Abstract
Preterm infants are most vulnerable to pertussis. Whether they might benefit from maternal immunization is unknown. Extending our previous results in term neonates, this observational study demonstrates that second- rather than third-trimester maternal vaccination results in higher birth anti–pertussis toxin titers in preterm neonates.
Keywords: pertussis, maternal immunization, maternal antibodies, preterm, neonates
Neonatal pertussis remains an important public health concern, and maternal vaccination is the sole way to protect this fragile population from complications and death [1]. Most countries recommended maternal pertussis immunization during the third trimester (>26 gestational weeks) on an empirical basis, given insufficient data on optimal timing [2]. Recently, we showed in a large observational study that antibody transfer to term neonates was superior following maternal pertussis immunization between gestational week (GW) 13 0/7 and 25 6/7 compared with after GW 25 6/7 [3].
Little is known concerning the kinetics and efficiency of transplacental antibody transport during early gestation. Maternal antibody transfer was reported as starting between GW 13 and 17 [4] and being most active after GW 32 [5]. At birth, fetomaternal ratios of pertussis toxin (PT) antibodies were reported as 0.55 before GW 32, 0.79 between GW 32 and 37, and 1.07 after GW 37 [6].
The extreme vulnerability of preterm infants to severe pertussis [7] led us to investigate whether maternal pertussis immunization during the second rather than third trimester could increase birth antibody titers in preterm neonates.
METHODS
This single-center prospective observational study took place between 15 July 2014 and 29 February 2016, following approval from the Geneva Ethics Commission (CCER 14–096).
Eligible participants were consenting pregnant women delivering before GW 37 0/7 at the University Hospitals of Geneva, Switzerland. They had previously been vaccinated during one of their routine follow-up visits with tetanus-diphtheria- acellular pertussis (Tdap) according to the official Swiss recommendations (any time after GW 13 0/7). All women received Boostrix (GlaxoSmithKline), which contains 8 µg of pertussis toxoid, 8 µg of filamentous hemagglutinin, and 2.5 µg of pertactin. None recalled having received a pertussis booster within the 5 previous years. Gestational age, determined by early ultrasound, and vaccination date were obtained from the women’s medical records. Exclusion criteria and data collection methods have been described previously [3].
The sample size was based upon the expected number of preterm births in the study hospital within a continuous 19-month period to avoid influence of a potential change in pertussis prevalence over time. We thus intended to include 100 patients.
We collected cord blood samples of preterm neonates after maternal Tdap immunization performed between GW 13 0/7 and 25 6/7 (second trimester) or after GW 25 6/7 (third trimester). We measured anti-PT and anti–filamentous hemagglutinin (FHA) antibodies by enzyme-linked immunosorbent assay and calculated geometric mean concentrations (GMCs) with their 95% confidence intervals (CIs). Seronegativity was defined as anti-PT ≤5 EU/mL.
Characteristics of women in second- and third-trimester groups were described and compared using Student t test, Fisher exact test, or χ2 test where appropriate. Student t test with unequal variances was employed for comparisons of antibody GMCs and Fisher exact test for seronegativity rates.
We also performed a multivariable linear regression analysis to compare GMC ratios using potential confounding factors identified in our large study of term neonates [3] and clinical considerations; all unbalanced epidemiological characteristics between the second- and third-trimester term groups (maternal age, parity, socioeconomic status) [3] and gestational age at birth [6] were introduced in the model. Antibody titers were transformed with the base-10 logarithmic function; the regression coefficients were back-transformed and reported as GMC ratios. The distribution of residuals was visually inspected.
Similarly, we determined the association of predefined time intervals between vaccination and delivery and birth antibody titers by analyzing the ratio of GMCs with 95% CIs [3]. No data were missing. A P value <.05 was considered statistically significant. All statistical tests were 2-sided. Stata software version 13.0 was used for statistical analysis and GraphPad Prism version 7 for graphs.
RESULTS
Between August 2014 and February 2016, 544 women delivered before term at the University Hospitals of Geneva (24–29 GW, 67 [11%]; 30–33 GW, 116 [20%]; 34–37 GW, 361 [63%]), with a distribution of the gestational ages following the general epidemiology of preterm births in Switzerland [8]. Eighty-five consenting Tdap-immunized mother–preterm newborn pairs were included: 68 (80%) were born between GW 34 0/7 and 36 6/7, and 17 (20%) between GW 30 0/7 and 33 6/7.
Among 85 mothers, 37 had been immunized during the second trimester, and 48 during the third trimester. The mean intervals between vaccination and delivery were 97.1 (standard deviation [SD], 25.5) days for the second trimester and 29.6 (SD, 21.9) days for third-trimester immunization. There were no statistically significant differences between the baseline clinical characteristics in the early (second trimester) and late pregnancy (third trimester) vaccination group (Supplementary Table 1).
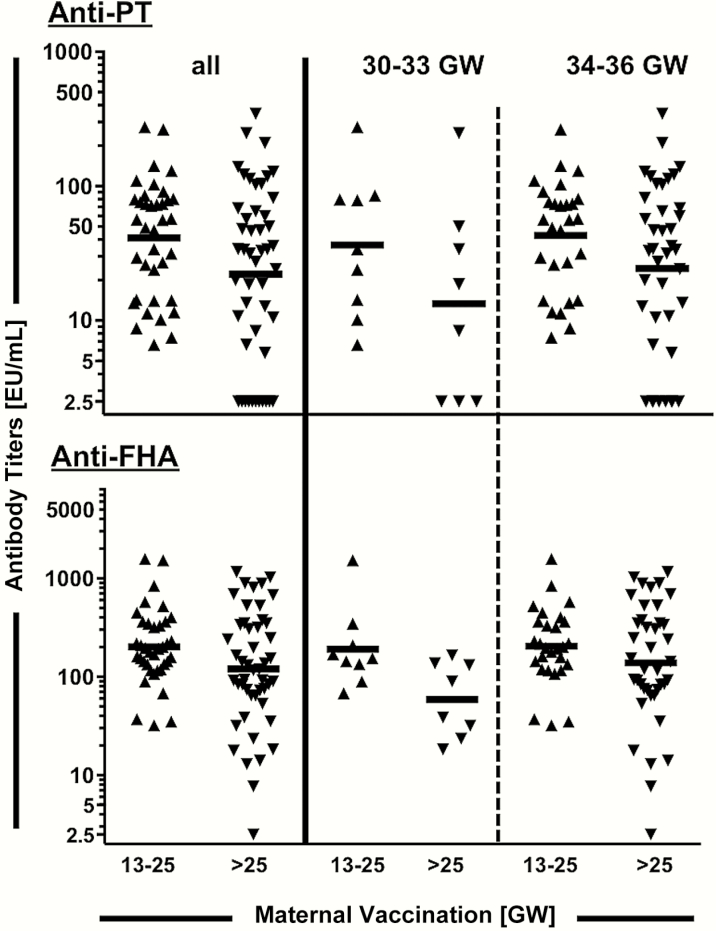
Birth antibody GMCs were significantly higher after second- compared to third-trimester immunization for both anti-PT (41.3 [95% CI, 29.6–57.5] EU/mL vs 22.1 [95% CI 14.3–34.2] EU/mL; P = .024) and anti-FHA antibodies (201.1 [95% CI, 149.7–270.1] EU/mL vs 120.2 [95% CI, 80.6–179.2] EU/mL; P = .040) (Figure 1). The ratio of second- to third-trimester anti-PT antibodies was significantly higher (1.87 [95% CI, 1.06–3.29]; P = .032), even after adjustment for maternal age, gestational age at birth, parity, and socioeconomic status (2.04 [95% CI, 1.15–3.61]; P = .016). For anti-FHA antibodies, the GMC ratio was 1.67 (95% CI, 1.00–2.81; P = .051), with an adjusted ratio of 1.57 (95% CI, .93–2.67; P = .092) (Supplementary Table 2). Dividing the population into early (GW 30 0/7–33 6/7) and late (GW 34 0/7–36 6/7) preterm neonates, even in those few born between GW 30 and 33, a second-trimester maternal immunization seemed to elicit higher birth anti-PT and anti-FHA antibody titers (Figure 1).
Figure 1.
Anti–pertussis toxin (PT) and anti–filamentous hemagglutinin (FHA) cord blood antibody concentrations by trimester of maternal immunization and gestational age at birth. Individual birth anti-PT and anti-FHA antibody concentrations after maternal tetanus-diphtheria-acellular pertussis immunization during the second trimester (gestational week [GW] 13 0/7–25 6/7) or third trimester (after GW 25 6/7) for all preterm neonates (left) or divided into early (GW 30 0/7–33 6/7, middle) and late (GW 34 0/7–36 6/7, right) preterm neonates; each point corresponds to 1 patient. Geometric mean concentrations are indicated with a horizontal line for each group. Seropositivity is defined as anti-PT ≥ 5 EU/mL.
None of the 37 preterm neonates born after second-trimester maternal immunization were seronegative, compared with 11 of 48 (22.9%; P = .002) in the third-trimester group. Following third-trimester immunization, the proportion of seronegative preterm neonates was high in both age groups (GW 30 0/7–33 6/7, 38%; GW 34 0/7–36 6/7, 20%). These differences persisted at higher antibody titer cutoffs (Supplementary Table 3).
We finally assessed the time interval between vaccination and delivery required to maximize maternofetal antibody transfer. An interval of 15 days was sufficient to observe significantly higher cord antibody titers in this preterm population (Supplementary Table 4). A growing number of seronegative neonates was observed as gestational age increased (Supplementary Figure 1).
DISCUSSION
This report is the first to show that preterm neonates benefit from second- rather than third-trimester Tdap maternal immunization. This effect is already seen for preterm births between GW 30 and 33 when antibody transfer was thought to be inefficient.
These results are unexpected, as the greater efficacy of placental transfer during the third trimester is well established [5]. This transfer is mediated through the neonatal Fc receptor (FcRn), expressed in the syncytiotrophoblast as of GW 13, when antibody transfer begins [9]. FcRn-mediated transport efficacy, which is both active and saturable, slowly increases with time as the cytotrophoblast becomes discontinuous. Consequently, the proportion of fetal to maternal total immunoglobulin G (IgG) antibody concentrations at birth increases steadily from 10% (GW 17–22) to 50% (GW 28–32) to ≥100% of maternal concentrations at term [4]. Following early pregnancy vaccination, maternal antibodies are thus likely transferred to the fetus with lower efficiency. We postulate this reduced daily transfer is compensated by the longer total transfer time, resulting in antibody accumulation in the fetal circulation. This longer transfer time may be the key element of the higher antibody levels following earlier maternal immunization.
Nevertheless, the half-life of anti-PT antibodies is short [10], such that antibodies elicited by maternal immunization early during pregnancy could wane prior to delivery and counterbalance the benefit of a prolonged in utero transfer. This suggests an additional mechanism protecting IgG antibodies from degradation better in the fetal than the maternal compartments. The catabolism vs recycling of serum IgG occurs in endothelial cells: only FcRn-bound IgG antibodies are protected from lysosomal degradation and transported back into the serum. As FcRn-mediated transport is saturable, the catabolism of IgG is faster at higher IgG serum concentrations [11]. We hypothesize that the low concentration of fetal total IgG does not saturate the FcRn receptors, such that most maternal IgG antibodies successfully bind to FcRn and are largely protected from degradation [12].
The relative contribution of antibody transfer duration and protection against degradation may not be directly tested: whether this is valid for most or all antigens will come from impending studies comparing early vs late maternal immunization against other pathogens such as respiratory syncytial virus and group B Streptococcus.
This study has limitations. First, its observational rather than randomized design does not rule out confounding factors influencing antibody transfer. Such factors were unidentified in our larger study including term infants [3], but nevertheless may exist. Information on prior Tdap vaccination is limited to the last 5 years, and may not exclude occult exposure to pertussis. Second, it does not include data for preterm neonates born before GW 30 0/7 and lacks power to detect an effect for preterm births between GW 30 0/7 and 33 6/7. Further studies are warranted to assess the benefits from early maternal immunization for very early preterm neonates. Recruitment was based on recruitment capacity and was interrupted after 19 months so as not to increase heterogeneity in the baseline risks of maternal exposure to pertussis. No increase in the rate of pertussis cases was observed during this period [13]. The effect of trimester on antibody titers remained statistically significant in the multivariable analysis, supporting the validity of our observations. We only measured antibody levels as surrogates for infant pertussis protection; demonstrating the effectiveness will require large ecological studies.
In conclusion, the transplacental transfer of maternal antibodies is effective sufficiently early in gestation so that the majority of preterm neonates born between 30 0/7 and 36 6/7 GW benefit from a maternal Tdap immunization performed during the second trimester of pregnancy. This extends our previous results in term neonates. Future recommendations on the optimal timing of maternal Tdap immunization should give consideration to these findings to hopefully increase protection against pertussis in both term and preterm neonates.
Supplementary Material
Notes
Acknowledgments. We thank all the women who participated in the study and the midwives who performed repeated blood sampling. We also thank Gianna Cadau, Paolo Valenti, and Stéphane Grillet for excellent technical assistance and Corinne Oberson and Suzanne Duperret-Vonlanthen for excellent administrative support.
Financial support. This work was supported by the Center for Vaccinology and Neonatal Immunology, University of Geneva, Switzerland.
Potential conflicts of interest. C.-A. S. has received grant support for preclinical and clinical studies from several vaccine manufacturers and has been part of the scientific advisory board of BioNet-Asia since March 2016. J. P. is the co-founder and chief scientific officer of BioNet-Asia and has filed a patent for a recombinant Bordetella pertussis strain. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Amirthalingam G, Campbell H, Ribeiro S, et al. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis 2016; 63(suppl 4):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–5. [PMC free article] [PubMed] [Google Scholar]
- 3. Eberhardt CS, Blanchard-Rohner G, Lemaître B, et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis 2016; 62:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol 1996; 36:248–55. [DOI] [PubMed] [Google Scholar]
- 5. Heininger U, Riffelmann M, Leineweber B, Wirsing von Koenig CH. Maternally derived antibodies against Bordetella pertussis antigens pertussis toxin and filamentous hemagglutinin in preterm and full term newborns. Pediatr Infect Dis J 2009; 28:443–5. [DOI] [PubMed] [Google Scholar]
- 6. Ercan TE, Sonmez C, Vural M, Erginoz E, Torunoğlu MA, Perk Y. Seroprevalance of pertussis antibodies in maternal and cord blood of preterm and term infants. Vaccine 2013; 31:4172–6. [DOI] [PubMed] [Google Scholar]
- 7. Berger JT, Carcillo JA, Shanley TP, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Collaborative Pediatric Critical Care Research Network (CPCCRN) Critical pertussis illness in children: a multicenter prospective cohort study. Pediatr Crit Care Med 2013; 14:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swiss Federal Statistical Office. Distribution of births by gestational age. Available at: https://www.bfs.admin.ch/bfs/en/home/statistics/health.assetdetail.212026.html. Accessed 13 February 2017.https://www.bfs.admin.ch/bfs/en/home/statistics/health.gnpdetail.2016-0427.html [Google Scholar]
- 9. Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol 1996; 26:1527–31. [DOI] [PubMed] [Google Scholar]
- 10. Van Savage J, Decker MD, Edwards KM, Sell SH, Karzon DT. Natural history of pertussis antibody in the infant and effect on vaccine response. J Infect Dis 1990; 161:487–92. [DOI] [PubMed] [Google Scholar]
- 11. Xiao JJ. Pharmacokinetic models for FcRn-mediated IgG disposition. J Biomed Biotechnol 2012; 2012:282989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J Immunol 2015; 194:4595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swiss Federal Statistical Office. Epidemiology of Pertussis between June 1991 and August 2015 "Sentinella-Meldungen Juni 1991 bis August 2015" (German). Bull BAG 2016; 08:137–9 http://www.bag.admin.ch/themen/medizin/00682/00684/01082/index.html?lang=fr . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.