with a higher risk of pneumonia in multivariable analysis included older age (HR, 1.02; 95% CI, 1.00–1.03; p=0.05), presence of COPD (HR, 2.17; 95% CI, 1.47–3.21; p<0.001), and recent steroid use (HR, 1.91; 95% CI, 1.01–3.59; p=0.05). Higher hemoglobin levels at baseline were protective (HR, 0.88; 95% CI, 0.79–0.97; p=0.01) (Table 2). Smoking history did not increase the risk of pneumonia in the multivariable model. Approximately 50% of patients were admitted on the same day of surgery, and the majority of the other patients were admitted within one week prior to surgery. Preoperative length of stay was not associated with the development of pneumonia after accounting for other risk factors. Operative variables associated with greater pneumonia risk included LVAD implantation or heart transplantation (HR, 2.79; 95% CI, 1.50–5.17; p=0.001), and longer operative time (HR, 1.38; 95% CI, 1.25–1.53; p<0.001).
Table 2.
Patient and Procedure Characteristics Associated With Pneumonia
| Baseline Variable | HR (95% CI) | P Value |
|---|---|---|
| Age (year) | 1.02 (1.00, 1.03) | 0.05 |
| COPD (yes/no) | 2.17 (1.47, 3.21) | <0.001 |
| Corticosteroids (yes/no) | 1.91 (1.01, 3.59) | 0.05 |
| Hemoglobin, g/dL | 0.88 (0.79, 0.97) | 0.01 |
| LVAD/Tx (yes/no) | 2.79 (1.50, 5.17) | 0.001 |
| Duration of surgery (hour) | 1.38 (1.25, 1.53) | <0.001 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease;
HR, hazard ratio; LVAD/Tx, left ventricular assist device or heart transplant surgery
Management Practices Related to Pneumonia
Controlling for baseline risk factors, management practices that were associated with an increased risk of pneumonia included the use of a nasogastric (NG) tube postoperatively (HR, 1.80; 95% CI, 1.10–2·94; p=0.02), and transfusion of packed red blood cells (PRBCs) (for each unit: HR, 1.16; 95% CI, 1.08–1·26; p < 0.001; Table 3). However, platelet transfusion was protective (HR, 0.49, 95% CI, 0.30–0.79, p=0.004). Compared to postoperative ventilation of 24 hours or less, an intubation time of 24–48 hours was associated with more than double the risk of pneumonia (HR, 2.83; 95% CI, 1.72–4.66; p<0.001) and ventilation exceeding 48 hours was associated with more than a four-fold higher risk (HR, 4.67; 95% CI, 2.70–8.08; p<0.001). Peri-operative second generation cephalosporins (cefuroxime, cefoxitin, with or without concomitant vancomycin), which were used in 47% of patients, reduced the risk of pneumonia by more than 30% (HR, 0.66; 95% CI, 0.45–0.97; p=0.04). There was consistent use of head of bed elevation and secretion management postoperatively and thus, neither affected pneumonia risk. Placement of femoral lines, number of central lines, and hyperglycemia were not significant predictors in the multivariable model.
Table 3.
Management Practices Associated With Pneumonia
| Management Practice* | HR (95% CI) | P Value |
|---|---|---|
| Second generation CEPH (yes/no) | 0.66 (0.45, 0.97) | 0.04 |
| Ventilation (vs. ≤24 hours) | ||
| 24 – 48 hours | 2.83 (1.72, 4.66) | <0.001 |
| > 48 hours | 4.67 (2.70, 8.08) | <0.001 |
| NG Tube (yes/no) | 1.80 (1.10, 2.94) | 0.02 |
| PRBC (unit) | 1.16 (1.08, 1.26) | <0.001 |
| Platelet (yes/no) | 0.49 (0.30, 0.79) | 0.004 |
Abbreviations: CI, confidence interval; CEPH, cephalosporin; HR, hazard ratio; NG, nasogastric tube; PRBC, packed red blood cells
Model adjusted for baseline risk factors in Table 2
Length of Stay and Readmissions
The observed mean length of stay after the initial surgical procedure was 32.4 ± 22.6 days for patients with pneumonia during the index hospitalization compared to 8.9 ± 6.5 days for those without it. Following their index surgery, patients who developed pneumonia stayed in the hospital for nearly two weeks longer (excess length of stay: 13.55 ± 1.95 days; bootstrap 95% CI, 10.31–16.58). In the overall parent cohort study, the readmission rate was 19% (n=945). Infection was the most common reason for readmission (16% of all readmissions) and pneumonia constituted 21.3% of this group.
Pneumonia and Mortality
Ninety-seven patients (1.9%) died during follow-up, with half the deaths occurring within 19 days of index surgery. Figure 1B depicts the unadjusted time-varying effect of pneumonia on mortality. Pneumonia was associated with a markedly higher risk of death (HR, 8.89; 95% CI, 5.02–15.75; p<0.001), after adjusting for patient’s age, sex, co-morbidities (diabetes and congestive heart failure), and baseline hemoglobin and creatinine levels (Table 4). The 65-day mortality rates (per 100 patient-months) for patients with and without pneumonia were 6 and 0.8, respectively.
Table 4.
Impact of Pneumonia on Mortality
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Pneumonia | 8.89 (5.02, 15.75) | <0.001 |
| Age (year) | 1.03 (1.01, 1.05) | <0.001 |
| Male | 0.60 (0.39, 0.91) | 0.02 |
| Diabetes* (yes/no) | 1.57 (1.03, 2.41) | 0.04 |
| Heart Failure (yes/no) | 1.86 (1.24, 2.80) | 0.003 |
| Creatinine, mg/dL | 1.17 (1.06, 1.30) | 0.002 |
| Hemoglobin, g/dL | 0.85 (0.75, 0.95) | 0.005 |
Abbreviations: CI, confidence interval; HR, hazard ratio
Insulin or oral medications.
COMMENT
In this large, multicenter, prospective cohort study, postoperative pneumonia was the most common infection that occurred within 65 days, with more than 30% of first events diagnosed after discharge from the index hospitalization14. Older age, COPD, steroid use, lower hemoglobin levels, LVAD/heart transplants, and longer duration of surgery were significantly associated with risk of pneumonia. Importantly, the use of second generation cephalosporins for peri-operative prophylaxis, shorter ventilation time (< 24 hours), avoidance of NG tube use, fewer PRBC transfusions, and receipt of platelet transfusions, were all associated with a decreased risk of post-operative pneumonia. Ultimately, postoperative pneumonia was associated with a nearly 9-fold increase in mortality and an additional 2 weeks of index hospital stay.
Impact of Pneumonia in Cardiac Surgery
Traditionally, surgical site infections have been perceived as the most common infection after cardiac surgery. Our study reveals pneumonia to be the most common serious infection post cardiac surgery and this finding is supported by other contemporary studies.14,21 In a recent analysis of over 19,000 patients, pneumonia was the most common postoperative infection (6%) whereas deep surgical site infections occurred in just 1%.22 In the present study, pneumonia was associated with an 8.9-fold increased risk of mortality even after adjusting for baseline patient characteristics, and resulted in a 65-day pneumonia mortality rate of 6 per 100 patient-months.
In addition to the effect on morbidity and mortality, pneumonia represents a significant burden on resource utilization. Our group has estimated the incremental cost associated with a major HAI to be $38,000 with infection related readmissions costing three times that of readmissions that were unrelated to infection.23 There have been few publications that focus on the economic impact of pneumonia (compared with undefined pneumonitis) in the cardiac surgery population. A recent analysis using the Nationwide Inpatient Sample in patients undergoing isolated mitral valve surgery estimated an average net increase in hospital cost of $29,692 for the 5.5% of individuals with postoperative pneumonia and an increase in the median LOS of 10 days.24 Thus, postoperative pneumonia represents not only a significant risk factor for morbidity and mortality but also a significant driver of resource utilization.
Timing of Pneumonia
In comparison to the published literature, which focuses mostly on the index hospitalization or on 30-day follow-up, patients in this study were followed for up to 65 days following the index operation. As such, this study revealed that a substantial proportion of first pneumonia events (over 30%) occurred after the index hospitalization, and 14% more than 30 days post-surgery.
While it would be expected that the majority of cases of pneumonia would manifest early in the post-operative setting due to postoperative pain, need for narcotics, and fluid shifts, there are some potential reasons why pneumonia may develop later. Since the average length of stay for many routine cardiac operations is 5–7 days, retrospective studies may underestimate the true prevalence of pneumonia. It is likely that the intensive pulmonary care that occurs in the hospital is not maintained after discharge. Also, many “late” pneumonias (after the first week) in our study occurred in patients with prolonged hospitalizations and/or difficult post-operative courses with multiple complications.
Modifiable Processes of Care
Given the significant association with mortality and increased resource utilization, there has been a strong effort over the past decade to reduce HAIs through improvements in management practices. This prospective study was the first to our knowledge to investigate the relationship between processes of care and the incidence of postoperative pneumonia following cardiac operations. National guidelines have been developed and implemented to avert infections, and among these is the Surgical Care Improvement Project (SCIP).25 The SCIP guidelines include standardizing peri-operative antibiotic use to decrease postoperative surgical site infections. Specific SCIP measures addressing the risk of infection include 1) the administration of appropriate antibiotics within 60 minutes of incision, 2) discontinuation of antibiotics within 48 hours following cardiac surgery, and 3) controlled blood glucose (<200mg/dl) at 6 AM postoperative day one. The STS has also published practice guidelines related to antibiotic prophylaxis.26 In these guidelines, 1st or 2nd generation cephalosporins are considered first line therapy for prophylaxis with no clear data supporting 1st generation over 2nd generation cephalosporins. In patients with significant allergy to β-lactam antibiotics, vancomycin with or without an aminoglycoside is recommended. In multivariable analysis, the use of second generation cephalosporins with broader Gram negative coverage was associated with a lower risk of pneumonia. However, neither first nor second-generation cephalosporins have activity against Pseudomonas, which was the most common organism observed in patients with pneumonia. Therefore, consideration might be given to optimizing perioperative antibiotic regimens for patients who may be high risk for developing postoperative pneumonia, though any recommendation of this type needs to be carefully balanced against the risk of promoting the emergence of multi-drug resistant nosocomial organisms.
Numerous studies have shown that prolonged intubation affects postoperative pneumonia, length of stay, and complications. As such, early extubation after surgery has been a prominent focus of cardiac surgical quality improvement for several years, often with reimbursement linked to postoperative extubation time. The present study reinforces the importance of timely postoperative extubation, with patients intubated between 24–48 hours or >48 hours having 2.83 and 4.67-fold increased risk of pneumonia, respectively, compared with patients who are extubated within 24 hours. Likewise, we have previously demonstrated the dose-dependent association between red blood cell transfusion and risk of postoperative infections and, as such, efforts need to continue to improve blood conservation in cardiac surgery.27
The use of a nasogastric (NG) tube was associated with risk of pneumonia. Previous reports have supported this finding. A randomized study comparing the use of an NG tube to no NG tube following cardiac surgery demonstrated no benefit to routine NG use.28 Another study identified the use of an NG tube to be the strongest risk factor for developing nosocomial pneumonia (OR=6.48 [2.11, 19.82] in patients intubated longer than 72 hours.29 The fact that many of the organisms cultured in our study were enteral flora supports this observation. It is possible that some patients who received an NG tube were ill for a long period of time postoperatively and, therefore, the NG tube was a marker of illness severity and not the dominant predisposing factor. Nonetheless, it was surprising that 73% of all patients in this study had an NG tube after surgery; NG tube management may be an important target for process improvement investigations.
Limitations
We identified the timing of infections and included in the analysis only those management practices that were used prior to the diagnosis of pneumonia, except transfusions, the timing of which was sometimes unavailable. We cannot definitively exclude practices that were used on the basis of a suspicion of infection. Absence of an association between pneumonia and head elevation and secretion management reflects the fact that there was nearly uniform adoption of these practices across sites. Randomized trials are challenging to conduct in this area. However, the standardization of infection definitions and data collection procedures, as well as site monitoring and adjudication of infections by an expert committee, improve the validity of this observational study. Finally, while we were able to garner much insight from these 123 patients with pneumonia, the relatively low number of outcome events limits our ability to assess more precisely the impact of all measured process of care variables.
CONCLUSION AND IMPLICATIONS
In this observational study of cardiac surgical patients, pneumonia was the most prevalent infection and had a significant, deleterious impact on length of stay and mortality. We identified various processes of care measures that were independently associated with the development of pneumonia, including choice of antibiotic prophylaxis, the use of a nasogastric tube, length of mechanical ventilation after surgery, and the use of red blood cell and platelet transfusions. Despite substantial efforts to reduce the incidence of surgery-associated pneumonia, gaps remain in the application of quality improvement strategies. Given the high incidence of post-discharge pneumonia, efforts should also be directed towards identifying high risk patients and developing and evaluating transitional programs to address this issue.
Supplementary Material
Central Message.
Pneumonia is the most common infection following cardiac surgery. Several specific modifiable management practices were associated with pneumonia, which offer targets for quality improvement.
Perspective.
Pneumonia is a common complication after cardiac surgery and previous studies have identified several patient-specific risk factors. Ours, however, is the first multicenter, prospective study of both risk factors and management practices on the risk of pneumonia. This analysis provides knowledge and targets for future quality improvement initiatives.
Acknowledgments
Sources of Funding: A cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke of the NIH and the Canadian Institutes of Health Research. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services.
ABBREVIATIONS
- CDC
Centers for Disease Control and Prevention
- CTSN
Cardiothoracic Surgical Trials Network
- HAIs
Healthcare-Associated Infections
- NHSN
National Healthcare Safety Network
Footnotes
Conflicts of Interest: Dr. Gillinov reports personal fees from Edwards Lifesciences, personal fees from Medtronic, personal fees from On-X, grants and personal fees from St. Jude Medical, personal fees from Abbott, personal fees and other from AtriCure, personal fees from ClearFlow, outside the submitted work. Dr. Alexander reports grants from National Institutes of Health/NHLBI, during the conduct of the study. Dr. Smith reports grants from NIH/CTSN , during the conduct of the study; personal fees from Abbott Vascular, personal fees from Edwards Lifesciences, grants from Edwards Lifesciences, personal fees from St Jude , outside the submitted work. All other authors have no conflicts of interest to disclose.
Clinical Trial Registry Number: NCT01089712
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He S, Chen B, Li W, Yan J, Wang X, Xiao Y. Ventilator-associated pneumonia after cardiac surgery: a meta analysis and systematic review. J Thorac Cardiovasc Surg. 2014 Dec;148:3148–55. doi: 10.1016/j.jtcvs.2014.07.107. [DOI] [PubMed] [Google Scholar]
- 2.Kollef MH, Sharpless L, Vlasnik J, Pasque C, Murphy D, Fraser VJ. The impact of nosocomial infections on patient outcomes following cardiac surgery. Chest. 1997;112:666–675. doi: 10.1378/chest.112.3.666. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007 Mar;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. VAP Outcomes Scientific Advisory Group. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 5.Scott RD. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention, 2009. Centers for Disease Control and Prevention; Feb, 2009. Available from: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. [Google Scholar]
- 6.Thomas CP, Ryan M, Chapman JD, Stason WB, Tompkins CP, Suaya JA, et al. Incidence and cost of pneumonia in medicare beneficiaries. Chest. 2012 Oct;142:973–81. doi: 10.1378/chest.11-1160. [DOI] [PubMed] [Google Scholar]
- 7.Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012 Mar;33:250–256. doi: 10.1086/664049. [DOI] [PubMed] [Google Scholar]
- 8.Asimakopoulos G, Smith PL, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg. 1999 Sep;68:1107–15. doi: 10.1016/s0003-4975(99)00781-x. [DOI] [PubMed] [Google Scholar]
- 9.Banbury MK, Brizzio ME, Rajeswaran J, Lytle BW, Blackstone EH. Transfusion increases the risk of postoperative infection after cardiovascular surgery. J Am Coll Surg. 2006;202:131–8. doi: 10.1016/j.jamcollsurg.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Sheng W, Xing QS, Hou WM, Sun L, Niu ZZ, Lin MS, et al. Independent risk factors for ventilator-associated pneumonia after cardiac surgery. J Invest Surg. 2014 Oct;27:256–61. doi: 10.3109/08941939.2014.892652. [DOI] [PubMed] [Google Scholar]
- 11.Allou N, Bronchard R, Guglielminotti J, Dilly MP, Provenchere S, Lucet JC, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score*. Crit Care Med. 2014 May;42:1150–6. doi: 10.1097/CCM.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 12.Bonten MJ. Healthcare epidemiology: Ventilator-associated pneumonia: preventing the inevitable. Clin Infect Dis. 2011 Jan 1;52:115–21. doi: 10.1093/cid/ciq075. [DOI] [PubMed] [Google Scholar]
- 13.Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care. 2005 Jun;50:725–39. discussion 739–41. [PubMed] [Google Scholar]
- 14.Gelijns AC, Moskowitz AJ, Acker MA, Argenziano M, Geller NL, Puskas JD, et al. Cardiothoracic Surgical Trials Network (CTSN) Management practices and major infections after cardiac surgery. J Am Coll Cardiol. 2014 Jul 29;64:372–81. doi: 10.1016/j.jacc.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–10. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 16.Harrel FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Allignol A, Schumacher M, Beyersmann J. Estimating summary functionals in multistate models with an application to hospital infection data. Comput Stat. 2011;26:181–197. [Google Scholar]
- 19.Allignol A, Schumacher M, Beyersmann J. Empirical Transition Matrix of Multi-State Models: The etm Package. J Stat Softw. 2011;38:1–15. http://www.jstatsoft.org/v38/i04/ [Google Scholar]
- 20.Snappin SM, Jiang Q, Iglewitz B. Illustrating the impact of time varying covariate with an extended Kaplan-Meier estimator. The American Statistician. 2005;59:301–307. [Google Scholar]
- 21.Likosky DS, Wallace AS, Prager RL, Jacobs JP, Zhang M, Harrington SD, et al. Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative. Sources of Variation in Hospital-Level Infection Rates After Coronary Artery Bypass Grafting: An Analysis of The Society of Thoracic Surgeons Adult Heart Surgery Database. Ann Thorac Surg. 2015 Nov;100:1570–6. doi: 10.1016/j.athoracsur.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mocanu V, Buth KJ, Johnston LB, Davis I, Hirsch GM, Légaré JF. The Importance of Continued Quality Improvement Efforts in Monitoring Hospital-Acquired Infection Rates: A Cardiac Surgery Experience. Ann Thorac Surg. 2015 Jun;99:2061–9. doi: 10.1016/j.athoracsur.2014.12.075. [DOI] [PubMed] [Google Scholar]
- 23.Greco G, Shi W, Michler RE, Meltzer DO, Ailawadi G, Hohmann SF, et al. Costs associated with health care-associated infections in cardiac surgery. J Am Coll Cardiol. 2015 Jan 6;65:15–23. doi: 10.1016/j.jacc.2014.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iribarne A, Burgener JD, Hong K, Raman J, Akhter S, Easterwood R, et al. Quantifying the incremental cost of complications associated with mitral valve surgery in the United States. J Thorac Cardiovasc Surg. 2012 Apr;143:864–72. doi: 10.1016/j.jtcvs.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Munday GS, Deveaux P, Roberts H, Fry DE, Polk HC. Impact of implementation of the Surgical Care Improvement Project and future strategies for improving quality in surgery. Am J Surg. 2014 Nov;208:835–40. doi: 10.1016/j.amjsurg.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, et al. Workforce on Evidence-Based Medicine, Society of Thoracic Surgeons. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann Thorac Surg. 2007 Apr;83:1569–76. doi: 10.1016/j.athoracsur.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 27.Horvath KA, Acker MA, Chang H, Bagiella E, Smith PK, Iribarne A, et al. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg. 2013 Jun;95:2194–201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell GN, Yam PC, Tran J, Innes P, Thomas SD, Berry PD, et al. Gastroesophageal reflux and tracheobronchial contamination after cardiac surgery: should a nasogastric tube be routine? Anesth Analg. 1996 Aug;83:228–32. doi: 10.1097/00000539-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Joshi N, Localio AR, Hamory BH. A predictive risk index for nosocomial pneumonia in the intensive care unit. Am J Med. 1992 Aug;93:135–42. doi: 10.1016/0002-9343(92)90042-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.