Abstract
Background
Inflammation within the central nervous system (CNS) is frequently comorbid with anxiety. Importantly, the pro-inflammatory cytokine most commonly associated with anxiety is IL-1β. The bioavailability and activity of IL-1β is regulated by caspase-1-dependent proteolysis vis-a-vis the inflammasome. Thus, interventions regulating the activation or activity of caspase-1 should reduce anxiety especially in states that foster IL-1β maturation.
Methods
Male C57BL/6j, C57BL/6j mice treated with the capase-1 inhibitor biotin-YVAD-cmk, caspase-1 knockout (KO) mice and IL-1R1 KO mice were fasted for 24 hours or allowed ad libitum access to food. Immediately after fasting, caspase-1 activity was measured in brain region homogenates while activated caspase-1 was localized in the brain by immunohistochemistry. Mouse anxiety-like behavior and cognition were tested using the elevated zero maze and novel object/object location tasks, respectively.
Results
A 24 h fast in mice reduced the activity of caspase-1 in whole brain and in the prefrontal cortex, amygdala, hippocampus, and hypothalamus by 35%, 25%, 40%, 40%, and 40% respectively. A 24 h fast also reduced anxiety-like behavior by 40% and increased novel object and object location recognition by 21% and 31%, respectively. IL-1β protein, however, was not reduced in the brain by fasting. ICV administration of YVAD decreased caspase-1 activity in the prefrontal cortex and amygdala by 55%, respectively leading to a 64% reduction in anxiety like behavior. Importantly, when caspase-1 KO or IL1-R1 KO mice are fasted, no fasting-dependent reduction in anxiety-like behavior was observed.
Conclusions
Results indicate that fasting decrease anxiety-like behavior and improves memory by a mechanism tied to reducing caspase-1 activity throughout the brain.
Keywords: IL-1β, Caspase-1, Anxiety, Fasting, Calorie Restriction, Neuroinflammation
Introduction
In the cell, the cysteine-dependent protease caspase-1 is critical to the maturation of pro-IL-1β to its secretable form [1, 2]. In order for caspase-1 to become active, it requires scaffolding within the multi-protein containing inflammasome [3]. Canonically, activation/danger signals are sensed by the Nod-like receptor (NLR) family or the absent in melanoma 2-like (AIM2) receptors. The most well studied of these caspase-1 activators being NLRP3 (NOD-, LRR, and pyrin domain-containing 3), which is normally in an auto-repressed state [4]. Relief of LRR-mediated auto-repression exposes the central nucleotide-binding domain (NBD) and the N-terminal pyrin domain (PYD). NLRP3 oligomerization via a homotypic NBD interaction results in the recruitment of the PYD and caspase recruitment domain (CARD) containing adapter protein (ASC) that then recruits pro-caspase-1 [5, 6]. Clustering of pro-caspase-1 in the assembled inflammasome results in auto-processing-dependent activation of caspase-1 and cleavage/maturation of pro-IL-1β [7, 8].
The pro-inflammatory cytokine IL-1β is a well-recognized anxiogenic [9–11]. Most work examining the behavioral effects of IL-1 are developed from the sickness behavior paradigm in which infection, often modelled by peripheral lipopolysaccharide (LPS) administration, induces brain-based production of IL-1 [12]. While agreement exists as to the importance of brain-generated IL-1 to certain types of behavioral dysfunction, the mechanisms producing IL-1β in the brain are not clearly understood. IL-1β is normally present in the brain, but the appearance of IL-1β is generally tied to inflammation-inducing conditions [13]. In support, pharmacological and genetic therapies targeting the IL-1 arm of the immune system are proven effective at treating some neurodegenerative conditions. In contrast, adversely impacting brain-based IL-1β concentration or signaling appears to disrupt memory in animals without disease, implicating a role for IL-1β in the healthy state [14, 15].
As a group, anxiety disorders are among the most common mental illnesses suffered by the general population [16]. The etiology of anxiety disorders is not known, but both genetic and environmental stressors appear to contribute [17–19]. Increasingly, inflammatory conditions, including childhood obesity, adult obesity, metabolic syndrome and type 2 diabetes (T2D) are co-morbid with anxiety disorders [20–22]. Since low-grade systemic inflammation is a key feature of the diabetes disease spectrum, it is hypothesized that inflammation is important to the psychophysiology of anxiety disorders. In fact, various SNPs in genes coding for both IL-1β and IL-1R2 have been linked to increases in both state and trait anxiety [23]. IL-1β gene polymorphisms also affect an individual’s response to stress events to predispose individuals to anxiety and depression disorders [24] underscoring the importance of IL-1β to anxiety and mood disorders in humans. Given that fasting is known to reduce some of the sickness symptoms associated with the peripheral administration of IL-1β [25], we sought to show, that in the healthy state, fasting has a beneficial impact on anxiety, and this is mechanistically tied to inhibition of IL-1β priming and activation within the brain. The translational rational for this idea is that calorie restriction in humans, whether achieved through intermittent fasting (IF), alternate-day fasting or starvation, is associated with self-perceived improvements in well-being.
1. Methods
2.1 Materials
All reagents were purchased from Sigma-Aldrich (St Louis, MO).
2.2 Animals
Animal use was performed according to protocols approved by the Institutional Animal Care and Use Committee at the University of Illinois. Wild-type C57BL/6J (WT), interleukin (IL)-1 Receptor 1 (IL1R1), and Caspase-1 (Casp-1) KO mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in-house. Mice were provided food and water ad libitum in standard shoebox cages unless otherwise stated. Animals were group housed (up to 8 per cage), then moved to individual housing the day prior to testing unless noted otherwise. Housing temperature (72 °F) and humidity (45–55%) were controlled, and a 12/12h reversed dark-light cycle (2200–1000 h) was maintained. Animal behavior was video recorded using a Sony HDR-XR500V Night Shot capable video camera (Tokyo, Japan). All mice used were males, between 9 and 15 weeks of age. All behavior testing was completed under red lights in the dark cycle unless otherwise indicated. 283 mice were used in total.
2.3 Fasting, refeeding, body weight, and food intake
As we have described [26], 2 days prior to fasting, mice were individually housed. Fasting was initiated by placing mice in a new cage without food but with ad libitum water. Mice were fasted for 24 h beginning within 1 hour of the beginning of the dark cycle. Fed mice were treated identically to fasted mice but were provided ad libitum access to food (fed mice). For refeeding, fasted mice were provided ad lib access to food immediately following the 24 hour fasting period, and fed mice were continued on ad lib feeding. Mouse weight was recorded at the time points indicated using an Ohaus Adventurer Pro digital scale (Parsippany, NJ). Food weight was measured from petri dishes placed inside mouse cages.
2.4 Locomotion
Spontaneous locomotor activity was measured as we have previously described [26, 27]. Mice were video recorded in modified home cages for the entirety of the active cycle using a Sony Night Shot capable video camera (Minato-ku, Tokyo) Distance moved was quantified using Noldus Information Technology EthoVision XT 7 automated tracking software (Leesburg, VA).
2.5 Injectables
Biotin-YVAD-cmk (YVAD) was obtained from AnaSpec (Fremont, CA). Intracerebroventricular (ICV) YVAD was administered at a dose of 150 ng/mouse.
2.6 Determination of caspase-1 activity
Caspase-1 activity was determined as we have previously described [28]. Briefly, mice we euthanized with CO2 then transcardially perfused with ice cold PBS. Brains were removed and brain regions were excised using a Mouse Brain Slicer (Zivic Insttruments, Pittsburgh, PA). Samples were immediately placed in liquid nitrogen and then freeze fractured using a Qiagen Tissuelyser II (Valencia, CA) in a buffer containing 50 mM CaCl, 10% glycerol, 1 mM DTT, 1 mM EDTA, 1 mM bestatin, 1 mM pepstatin, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride and 50 mM HEPES, pH 7.4. Lysates were clarified at 16,000g for 15min at 4°C and the supernatant protein concentrations determined using the Bio-Rad DC Protein Assay (Hercules, CA) and a BioTek ELx800 Absorbance Microplate Reader (Winooski, VT). Supernatant protein concentrations were normalized with reaction buffer. Caspase-1 activity was determined colorimetrically in the clarified lysates using the caspase-1 substrate Ac-YVAD-p-nitroaniline (p-NA) (Enzo Life Science, Farmingdale, NY) at a final concentration of 4mM. Substrate incubation was at 37 °C for the times indicated. Moles of p-NA liberated were determined by a standard curve ranging from 0.075 to 0.3 mM p-NA (Enzo Life Science, Farmingdale, NY). Caspase-1 activity was calculated as (Δ[p-NA]/Δ time)/(total protein). Results are expressed as nM p-NA/min/μg of protein.
2.7 ICV Surgeries
As we have described [29, 28] mice were deeply anesthetized with an IP injection of sodium ketamine hydrochloride (80mg/mL)/xylazine (12mg/mL) solution. The top of the skull was shaved to remove fur and animals were then placed in a David Kopf Instruments stereotaxic device (Tujunga, CA). Surgical site was swabbed three times with betadine solution and then with 70% ethanol. A small hole was made at 0.6 mm posterior and 1.5 mm lateral to the bregma. For behavioral experiments a Plastics One mouse-specific brain infusion cannula was inserted and fixed to the skull with Plastics One cyanoacrylate gel adhesive and protected by a Plastics One guard. Mice were allowed to recover a minimum of three days before experimentation.
2.8 Activated caspase-1 labeling
Following fasting, mice were deeply anesthetized with ketamine/xylazine (80mg/kg/12mg/kg) then were injected with 100 ng of biotin-YVAD-cmk ICV using a microinjection unit attached to a Kopf stereotaxic. 2 Hours following biotin-YVAD-cmk injection mice were euthanized using CO2 and perfused with ice cold PBS followed by 4% paraformaldehyde. Brains were sectioned at 1.5 and 3.5 mm from the interaural coronally using a Mouse Brain Slicer (Zivic Insttruments, Pittsburgh, PA). Slices were fixed in 4% paraformaldehyde for 24 hours then paraffin embedded. 5μm slices from the two sections were blocked with hydrogen peroxide (Peroxidazed (PX968)) and labeled with SS HRP Label (4+ Strep HRP Label (HP604)), stained with liquid 3.3′-diaminobenzidine (DAB) chromogen (IP FLX DAB (IPK5010)), counterstained with hematoxylin (Cat Hematoxylin (CATHE)) and mounted and cover-slipped with the BioCare IntelliPATH. The entire slide was imaged at 40x with a NanoZoomer 2.0-HT (Hamamatsu, Bridgewater, NJ). Area of staining was measured by color thresholding in ImageJ (National Institute of Health) using a macro (sup figure. 1.) by a blinded observer.
2.9 Quantitative PCR (qPCR)
As we have described [27], animals were transcardially perfused with 30 mL of ice cold PBS and RNA isolated from homogenized tissues using Qiagen RNeasy Lipid Tissue Mini Kits (Valencia, CA). RNA was reverse transcribed using the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit. The TaqMan Gene Expression primers used were [IL-1β: mm00434228_m1, caspase-1: mm00438023_m1, nlrp3: mm00840904_m1, pycard: mm00445747_g1]. qPCR was performed on a Applied Biosystems 7900 HT Fast Real-Time PCR System using Applied Biosystems TaqMan Universal PCR Master Mix. To compare gene expression, a parallel amplification of endogenous RPS3 (Mm00656272_m1) was performed. Relative quantitative evaluation of target gene to RPS3 was performed by comparing ΔCts, where Ct is the threshold concentration.
2.10 Quantification of IL-1β protein
Regional IL-1β was determined colorimetrically using a Quantikine ELISA kit from R&D Systems. The minimum detectable level was measured to be 0.46–4.8 pg/mL. Brain regions from 3 mice were pooled and frozen in liquid nitrogen, then freeze fractured in a buffer containing 50 mM CaCl, 10% glycerol, 1 mM DTT, 1 mM EDTA, 1 mM bestatin, 1 mM pepstatin, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride and 50 mM HEPES, pH 7.4. Lysates were clarified at 16,000xg for 15 min. Supernatant protein concentrations were determined using the Bio-Rad DC Protein Assay (Hercules, CA). IL-1β was determined in 50 uL of supernatant. Data is expressed as pg/mg total protein.
2.11 Elevated Zero Maze
As previously described [30], a zero maze consisting of two walled arms (14 cm) and 2 open arms was under white light illumination. Mice were placed in one of the high walled arms to begin testing and video recorded for 5 min. Time spent in the non-high wall area (open arm) was quantified using Noldus Information Technology EthoVision XT 7 automated tracking software (Leesburg, Va). Data is expressed as % time in the open arms.
2.12 Novel Object Recognition
Novel object recognition was examined as previously described [28, 31]. Mice were placed in a rat cage-sized arena (26 cm × 48 cm × 21 cm) containing two identical objects on opposite ends of the arena (training). After 30 minutes, objects were removed and cleaned and sanitized with ethanol. Testing was initiated 2, 24, and 48 hours following the end of training. During testing, two objects were replaced in the cage, one seen during training (familiar object) and one unfamiliar object (novel object). A new novel object was introduced during each testing period. Object investigation was video recorded for 5 min and evaluated by a blinded observer. Novel and familiar object % investigation was calculated by dividing the time spent examining each object by the total object investigation time.
2.13 Object Location Recognition
Novel object location recognition was assessed in a method adapted from previously described novel object recognition test [28, 31]. Briefly, mice were placed in a large rat-sized cage (26 cm × 48 cm × 21 cm) arena with spatial cues marked on three sides. During the training phase, two identical objects were presented to the animal on one side of the arena. After 30 minutes of training, the objects were removed from the testing arena and sanitized with ethanol. Testing was initiated 2, 24, and 48 hours following the end of the training phase. During testing, one of the objects was placed in the same location as during training (familiar location), and the other object was placed on the opposite end of the arena (novel location). A new novel location was chosen for each testing period. Investigation was video recorded for 5 min and evaluated by a blinded observer. Novel and familiar location % investigation was calculated by dividing the time spent examining each location by the total investigation time to determine the degree to which the mouse investigated the novel location.
2.14 Statistics
Data are expressed as mean ± SEM. Analysis was conducted using Sigma Plot 11.2 (Systat Software, Chicago, IL). To test for statistical differences, one-way and two-way ANOVAs were used as indicated. Novel object recognition and object location recognition were analyzed using one-sample t-test comparing novel object preference to a chance level of 0.50. Data were transformed where necessary to attain normality or equal variance. Post-hoc comparison used Tukey’s test. Statistical significance was determined as p<0.05.
2. Results
3.1 Fasted mice weigh less, eat more, but move the same distance as fed mice
Mice were examined after a 24 h fast and then 24 h after being refed. Table 1 shows that when mice are fasted (0–24 h) they are 19% lighter than mice allowed ad libitum access to food. During the period of no food (0–24 h), fasted mice moved a similar distance when compared to fed mice. During the refeeding period (24–48 h), fasted mice ingested 19% more food and gained 17% more weight. Locomotor activity was similar in fasted and fed mice during the refeeding period (24–48 h).
TABLE 1.
Body weight (g), Body weight change (% loss), Food intake (g), Locomotor activity (m)
| 0–24 Hours | 24–48 Hours | |||
|---|---|---|---|---|
|
| ||||
| Fed | Fast | Fed | Fast | |
| Body weight | 27.41 ± 0.94 | 22.30 ± 0.57* | 26.99 ± 0.94 | 25.65 ± 0.77 |
| Body Weight Change | 0.75 ± 1.0 | 16.93 ± 0.77* | 1.58 ± 0.71 | −15.085 ± 1.22* |
| Food Intake | 4.57 ± 0.62 | 0 ± 0.0* | 5.22 ± 0.30 | 6.23 ± 0.35* |
| Locomotor Activity | 55.97 ± 6.11 | 67.23 ± 5.68 | 54.77 ± 6.20 | 52.97 ± 3.53 |
Results are expressed as mean ± SEM, n=7–8.
p<0.05 between fed and fast groups
2.2 Fasting reduces caspase-1 activity in the brain
With respect to neuroimmunity, calorie restriction appears to be anti-inflammatory [25, 26] providing resistance to sickness symptoms generated by exposure to LPS [32]. To determine if fasting impacted caspase-1, enzymatic activity was measured in mouse whole brain and brain regions after a 24 h fast. Fig.1 A shows that fasting reduced caspase-1 activity by 35% (nM/min/μg protein fed vs. fasted, 1.09 ± 0.12 vs 0.71 ± 0.09, p<0.001) in whole brain and by 25% (nM/min/μg protein fed vs. fasted, 0.733 ± 0.06 vs. 0.49 ± 0.07, p=0.016), 40% (nM/min/μg protein fed vs. fasted, 0.65 ± 0.14 vs. 0.33 ± 0.06), 42% (nM/min/μg protein fed vs. fasted, 0.44 ± 0.09 vs. 0.23 ± 0.04, p=0.49) and 38% (nM/min/μg protein fed vs. fasted, 1.32 ± 0.14 vs. 0.75 ± 0.14, p=0.007) in prefrontal cortex, amygdala, hippocampus and hypothalamus, respectively. To better understand the morphological location of caspase-1 activity in the brain, activated caspase-1 was labeled in fed and fasted mice. Fig.1 B demonstrates, in a representative fashion, where caspase-1 activity is localized regionally. Fig.1C shows that fasting reduced active caspase-1 labeling by 71% (% area stained/100 cells fed vs. fasted, 3.48 ± 0.71 vs. 0.96 ± 0.44), 88% (% area stained/100 cells fed vs. fasted, 2.68 ± 0.13 vs. 0.38 ± 0.06), 66% (% Area stained/100 cells fed vs. fasted 1.61 ± 0.48 vs. 0.38 ± 0.13) and 55% (% Area stained/100 cells fed vs. fasted, 2.22 ± 0.22 vs. 1.08 ± 0.33) in prefrontal cortex, amygdala, hippocampus and hypothalamus, respectively. Table 2 shows that fasting significantly reduced IL-1β gene expression in whole brain and in prefrontal cortex, amygdala and hypothalamus (Table 2). No change was seen in gene expression for caspase-1 or NLRP3. A decrease in pycard expression was observed in the prefrontal cortex and in the hypothalamus of fasted mice. Fig. 1D shows no differences in IL-1β protein levels between fed and fasted mice.
Figure. 1. Fasting reduces caspase-1 activity in the brain.
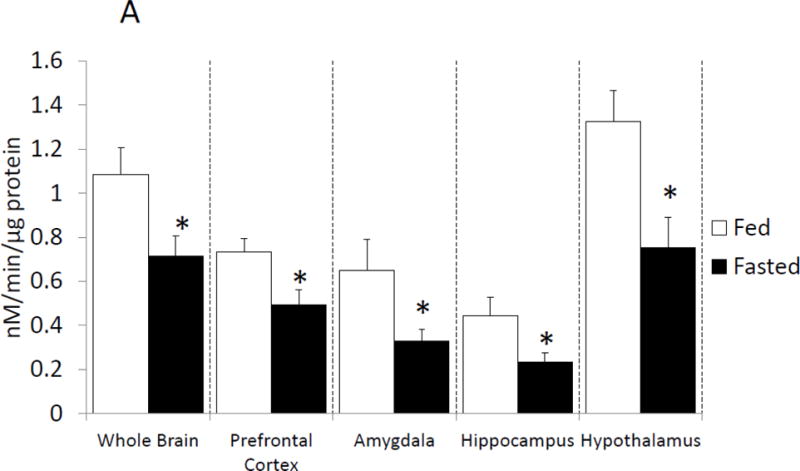
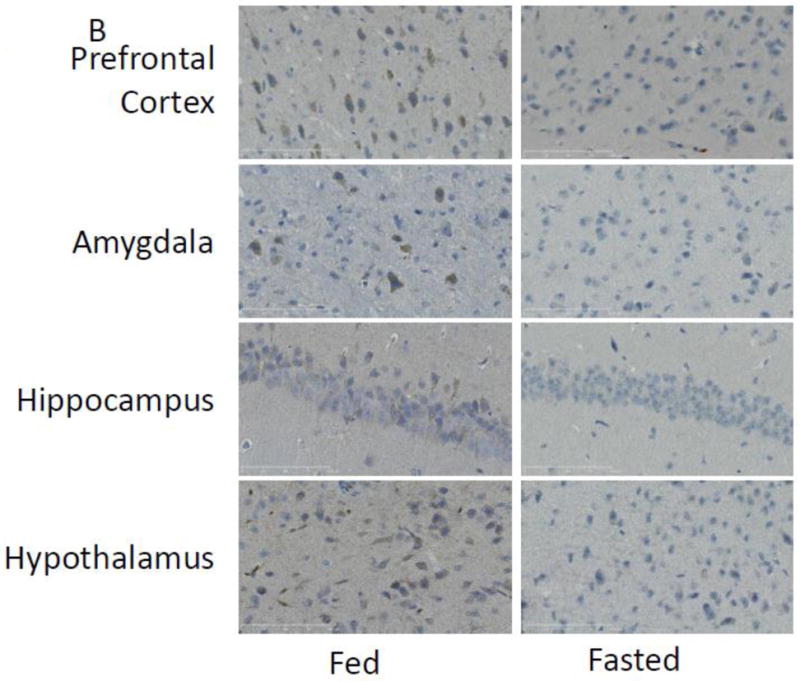
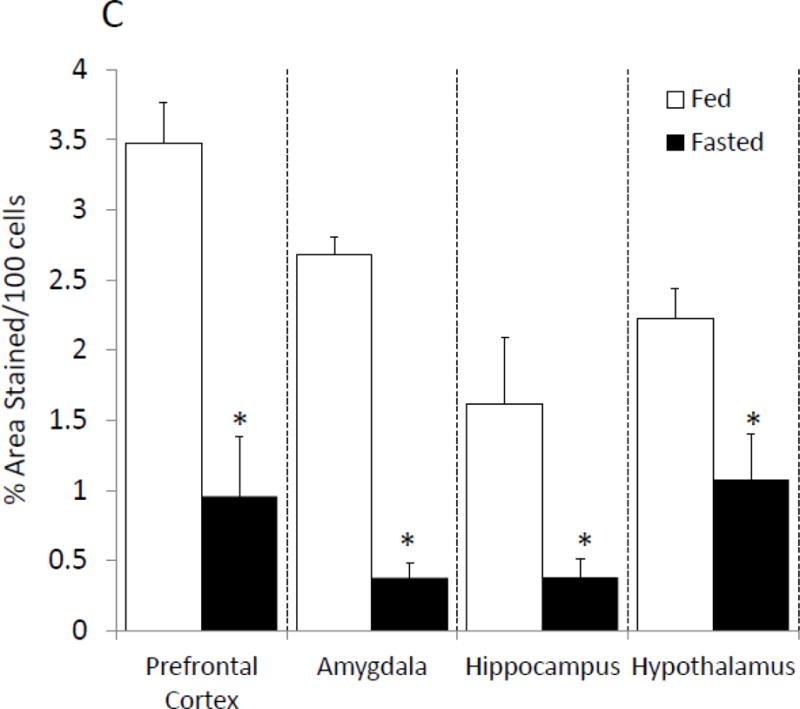
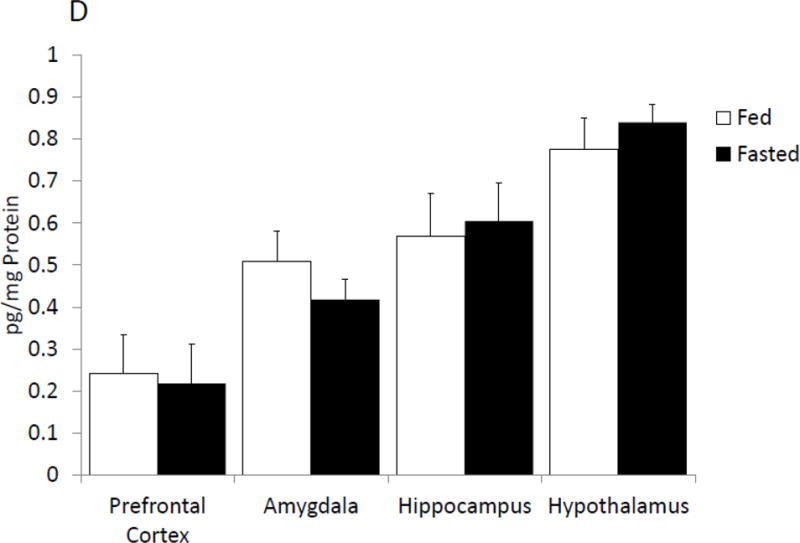
(A) Mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Caspase-1 activity was measured in whole brain and the brain regions indicated. Results are expressed as means ± SEM nM/min/μg total protein; n=7–10. (*p<0.05) (B) As in (A), mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Activated caspase-1 was examined in formalin-fixed paraffin embed sections by immunohistochemistry. Results are representative, 40X. (C) As in (A), mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). As in (B), activated caspase-1 was examined in formalin-fixed paraffin embed sections by immunohistochemistry. Activated caspase-1 was quantified by image analysis. Results are expressed as means ± SEM % area stained/100 cells; n=4. (*p<0.05) (D) As in (A), mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). IL-1β protein levels were measured by ELISA. Results are expressed as means ± SEM pg/mg Protein; n=7–10.
TABLE 2.
Impact of Fasting on IL-1β, Caspase-1, NLRP3, Pycard gene expression in whole brain, prefrontal cortex, amygdala, hippocampus, and hypothalamus of mice after a 24 hour fast.
| Gene | |||||
|---|---|---|---|---|---|
|
| |||||
| IL-1β | Caspase-1 | NLRP3 | Pycard | ||
| Whole Brain | Fed | 1.00 (0.23, 0.19) | 1.00 (0.11, 0.09) | 1.00 (0.08, 0.07) | 1.00 (0.10, 0.09) |
| Fast | 0.58* (0.07, 0.06) | 1.07 (0.32, 0.24) | 1.49 (0.40, 0.31) | 0.95 (0.29, 0.22) | |
| Prefrontal Cortex | Fed | 1.00 (0.20, 0.17) | 1.00 (0.10, 0.09) | 1.00 (0.08, 0.07) | 1.00 (0.07, 0.07) |
| Fast | 0.41* (0.09, 0.07) | 0.93 (0.07, 0.06) | 1.09 (0.08, 0.07) | 0.61* (0.05, 0.04) | |
| Amygdala | Fed | 1.00 (0.45, 0.31) | 1.00 (0.11, 0.10) | 1.00 (0.12, 0.10) | 1.00 (0.11, 0.10) |
| Fast | 0.29* (0.09, 0.07) | 1.04 (0.09, 0.09) | 0.96 (0.09, 0.08) | 0.90 (0.09, 0.08) | |
| Hippocampus | Fed | 1.00 (0.31, 0.24) | 1.00 (0.13, 0.12) | 1.00 (0.10, 0.09) | 1.00 (0.20, 0.16) |
| Fast | 0.98 (0.24, 0.19) | 1.37 (0.27, 0.23) | 0.86 (0.05, 0.05) | 0.87 (0.09, 0.08) | |
| Hypothalamus | Fed | 1.00 (0.14, 0.12) | 1.00 (0.06, 0.06) | 1.00 (0.16, 0.14) | 1.00 (0.06, 0.05) |
| Fast | 0.34* (0.09, 0.07) | 1.09 (0.15, 0.13) | 1.06 (0.15, 0.13) | 0.70* (0.15, 0.13) | |
Results are expressed as relative fold change in mRNA (ΔmRNA), means (SEM upper, SEM lower); n=7–10
p<0.05
3.3 Fasting lessens anxiety-like behaviors and improves memory
Fasted mice and fed mice were examined for changes in prefrontal cortex, amygdala and hippocampus sensitive behaviors. In the elevated zero maze, fasted mice spent 70% (fed vs. fasted, 24.41% ± 2.53 vs. 35.07% ± 1.596, p=0.002; Figure 2A) more time in the open than unfasted mice. Locomotor activity during elevated zero maze testing was comparable between fasted and fed mice (fed vs. fasted, 2234.67 cm ± 254.45 vs. 2435.62 cm ± 220.71, p=0.557; Figure 2B). In the novel object recognition (NOR) task fed and fasted mice investigated the novel object similarly at 2 h post-training, which immediately followed fasting (fed vs. fasted, 62.8% ± 2.23 vs. 68.06% ± 1.82, p=0.089; one-sample t-test: fed- p<0.001, fasted- p<0.001; Figure 2C). At 24 h post-training, fasted mice investigated the novel object 21% (fed vs. fasted, 52.98% ± 2.28 vs. 64.34% ± 3.78, p=0.028; one-sample t-test: fed- p=0.211, fasted- p=0.007; Figure 2C) more than fed mice. Fed and fasted mice investigated the novel object comparably after 48 h post-training (fed vs. fasted, 55.88% ± 4.89 vs. 47.62% ± 5.58, p=0.284; one-sample t-test: fed- p=0.268, fasted- p=0.683; Figure 2C). In the object location recognition (OLR) task, fed and fasted mice investigated the object in the novel position similarly at 2 h post-training (fed vs. fasted, 60.33% ± 3.93 vs. 69.09% ± 1.96, p=0.066; one-sample t-test: fed- p=0.034, fasted- p<0.001; Figure 2D). At 24 h post-training, the fasted mice investigated the object in the novel position 32% (fed vs. fasted, 49.21% ± 1.43 vs. 64.93% ± 2.81, p<0.001; one-sample t-test: fed- p=0.598, fasted- p=0.001; Figure 2D) more than fed mice. As with NOR, fed and fasted mice investigated both novel and familiar similarly after 48 h after training (fed vs. fasted, 46.08% ± 3.51 vs. 48.94% ± 2.69, p=0.529; one-sample t-test: fed- p=0.801, fasted- p=0.954; Figure 2D).
Figure. 2. Fasting lessens anxiety-like behaviors and improves memory.
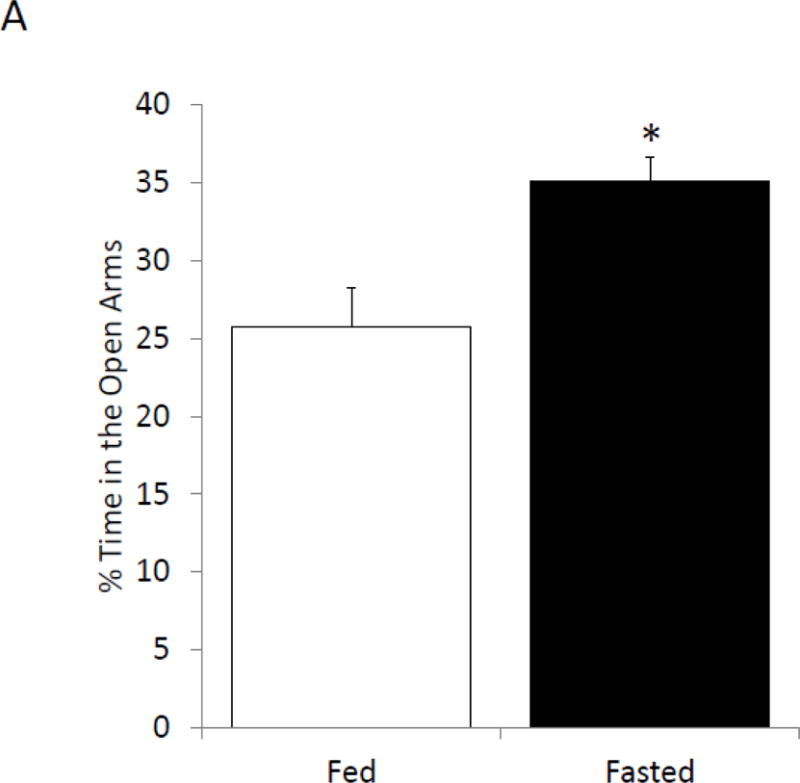
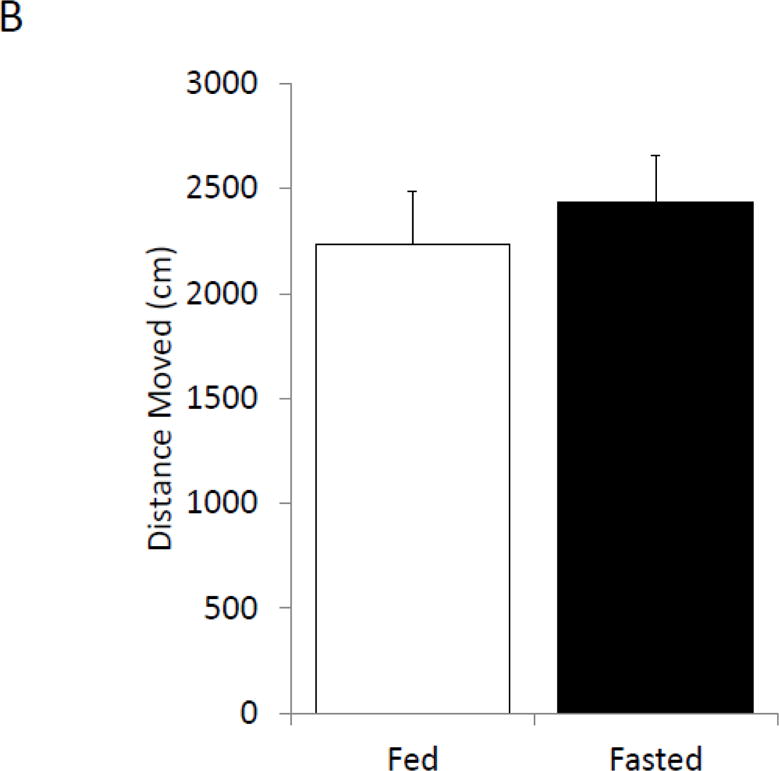
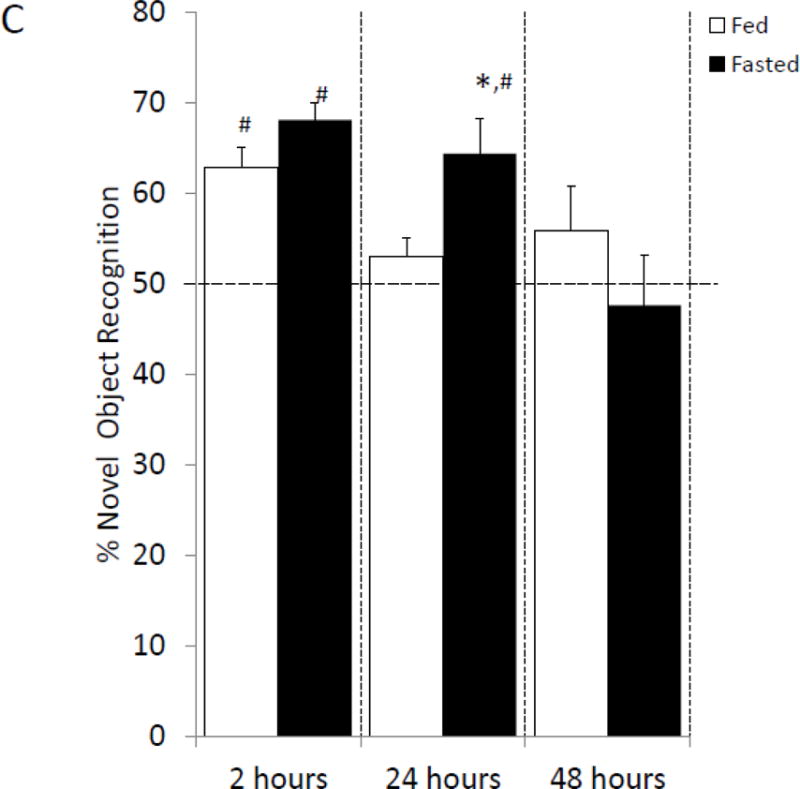
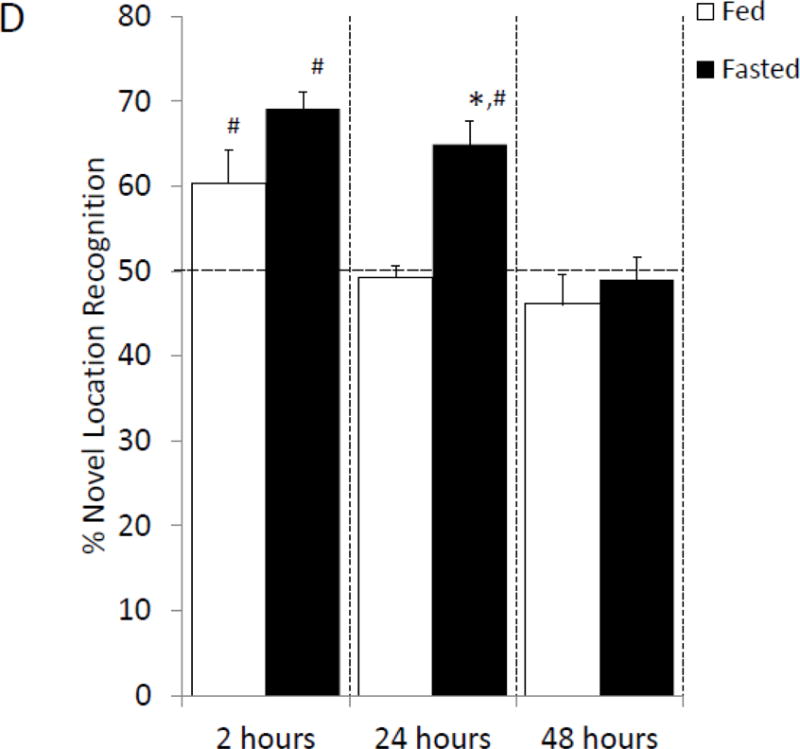
(A) Mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Total time spent in the open arms of the elevated zero maze was measured. Results are expressed as % time in the open arms; n=12. (B) As in (A), mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Total distance moved while in the elevated zero maze was measured. Results are expressed as distance moved in cm; n= 12. (C) As in (A), mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Mice were then refed for an additional 24 hours (48 hrs). Novel object recognition was performed at the time points indicated. Results are expressed as % novel object recognition; n=7–8. (D) As in (C), mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Mice were then refed for an additional 24 hours (48 hrs). Novel location recognition was performed at the time points indicated. Results are expressed as % novel location recognition; n=7–8. In (A–D), all results are expressed as means ± SEM; values with * are significant at p<0.05 using a one-way ANOVA between fed vs. fast, values with # are significant at p<0.05 using one-sample t-test with novel object preference compared with chance level of 0.5.
3.4 The caspase-1 inhibitor, YVAD, reduces caspase-1 activity in the brain and anxiety-like behavior
To determine if pharmacologic inhibition of caspase-1 activity mimics fasting, mice were ICV injected with the caspase-1 inhibitor YVAD. Table 3 shows that mice administered YVAD have no difference in weight, food intake or locomotor activity when compared to saline injected control mice. Caspase-1 activity was measured 24 hours after YVAD injection. Figure 3A shows that YVAD reduced caspase-1 activity by 55% (nM/min/μg protein saline vs. YVAD, 1.69 ± 0.21 vs. 0.76 ± 0.21, p=0.014) and 54% (nM/min/μg protein saline vs. YVAD, 1.16 ± 0.27 vs. 0.54 ± 0.06, p=0.012) in the prefrontal cortex and amygdala, respectively. No significant reduction in caspase-1 activity was seen in the hippocampus (nM/min/μg saline vs. YVAD, 0.96 ± 0.15 vs. 0.71 ± 0.07) and hypothalamus (nM/min/μg protein saline vs. YVAD, 2.11 ± 0.58 vs. 2.02 ± 0.66). YVAD, in comparison to saline, increased time spent in the open arms of the elevated zero maze by 65% (% time in open arms saline vs. YVAD, 9.14 ± 1.68 vs. 14.99 ± 1.71, p=0.023; Figure 3B). This increase was not associated with an increase in locomotion in the maze (cm moved saline vs. YVAD, 2017.46 ± 204.21 vs. 2022.35 ± 147.71, p=0.98).
TABLE 3.
Body weight (g), Body weight change (% loss), Food intake (g), Locomotor activity (m)
| 0–24 Hours
|
||
|---|---|---|
| Saline | YVAD | |
| Body weight | 25.14 ± 0.98 | 24.79 ± 0.52 |
| Body Weight Change | −0.54 ± 0.48 | −0.39 ± 0.67 |
| Food Intake | 3.70 ± 0.27 | 3.92 ± 0.12 |
| Locomotor Activity | 50.14 ± 5.12 | 50.35 ± 4.43 |
Results are expressed as mean ± SEM, n=7–8.
p<0.05 between Saline and YVAD groups
Figure. 3. The caspase-1 inhibitor, YVAD, reduces caspase-1 activity in the brain and anxiety like behavior.
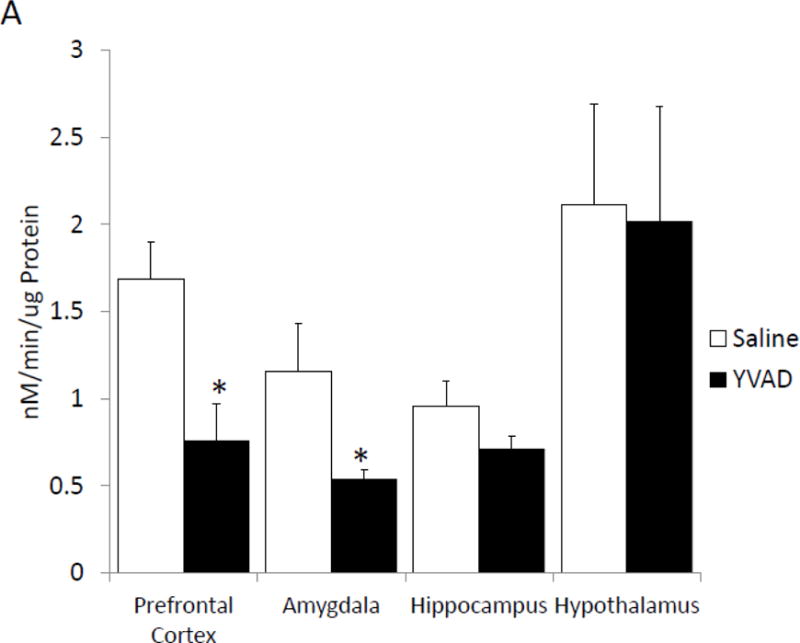
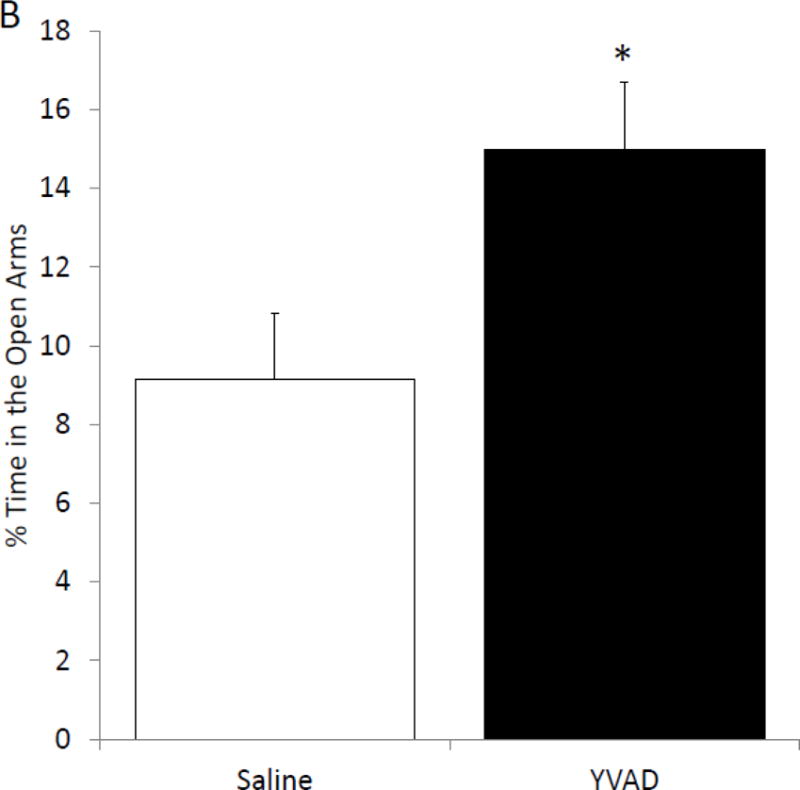
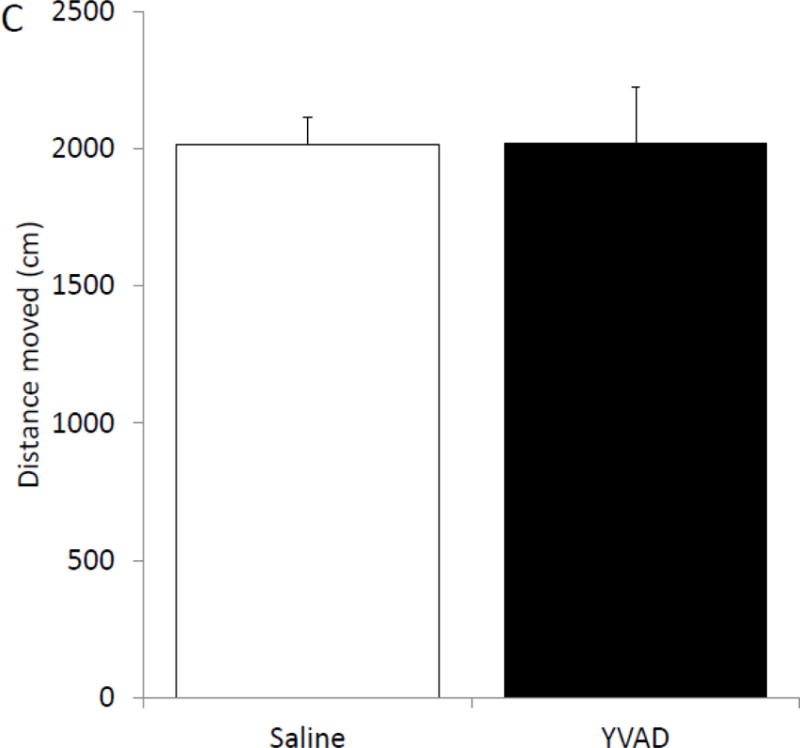
(A) Mice were administered ICV YVAD. 24 hours after YVAD administration caspase-1 activity was measured in the brain regions indicated. Results are expressed as means ± SEM nM/min/μg total protein; n=6-8 (*p<0.05). (B) Mice were treated as in (A). Total time spent in the open arms of the elevated zero maze was measured 24 hours after YVAD administration. Results are expressed as % time in the open arms of the maze; n=12–13 (*p<0.05). (C) Mice were treated as in (A). Total distance moved while in the elevated zero maze was measured 24 hours after YVAD administration. Results are expressed as distance moved in cm; n=12–13 (*p<0.05).
3.5 Caspase-1 and IL1-R1 knockout (KO) mice are resistant to fasting-induced improvements in anxiety-like behavior
To further determine if anxiety-like behavior is dependent on the IL-1β system caspase-1 and IL1-R1 KO mice were examined with or without fasting. Figure 4A shows that in the elevated zero maze caspase-1 KO mice have no fasting-induced change in anxiety-like behavior, while wild-type mice have a 60% increase in time spent in the open arms (% time in open arms fed wildtype 12.63 ± 1.64 vs. fasted wildtype 20.44 ± 3.18 vs. fed caspase-1 KO 18.72 ± 2.43 vs. fasted caspase-1 KO 14.57 ± 1.23); with a significant treatment-genotype interaction (p=0.019)). Figure 4B shows that caspase-1 KO mice move less during the task than wildtype mice (cm moved fed wildtype 1811.95 ± 180.07 vs. fasted wildtype 175.93 ± 190.06, vs. fed caspase-1 KO 1353.65 ± 115.96 vs. fasted caspase-1 KO 1470.53 ± 74.79); significant effect of genotype (p=0.001)). Figure 4C demonstrates that IL-1R1 KO mice are resistant to fasting induced changes in anxiety-like behavior (% time in open arms fed wildtype 20.93 ± 2.35 vs. fasted wildtype 33.50 ± 5.07 vs. fed IL-1R1 KO 28.54 ± 4.04 vs. fasted IL-1R1 34.30 ± 3.64); significant main effect of treatment (p=0.032)). Changes in time spent in the open arms of the zero maze were independent of changes in locomotor activity within the task (cm moved fed wildtype 2866.97 ± 263.28 vs. fasted wildtype 2721.55 ± 209.89 vs. fed IL-1R1 KO 2514.25 ± 379.15 vs. fasted IL-1R1 KO 2466.17 ± 284.49)).
Figure. 4. Caspase-1 and IL1-R1 knockout (KO) mice are resistant to fasting-induced improvements in anxiety-like behavior.
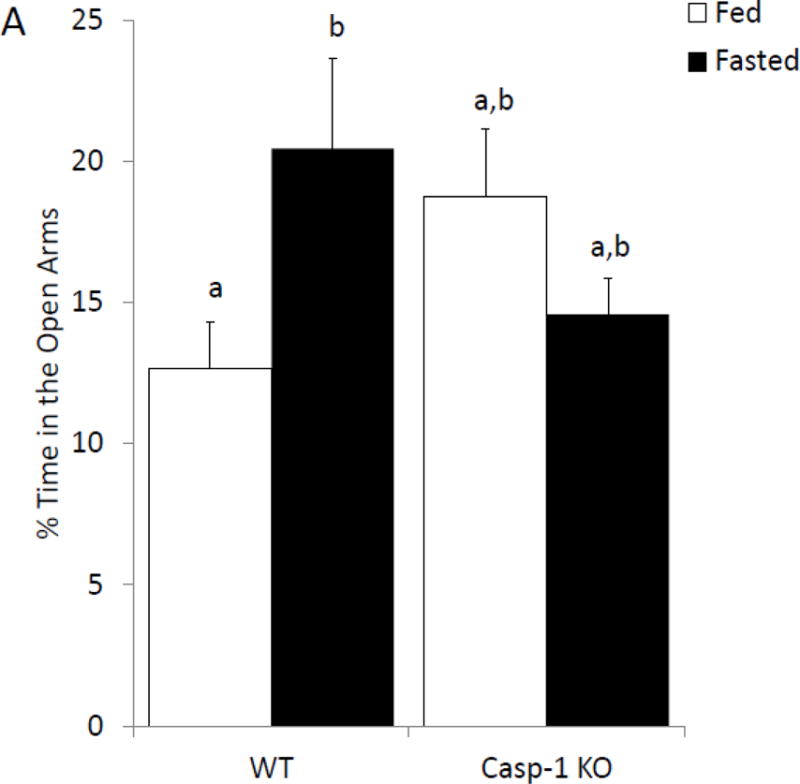
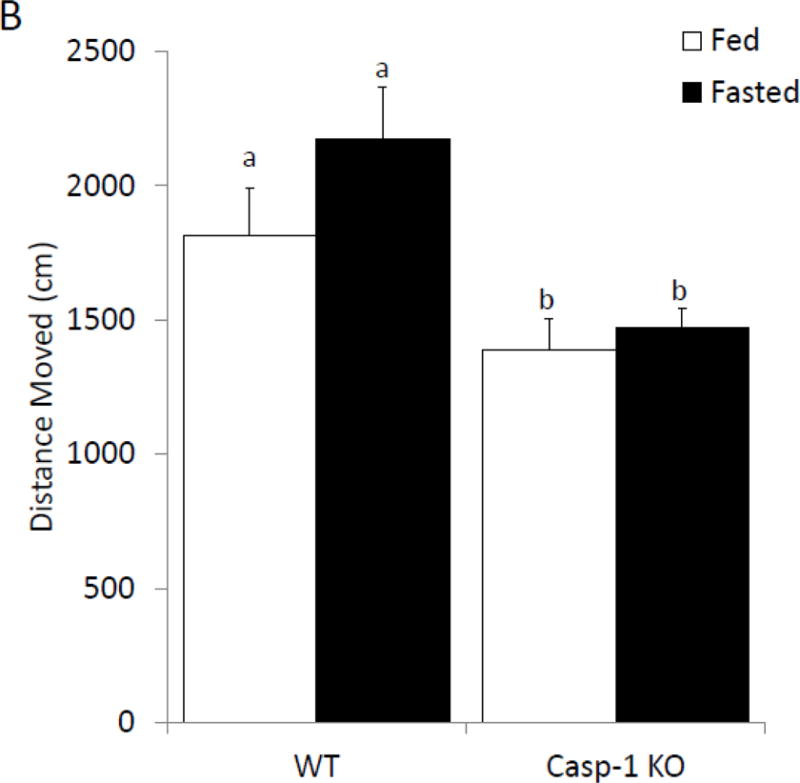
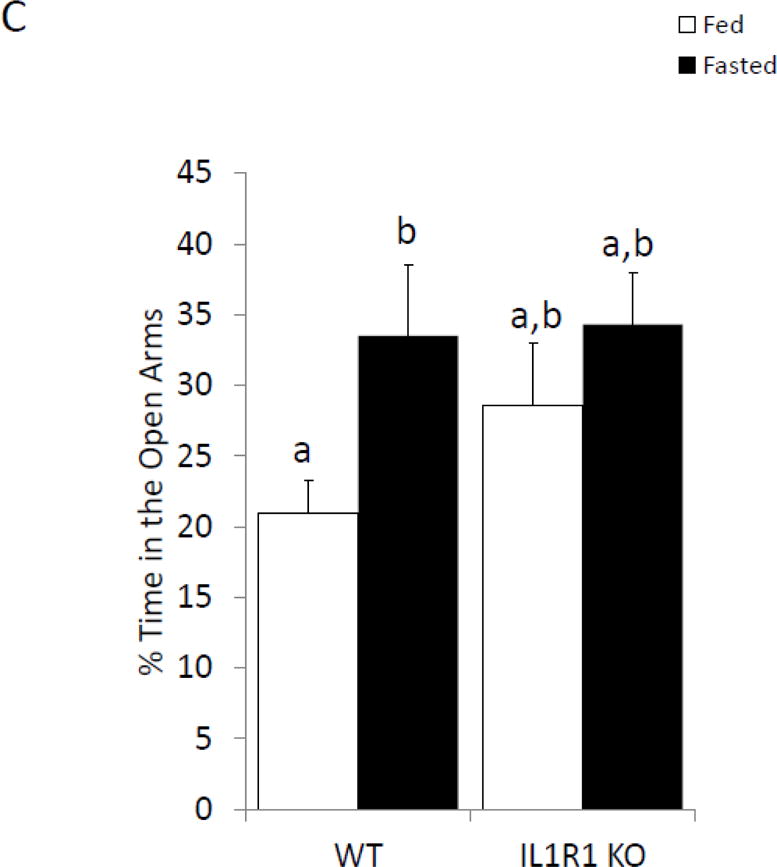
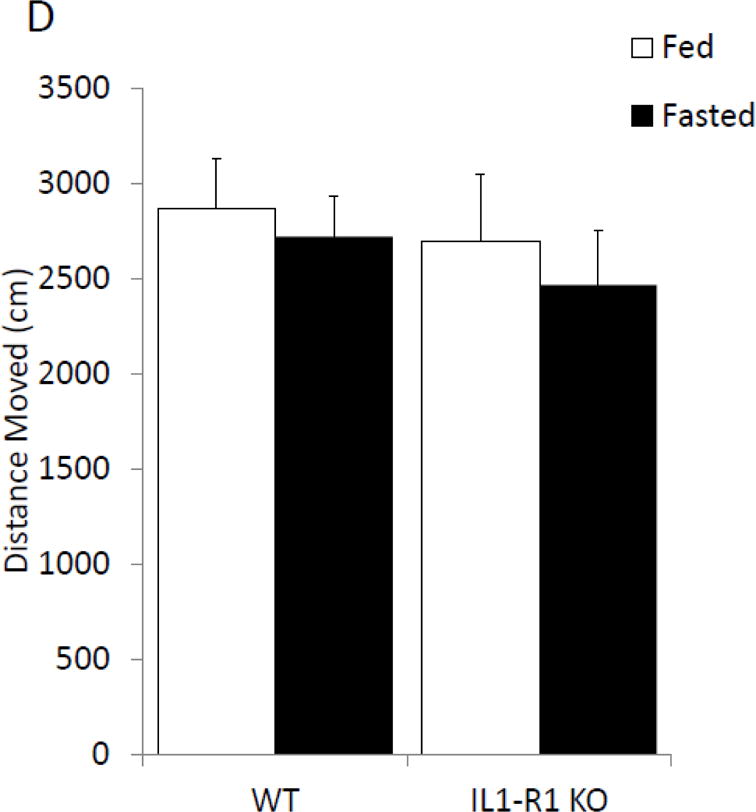
(A) Wild type (WT) or caspase-1 KO (Casp-1 KO) mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Total time spent in the open arms of the elevated zero maze was measured. Results are expressed as % time in the open arms of the maze; n=7–10. (B) As in (A), WT or Casp-1 KO mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Total distance moved while in the elevated zero maze was measured. Results are expressed as distance moved in cm; n=7–10. (C) WT or IL-1R1 mice (R1) mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Total time spent in the open arms of the elevated zero maze was measured. Results are expressed as % time in the open arms of the maze; n=8. (D) As in (C), WT or R1 mice were given ad lib access to food (Fed) or fasted for 24 hours (Fasted). Total distance moved while in the elevated zero maze was measured. Results are expressed as distance moved in cm; n=8. For (A–D) results are expressed as means ± SEM and values without a common superscript are significant at p<0.05.
4. Discussion
The starvation response in animals is critical to maintain homeostasis in the event of nutrient deprivation. This reaction integrates several metabolic and hormonal signals that regulate energy levels [33]. Maintenance of locomotor activity and a reduction in anxiety-like behavior would appear advantageous to exploratory behavior required for nutrient acquisition. Likewise, improved memory could facilitate both exploration and revisitation. The pro-inflammatory cytokine IL-1β directly impacts movement, anxiety and memory [34], and, in the sickness paradigm, decreases locomotion while promoting anxiety-like behavior and memory impairment in aged animals [35–37]. We previously found that a high-fat diet causes behavioral changes similar to those triggered by IL-1 administration including anxiety and cognitive impairment [30, 38]. Importantly, administration of the ATP-sensitive K+ (KATP) channel blocker glyburide rapidly ameliorates the anxiety-like and cognitive consequences of a high-fat diet [38]. Since inflammasome activation can be triggered by a drop in the intracellular concentration of K+ [39–41], our previous work suggests that dietary restriction might reduce the activity of caspase-1 and that such changes would likely be present in the brain.
Overnutrition can increase caspase-1 activity in the periphery [42]. Such increases in caspase-1 appear associated with high-fat diets and the inflammatory consequences of obesity outside the CNS [43, 44]. While high-fat diets have been shown to increase NLRP3 expression in the hippocampus [45], caspase-1 regulation throughout the rest of the brain is currently untied to diet. Figure 1 and Table 2 illustrate that undernutrition reduces caspase-1 activity in the brain in concert with a marked reduction in IL-1β gene expression in the prefrontal cortex, amygdala and hypothalamus. Surprisingly, figure 1D shows that, despite changes in IL-1β gene expression, there is no change in IL-1β protein concentration. This lack of change in protein levels could be due to the sequestering of pro-IL-1β in vesicles [46]. As IL-1β secretion can be acutely increased in as little 10 to 20 minutes following administration of a danger signal such as ATP [47], it is vital to the inflammatory response of the cell to maintain stable levels of IL-1β protein. Several studies have shown this ability to store IL-1β for future release, rather than degrading it [48, 49]. While a reduction in the ability of the hypothalamus to generate IL-1β makes teleological sense when paired with starvation and the anorexigenic properties of IL-1, such outcomes were unexpected in other brain areas. Canonically, inflammasome activation and IL-1β gene expression are regulated by two independent signals. IL-1β is activated by caspase-1 cleavage of pro-IL-1β, while IL-1β transcription is usually secondary to NFкb as part of TLR4 stimulation [50, 51]. Caspase-1 activation is generally driven by NLRs [52], following sensing of a danger associated molecular pattern (DAMP) or pattern associated molecular pattern (PAMP). As a responder to intracellular danger signals, caspase-1 is largely constitutively expressed, and is further expressed after induction [53], which would explain why we saw little change in expression of the genes comprising the inflammasome. However, this is in contrast to our finding in Figure 1 A–C, where we saw decreased caspase-1 activity, which suggests that suppression of caspase-1 activity may be due to factors affecting the assembly of the inflammasome complex, such as the effects of fasting on oxidative state [54]. Likewise, autophagy directly targets the inflammasome for destruction [55–57] and 24 hours of fasting can trigger autophagy in mouse [58]. Similar to glyburide, the ketone body β-hydroxybutyrate inhibits the inflammasome by preventing potassium efflux [59] and ketone bodies are elevated by fasting in mice [60]. Thus, the ability of fasting to both reduce IL-1β gene expression and caspase-1 activity, while having no effect on IL-1β protein, could be a sign of both short, and long-term inhibition of IL-1 signaling in the brain.
YVAD-biotin labeling in the brain demonstrated that active caspase-1 was primarily localized to cells morphologically consistent with neurons in the prefrontal cortex, amygdala and hippocampus. In the hypothalamus, cell type was less discernable. While resident immune cells, such as microglia, are principally thought to produce and elaborate inflammatory cytokines, several studies show that human neurons contain an inflammasome [61, 62]. In cultured primary cortical neurons, ethanol can induce caspase-1 activation and HMGB1 release [63]. Primary cortical neuron cultures demonstrate inflammasome activation mediated by K+ efflux in response to acidosis [64]. In vivo, an ability of neurons to possess and activate the inflammasome is seen in human patients with temporal lobe epilepsy [65]. Outside the brain, NALP3-induced caspase-1 activation is observed in neurons of the trigeminal ganglia [66]. What role brain-based caspsae-1 plays in health and disease is still not clear and this is especially true for neuronal caspase-1, but endogenous IL-1 is important to certain aspects of anxiety-like behaviors, learning [14] and memory [15, 67] in the healthy state [68]. As an example, changes in proteins responsible for IL-1 production and/or its signaling influence brain function with the best evidence being gleaned from studies using IL-1 antagonists [69, 70]. Pertinent to our findings, undernutrition has been shown to positively impact behavior in that intermittent fasting has been shown to improve Barnes maze performance and fear conditioning [71]. In contrast, some previous work looking at calorie restriction or intermittent fasting on elevated plus maze performance does not demonstrate an impact of these interventions [72]. This is likely related to the acuteness of the fasting paradigm which here resulted in a near 23% drop in body weight. Such drastic reductions in body weight may limit the translatability of this work. In one study of the starvation response in humans, it was found that 24 hours of fasting only reduced body weight by 1.9%, and only by 9.5% over ten days [73]. Reviews on the topic of calorie restriction, however, (regardless of how it is implemented) appear to benefit certain cognitive tasks [74]. Thus, the model of fasting used here, provides meaning mechanistic insights into this phenomenon. In turn, recent work by Traba et al. [75] shows a decrease in NLRP3 inflammasome activation in primary leukocytes from a 24 hour fast in humans suggesting the potential general applicability of dietary-regulation of caspase-1. Finally, weight reductions in mice are tightly correlated to increases in IL-1R2 and IL-1RA in liver and fat, but not in serum [25] insinuating that an acute fast impacts IL-1 signaling peripherally and centrally.
As a potent inhibitor of caspase-1, YVAD affects a broad range of biobehaviors. YVAD markedly decreases depressive like behaviors in LPS-induced treated mice [76]. We, and others, find that YVAD can abrogate memory loss induced by hypoxia and aging [28, 77]. Interestingly, YVAD appears to be a potent anxiolytic reducing anxiety-like behaviors in the basal state (Figure.3). In regards to body weight, food intake and locomotor activity, centrally administered YVAD had little effect even though it inhibited caspase-1 activity in the prefrontal cortex and amygdala. The inability of YVAD to modify food intake or locomotor activity could be due to the absence of caspase-1 inhibition in the hypothalamus, as this region is the main regulator of energy homeostasis in the brain [78]. Support for this contention is observed in mouse models of obesity, where YVAD attenuates inflammation while failing to impact body weight, fat mass and glucose levels [79].
Finally, in mice lacking caspase-1 or IL-1R1, a fasting-dependent reduction in anxiety-like behaviors was not observed suggesting that caspase-1 and IL-1R1 are critical to anxiolytic effect in fasted mice. Although caspase-1 and IL-1R1 mice were not statistically different from WT mice in regards to anxiety-like behavior in the fed state using 2-way ANOVAs, t-testing demonstrated significance for caspase-1 mice. The inability of IL-1R1 knockout to improve anxiety-like behavior on its own could be due to several mechanisms. It is possible that expression of other IL-1 receptors is altered in these animals, since we have previously shown that fasting can increase IL-1R2 expression in the cortex [25]. The observation that caspase-1 KO mice do not show reduced anxiety could be due to compensation in IL-1β processing through caspase-1 independent production [80, 81], or a redundancy, aka IL-1α. It has been shown that caspase-1 KO mice still secrete IL-1α [82]. This compensatory effect of IL-1 can be seen in mouse models of ischemic brain damage, where animals lacking IL-1β or IL-1α were susceptible to damage, but mice lacking both were protected [83]. However, this finding that genetic deletion of core components of the IL-1 signaling pathway does not improve behavior at basal conditions is similar to previous work in which IL-1RA overexpressing mice demonstrated impaired spatial memory compared to WT controls [14].
As noted above, IL-1β gene polymorphisms can predispose patients to anxiety and depressive disorders [23, 24]. In addition, IL-1β and IL-1RA gene polymorphisms appear tied to cognitive ability in Caucasians and African-Americans [84]. Likewise, susceptibility to schizophrenia is coupled to perturbations within IL-1 gene family components [85]. Serum IL-1β levels are correlated with anxiety and mood disorders in those with rheumatoid arthritis [86]. In sum, the IL-1 system, in humans, is linked to psychiatric disturbances at both the gene and protein level. Given this comorbidity, nutritional and/or dietary strategies that target IL-1β functionality could foster novel clinical intervention for a subset of psychiatric illnesses that are notoriously hard to treat. Given the known limitations of translating animal-based discovers on neurological diseases to humans [87], especial those tied to the prefrontal cortex [88], further validation of this exciting preclinical work is required. Nevertheless, our data build upon our previous research by showing that acute fasting can induce IL-1β resistance by inhibiting caspase-1 activity in the brain and that this effect is particularly concentrated in neurons of the prefrontal cortex, amygdala and hippocampus. Reductions in brain caspase-1 activity reduce anxiety and improve memory. Thus, fasting could prove to be a potent intervention to improve brain function.
Supplementary Material
Highlights.
Acute fasting inhibits caspase-1 activation in distinct brain regions
Acute fasting reduces anxiety-like behavior and improves memory
The mechanism of behavioral change is dependent on caspase-1 and IL-1R1 signaling
Acknowledgments
Support: This research was supported by the National Institutes of Health (DK064862 to GGF)
Abbreviations
- CNS
Central nervous system
- WT
Wild-type
- KO
Knockout
- IL
interleukin
- IL1R1
IL-1 Receptor 1
- Casp-1
Caspase-1
- LPS
Lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cerretti DP, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 2.Thornberry NA, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–74. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 3.Martinon F, Burns K, Tschopp J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 4.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu A, et al. Unified polymerization mechanism for the assembly of asc-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J. 2013;449:613–21. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999;96:10964–7. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y. Caspase activation: Revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Rossi S, et al. Interleukin-1 Causes Anxiety by Interacting with the Endocannabinoid System. J Neurosci. 2012;32:13896–13905. doi: 10.1523/JNEUROSCI.1515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray CL, Obiang P, Bannerman D, Cunningham C. Endogenous IL-1 in cognitive function and anxiety: a study in IL-1RI-/- mice. PLoS One. 2013;8:e78385. doi: 10.1371/journal.pone.0078385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu GS, et al. Adenosine through the A2A adenosine receptor increases IL-1beta in the brain contributing to anxiety. Brain Behav Immun. 2014;41:218–231. doi: 10.1016/j.bbi.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spulber S, Bartfai T, Schultzberg M. IL-1/IL-1ra balance in the brain revisited — evidence from transgenic mouse models. Brain Behav Immun. 2009;23:573–9. doi: 10.1016/j.bbi.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Goshen I, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Yirmiya R, Winocur G, Goshen I. Brain Interleukin-1 Is Involved in Spatial Memory and Passive Avoidance Conditioning. Neurobiol Learn Mem. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]
- 16.Somers JM, Goldner EM, Waraich P, Hsu L. Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J psychiatry Revue Can Psychiatr. 2006;51:100–113. doi: 10.1177/070674370605100206. [DOI] [PubMed] [Google Scholar]
- 17.Markovitz AA, et al. Toxoplasma gondii and anxiety disorders in a community-based sample. Brain Behav Immun. 2015;43:192–197. doi: 10.1016/j.bbi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Otowa T, Gardner CO, Kendler KS, Hettema JM. Parenting and risk for mood, anxiety and substance use disorders: A study in population-based male twins. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1841–1849. doi: 10.1007/s00127-013-0656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakolsky DJ, McCracken JT, Nurmi EL. Genetics of Pediatric Anxiety Disorders. Child and Adolescent Psychiatric Clinics of North America. 2012;21:479–500. doi: 10.1016/j.chc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 21.Bankier B, Barajas J, Martinez-Rumayor A, Januzzi JL. Association between C-reactive protein and generalized anxiety disorder in stable coronary heart disease patients. Eur Heart J. 2008;29:2212–2217. doi: 10.1093/eurheartj/ehn326. [DOI] [PubMed] [Google Scholar]
- 22.Smith KJ, et al. Association of diabetes with anxiety: A systematic review and meta-analysis. Journal of Psychosomatic Research. 2013;74:89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Miaskowski C, et al. Cytokine gene variations associated with trait and state anxiety in oncology patients and their family caregivers. Support Care Cancer. 2015;23:953–965. doi: 10.1007/s00520-014-2443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovacs D, et al. Effects of IL1B single nucleotide polymorphisms on depressive and anxiety symptoms are determined by severity and type of life stress. Brain Behav Immun. 2016;56:96–104. doi: 10.1016/j.bbi.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Joesting JJ, et al. Fasting Induces IL-1 Resistance and Free-Fatty Acid-Mediated Up-Regulation of IL-1R2 and IL-1RA. Front Immunol. 2014;5:315. doi: 10.3389/fimmu.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavin DN, et al. Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obesity (Silver Spring) 2011;19:1586–1594. doi: 10.1038/oby.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.York JM, et al. The biobehavioral and neuroimmune impact of low-dose ionizing radiation. Brain Behav Immun. 2012;26:218–227. doi: 10.1016/j.bbi.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu GS, et al. Hypoxia/reoxygenation impairs memory formation via adenosine-dependent activation of caspase 1. J Neurosci. 2012;32:13945–13955. doi: 10.1523/JNEUROSCI.0704-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson DR, O’Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system, which diabetes exacerbates. J Neurosci. 2007;27:1161–1166. doi: 10.1523/JNEUROSCI.4560-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaczmarczyk MM, et al. Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. Psychoneuroendocrinology. 2013;38:1553–1564. doi: 10.1016/j.psyneuen.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.York JM, Blevins NA, Baynard T, Freund GG. Mouse testing methods in psychoneuroimmunology: an overview of how to measure sickness, depressive/anxietal, cognitive, and physical activity behaviors. Methods Mol Biol. 2012;934:243–276. doi: 10.1007/978-1-62703-071-7_13. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald L, Radler M, Paolini AG, Kent S. Calorie restriction attenuates LPS-induced sickness behavior and shifts hypothalamic signaling pathways to an anti-inflammatory bias. Am J Physiol Regul Integr Comp Physiol. 2011;301:R172–R184. doi: 10.1152/ajpregu.00057.2011. [DOI] [PubMed] [Google Scholar]
- 33.Longo VD, Mattson MP. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 35.Savignac HM, et al. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav Immun. 2016;52:120–131. doi: 10.1016/j.bbi.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biesmans S, et al. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm. 2013;2013:271359. doi: 10.1155/2013/271359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarr AJ, et al. The effects of age on lipopolysaccharide-induced cognitive deficits and interleukin-1β expression. Behav Brain Res. 2011;217:481–485. doi: 10.1016/j.bbr.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 38.Gainey SJ, et al. Short-Term High-Fat Diet (HFD) Induced Anxiety-Like Behaviors and Cognitive Impairment Are Improved with Treatment by Glyburide. Front Behav Neurosci. 2016;10:156. doi: 10.3389/fnbeh.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perregaux D, Gabel CA. Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 40.Franchi L, Kanneganti TD, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 41.Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol. 2015;194:3937–52. doi: 10.4049/jimmunol.1402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohamed IN, et al. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia. 2014;57:413–423. doi: 10.1007/s00125-013-3101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo B, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One. 2014;9:e104771. doi: 10.1371/journal.pone.0104771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solini A, et al. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: Possible role of NLRP3 inflammasome activation. J Pathol. 2013;231:342–353. doi: 10.1002/path.4237. [DOI] [PubMed] [Google Scholar]
- 45.Sobesky JL, et al. Glucocorticoids Mediate Short-Term High-Fat Diet Induction of Neuroinflammatory Priming, the NLRP3 Inflammasome, and the Danger Signal HMGB1. eNeuro. 2016;3:1–17. doi: 10.1523/ENEURO.0113-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 48.Andrei C, et al. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–75. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990;9:1503–10. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodrigues A, et al. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia Coli. Biol Reprod. 2008;79:1135–47. doi: 10.1095/biolreprod.108.069930. [DOI] [PubMed] [Google Scholar]
- 51.Garía Bueno B, Caso JR, Madrigal JLM, Leza JC. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neuroscience and Biobehavioral Reviews. 2016;64:134–147. doi: 10.1016/j.neubiorev.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishra BB, et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 54.Johnson JB, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi CS, et al. Activation of autophagy by inflammatory signals limits IL-1beta] production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris J, et al. Autophagy Controls IL-1Î2 Secretion by Targeting Pro-IL-1Î2 for Degradation. J Biol Chem. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1bgr] production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 58.Alirezaei M, et al. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6:702–710. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Youm YH, et al. The ketone metabolite beta]-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGarry JD, Foster DW. Regulation of Hepatic Fatty Acid Oxidation and Ketone Body Production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 61.Ward R, Ergul A. Relationship of endothelin-1 and NLRP3 inflammasome activation in HT22 hippocampal cells in diabetes. Life Sci. 2016;159:97–103. doi: 10.1016/j.lfs.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaushal V, et al. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015;22:1–11. doi: 10.1038/cdd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, et al. Ethanol directly induced HMGB1 release through NOX2/NLRP1 inflammasome in neuronal cells. Toxicology. 2015;334:104–110. doi: 10.1016/j.tox.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Wang YC, et al. Acid-sensing ion channel 1a contributes to the effect of extracellular acidosis on NLRP1 inflammasome activation in cortical neurons. J Neuroinflammation. 2015;12:246. doi: 10.1186/s12974-015-0465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan CC, et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model. J Neuroinflammation. 2015;12:1–12. doi: 10.1186/s12974-014-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen L, et al. Chemical stimulation of the intracranial dura activates NALP3 inflammasome in trigeminal ganglia neurons. Brain Res. 2014;1566:1–11. doi: 10.1016/j.brainres.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 67.Avital A, et al. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- 68.Schneider H, et al. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–83. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert Opin Ther Targets. 2012;16:1097–112. doi: 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- 70.Neveu PJ, Liège S. Mechanisms of behavioral and neuroendocrine effects of interleukin-1 in mice. Ann N Y Acad Sci. 2000;917:175–85. doi: 10.1111/j.1749-6632.2000.tb05382.x. [DOI] [PubMed] [Google Scholar]
- 71.Li L, Wang Z, Zuo Z. Chronic Intermittent Fasting Improves Cognitive Functions and Brain Structures in Mice. PLoS One. 2013;8:e66069. doi: 10.1371/journal.pone.0066069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto Y, et al. Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol Genomics. 2009 doi: 10.1152/physiolgenomics.00082.2009. [DOI] [PubMed] [Google Scholar]
- 73.Consolazio CF, Matoush LO, Johnson HL, Nelson RA, Krzywicki HJ. Metabolic aspects of acute starvation in normal humans (10 days) Am J Clin Nutr. 1967;20:672–83. doi: 10.1093/ajcn/20.7.672. [DOI] [PubMed] [Google Scholar]
- 74.Cherif A, Roelands B, Meeusen R, Chamari K. Effects of Intermittent Fasting, Caloric Restriction, and Ramadan Intermittent Fasting on Cognitive Performance at Rest and During Exercise in Adults. Sports Med. 2016;46:35–47. doi: 10.1007/s40279-015-0408-6. [DOI] [PubMed] [Google Scholar]
- 75.Traba J, et al. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest. 2015;125:4592–600. doi: 10.1172/JCI83260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, et al. Involvement of Inflammasome Activation in Lipopolysaccharide-induced Mice Depressive-like Behaviors. CNS Neurosci Ther. 2014;20:119–124. doi: 10.1111/cns.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur J Neurosci. 2005;22:1751–1756. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- 78.Yamanaka A, et al. Hypothalamic Orexin Neurons Regulate Arousal According to Energy Balance in Mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 79.Morrison MC, et al. Intervention with a caspase-1 inhibitor reduces obesity-associated hyperinsulinemia, non-alcoholic steatohepatitis (NASH) and hepatic fibrosis in LDLR-/-Leiden mice. Int J Obes (Lond) 2016;40:1416–23. doi: 10.1038/ijo.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayer-Barber KD, et al. Cutting Edge: Caspase-1 Independent IL-1 Production Is Critical for Host Resistance to Mycobacterium tuberculosis and Does Not Require TLR Signaling In Vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Provoost S, et al. NLRP3/Caspase-1-Independent IL-1 Production Mediates Diesel Exhaust Particle-Induced Pulmonary Inflammation. J Immunol. 2011;187:3331–3337. doi: 10.4049/jimmunol.1004062. [DOI] [PubMed] [Google Scholar]
- 82.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–3. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 83.Boutin H, et al. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–34. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benke KS, et al. The association of genetic variants in interleukin-1 genes with cognition: findings from the cardiovascular health study. Exp Gerontol. 2011;46:1010–9. doi: 10.1016/j.exger.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshida M, et al. Haplotypes in the expression quantitative trait locus of interleukin-1β gene are associated with schizophrenia. Schizophr Res. 2012;140:185–191. doi: 10.1016/j.schres.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 86.El-Tantawy AM, El-Sayed AE, Kora BA, Amin RT. Psychiatric morbidity associated with some cytokines (IL-1beta, IL-12, IL-18 and TNF-alpha) among rheumatoid arthritis patients. Egypt J Immunol. 2008;15:1–11. [PubMed] [Google Scholar]
- 87.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–79. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mackey WE, Devinsky O, Doyle WK, Meager MR, Curtis CE. Human Dorsolateral Prefrontal Cortex Is Not Necessary for Spatial Working Memory. J Neurosci. 2016;36 doi: 10.1523/JNEUROSCI.3618-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


