Abstract
Purpose of review
Although the liver possesses a unique, innate ability to regenerate through mass compensation, transplantation remains the only therapy when damage outpaces regeneration, or liver metabolic capacity is irreversibly impacted. Recent insight from developmental biology has greatly influenced the advancement of alternative options to transplantation in these settings.
Recent findings
Factors known to direct liver cell specification, expansion, and differentiation have been used to generate hepatocyte-like cells from stem and somatic cells for cell therapies. Additionally, interactions between hepatic epithelial and non-epithelial cells key to establishing hepatic architecture have been used in tissue engineering approaches to develop self-organizing hepatic organoids and bio-artificial liver devices. Simultaneously, recent clinically applicable advances in human hepatocyte transplantation and promotion of innate hepatic regeneration have been limited.
Summary
Although mature hepatocytes have the potential to bridge or replace whole organ transplantation, limits in the ability to obtain healthy cells, stabilize in vitro expansion, cryopreservation, and alleviate rejection, still exist. Alternative sources for generating hepatocytes hold promise for cell therapy and tissue engineering. These may allow generation of autologous or universal donor cells that eliminate the need for immunosuppression; however, limits exist regarding hepatocyte maturity and efficacy at liver repopulation, as well as applicability to human chronic liver disease.
Keywords: liver transplantation, hepatocyte, stem cell, organoid
INTRODUCTION
Since the early 1960s, liver transplantation has become a life-saving option for adults and children with end-stage liver disease. With improvement in surgical techniques, including use of partial livers and live donors, and management of post-transplant immunosuppression, the biggest obstacle in solid organ transplantation remains the limited availability of quality organs. While every other aspect of organ transplantation has evolved, there has been little to no movement in organ donation rates in the Western world in the last 50 years, and in Asia and Eastern Europe organ donation is still largely considered culturally taboo.
Advances in our understanding of liver organogenesis, biotechnology and bioengineering offer the hope of not only expanding the utility of donated organs, but also the dream of abolishing the need for donor organs by creating laboratory generated organs/organoids. Although in its infancy, when this technology fulfills its potential it could end the need for a transplant waiting list by providing size matched, genetically identical, immunologically privileged, healthy organs on-demand. Furthermore, advances in cell based therapy may eventually result in non-transplant options for treating a broad range of liver diseases and liver failure.
We will discuss current options and obstacles for maximizing available donor livers for transplantation using our current knowledge of liver organogenesis, the emerging practices that promise to expand the utility of currently available donated livers, and new technologies on the horizon for engineering livers for both clinical use and research.
REGENERATIVE CAPACITY OF THE HEPATOCYTE
The liver is alone among solid organs in its ability to regenerate full mass and function following extensive acute injury (as in acute liver failure) or partial resection (as in living donation/transplantation). The adult liver contains two main epithelial cell types, hepatocytes and cholangiocytes (i.e., bile duct epithelial cells). During development these two cell types are derived from the hepatoblast, a bipotential progenitor (1)(Figure 1A). Hepatocytes perform liver metabolic functions, including secretion of bile, and bile duct epithelial cells serve as a conduit for bile to drain from the liver as well as playing an active role in modifying bile composition (2, 3). Mouse studies using genetic reporters to trace hepatocyte lineage demonstrate that adult hepatic cell turnover is slow. Further, these studies reveal no evidence of a contribution from a non-hepatocyte stem cell during injury, suggesting that hepatocyte homeostasis does not require a rare stem cell held in reserve from development (4).
Figure 1.
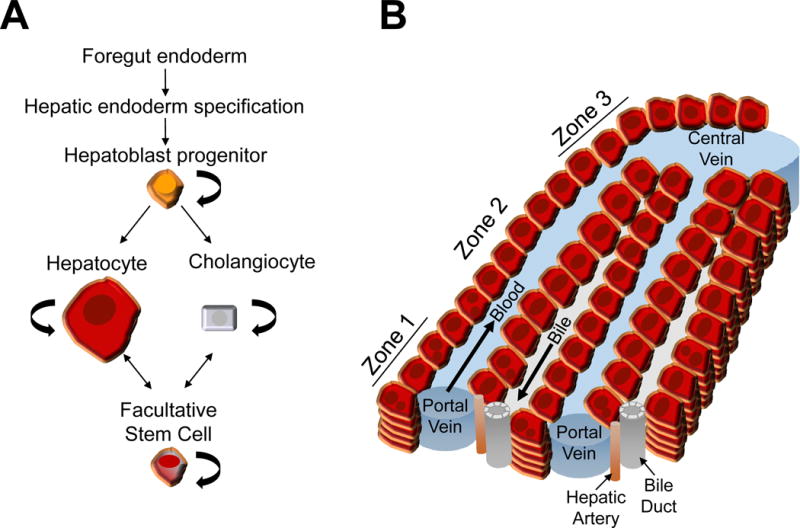
A. Developmental and regenerative cell lineage relationships of the liver.
B. Hepatic architecture and hepatocyte functional zones of the hepatic lobule. Hepatocyte cords run between portal and central veins. Bile is secreted from hepatocytes into canalicular channels and transported to the bile duct. Sinusoidal capillaries carry higher oxygenated blood supplied by the portal vein and hepatic artery past the hepatocytes toward the central vein. Hepatocytes are arranged into three zones. Zone 1 is composed of periportal hepatocytes and zone 3 is composed of pericentral hepatocytes. Zone 1 hepatocytes specialize in gluconeogenesis and urea formation, while zone 3 hepatocytes specialize in liponeogenesis, glutamine synthesis, and glycolysis.
Hepatocytes perform a wide variety of tasks, such as gluconeogenesis, urea genesis, beta-oxidation, and liponeogenesis (5). These functions are segregated between different zones of the liver functional unit (Figure 1B). Interestingly, mouse studies using refined genetic reporters to lineage trace either periportal (zone 1) or pericentral (zone 3) hepatocytes suggest that both sub-populations are involved in hepatocyte homeostasis (6, 7). Therefore, at this time, the primary hepatocyte population chosen for cell repopulation may not need to be refined. Of note, periportal hepatocytes have been shown experimentally to undergo extensive proliferation without giving rise to hepatocellular carcinoma (6).
The mechanisms which govern, and limit, liver regenerative capacity include blood born soluble growth factors and cytokines found in the portal and systemic circulation, the influence of extracellular matrix components, interactions between hepatocytes and other resident liver cells (biliary epithelium, vascular endothelium, Kuppfer cells, etc), and changes in portal vein hemodynamics (8). The complex interplay of these variables is illustrated by the fact that when mature hepatocytes are removed from the native microenvironment they lose their ability to carry out liver-specific functions and their replication is stunted. For this reason, current use of mature human hepatocytes is limited. Hepatocyte cell transplantation involves replacing approximately 5–10% of the native liver volume with human hepatocytes harvested from donor livers which, for a variety of reasons, are unsuitable for conventional orthotopic liver transplantation (9). This method, first introduced more than 15 years ago, is appropriate for situations in which the metabolic function of the liver is compromised but the hepatic scaffolding and microenvironment remain intact – including genetic defects in key enzymes (liver-based metabolic disorders such as urea cycle defects) and sudden and extreme loss of functional hepatocyte mass (acute liver failure). Replacement of metabolically active hepatocytes can either completely correct the defect or, at a minimum, serve as a bridge to conventional transplant.
This type of cell-based therapy is limited by the availability of quality livers as a source for hepatocyte isolation, the advanced technology necessary to expand and maintain harvested hepatocytes, the potential loss of functionality when human hepatocytes are cryopreserved (vs. used immediately), the need for ongoing immunosuppression following hepatocyte transplant, which carries a higher risk of rejection than an intact organ, and the reported loss of functionality of transplanted hepatocytes over time (10, 11).
Unfortunately, the same factors that dictate whether an intact liver is suitable for transplantation (ABO compatibility, donor age, presence of steatosis, infectious risk, etc) are equally relevant for livers donated for hepatocyte isolation. Thus, alternate sources of hepatocytes from liver resections, discards from split liver grafts, livers from donors after cardiac death, and neonatal livers may represent better long-term sources for primary human hepatocytes (10, 12).
ALTERNATIVE SOURCES OF FUNCTIONAL HEPATOCYTES
If the progress in primary human hepatocyte transplantation has, by virtue of its requirement for clinical trials in fragile populations, been understandably slow, the progress in identifying other sources of functional hepatocytes amenable to transplantation (cellular therapy) and tissue engineering has been the opposite. In the absence of a bona fide liver stem cell, there has been increased focus on isolation of a bipotential facultative stem cell and the generation of hepatocyte-like cells in the laboratory.
Facultative stem cell source
When hepatocyte proliferative capacity is exhausted by massive acute hepatocyte loss or indolent, chronic damage, a hypothesized facultative stem cell (FSC) population emerges (13). The origin of this postulated FSC has been a major focus to identify a potential alternative source for hepatocyte production. The FSCs are hypothesized to be quiescent cells residing in the periportal regions within terminal ductules (i.e., Canals of Hering) and when activated proliferate to produce ‘oval cells’ (14, 15). These ‘oval cells’ behave as a transient amplifying population with an intermediate phenotype expressing markers of both hepatocytes and cholangiocytes. Mouse studies using genetic reporters to lineage trace the contribution of cholangiocyte-derived FSCs to hepatocyte replacement has revealed no significant contribution in multiple chemical injury models (16–19). However, complimentary lineage tracing experiments revealed that pre-existing hepatocytes are the dominant source of newly formed hepatocytes (17, 19–23). Further, when the injury is relieved, the lineage traced ‘oval cells’ revert back to hepatocytes (20, 22). Remarkably, lineage traced hepatocytes also contributed to a limited amount of cholangiocytes (20, 22, 23). It is possible that if a therapeutic hepatocyte population is identified, it might also be able to generate cholangiocytes given the correct signals and microenvironment. Overall, these mouse studies suggest that the ‘oval cell’ population represents hepatocyte metaplasia providing a way for hepatocytes to evade hepatocyte specific damage. Alternatively, the chemical injury models used in these mouse studies may not have provided the type or degree of injury necessary to stimulate the full potential of the hypothesized FSC. Indeed, in a genetic mouse model which induced 98% hepatocyte senescence by specifically deleting Mdm2 in hepatocytes, an expansion of FSCs was observed that contributed to liver regeneration (24**). This population of FSCs can be expanded in vitro and is capable of repopulating the hepatocyte Mdm2 deficient mouse model (24**). Although this work is a big step forward in identifying an alternative to primary human hepatocytes that can be expanded in vitro and has potential in vivo clinical benefit, it is unknown whether the extreme survival selection pressure generated in this specific model is relevant to human disease. Additionally, as implied by their definition as FSCs, these populations can only be isolated from an injury condition that carries with it the risk of potential genomic alterations. From a developmental perspective, these studies suggest that the adult hepatic epithelial differentiation state is not fixed but very plastic and can change depending on the injury type or severity, again highlighting the remarkable regenerative ability of the liver.
Lgr5+ organoid source
An interesting population of hepatic clonogenic cells are marked by the winged helix transcription factor Foxl1 and the leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) (25, 26). Only upon chemical injury are Foxl1- and Lgr5-positive cells detectable by genetic reporter lineage tracing in adult mice. These cells are cholangiocyte in character, demonstrate hepatoblast-like bipotential differentiation in vitro, but have low engraftment potential. However, Huch et al., used their knowledge of isolating and expanding Lgr5+ mouse liver and human intestinal organoids to develop a culture system for expansion of genomically stable primary human hepatic epithelial cells from a biopsy (27, 28). Like Foxl1 and Lgr5 cells, these cells are cholangiocyte in character, demonstrate bipotential ability in vitro, and have very low engraftment ability. Because there is great benefit to their potential for in vitro expansion, future studies will need to fully evaluate the potential to substantially contribute to new engrafted hepatocytes.
Laboratory generated hepatocyte-like cell sources
The goal of multiple laboratories has been to generate hepatocyte-like cells from pluripotent stem, mesenchymal stem or somatic cells by differentiating them through a series of developmental cues in culture or by reprogramming them using key developmental transcriptions factors important for hepatocyte differentiation (29–35).
The most common approach to generate hepatocytes is to differentiate induced pluripotent stem cells (iPSC-Heps) derived from reprogrammed fibroblasts. The differentiation protocols use a combination of growth factors and cytokines supplied in the culture growth media that are known to induce hepatocyte differentiation during development (36–40). iPSCs are differentiated in a stepwise fashion from foregut endoderm through hepatic specification, hepatoblasts and finally to hepatocytes. Directed differentiation of iPSCs has also been used to generate cholangiocyte-like cells taking advantage of a similar approach with additions of extracellular matrices (41). Until a recent study by Carpentier et al., significant liver repopulation and detection of human serum albumin have been an issue using transplanted iPSC-Heps (42). New approaches to precondition rodent recipients by using irradiation to locally stimulate liver regeneration and providing hepatocyte growth factor to molecularly stimulate hepatocyte proliferation has proven successful in using iPSC-Heps to treat a rat model of Crigler-Najjar syndrome (43). A similar approach to generate hepatocytes bypasses the need to fully induce a pluripotent state. Instead, hepatocytes are differentiated from induced multipotent progenitor cells (iMPC-Heps) derived from fibroblasts (44). Additionally, directed differentiation protocols taking advantage of growth factors and cytokines in culture media can be used to force hepatocyte differentiation of spherical adipocyte-derived stem cells aggregates (Sci-Heps) (45). These approaches demonstrate the ability of directing the differentiation of different types of stem/progenitor cells using developmental cues in vitro and the success of up to 20% liver repopulation (33).
Direct reprogramming can also be used to generate hepatocytes from somatic or stem/progenitor cell populations (iHeps) (46, 47). Combinations of hepatic specification factors, such as HNF1, HNF4A, HNF6, FOXA3, ATF5, PROX1 and/or CEBPA, are virally expressed in fibroblasts to obtain hepatocyte-like cells within 2 to 4 weeks in culture. iHeps can readily be expanded in culture, demonstrate up to 30% liver repopulation, and improve survival from liver injury in mouse models (46, 47).
The critical barriers to successful cell therapy are obtaining sufficient cells with hepatocyte function, efficient engraftment and proliferation to achieve liver repopulation. The amount of liver repopulation to alleviate disease, potentially as low as 7.5%, may vary considerably depending on the specific disease (43). Additionally, the laboratory generated hepatocyte-like cells are incompletely differentiated in vitro and therefore need to complete differentiation once they are engrafted. The contrast of global gene expression analysis comparing primary hepatocytes versus iMPC-Heps before and after engraftment demonstrate the change in the iMPC-Heps expression profile from more fetal to resembling primary hepatocytes once engrafted (44). It is unclear whether and how efficiently different laboratory generated hepatocyte-like cells can mature once engrafted. This may explain some of the differences between human serum albumin levels and liver repopulation efficiencies of different laboratory generated hepatocyte-like cells (33). In addition, the type of pre-conditioning that has been used in animal models to maximize proliferation and repopulation may not be appropriate in human recipients, particularly the fragile populations in most need of alternative therapies.
Although the above strategies may eventually be useful for the same groups of patients as primary hepatocyte transplantation, that is, those with acute liver failure or metabolic disorders, their utility in patients with extensive hepatic fibrosis/cirrhosis with or without portal hypertension is questionable. Limited data on stem cell therapy in this setting has shown that it is feasible and may delay transplantation and decrease liver fibrosis, but long-term benefit remains to be seen, and there are ongoing concerns about tumorigenic potential (48, 49).
MAXIMIZING INNATE HEPATIC REGENERATION
Advances in our understanding of mechanisms which govern, and limit, hepatocyte regeneration have the potential to influence clinical care on multiple fronts. The ability to influence the hepatic microenvironment in vivo, to promote rapid regeneration, could be used in acute liver failure, to facilitate rapid regeneration of the native liver and avoid the need for transplantation. The same techniques might be useful to expand the current pool of donor organs by allowing the use of marginal grafts and/or lowering the minimum graft weight necessary for transplantation without increasing recipient risk (i.e., small-for-size syndrome). In order for these techniques to be clinically relevant, they will need to be rapidly employed and effective.
Although the basics of regeneration in a normal liver, as exemplified in animal partial hepatectomy models, have been described for decades, there is little clinically applicable data on how to manipulate these processes to improve or accelerate regeneration in humans, and specifically in cirrhotic livers and the setting of transplantation (50). To date, attempts to support liver regeneration in vivo have depended upon augmentation of total liver mass via partial auxiliary partial orthotopic liver transplantation in cases of acute liver failure (51). More recently, a two-stage liver transplantation method has been proposed for adults with chronic liver disease. In the first stage, a left lateral segment graft, which alone would provide insufficient liver mass and lead to small for size syndrome, is transplanted, the left lateral segment of the recipient is resected, and the right native liver is left in place to “nurse” the hyper-small graft. Weeks later, after sufficient hypertrophy of the graft, hepatectomy of the remaining native liver is completed (52**).
Another approach that has been successfully used in experimental models to help native livers regenerate is in vivo reprogramming of myofibroblasts to generate hepatocytes (53**–55). The method of using adeno-associated virus (AAV) vectors with AAV6 capsids to specifically infect myofibroblasts and induce expression of hepatic transcription factors has the potential to translate into a clinical therapy for liver fibrosis (33). This process not only produces more functional hepatocytes, but in the process reduces fibrosis. Future investment in this strategy may provide the liver just enough relief from fibrosis to help restore the native liver’s balance of hepatocyte proliferation versus damage and provide treatment of chronic liver disease without liver transplantation.
LIVER TISSUE ENGINEERING
For patients with chronic liver disease and those with hepatic malignancies, total organ replacement is a more realistic option than isolated hepatocyte transplantation. Advances in whole organ bioengineering have included the use of synthetic scaffolds, and more recently, whole-liver decellularization to create a structural and molecular environment that mimics that of the native liver and is amenable to repopulation with healthy liver cells (56). Although techniques to optimize the decellularization process have been refined, several challenges remain. These include identification of the most appropriate cell source to repopulate an entire functional liver, and the ability to do so on a scale relevant to human transplantation. Recent advances in this field have included the creation of porcine liver scaffolds repopulated with parenchymal cells and re-endothelialized vasculature that withstands in vivo perfusion (57, 58**). Creation of a long-lived and fully functional transplantable liver based on a porcine scaffold, and defining the immunologic impact of this type of organ, are future objectives.
An alternative approach to potentially overcome issues with repopulating decellularized scaffolds are tissue engineering methods that harness the inherent self-organizing response of cellular interactions during liver organogenesis. ‘Liver buds’/organoids containing iPSC-derived human hepatic endoderm (iPSC-HE), human umbilical vein endothelial cells and human mesenchymal stem cells can be produced in vitro and transplanted into extrahepatic anatomical sites (59**). Once the cells are combined in a two-dimensional culture, the complex cellular interactions direct morphogenesis into a three-dimensional organoid within 48 hours. The engineered microenvironment promotes vascularization and further hepatocyte differentiation of the iPSC-HEs (60). Transplantation of multiple organoids under the kidney capsule or into the mesentery are capable of recruiting host vasculature and effective in protecting mice from experimentally induced liver failure (60). One potential draw-back is the absence of cholangiocytes in the organoid. Therefore, the bile produced by the organoid would presumably be released into the circulation. The state of the recipient’s liver to perform excretory function may determine whether this is a confounding issue (61). Because liver organoids can be transplanted into extrahepatic sites they have the potential to overcome cell therapy obstacles for patients with chronic liver disease due to a hostile fibrotic environment and those with hepatic malignancies due to the absence of an environment post-resection for cell engraftment. Further advancement of the liver organoid system in combination with iPSC differentiation will enlighten the critical cellular and molecular mechanisms required for both liver organogenesis and potential liver replacement therapies (62).
CONCLUSION
In conclusion, the enormous recent advances in liver cell therapy and tissue engineering raise the hope that liver transplantation, as we currently know it, will someday be obsolete. Future research objectives include: translating these findings from animal models of acute liver injury to models more accurately recapitulating human end-stage liver disease, which often develops over decades, with ongoing tissue injury and fibrosis; scaling up current models to realistically replace the adult liver; and defining appropriate endpoints for clinical trials of novel liver cell therapies.
KEY POINTS.
Recent advances in hepatic epithelial cell lineage tracing tools, directed differentiation of stem cell populations, and reprogramming somatic cells have focused the search for alternative sources of functional hepatoctyes.
Understanding the mechanisms which govern and limit hepatocyte regeneration have led to maximizing innate hepatic regeneration through two-stage liver transplantation methods and in vivo reprogramming of myofibroblasts to generate hepatocytes.
Liver tissue engineering options, such as decellularized scaffolds and self-organizing organoids, composed of multiple cells types hold the potential for developing extrahepatic site transplantation procedures.
Acknowledgments
None.
Financial support and sponsorship
This work was supported in part by grants from the National Institutes of Health to S.S.H. (R01DK107553) and the Cincinnati Children’s Research Foundation.
Abbreviations
- AAV
adeno-associated virus
- FSC
facultative stem cell
- iPSC-Heps
induced pluripotent stem cell-hepatocytes
- iMPC-Heps
induced multipotent stem cell-hepatocytes
- Sci-Heps
spherical culture induced-hepatocytes
- iHeps
induced hepatocytes
- iPSC-HE
induced pluripotent stem cell-hepatic endoderm
Footnotes
Conflicts of interest
S.S.H. and K.M.C. have no conflicts of interest to report.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as ** of outstanding interest
- 1.Carpentier R, Suner RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141(4):1432–8. 8 e1–4. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maroni L, Haibo B, Ray D, Zhou T, Wan Y, Meng F, et al. Functional and structural features of cholangiocytes in health and disease. Cellular and molecular gastroenterology and hepatology. 2015;1(4):368–80. doi: 10.1016/j.jcmgh.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Hara SP, Tabibian JH, Splinter PL, LaRusso NF. The dynamic biliary epithelia: molecules, pathways, and disease. Journal of hepatology. 2013;58(3):575–82. doi: 10.1016/j.jhep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malato Y, Naqvi S, Schurmann N, Ng R, Wang B, Zape J, et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. The Journal of clinical investigation. 2011;121(12):4850–60. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cast AE, Walter TJ, Huppert SS. Vascular patterning sets the stage for macro and micro hepatic architecture. Developmental dynamics: an official publication of the American Association of Anatomists. 2015;244(3):497–506. doi: 10.1002/dvdy.24222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162(4):766–79. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524(7564):180–5. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 9.Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. Journal of internal medicine. 2012;272(3):201–23. doi: 10.1111/j.1365-2796.2012.02574.x. [DOI] [PubMed] [Google Scholar]
- 10.Oldhafer F, Bock M, Falk CS, Vondran FW. Immunological aspects of liver cell transplantation. World journal of transplantation. 2016;6(1):42–53. doi: 10.5500/wjt.v6.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell transplantation. 2012;21(1):1–10. doi: 10.3727/096368911X566208. [DOI] [PubMed] [Google Scholar]
- 12.Ibars EP, Cortes M, Tolosa L, Gomez-Lechon MJ, Lopez S, Castell JV, et al. Hepatocyte transplantation program: Lessons learned and future strategies. World journal of gastroenterology. 2016;22(2):874–86. doi: 10.3748/wjg.v22.i2.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell stem cell. 2014;14(5):561–74. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47(6):1994–2002. doi: 10.1002/hep.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30(6):1425–33. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 16.Espanol-Suner R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143(6):1564–75 e7. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Schaub JR, Malato Y, Gormond C, Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell reports. 2014;8(4):933–9. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarlow BD, Finegold MJ, Grompe M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology. 2014;60(1):278–89. doi: 10.1002/hep.27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell stem cell. 2014;15(3):340–9. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekiya S, Suzuki A. Hepatocytes, rather than cholangiocytes, can be the major source of primitive ductules in the chronically injured mouse liver. The American journal of pathology. 2014;184(5):1468–78. doi: 10.1016/j.ajpath.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Tanimizu N, Nishikawa Y, Ichinohe N, Akiyama H, Mitaka T. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM-) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. The Journal of biological chemistry. 2014;289(11):7589–98. doi: 10.1074/jbc.M113.517243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell stem cell. 2014;15(5):605–18. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes & development. 2013;27(7):719–24. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nature cell biology. 2015;17(8):971–83. doi: 10.1038/ncb3203. This study re-opens the door for a potential liver facultative stem cell compartment that is independent of the hepatocyte. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494(7436):247–50. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, et al. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes & development. 2011;25(11):1185–92. doi: 10.1101/gad.2027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160(1–2):299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–72. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 29.Alejandra MR, Juan AB, Ana SR. Cell therapy for liver diseases: current medicine and future promises. Expert review of gastroenterology & hepatology. 2015;9(6):837–50. doi: 10.1586/17474124.2015.1016913. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Science translational medicine. 2014;6(245):245sr2. doi: 10.1126/scitranslmed.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantz T, Sharma AD, Ott M. Concise review: cell therapies for hereditary metabolic liver diseases-concepts, clinical results, and future developments. Stem cells. 2015;33(4):1055–62. doi: 10.1002/stem.1920. [DOI] [PubMed] [Google Scholar]
- 32.Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. Journal of hepatology. 2015;62(1 Suppl):S157–69. doi: 10.1016/j.jhep.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 33.Rezvani M, Grimm AA, Willenbring H. Assessing the therapeutic potential of lab-made hepatocytes. Hepatology. 2016;64(1):287–94. doi: 10.1002/hep.28569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiota G, Itaba N. Progress in stem cell-based therapy for liver disease. Hepatology research: the official journal of the Japan Society of Hepatology. 2016 doi: 10.1111/hepr.12747. [DOI] [PubMed] [Google Scholar]
- 35.Sokal EM. Treating inborn errors of liver metabolism with stem cells: current clinical development. Journal of inherited metabolic disease. 2014;37(4):535–9. doi: 10.1007/s10545-014-9691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannan NR, Segeritz CP, Touboul T, Vallier L. Production of hepatocyte-like cells from human pluripotent stem cells. Nature protocols. 2013;8(2):430–7. doi: 10.1038/nprot.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loh KM, Ang LT, Zhang J, Kumar V, Ang J, Auyeong JQ, et al. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell stem cell. 2014;14(2):237–52. doi: 10.1016/j.stem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma X, Duan Y, Tschudy-Seney B, Roll G, Behbahan IS, Ahuja TP, et al. Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem cells translational medicine. 2013;2(6):409–19. doi: 10.5966/sctm.2012-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. The Journal of clinical investigation. 2010;120(9):3127–36. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghanekar A, Kamath BM. Cholangiocytes derived from induced pluripotent stem cells for disease modeling. Current opinion in gastroenterology. 2016;32(3):210–5. doi: 10.1097/MOG.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 42.Carpentier A, Tesfaye A, Chu V, Nimgaonkar I, Zhang F, Lee SB, et al. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. The Journal of clinical investigation. 2014;124(11):4953–64. doi: 10.1172/JCI75456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Li Y, Wang X, Zhang W, Sauer V, Chang CJ, et al. Amelioration of Hyperbilirubinemia in Gunn Rats after Transplantation of Human Induced Pluripotent Stem Cell-Derived Hepatocytes. Stem cell reports. 2015;5(1):22–30. doi: 10.1016/j.stemcr.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, et al. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508(7494):93–7. doi: 10.1038/nature13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu D, Nishimura T, Zheng M, Wu M, Su H, Sato N, et al. Enabling autologous human liver regeneration with differentiated adipocyte stem cells. Cell transplantation. 2014;23(12):1573–84. doi: 10.3727/096368913X673432. [DOI] [PubMed] [Google Scholar]
- 46.Du Y, Wang J, Jia J, Song N, Xiang C, Xu J, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell stem cell. 2014;14(3):394–403. doi: 10.1016/j.stem.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell stem cell. 2014;14(3):370–84. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Qi X, Guo X, Su C. Clinical outcomes of the transplantation of stem cells from various human tissue sources in the management of liver cirrhosis: a systematic review and meta-analysis. Current stem cell research & therapy. 2015;10(2):166–80. doi: 10.2174/1574888x09666141112114011. [DOI] [PubMed] [Google Scholar]
- 49.Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016 doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 50.Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nature reviews Gastroenterology & hepatology. 2016;13(8):473–85. doi: 10.1038/nrgastro.2016.97. [DOI] [PubMed] [Google Scholar]
- 51.Rela M, Kaliamoorthy I, Reddy MS. Current status of auxiliary partial orthotopic liver transplantation for acute liver failure. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2016;22(9):1265–74. doi: 10.1002/lt.24509. [DOI] [PubMed] [Google Scholar]
- 52**.Scatton O, Cauchy F, Conti F, Perdigao F, Massault PP, Goumard C, et al. Two-stage liver transplantation using auxiliary laparoscopically harvested grafts in adults: Emphasizing the concept of “hypersmall graft nursing”. Clinics and research in hepatology and gastroenterology. 2016 doi: 10.1016/j.clinre.2016.03.002. This is the first description of a new model in auxiliary liver transplant, in which a portion of the diseased liver is left in place to “nurse” the liver graft Effective use of this model could expand the pool of potential livers for transplantation by significantly decreasing the necessary graft to recipient ratio. [DOI] [PubMed] [Google Scholar]
- 53**.Rezvani M, Espanol-Suner R, Malato Y, Dumont L, Grimm AA, Kienle E, et al. In Vivo Hepatic Reprogramming of Myofibroblasts with AAV Vectors as a Therapeutic Strategy for Liver Fibrosis. Cell stem cell. 2016;18(6):809–16. doi: 10.1016/j.stem.2016.05.005. This study is the first demonstration of in vivo reprogramming myofibroblasts into hepatoyctes using adeno-associated virus vectors expressing hepatocyte transcription factors. This strategy has the potential to be clinically translated into a therapy for liver fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, et al. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell stem cell. 2016;18(6):797–808. doi: 10.1016/j.stem.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Zhu S, Wang H, Ding S. Reprogramming fibroblasts toward cardiomyocytes, neural stem cells and hepatocytes by cell activation and signaling-directed lineage conversion. Nature protocols. 2015;10(7):959–73. doi: 10.1038/nprot.2015.059. [DOI] [PubMed] [Google Scholar]
- 56.Peloso A, Dhal A, Zambon JP, Li P, Orlando G, Atala A, et al. Current achievements and future perspectives in whole-organ bioengineering. Stem cell research & therapy. 2015;6:107. doi: 10.1186/s13287-015-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hussein KH, Park KM, Kang KS, Woo HM. Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta biomaterialia. 2016;38:82–93. doi: 10.1016/j.actbio.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 58**.Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials. 2015;40:72–9. doi: 10.1016/j.biomaterials.2014.11.027. This study is the first to describe a bioengineered porcine liver that withstands the sheer forces of vascular flow in vivo without thrombosis or obvious early vascular injury. [DOI] [PubMed] [Google Scholar]
- 59**.Shinozawa T, Yoshikawa HY, Takebe T. Reverse engineering liver buds through self-driven condensation and organization towards medical application. Dev Biol. 2016 doi: 10.1016/j.ydbio.2016.06.036. This manuscript highlights the biophysical properties used by self-organizing organ buds to incorporate multiple cells types This is a step forward to model aspects of hepatic organogenesis and disease Additionally, these organoids hold the potential for developing extrahepatic site transplantation procedures. [DOI] [PubMed] [Google Scholar]
- 60.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–4. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 61.Hoppo T, Komori J, Manohar R, Stolz DB, Lagasse E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology. 2011;140(2):656–66 e2. doi: 10.1053/j.gastro.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rashid T, Takebe T, Nakauchi H. Novel strategies for liver therapy using stem cells. Gut. 2015;64(1):1–4. doi: 10.1136/gutjnl-2014-307480. [DOI] [PubMed] [Google Scholar]


