Abstract
Objectives
High-dose vitamin D3 increases plasma total 25-hydroxyvitamin D [25(OH)D] in critically ill, ventilated patients, but the impact on plasma levels of free (non-protein bound) 25(OH)D, has not been investigated in critical illness. Moreover, the relationship of free 25(OH)D and regulation of endogenous antimicrobial peptides (AMPs) is unknown.
Research Methods & Procedures
In a double blind, randomized controlled trial, critically ill ventilator-dependent adults (n=30) received enteral vitamin D3 (250,000 or 500,000 IU total over 5 days) or placebo. Plasma was obtained serially for concentrations of free 25(OH)D, cathelicidin (LL-37), human beta-defensin-2 (hBD-2) and expression of peripheral blood mononuclear cell (PBMC) human cationic antimicrobial protein (hCAP18) mRNA. Total 25(OH)D and LL-37 concentrations and alveolar macrophage phagocytosis were determined in bronchoalveolar lavage fluid (BALF).
Results
Plasma concentrations of free 25(OH)D over time were correlated with total 25(OH)D levels (ρ=0.82, p< 0.001). The increase in free 25(OH)D was greatest with the 500,000 IU vitamin D3 dose compared to the lower dose. The percent change in mRNA expression of hCAP18 was positively associated with percent change in free 25(OH)D at day 7 and 14 (ρ=0.48, p=0.04 and ρ=0.59, p=0.03, respectively). In addition, plasma LL-37 levels correlated with the percentage of alveolar macrophages exhibiting phagocytosis (ρ=0.51, p = 0.04).
Conclusions
We found a dose-related increase in plasma free 25(OH)D levels, which was associated with increasing circulating mRNA expression of hCAP18 over time. There were no correlations between changes in total and free 25 (OH)D, and plasma LL-37 and hBD-2 concentrations. Larger studies appear warranted to determine the impact of high-dose vitamin D3 administration on endogenous AMPs.
Keywords: Antimicrobial peptides, critical care, LL-37, respiratory failure, vitamin D
INTRODUCTION
The potential benefit of vitamin D administration in critically ill patients is being investigated given the high rate of vitamin D deficiency in this patient population (1–5) and its strong association between low blood levels of total 25-hydroxyvitamin D [25(OH)D] and adverse clinical outcomes, such as increased risk of mortality (6–13). Recently, several randomized clinical trials (RCT) have reported clinical effects of administration of vitamin D in adult intensive care unit (ICU) patients (5, 14–16). Three of the recent RCTs studied various high-dose regimens of vitamin D3 (cholecalciferol) and reported some beneficial effects on secondary clinical endpoints, including mortality (15) and hospital length of stay (LOS) (5, 16), concomitant with increased total 25(OH)D levels in blood.
Vitamin D administration has been a focus for intervention because of benefits to the immune system. 1,25(OH)2D has pleotropic effects on immune cells (17), and in many studies upregulates expression of the endogenous antimicrobial peptide (AMP) cathelicidin (LL-37) in human skin, plasma, monocytes and macrophages (12, 16, 18–21). LL-37, the C-terminal peptide fragment of human cationic antimicrobial protein (hCAP18) and human beta-defensin 2 (hBD-2) (the most abundant beta-defensin in the lung) is induced in respiratory and other epithelia and immune cells in response to infection and inflammation (22–26). LL-37 and other AMPs, such as defensins, exhibit direct antimicrobial effects against microorganisms and also modulate various innate and adaptive immune functions, including chemotaxis and phagocytosis (24, 27). hBD-2 is up-regulated in epithelia during infections and/or inflammation (24, 26). Studies in humans with respiratory infections demonstrate upregulation of these AMPs in lung (28–30). Limited data suggests that 1,25(OH)2D may upregulate hBD-2 (17, 31, 32). This is relevant to studies of vitamin D administration in ICU patients because induction of hBD-2 is impaired in patients with severe sepsis (33). In addition, no studies have explored the relationship between circulating AMPs and lung macrophage phagocytosis.
In catabolic patients, some studies have associated increased blood total 25(OH)D levels with upregulated LL-37 protein and mRNA expression of hCAP18, suggesting a possible mechanistic relationship between vitamin D status and infection (1, 14, 16, 34). However, other studies in patients with infections or lung disease did not show a relationship between blood total 25(OH)D and LL-37 concentrations (35–37). Thus far, the strongest evidence supporting vitamin D administration in critically ill patients are from the large randomized controlled trial of Amrein et al (15) and a meta-analysis of vitamin D trials in ICUs by Putzu et al (38). Taken together, these data suggest that administration of high-dose vitamin D may be associated with reduced mortality in some patient subgroup (e.g. those with frank vitamin D deficiency) without improvement in other clinical outcomes; thus, vitamin D therapy in this setting remains controversial and there is a need for further investigations (15, 38). In our recent RCT in mechanically ventilated adults, high-dose vitamin D3 increased plasma total 25(OH)D levels by 2 to 3-fold, but did not alter plasma LL-37 concentrations (5).
Total 25(OH)D concentrations in blood reflects 25(OH)D tightly bound to vitamin D binding protein (DBP), the major 25(OH)D carrier protein, the rest is loosely bound to albumin or is free in circulation, defined as bioavailable 25(OH)D (39, 40). Recently, immunoassays have been developed to accurately and directly measure free (non-protein bound) 25(OH)D in human blood (39). Direct measurement of free 25(OH)D may be advantageous in ICU patients, in whom the catabolic response results in a marked decline in blood albumin concentrations and circulating levels of DBP (1,41). Thus far, no studies in ICU patients have evaluated the impact of vitamin D administration on directly measured free 25(OH)D levels or relationships to AMP expression in blood. Here we determined in critically ill adults with respiratory failure: 1) the impact of our previous high-dose regimens of vitamin D3 on free 25(OH)D concentrations, 2) the relationship of free 25(OH)D with circulating LL-37 and hBD-2, and 3) associations between plasma levels of free 25(OH)D and these AMPs to alveolar macrophage phagocytosis function.
MATERIALS AND METHODS
Trial Design
The parent RCT was approved by the Emory University Institutional Review Board (www.clinicaltrials.gov NCT01372995) and published previously (5). Written informed consent was obtained from all subjects or their legally authorized representative. Subjects were enrolled at two Emory University School of Medicine teaching hospitals, Emory University Hospital Midtown and Emory University Hospital. Full details of trial design, inclusion and exclusion criteria, safety criteria and other methodological details are provided in the previous publication (5).
Participant Selection
Briefly, major inclusion criteria were 1) age > 18 years; 2) respiratory failure requiring mechanical ventilation for at least 72 hours after study entry; and 3) anticipated stay in the ICU for at least 96 hours after entry. Major exclusion criteria were 1) use of high-dose vitamin D3 supplementation (≥ 50,000 IU a week) to treat vitamin D deficiency within the prior 6 months; 2) history of medical disorders associated with hypercalcemia, chronic renal failure requiring dialysis, cirrhosis or HIV infection; and 3) hypercalcemia (albumin-corrected serum calcium > 10.8 mg/dL or ionized calcium > 5.2 mg/dL).
Study subjects were block-randomized into the respective double-blind treatment groups according to hospital study site and Acute Physiology and Chronic Health Evaluation II score (APACHE II) score >15 or ≤ 15 (42).
Intervention
After baseline sample collection (see below), either a daily dose of 50,000 IU vitamin D3, 100,000 IU vitamin D3, or matching placebo was administered enterally via nasal tube for 5 consecutive days by the primary nurse. Thus, the two high-dose vitamin D3 regimens provided a total dose of 250,000 IU or 500,000 IU. Capsules containing 50,000 IU of vitamin D3 were manufactured from Tischon (Westbury, NY) and Biotech (Fayetteville, AR). Bioavailability testing by an independent commercial laboratory showed vitamin D3 content within ± 10% of the expected dose.
Measurements
Clinical and demographic data collection
Details on methodology used are outlined in the parent RCT publication (5).
Blood sampling
Twenty mL of venous blood for isolation of plasma was collected at baseline just prior to study drug administration and again 7 and 14 days later while subjects were still hospitalized.
Bronchoalveolar lavage (BAL) collection
Conventional methods to obtain BAL fluid (BALF) at baseline in intubated subjects were performed. BAL involved bronchoscopy and instillation of 30 mL aliquots of normal saline into a pulmonary segment followed by suction (5). Bronchoscopy was performed by either JEH or GSM in 19 of the 30 subjects.
Plasma free and total 25(OH)D
Free concentrations of 25(OH)D in serial plasma samples was measured with a competitive enzyme-linked two-step immunosorbent assay (ELISA), calibrated against a symmetric dialysis method (DIAsource ImmunoAssays, Louvain-la-Neuve, Belgium). Total 25(OH)D (representing DBP-bound, albumin-bound and free fractions) was measured using a chemiluminescent-based automated method (IDS-iSYS; Immunodiagnostic Systems, Scottsdale, AZ) (5).
Plasma LL-37 and hBD-2
ELISA kits were used to measure plasma concentrations of LL-37 (Hycult Biotech; Uden, The Netherlands) and hBD-2 (Phoenix Pharmaceuticals, Inc.; Burlingame, CA).
BALF LL-37 and total 25(OH)D
BALF was concentrated 5- to 10-fold and analyzed by ELISA for LL-37 and total 25(OH)D concentrations (5). To control for dilution by the lavage procedure, the LL-37 and total 25(OH)D concentrations were normalized using the urea method (43). Urea nitrogen was measured in the plasma and BALF supernatant by a quantitative colorimetric assay (Pointe Scientific, Canton, MI) and dilution of the BALF was calculated from ([urea]plasma/[urea]BALF) (44).
mRNA expression of hCAP18
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using cell preparation tubes containing Ficoll (Becton-Dickinson and Co., Franklin Lakes, NJ) and stored in RNA preservation solution at −80°C before RNA isolation by standard methods. Expression of hCAP18 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA in PBMCs were quantitated by real-time polymerase chain reaction (RT-qPCR). GAPDH was used as a reference gene to calculate the fold change of gene expression using the AACT method. Primers used for RT-qPCR reactions were: hCAP18;CTTCACCAGCCCGTCCTTC and CCAGGACGACACAGCGTCA, GAPDH; CTTAGCACCCCTGGCCAAG and TGGTCATGAGTCCTTCCACG. TaqMan probes were: hCAP18; 6-FAM CAG AGG ATT GTG ACT TCA MGBNFQ, GAPDH; VIC-CAT CCA TGA CAA CTT TGG TA MGBNFQ. We were unable to detect hBD-2 mRNA in PBMCs using specific primers for this defensin.
Alveolar macrophage percent positive analysis and phagocytosis index
Macrophage phagocytosis was determined using methods as previously described in humans (44). Briefly, BALF was centrifuged and total cell counts determined in the cell pellets with a hemocytometer. Diff-Quick staining (Andwin Scientific, Addison, Ill) was used to determine cellular differential counts from 300 consecutive cells. Cell pellets with <85% alveolar macrophage purity were excluded from further analysis (n=3), leaving a total of 16 subjects with macrophage phagocytosis data.
Fluorescence of phagocytized S. aureus was determined by quantitative computer analysis. Macrophages with any internalized bacteria were considered positive for phagocytosis. Phagocytosis was quantified by the percentage (%) of cells positive for phagocytosis and phagocytic index, defined the % of cells positive for phagocytosis multiplied by the mean relative fluorescence units of S. aureus per cell (44).
CALCULATIONS
Descriptive statistics were performed by treatment group. Group differences in continuous variables at baseline were assessed with analysis of variance (ANOVA) or a Kruskal-Wallis test depending on the normality of the distribution, and differences in categorical variables were assessed with a Chi2 test. Data are reported as mean (± SD) for normally distributed variables and median (IQR) for non-normally distributed variables. Differences by treatment, over time, and the treatment by time interaction (treatment × time) for free 25(OH)D were assessed with mixed-model repeated measures ANOVA with Tukey’s post-hoc analyses; normal probability plots of residuals were visually assessed to ensure normal distributions. Percent changes in variables were compared by group using the Kruskal-Wallis test. Bivariate relationships were assessed using Spearman or Pearson correlations based on the distributions of the variables. Analyses were performed with the JMP® program (version 12.0.1, SAS Institute Inc., Cary, NC) using 2-sided tests. P-values < 0.05 were considered statistically significant.
RESULTS
The CONSORT diagram, clinical outcomes and concentrations of total 25(OH)D and LL-37 in plasma over time and in baseline BALF are previously published (5). For illustration purposes, selected baseline demographic characteristics of the same subjects in this sub-study, and the baseline vitamin D and AMP endpoints from plasma and BALF are shown in Table 1. Baseline clinical, demographic indexes, baseline plasma total 25(OH)D and LL-37 concentrations were similar between groups at baseline, as previously reported (5). There were no differences between groups for baseline plasma free 25(OH)D, hBD-2 concentrations, and for PBMC hCAP18 mRNA expression (Table 1). BALF concentrations for total 25(OH)D and LL-37 concentrations obtained at baseline were also similar between groups (Table 1).
Table 1.
Baseline Demographics, 25 (OH)D and Antimicrobial Peptide Indexes
| Variables | Placebo | Vitamin D3 250,000 IU | Vitamin D3 500,000 IU | p-value |
|---|---|---|---|---|
| N=10 | N=9 | N=11 | ||
| Age (yr) | 64.8 ± 17.5 | 56.4 ± 15.4 | 68.1 ± 18.6 | 0.33 |
| Male | 6 (60%) | 5 (56%) | 8 (73%) | 0.72 |
| African American | 4 (40%) | 7 (78%) | 3 (27%) | 0.09 |
| Surgical ICU | 3 (30%) | 5 (56%) | 8 (73%) | 0.16 |
| BMI, (kg/m2) | 28.2 ± 9.9 | 33.4 ± 6.3 | 30.2 ± 6.1 | 0.62 |
| APACHE II score at entry | 19 ± 7 | 20 ± 10 | 23 ± 9 | 0.55 |
| SOFA Day 0, mean (SD) | 8.6 (4.3) | 8.9 (3.6) | 9.1 (3.1) | 0.47 |
| Plasma total 25(OH)D, (ng/mL) | 21.5 ± 1.2 | 23.2 ± 7.8 | 20.0 ± 7.3 | 0.75 |
| Plasma free 25(OH)D, (pg/mL) | 4.2 ± 2.4 | 5.8 ± 2.8 | 4.1 ± 1.5 | 0.20 |
| Plasma hBD-2, (pg/mL)* | 2212 (669, 8520) |
1489 (348, 2762) |
2310 (708, 6953) |
0.27 |
| Plasma LL-37, (ng/mL) * | 58 (37, 97) |
46 (41,77) |
58 (37, 284) |
0.42 |
| hCAP18 mRNA¥ | 1.3 (1.1, 1.6) |
1.2 (1.1, 1.4) |
1.1 (1.0, 1.3) |
0.44 |
| BALF LL-37, (ng/mL)† | 0.26 (0.19, 1.32) |
0.31 (0.17, 0.44) |
0.15 (0.12, 0.76) |
0.29 |
| BALF total 25(OH)D, (ng/mL)# | 14.6 ± 9.0 | 13.0 ± 7.0 | 10.4 ± 2.0 | 0.63 |
Data reported N (%) and as mean ± standard deviation (SD) for normal distributions, or median (25%–75% inter-quartile range) for non-normal distributions. P<0.05 = statistically significant. 25 (OH)D= 25-hydroxyvitamin D, APACHE II = Acute Physiology and Chronic Health Evaluation II score, BMI=Body Mass Index, BALF = bronchoalveolar lavage fluid; hBD-2 = human beta-defensin-2;ICU = intensive care units
placebo N=8; 250,000 IU N=9; 500,000 IU N=10
placebo N=9; 250,000 IU N=8; 500,000 IU N=7
placebo N=7; 250,000 IU N=2; 500,000 IU N=7
placebo N=7; 250,000 IU N=5; 500,000 IU N=7
Baseline plasma free 25(OH)D and total 25(OH)D concentrations
There was a strong correlation between baseline plasma free 25(OH)D and total 25(OH)D concentrations in the 30 study subjects (ρ= 0.82, p < 0.001) (Figure 1).
Figure 1. Baseline Correlation between Free and Total 25-hydroxyvitamin D (25(OH)D).
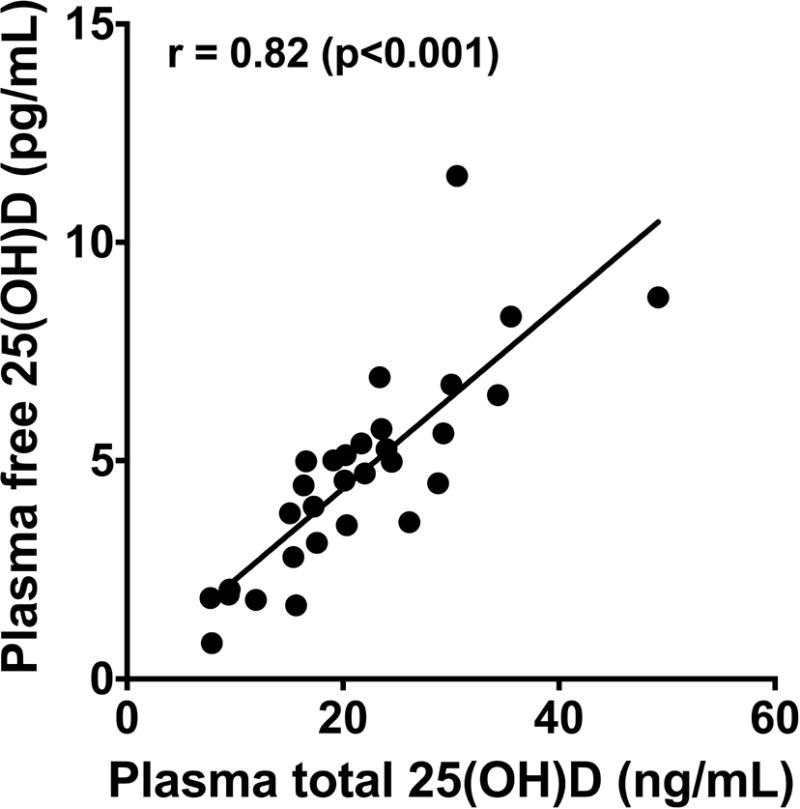
At baseline (prior to study drug administration), there was a strong positive correlation between plasma free 25(OH)D and total 25(OH)D concentrations in 30 critically ill adult patients with respiratory failure, ρ=0.82, p<0.0001. p<0.05 considered statistically significant.
High-dose vitamin D3 on plasma free 25(OH)D concentrations over time
In the three study groups, repeated measures ANOVA showed significant effects of treatment, time and treatment × time interaction on plasma free 25(OH)D concentrations (each p<0.001) (Figure 2). Free 25(OH)D levels in plasma remained unchanged in the placebo group. In contrast, both high-dose vitamin D3 regimens significantly increased plasma free 25(OH)D levels from baseline values by day 7. Free 25(OH)D levels rose further by day 14 in the group administered 500,000 IU vitamin D3. The magnitude of the plasma free 25(OH)D was dose-responsive. With the lower 250,000 IU dose, levels rose approximately 2-fold from baseline by day 7 and then decreased slightly by day 14. The plasma free 25(OH)D response to the higher 500,000 IU dose was more robust; levels rose more than 4-fold from baseline by day 7 and were nearly 5-fold greater than baseline by day 14 (Figure 2). The percent change from baseline to day 14 in free and total 25(OH)D in the three groups were highly correlated in the 19 subjects in whom plasma was available for analysis at both time points after placebo or vitamin D3 administration (Figure 3).
Figure 2. Dose-Related Effect of High-Dose Vitamin D3 Administration on Free 25(OH)D.
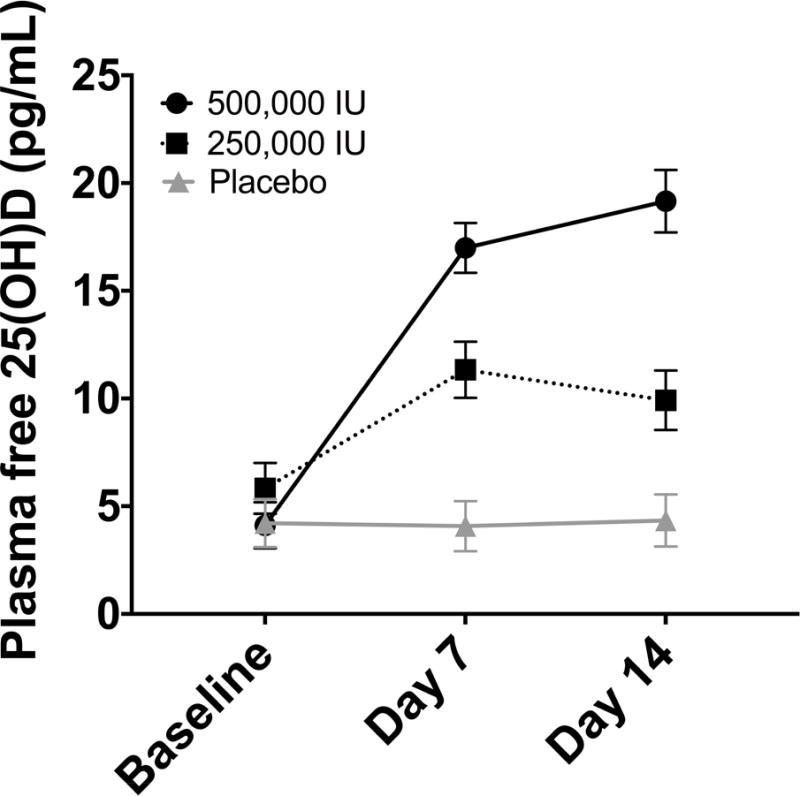
Significant effects of treatment, time and treatment × time interaction on plasma free 25(OH)D concentrations were demonstrated (each p<0.001). With the lower 250,000 IU vitamin D3 dose, plasma free 25(OH)D levels rose approximately 2-fold from baseline by day 7 and then decreased slightly by day 14. In contrast, with the 500,000 IU dose, plasma free 25(OH)D concentrations were more than 4-fold greater than baseline values by day 7 and nearly 5-fold greater by day 14.
Figure 3. Correlation of Changes in Serial Concentrations of Free and Total 25(OH) after High-dose Vitamin D3 Administration.
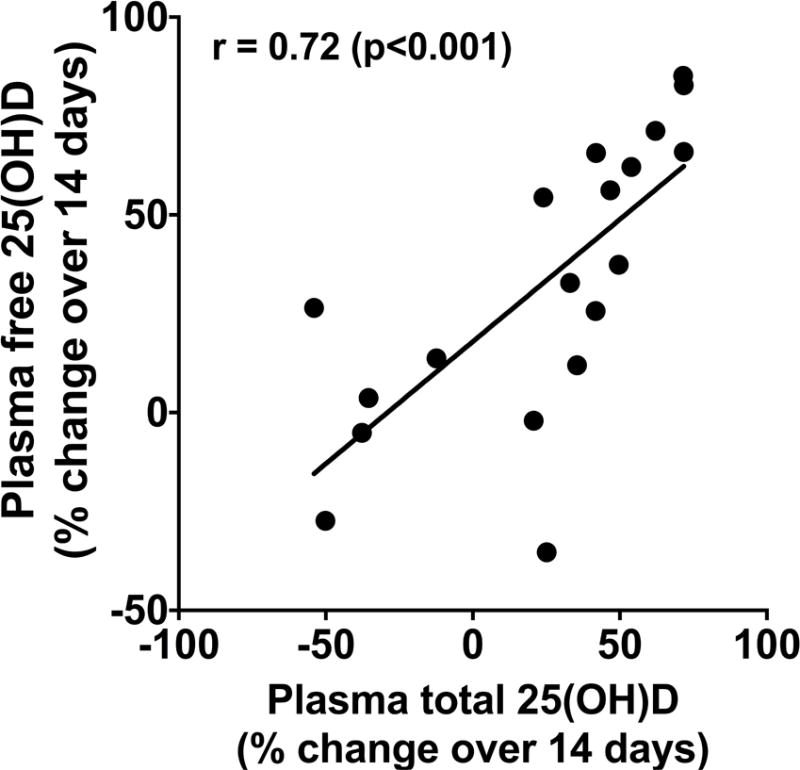
Data were derived from 19 adults with ventilator-dependent critical illness in whom plasma was available for analysis for both vitamin D indexes at baseline and 14 days after placebo or vitamin D3 administration, ρ=0.72, p<0.001.
Plasma hBD-2 and PBMC hCAP18 mRNA expression
In our previous report, we found no change over time in plasma LL-37 concentrations despite a 2-3-fold increase in plasma total 25(OH)D levels (5). Despite the marked increase in free 25(OH)D concentrations with high-dose vitamin D3 administration in this study, there was similarly no change in plasma levels of hBD-2 or hCAP18 mRNA expression from baseline between groups over time (not shown). There was also no difference between groups for percent change in values from baseline to day 7 for plasma hBD-2 concentrations and hCAP18 mRNA expression (Table 2). Similarly, there was no difference between groups for percent change in plasma LL-37 concentrations at days 7 and 14 (Table 2).
Table 2.
Percent Change from Baseline in Circulating Antimicrobial Peptide Expression
| Percent (%) change from baseline to: | Placebo | 250,000 IU D3 | 500,000 IU D3 | p-value |
|---|---|---|---|---|
| Plasma hBD-2 Day 7* |
−47.9 (−651.4, 9.8) | 18.6 (−193, 59.9) | 3.9 (−9.7, 37.8) | 0.19 |
| hCAP18 mRNA Day 7¥ |
−14.9 (−40.6, 8.3) | −2.2 (−14.0, 8.4) | 11.4 (−10.5, 15.7) | 0.16 |
| Plasma LL-37 Day 7± |
−11.5 (−34.2, 30.7) | 9.13 (−21.3, 22.3) | −22.4 (−31.3, 40.5) | 0.48 |
| Plasma LL-37 Day 14# |
0.8 (−17.9, 27.2) | −12.3 (−52.6, 25.7) | −10.8 (−33.9, 34.7) | 0.85 |
Reported as median (25%–75% inter-quartile range) analyzed using Kruskal-Wallis test. hBD-2 = human beta-defensin-2.
Placebo N=7; 250,000 IU N=7; 500,000 IU N=9
Placebo N=8; 250,000 IU N=6; 500,000 IU N=6
Placebo N=9; 250,000 IU N=7; 500,000 IU N=9
Placebo N=8; 250,000 IU N=6; 500,000 IU N=5
Correlation analysis
Spearman correlation analysis showed no statistically significant association between baseline plasma free 25(OH)D levels to plasma LL-37 (ρ=0.002, p=0.99) and plasma free 25(OH)D levels to plasma hBD-2 (ρ=−0.22, p=0.28). There was no difference in the correlation of percent change in plasma free or total 25(OH)D levels and percent change in plasma hBD-2 or plasma LL-37 levels at either days 7 or 14 (Table 3). In contrast, the percent increase in plasma free 25(OH)D, but not total 25(OH)D, was significantly associated with the percent increase in PBMC hCAP18 mRNA expression at both days 7 and day 14 (Table 3).
Table 3.
Correlations Between Changes in Total and Free 25(OH)D and Circulating Antimicrobial Peptides.
| Total 25(OH)D | Free 25(OH)D | |||
|---|---|---|---|---|
|
| ||||
| Day 7 | Day 14 | Day 7 | Day 14 | |
| Plasma hBD-2 | 0.40 (0.06) | 0.17 (0.52) | 0.32 (0.14) | −0.04 (0.88) |
| Plasma LL-37 | 0.07 (0.75) | 0.11 (0.65) | −0.10 (0.62) | −0.07 (0.76) |
| hCAP18 mRNA | 0.36 (0.14) | 0.31 (0.30) | 0.48 (0.04)* | 0.59 (0.03)* |
Reported as ρ (p-value) using Spearman’s rank test. Percent changes from baseline to day 7 and 14, in all subjects with respective values.
P<0.05=statistically significant. 25 (OH)D= 25-hydroxyvitamin D; AMP= antimicrobial peptide; hBD-2= human beta-defensin 2
We evaluated associations between vitamin D, hBD-2 and LL-37 measures and the two indices of alveolar macrophage phagocytosis function in 16 subjects with adequate BALF-derived macrophages isolated for analysis (Table 4). There were no correlations between concomitant plasma total 25 (OH)D, free 25(OH)D, plasma hBD-2, PBMC hCAP18 mRNA expression, BALF LL-37, BALF total 25(OH)D and either of the alveolar macrophage phagocytosis function indices. However, there was a significant positive correlation between plasma LL-37 concentrations and the percent positive alveolar macrophages undergoing phagocytosis (ρ= 0.51, p = 0.04), and a trend for a positive association between plasma LL-37 levels and the alveolar macrophage phagocytosis index (ρ= 0.44, p= 0.09) (Table 4).
Table 4.
Baseline Correlations Between Plasma Free and Total 25(OH)D, Antimicrobial Peptides and Alveolar Macrophage Phagocytosis
| Variable | Percent (%)of cells phagocytosis-positive | Phagocytosis Index |
|---|---|---|
| Plasma Total 25(OH)D (ng/mL), N=16 | 0.18 (0.51) | 0.23 (0.40) |
| Plasma Free 25(OH)D (pg/mL), N=16 | −0.08 (0.76) | 0.26 (0.32) |
| Plasma hBD-2 (pg/mL), N=15 | 0.37 (0.17) | 0.19 (0.49) |
| Plasma LL-37 (ng/mL), N=16 | 0.51 (0.04)* | 0.44 (0.09) |
| hCAP18 mRNA, N=13 | −0.15 (0.63) | −0.26 (0.39) |
| BALF LL-37 (pg/mL), N=9 | 0.30 (0.43) | 0.23 (0.55) |
| BALF total 25(OH)D (ng/mL), N=10 | −0.19 (0.60) | −0.14 (0.70) |
Reported as rho (p-value) using Spearman’s rank test.
P<0.05=statistically significant. BALF = bronchoalveolar lavage fluid; N= number of subjects studied
Phagocytosis index = % of cells positive for phagocytosis × mean relative fluorescence units of S. aureus per cell.
DISCUSSION
This pilot study was designed to demonstrate the impact of high-dose vitamin D3 administration (either 250,000 or 500,000 IU versus placebo) on serial plasma free 25(OH)D concentrations. At baseline (prior to vitamin D3 or placebo administration), plasma values ranged between 4.1–5.8 pg/mL, similar to serum free 25(OH)D levels in our recent report in healthy adults (4.7 pg/mL) and non-critically ill adults with cystic fibrosis (4.6–5.9 pg/mL) (39). Our three study groups were well matched at entry into the study. Baseline and change from baseline to day 14 plasma free 25(OH)D concentrations were highly correlated with total 25(OH)D levels, consistent with our recent report of serum levels in healthy and non-critically ill adults with cystic fibrosis (39). This suggests that changes in blood levels of total 25(OH)D, currently considered the best biomarker of vitamin D status (45, 46), may reflect changes in free 25(OH)D levels.
We found a robust increase in plasma free 25(OH)D levels at day 7 with both high-dose vitamin D3 regimens. A dose-response was clearly evident; these kinetic changes in free 25(OH)D plasma concentrations over time contrast with changes we found in plasma total 25(OH)D levels in the same subjects, where there was no statistically significant dose-response of plasma total 25(OH)D after vitamin D3 administration (5). Therefore, these pilot data suggest that higher doses of exogenous vitamin D3 supplementation in adult ICU patients increase free 25(OH)D to a greater extent than total 25(OH)D plasma concentrations.
A significant proportion (estimated to be 85–90%) of 25(OH)D is tightly bound to DBP. The combined albumin-bound and smaller free 25(OH)D fractions, is considered the “bioavailable” circulating 25(OH)D pool (45–47). Bioavailable 25(OH)D levels are estimates calculated using equations incorporating blood levels of total 25(OH)D, DBP and albumin that were developed in healthy subjects and thus may not be completely applicable to ICU and other types of catabolic patients. In critical illness, blood levels of both albumin and DBP may be altered due to insufficient dietary protein intake, accelerated protein breakdown, malabsorption, decreased synthesis, leakage of blood proteins into the interstitial space, changes in urinary excretion and/or fluid status, thus affecting calculated bioavailable 25(OH)D estimates (1, 41, 47–50). Further, ethnic differences in DBP affinity for 25(OH)D binding due to genetic DBP polymorphisms may obscure true vitamin D bioavailability in some subjects (39, 50).
In a pilot study similar to ours, Quraishi et al administered a one-time enteral dose of vitamin D3 (either 200,000 IU or 400,000 IU) or placebo in adults with new onset of severe sepsis or septic shock (16). Compared to baseline values, these investigators showed a modest ≈ 50% to 70% increase in plasma total 25(OH)D levels from baseline to day 5 of study with the one-time 200,000 IU and 400,000 IU vitamin D3 doses, respectively. However, no change from baseline occurred in calculated bioavailable 25(OH)D from baseline to day 5 with the lower vitamin D3 dose, while the 400,000 IU dose resulted in a significant 60% increase in calculated bioavailable 25(OH)D over this time frame (16). The reasons for the more robust response of total and free 25(OH)D over time in our study, compared to the magnitude of change in total and bioavailable 25(OH)D levels in the previous study (16) are unclear, but may be related to decreased plasma albumin and/or DBP levels in the septic patients or other differences in clinical characteristics. Also, the timing of serial blood sample collection and vitamin D3 bioavailability comparing a one-time dose by Quraishi (16) versus 5 days in the current study may have resulted in different circulating vitamin D responses.
In contrast to the reports cited earlier, some studies in critically ill patients, including our previous report, show no link between total 25(OH)D levels and rates of sepsis or infection (51, 52). This may reflect the impact of altered circulating albumin and DBP on total 25(OH)D measurements (47, 53). Although limited data suggest that free 25(OH)D levels in blood may more accurately reflect changes in vitamin D-mediated metabolic responses (46, 54), further studies are required to determine whether circulating free 25(OH)D values are superior to total 25(OH)D levels as a marker of vitamin D status and metabolic responses in ICU patients.
Given the associations between activated vitamin D and AMP expression, in particular LL-37 (17–19, 55), another goal was to explore potential relationships between high-dose vitamin D3 administration and free plasma 25(OH)D with circulating AMPs over time. We report here serial plasma levels of the major lung defensin, hBD-2, and hCAP18 mRNA expression in PBMCs. There was no difference between groups over time in hBD-2 protein, hCAP18 mRNA expression, plasma LL-37, hBD-2 or in percent change from baseline. However, the percent change from baseline in plasma free 25(OH)D concentrations was positively and significantly associated with the percent change in PBMC hCAP18 mRNA expression at both days 7 and 14 after study entry. This result lends credence to the prevailing concept that vitamin D may regulate LL-37, although these pilot data should be considered hypothesis-generating. We were also unable to detect hBD-2 mRNA in PBMCs or hBD-2 protein in BALF; this may be due to hBD-2 being primarily expressed in epithelia (24, 26), although monocytes may also express hBD-2 (17).
The AMP data in this report nonetheless complement and extend earlier data. Leaf et al (14) gave a single 2 μg intravenous dose of 1,25(OH)2D (calcitriol) to 36 adults with severe sepsis compared to 31 subjects who received placebo injections. Blood samples were obtained for LL-37 protein levels by ELISA and whole blood leukocyte LL-37 mRNA at 6, 24 and 48 h after calcitriol. No changes in LL-37 protein occurred in this acute period of observation; in contrast there was a significant (≈ 3-fold) increase in leukocyte LL-37 mRNA expression at the 24-h time point in the calcitriol-treated group (14). In the study of high-dose vitamin D3 in adult septic patients, the higher dose of 400,000 IU significantly increased LL-37 protein levels by ≈30% from baseline to day 5 (16). In that study there was no significant correlation between the change in plasma total 25(OH)D levels and the change in plasma LL-37 levels; however, there was a significant correlation between the change in calculated bioavailable 25(OH)D levels and the change in plasma LL-37 levels (16).
This pilot RCT also evaluated alveolar macrophage phagocytosis indices given that several lines of evidence link AMP expression and vitamin D status with human monocyte/macrophage phagocytosis (17, 18, 27, 56). However, surprisingly little data is available on lung macrophage phagocytosis in human critical illness (24, 44). Our group and others have previously demonstrated that alveolar macrophage function is impaired in humans with poorly controlled asthma (44, 57) and in adults with chronic obstructive lung disease prone to frequent exacerbations (58). MRNA expression of hCAP18 significantly correlated with the percentage of isolated BALF alveolar macrophages exhibiting phagocytosis. No correlation was found between plasma free 25(OH)D levels, plasma levels of hBD-2, PBMC mRNA expression of hCAP18, BALF LL-37 or BALF total 25(OH)D levels and either of the alveolar macrophage phagocytosis endpoints. Nonetheless, these hypothesis-generating data are novel and support the concept that plasma LL-37 levels may impact alveolar macrophage phagocytic function.
The major limitation of this RCT is the small number of patients enrolled, such that the overall study should be considered hypothesis-generating. Our serial endpoint measures were obtained at baseline and days 7 and 14 after entry; earlier and more frequent determination of our endpoints would provide needed insight into both vitamin D and AMP responses to our dosing regimens. As outlined in our parent RCT paper (5), we followed subjects for longer than 14 days, but the increasingly diminished subject availability over time preclude meaningful analysis of the longer-term data.
CONCLUSIONS
We here show the first data on directly measured, non-protein bound, free 25(OH)D levels in response to high-dose vitamin D3 in critically ill adult patients with respiratory failure. Additionally we relate the administration of vitamin D3 to changes in circulating anti-microbial molecules that may have an impact in critical illness infectious and inflammatory outcomes. Additionally, we find that plasma LL-37 levels may impact alveolar macrophage function. Taken together, our results suggest that additional research should focus on the impact of high-dose vitamin D regimens on clinical outcomes, coupled with changes in vitamin D availability and on anti-microbial peptide responses.
Highlights.
First study to determine free vitamin D [25(OH)D] level in critical illness.
Plasma free 25(OH)D increased with vitamin D3 treatment in a dose-response manner.
Plasma free 25(OH)D levels correlated with percent change in mRNA expression of human cationic antimicrobial protein (hCAP-18).
Plasma total 25 (OH)D did not correlate with antimicrobial peptide expression.
Plasma cathelicidin (LL-37) levels significantly correlated with alveolar macrophage phagocytosis.
Acknowledgments
We would like to acknowledge Frank Harris for his technical assistance. Statement of Authorship: Conception, design, conduct of trial, analysis, and interpretation, drafting and revising manuscript: JEH, JAA, JLJ, LAB, VT, GSM, TRZ
Conduct of trial, interpretation, drafting work of intellectual content: MAB, LH
Analysis and interpretation and drafting work of intellectual content: JEH, JAA, LAB, JLJ, VT, GSM, TRZ.
Primary Source of Funding: Supported, in part, by National Institutes of Health grants: NIH R21 HL110044 (GSM, TRZ), K24 DK096574 (TRZ), UL1 TR000454 (JEH, GSM, TRZ, VT), K01 DK102851 (JAA), T32 DK007298 (JLJ), T32 AA013528 (JEH).
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- AMP
Antimicrobial peptide
- APACHE II
Acute Physiology and Chronic Health Evaluation II score
- BALF
Bronchoalveolar lavage fluid
- DBP
vitamin D binding protein
- hBD-2
Human beta-defensin-2
- hCAP-18
human cationic antimicrobial protein
- IQR
Inter-quartile range
- LL-37
cathelicidin
- PBMC
Peripheral blood mononuclear cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Clinical Trial Registration: www.clinicaltrials.gov (NCT01372995)
References
- 1.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. Journal of translational medicine. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. The New England journal of medicine. 2009;360:1912–1914. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 3.Higgins DM, Wischmeyer PE, Queensland KM, Sillau SH, Sufit AJ, Heyland DK. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN Journal of parenteral and enteral nutrition. 2012;36:713–720. doi: 10.1177/0148607112444449. [DOI] [PubMed] [Google Scholar]
- 4.Hebbar KB, Wittkamp M, Alvarez JA, McCracken CE, Tangpricha V. Vitamin D Deficiency in Pediatric Critical Illness. Journal of clinical & translational endocrinology. 2014;1:170–175. doi: 10.1016/j.jcte.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han JE, Jones JL, Tangpricha V, Brown MA, Brown LA, Hao L, Hebbar G, Lee MJ, Liu S, Ziegler TR, Martin GS. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. Journal of clinical & translational endocrinology. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair P, Lee P, Reynolds C, Nguyen ND, Myburgh J, Eisman JA, Center JR. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive care medicine. 2013;39:267–274. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 7.Braun AB, Gibbons FK, Litonjua AA, Giovannucci E, Christopher KB. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Critical care medicine. 2012;40:63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quraishi SA, Bittner EA, Blum L, McCarthy CM, Bhan I, Camargo CA., Jr Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Critical care medicine. 2014;42:1365–1371. doi: 10.1097/CCM.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett N, Zhao Z, Koyama T, Janz DR, Wang CY, May AK, Bernard GR, Ware LB. Vitamin D deficiency and risk of acute lung injury in severe sepsis and severe trauma: a case-control study. Annals of intensive care. 2014;4:5. doi: 10.1186/2110-5820-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amrein K, Zajic P, Schnedl C, Waltensdorfer A, Fruhwald S, Holl A, Urbanic Purkart T, Wunsch G, Valentin T, Grisold A, Stojakovic T, Amrein S, Pieber TR, Dobnig H. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Critical care (London, England) 2014;18:R47. doi: 10.1186/cc13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNally JD, Menon K, Chakraborty P, Fisher L, Williams KA, Al-Dirbashi OY, Doherty DR. The association of vitamin D status with pediatric critical illness. Pediatrics. 2012;130:429–436. doi: 10.1542/peds.2011-3059. [DOI] [PubMed] [Google Scholar]
- 12.Leaf DE, Croy HE, Abrahams SJ, Raed A, Waikar SS. Cathelicidin antimicrobial protein, vitamin D, and risk of death in critically ill patients. Critical care (London, England) 2015;19:80. doi: 10.1186/s13054-015-0812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Pascale G, Vallecoccia MS, Schiattarella A, Di Gravio V, Cutuli SL, Bello G, Montini L, Pennisi MA, Spanu T, Zuppi C, Quraishi SA, Antonelli M. Clinical and microbiological outcome in septic patients with extremely low 25-hydroxyvitamin D levels at initiation of critical care. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2016;22:456.e457–456.e413. doi: 10.1016/j.cmi.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. American journal of respiratory and critical care medicine. 2014;190:533–541. doi: 10.1164/rccm.201405-0988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, Urbanic Purkart T, Waltensdorfer A, Munch A, Warnkross H, Stojakovic T, Bisping E, Toller W, Smolle KH, Berghold A, Pieber TR, Dobnig H. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. Jama. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 16.Quraishi SA, De Pascale G, Needleman JS, Nakazawa H, Kaneki M, Bajwa EK, Camargo CA, Jr, Bhan I. Effect of Cholecalciferol Supplementation on Vitamin D Status and Cathelicidin Levels in Sepsis: A Randomized, Placebo-Controlled Trial. Critical care medicine. 2015;43:1928–1937. doi: 10.1097/CCM.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Frontiers in physiology. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science (New York, NY) 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 19.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. The Journal of investigative dermatology. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 21.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, Kanada K, Yamasaki K, Alexandrescu D, Gallo RL. Administration of oral vitamin D induces cathelicidin production in atopic individuals. The Journal of allergy and clinical immunology. 2008;122:829–831. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Experimental lung research. 2007;33:537–542. doi: 10.1080/01902140701756687. [DOI] [PubMed] [Google Scholar]
- 23.Tecle T, Tripathi S, Hartshorn KL. Review: Defensins and cathelicidins in lung immunity. Innate immunity. 2010;16:151–159. doi: 10.1177/1753425910365734. [DOI] [PubMed] [Google Scholar]
- 24.Doss M, White MR, Tecle T, Hartshorn KL. Human defensins and LL-37 in mucosal immunity. Journal of leukocyte biology. 2010;87:79–92. doi: 10.1189/jlb.0609382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaumont PE, McHugh B, Gwyer Findlay E, Mackellar A, Mackenzie KJ, Gallo RL, Govan JR, Simpson AJ, Davidson DJ. Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PloS one. 2014;9:e99029. doi: 10.1371/journal.pone.0099029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hielpos MS, Ferrero MC, Fernandez AG, Bonetto J, Giambartolomei GH, Fossati CA, Baldi PC. CCL20 and Beta-Defensin 2 Production by Human Lung Epithelial Cells and Macrophages in Response to Brucella abortus Infection. PloS one. 2015;10:e0140408. doi: 10.1371/journal.pone.0140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan M, van der Does AM, Tang X, Lindbom L, Agerberth B, Haeggstrom JZ. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. Journal of leukocyte biology. 2014;95:971–981. doi: 10.1189/jlb.0513304. [DOI] [PubMed] [Google Scholar]
- 28.Ashitani J, Mukae H, Hiratsuka T, Nakazato M, Kumamoto K, Matsukura S. Plasma and BAL fluid concentrations of antimicrobial peptides in patients with Mycobacterium avium-intracellulare infection. Chest. 2001;119:1131–1137. doi: 10.1378/chest.119.4.1131. [DOI] [PubMed] [Google Scholar]
- 29.Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, Davidson DJ, Donis RO. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PloS one. 2011;6:e25333. doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cakir E, Torun E, Gedik AH, Umutoglu T, Aktas EC, Topuz U, Deniz G. Cathelicidin and human beta-defensin 2 in bronchoalveolar lavage fluid of children with pulmonary tuberculosis. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2014;18:671–675. doi: 10.5588/ijtld.13.0831. [DOI] [PubMed] [Google Scholar]
- 31.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of immunology (Baltimore, Md: 1950) 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 32.Abdelmaksood R, Hashad D. The impact of topical calcipotriol and betamethasone on human beta-defensin 2 expression and serum level in psoriatic patients. Clinical laboratory. 2013;59:277–282. doi: 10.7754/clin.lab.2012.120207. [DOI] [PubMed] [Google Scholar]
- 33.Book M, Chen Q, Lehmann LE, Klaschik S, Weber S, Schewe JC, Luepertz M, Hoeft A, Stuber F. Inducibility of the endogenous antibiotic peptide beta-defensin 2 is impaired in patients with severe sepsis. Critical care (London, England) 2007;11:R19. doi: 10.1186/cc5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, Meddings J, O’Sullivan M. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: Results from a randomised double-blind placebo-controlled study. United European gastroenterology journal. 2015;3:294–302. doi: 10.1177/2050640615572176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tangpricha V, Judd SE, Ziegler TR, Hao L, Alvarez JA, Fitzpatrick AM, McComsey GA, Eckard AR. LL-37 concentrations and the relationship to vitamin D, immune status, and inflammation in HIV-infected children and young adults. AIDS research and human retroviruses. 2014;30:670–676. doi: 10.1089/aid.2013.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamshchikov AV, Kurbatova EV, Kumari M, Blumberg HM, Ziegler TR, Ray SM, Tangpricha V. Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. The American journal of clinical nutrition. 2010;92:603–611. doi: 10.3945/ajcn.2010.29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang YM, Guo YF, Zhang HS, Sun TY. Antimicrobial peptide LL-37 circulating levels in chronic obstructive pulmonary disease patients with high risk of frequent exacerbations. Journal of thoracic disease. 2015;7:740–745. doi: 10.3978/j.issn.2072-1439.2015.04.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, Landoni G. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. Journal of critical care. 2016;38:109–114. doi: 10.1016/j.jcrc.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Lee MJ, Kearns MD, Smith EM, Hao L, Ziegler TR, Alvarez JA, Tangpricha V. Free 25-Hydroxyvitamin D Concentrations in Cystic Fibrosis. The American journal of the medical sciences. 2015;350:374–379. doi: 10.1097/MAJ.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouillon R. Free or Total 25OHD as Marker for Vitamin D Status? Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2016;31:1124–1127. doi: 10.1002/jbmr.2871. [DOI] [PubMed] [Google Scholar]
- 41.Madden K, Feldman HA, Chun RF, Smith EM, Sullivan RM, Agan AA, Keisling SM, Panoskaltsis-Mortari A, Randolph AG. Critically Ill Children Have Low Vitamin D-Binding Protein, Influencing Bioavailability of Vitamin D. Annals of the American Thoracic Society. 2015;12:1654–1661. doi: 10.1513/AnnalsATS.201503-160OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13:818–829. [PubMed] [Google Scholar]
- 43.Brown LA, Perez JA, Harris FL, Clark RH. Glutathione supplements protect preterm rabbits from oxidative lung injury. The American journal of physiology. 1996;270:L446–451. doi: 10.1152/ajplung.1996.270.3.L446. [DOI] [PubMed] [Google Scholar]
- 44.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. The Journal of allergy and clinical immunology. 2008;121:1372–1378. 1378.e1371–1373. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tangpricha V, Kelly A, Stephenson A, Maguiness K, Enders J, Robinson KA, Marshall BC, Borowitz D. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the Cystic Fibrosis Foundation. The Journal of clinical endocrinology and metabolism. 2012;97:1082–1093. doi: 10.1210/jc.2011-3050. [DOI] [PubMed] [Google Scholar]
- 46.Herrmann M, Farrell CL, Pusceddu I, Fabregat-Cabello N, Cavalier E. Assessment of vitamin D status - a changing landscape. Clinical chemistry and laboratory medicine. 2016 doi: 10.1515/cclm-2016-0264. [DOI] [PubMed] [Google Scholar]
- 47.Quraishi SA, Camargo CA., Jr Vitamin D in acute stress and critical illness. Current opinion in clinical nutrition and metabolic care. 2012;15:625–634. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler TR. Parenteral nutrition in the critically ill patient. The New England journal of medicine. 2009;361:1088–1097. doi: 10.1056/NEJMct0806956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rebholz CM, Grams ME, Lutsey PL, Hoofnagle AN, Misialek JR, Inker LA, Levey AS, Selvin E, Hsu CY, Kimmel PL, Vasan RS, Eckfeldt JH, Coresh J. Biomarkers of Vitamin D Status and Risk of ESRD. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2016;67:235–242. doi: 10.1053/j.ajkd.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, Durazo-Arvizu R, Whitehead K, Feldman HI, Leonard MB. Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations Across Genotypes. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2016;31:1128–1136. doi: 10.1002/jbmr.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ala-Kokko TI, Mutt SJ, Nisula S, Koskenkari J, Liisanantti J, Ohtonen P, Poukkanen M, Laurila JJ, Pettila V, Herzig KH. Vitamin D deficiency at admission is not associated with 90-day mortality in patients with severe sepsis or septic shock: Observational FINNAKI cohort study. Annals of medicine. 2016;48:67–75. doi: 10.3109/07853890.2015.1134807. [DOI] [PubMed] [Google Scholar]
- 52.Kempker JA, West KG, Kempker RR, Siwamogsatham O, Alvarez JA, Tangpricha V, Ziegler TR, Martin GS. Vitamin D status and the risk for hospital-acquired infections in critically ill adults: a prospective cohort study. PloS one. 2015;10:e0122136. doi: 10.1371/journal.pone.0122136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venkatesh B, Davidson B, Robinson K, Pascoe R, Appleton C, Jones M. Do random estimations of vitamin D3 and parathyroid hormone reflect the 24-h profile in the critically ill? Intensive care medicine. 2012;38:177–179. doi: 10.1007/s00134-011-2415-x. [DOI] [PubMed] [Google Scholar]
- 54.Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almas B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scandinavian journal of clinical and laboratory investigation. 2014;74:177–183. doi: 10.3109/00365513.2013.869701. [DOI] [PubMed] [Google Scholar]
- 55.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future microbiology. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephan A, Batinica M, Steiger J, Hartmann P, Zaucke F, Bloch W, Fabri M. LL37:DNA complexes provide antimicrobial activity against intracellular bacteria in human macrophages. Immunology. 2016;148:420–432. doi: 10.1111/imm.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, Wenzel SE. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. American journal of respiratory and critical care medicine. 2005;172:972–979. doi: 10.1164/rccm.200501-035OC. [DOI] [PubMed] [Google Scholar]
- 58.Berenson CS, Kruzel RL, Eberhardt E, Dolnick R, Minderman H, Wallace PK, Sethi S. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax. 2014;69:811–818. doi: 10.1136/thoraxjnl-2013-203669. [DOI] [PubMed] [Google Scholar]


