Abstract
Large and growing segments of the U.S. population consume seafood or engage in marine recreation. These activities provide significant benefits but also bring risk of exposure to marine borne illness. To manage these risks, it is important to understand the incidence and cost of marine borne disease. We review the literature and surveillance/monitoring data to determine the annual incidence of disease and health consequences due to marine borne pathogens from seafood consumption and beach recreation in the United States. Using this data, we employ a cost-of-illness model to estimate economic impacts. Our results suggest that health consequences due to marine borne pathogens in the United States have annual costs on the order of $900 million. This includes $350 million due to pathogens and marine toxins specifically identified as causing food-borne disease, an estimated $300 million due to seafood borne disease with unknown etiology, $30 million from direct exposure to Vibrio vulnificus, V. parahaemolyticus and V. alginolyticus, and $300 million due to gastrointestinal illness from beach recreation. Although there is considerable uncertainty about the degree of underreporting of certain pathogen-specific acute marine-related illnesses, the conservative assumptions we have used in constructing our estimate suggest that it should be considered a lower bound on true costs.
Keywords: contaminated beach exposure, cost-of-illness model, health costs, marine borne disease, seafood borne illness, underreporting
Introduction
The marine environment contains millions of microbial pathogens and toxins that are both naturally occurring and foreign; and many of these microbial agents have been linked to human diseases (Thompson et al. 2005). As coastal urban communities grow and our reliance on marine environments for aquaculture and recreation increases, so do the risks of disease from these pathogens. The primary goal of this study is to produce an estimate of the annual human health costs for residents of the United States due to exposure to selected pathogens and toxins from the marine environment. This estimate will allow researchers and public health officials to target pathogens with the greatest economic impact to public health, and may lead to improvements in monitoring of marine waters by producing an economically optimal management strategy. We also identify areas for further research to develop a more complete economic analysis.
The pathogen-specific cost estimate in this paper includes two viral pathogens, fifteen bacterial pathogens, and four marine toxins. Many of these pathogens and toxins are endemic to the marine environment (e.g. the Vibrio species); others enter the water via fecal contamination (e.g. Norwalk virus, Salmonella and Campylobacter). Over the past 15 years, about 9% of seafood related outbreaks with known etiology were caused by viruses (predominately Norwalk virus), 25% were caused by bacteria and 64% were from marine toxins (CSPI 2007).
The pathogens selected for this analysis are those for which there is evidence of links to human disease, with documented cases in the United States. Seafood is thought to be the most common route of exposure, although this may depend in part on the limited data on illnesses due to direct exposure and the lack of reliable methods for detecting specific pathogens in marine waters. Our approach excludes marine agents for which there is too little surveillance to obtain accurate measurements, such as parasites, anthropogenic chemical agents, persistent organic pollutants, heavy metals and pharmaceutically active products. Potential chronic health effects due to these agents may contribute significantly to health costs (REF).
The two primary routes of transmission for marine borne disease are seafood consumption and direct exposure from beach recreational environments. Direct exposure includes accidental ingestion of contaminated wate exposure to skin, eyes, and ears during swimming and the inhalation of aerosolized toxins while at the beach. The methods used to estimate disease incidence and cost are different for each route of transmission, largely because of differences in case reporting and pathogen identification.
Seafood is a leading cause of foodborne disease with known etiology, responsible for 10–20% of outbreaks (2 or more cases caused by the same source) among all food types and about 5% of all individual illnesses (CSPI 2007; Huss et al. 2004). The Centers for Disease Control and Prevention (CDC) is the agency primarily responsible for tracking and monitoring foodborne disease, and maintains several surveillance systems that are the basis for incidence and cost estimates of seafood borne illness. The limitations of underreporting and unknown etiology in these data systems must be considered in interpreting the reported figures and calculating cost and incidence estimates.
Most surveillance systems record only 1%–10% of foodborne cases (CDC 1988; Huss et al. 2004) because in many of these cases medical help is not sought. Illnesses with mild symptoms (such as that caused by Norwalk virus) or those endemic to particular regions and thus familiar to local residents (such as CFP in the tropics) are more likely to go unreported. The reporting rate also depends on whether the surveillance system relies on healthcare providers to report a disease (passive surveillance) or if regular outreach to laboratories and hospitals encourages reporting (active surveillance).
Even when help is sought by the patient, health care professionals may either misdiagnose or fail to recognize the illness as marine borne, may not report the illness to public health officials, or may not obtain specimens for diagnosis. When specimens are available, laboratories are not always able to perform the necessary diagnostic tests. The lack of diagnostic techniques often makes it impossible to identify the pathogenic etiology (Olsen et al., 2000). In 1998 improvements in the laboratory method for detection of Norwalk virus resulted in increases in seafood-borne disease attributed to Norwalk (CSPI, 2007). Prior to the change in methodology, few cases of seafood borne illness were attributed to Norwalk virus. A recent study has shown that Staphylococcus aureaus, including MRSA and MSSA, has been found in the recreational marine environments from bather shedding (Plano et al 2011). Given the high number of Staphylococcus aureus infections in hospitals (Moran et al 2006; McCraig et al 2006) and number of deaths due to this bacteria (Klein et al. 2007), inclusion of these illnesses could significantly increase the cost estimate of marine borne illness.
While the incidence of seafood borne disease is largely estimated from surveillance data, illnesses from direct exposure are estimated primarily using modeling techniques. An exception is the Cholera and Other Vibrio Illness Surveillance (COVIS) system established in 1988 to record the number of illnesses from exposure to the bacterial Vibrio species. Although most Vibrio infections result from ingestion of contaminated seafood, 12%–28% are from direct exposure to marine water (CDC, 1999–2006) resulting in wound infections (Shapiro et al. 1998).
Most incidence estimates for disease from direct exposure model the number of excess cases of gastrointestinal illness using risk relationships between polluted water and illness. These relationships are derived from randomized trial studies (Fleisher et al. 1996) and prospective cohort studies (Cabelli et al. 1982), and show that swimmers exposed to high levels of fecal indicator bacteria experienced adverse health symptoms including gastrointestinal illness (Cabelli et al. 1982, Haile et al. 1999), respiratory illness, and infections in the eyes, ears and skin (Fleisher et al. 1998; Fleisher et al. 2010).
Using these relationships and an exposure index based on Enterococcus levels and swimming exposure, Given et al. (2006) estimated an excess 627,800 to 1,479,200 cases of gastrointestinal illnesses at 28 beaches in Southern California, with an economic loss of $21 to $51 million dollars. Similarly, 36,778 excess cases of gastrointestinal illnesses per year were found at two beaches in Southern California, resulting in a $3.3 million loss (Turbow et al. 2003). Although these studies were limited to a small region where recreational beach attendance is high, it is likely that public health costs exist at other recreational beaches around the country.
The adverse effect of ocean exposure on health outcomes is not limited to fecally contaminated waters. Aerosolized brevetoxins produced by the marine algae Karenia brevis has been associated with respiratory symptoms among asthmatics on beaches in Southern Florida. Studies have found a significant increase in intensity of respiratory symptoms and emergency room admissions for respiratory illnesses when an algae bloom was present Kirkpatrick et al. (2006).
The pathogenic etiology of most illnesses from direct exposure to ocean water or the marine environment is not well understood. Fecal coliform levels are typically used as indicators of fecal contamination; but research linking specific pathogens to disease outbreaks is complicated by the broad spectrum of pathogens in beach water (NRC 1999) and the high degree of temporal and spatial variability (Boehm 2007). For these reasons most studies to date have been limited to estimating costs associated with general beach exposure.
Methods
The first step to determine a cost estimate is to identify the marine borne pathogens and toxins that are major disease agents rather than those causing a few sporadic cases. Although many pathogens in the marine environment are capable of causing human illness, we focus on those that were likely to have a significant economic impact. Sporadic cases are unlikely to account for significant costs unless the disease is life threatening or requires extensive medical attention. We start by generating a broad list of pathogens that cause human disease (Thompson et al., 2005; Lees 2001; Feldhusen, 2000; CDC Outbreak Surveillance Summaries 1993–2006). We narrow the list to include only pathogens that have been documented to cause illnesses in the United States due to ocean exposure or seafood ingestion and that are likely to have significant costs because they are widespread or cause severe health consequences.
Estimating the annual human health costs requires an estimate of the incidence of disease and a cost per illness. We review the literature and surveillance and monitoring data to determine an annual incidence of marine borne disease and health consequences. Using this data, we employ a cost-of-illness model used by the United States Department of Agriculture’s (USDA) Economic Research Service (ERS) to estimate economic impacts. The model divides cases into four severity categories and assigns a cost to each (Frenzen et al 2005; Buzby and Roberts 1996). We multiply the total number of cases in each category by the corresponding category cost. The sum over all four categories represents the annual cost for each pathogen.
This approach acknowledges the bimodal nature of many marine borne diseases, with symptoms that are either mild or severe, and incorporates the level of medical and hospital services used. Cases are categorized by level of medical care: 1) did not visit a physician or seek medical treatment (lost productivity), 2) visited a physician, 3) were hospitalized, or 4) prematurely died. Given that many marine borne illnesses are not reported and are relatively mild in severity, assigning a lower cost to illnesses that do not require medical attention is a key aspect to this model.
Other than for Vibrio species and K. brevis, we find that there are insufficient data to estimate with enough accuracy the pathogen-specific costs of illness due to direct exposure. We therefore adopt a general health cost estimate for direct exposure by extrapolating findings from a beach exposure study in Southern California to the rest of the country.
Disease incidence
Seafood borne
We use information from the CDC’s surveillance systems to estimate the number of cases of seafood borne illness (table 1), taking into account limitations of surveillance data and underreporting. For each pathogen, we first determine an average annual number of surveillance cases caused by seafood consumption, either from outbreak data (which exclude sporadic cases or cases not identified as part of an outbreak) or from passive surveillance data (which include sporadic cases). For most pathogens, a scaling ratio is applied to estimate the total reported cases that takes into account the exclusion of sporadic cases and/or cases not captured by passive or outbreak surveillance. The method for determining the scaling ratio varies by pathogen and depends on the type of surveillance through which that pathogen is reportable.
Table 1.
Annual incidence estimates for diseases with known etiology from seafood or recreational exposure
| Surveillance cases | Scaling ratio | Total reported | Underreporting ratio* | Incidence estimate | |
|---|---|---|---|---|---|
| Norwalk | 4842o | 38 | 183,996 | ||
| Hepatis A | 5b,c | 5r | 23 | 3 | 68 |
| Campylobacter jejuni | 7b,c,d, | 250p | 1813 | 38 | 68,875 |
| Clostridium botulism | 6n | 6 | 2 | 12 | |
| Clostridium perfringens | 19c,d | 10o | 194 | 38 | 7,372 |
| Plesiomonas | 2b | 10o | 20 | 38 | 760 |
| Salmonella | 52b,c,d | 14p | 732 | 38 | 27,824 |
| Shigella | 8b,c,d | 100p | 798 | 20 | 15,950 |
| Staphylococcus aureus | 2c,d | 10o | 17 | 38 | 627 |
| V. alginolyticus -seafood | 4a | 2q | 8 | 20 | 160 |
| V. alginolyticus -direct | 26a,j,k | ||||
| V. cholera O1 | 6a | 6 | 2 | 12 | |
| V. cholerae non-O1 | 36a | 2q | 72 | 20 | 1440 |
| V. fluvialis | 19a | 2q | 38 | 20 | 760 |
| V. hollisae | 7a | 2q | 14 | 20 | 280 |
| V. mimicus | 6a | 2q | 12 | 20 | 240 |
| V. parahaemolyticus-seafood | 167a | 2q | 334 | 20 | 6,680 |
| V. parahaemolyticus-direct | 19a,j,k | ||||
| V. vulnificus-seafood | 57a | 2q | 114 | 2 | 228 |
| V. vulnificus-direct | 31a,j,k,l | ||||
| Alexandrium/Gymnodinium (PSP) | 8b,c,h,i,m | 10o | 75 | 10 | 750 |
| Gambierdiscus toxicus (CFP) | 65b,c,e,f,g,h,m | 10o | 651 | 67 | 43,588 |
| Karenia brevis (NSP) | 4c,i | 10o | 44 | 10 | 440 |
UR for category 1 and category 2; A UR of 1.3 is used for all pathogens for categories 3 and 4.
CDC, COVIS 1999–2006: We included all instances of Vibrio isolates in either stool or blood, the primary sites for seafood borne illness. When there was more than one isolate per patient, we applied the percentage of isolates that were seafood borne to the total number of patients.
CSPI, 2007 We assume an equal number of cases per outbreak for each pathogen and toxin. We applied the percentage of outbreaks attributable to CFP (22%) to the total number of cases
The National Botulism Surveillance System, 2001–2007
Ratio of cases in Outbreak Surveillance (CDC 1993–2006) to cases in FoodNet (CDC 2000–2004) from 2000–2004.
Ratio of cases in COVIS (CDC 1999–2006) to cases in FoodNet (CDC 2000–2004) from 2003–2004.
Ratio of cases in Outbreak Surveillance (CDC 1993–2006) to cases in NNDSS (CDC 2001–2005) from 2001–2005.
Pathogens only reportable through outbreak surveillance (Plesmonias, Staphylococcus, Clostridum Perfringens, CFP, NSP and PSP) are scaled by 10, a multiplier proposed by Mead et al. (1999) to account for sporadic cases. Some pathogens are reportable through outbreak surveillance as well as passive surveillance (Vibrio species, Clostridium botulism, Hepatitis A) or active surveillance (Vibrio species Campylobacter, Salmonella and Shigella). For these pathogens, we determine the ratio of outbreak cases to passive cases or to active cases over a five year period. This ratio is used to scale up the outbreak estimate. Because the Vibrio species is included in both passive and active surveillance, we use this method to scale up the cases from passive surveillance rather than from outbreak data. Clostridium botulism and Vibrio Cholera O1 are reportable through passive surveillance and tend to be severe illnesses so we assume most cases are included in the data and do not apply a scaling ratio.
Seafood borne Norwalk virus cases are subject to very limited surveillance and a high degree of suspected underreporting. We estimate that of the 27,171 reported outbreak cases of foodborne Norwalk virus from 1998–2002, 2% were from seafood (CDC Outbreak Surveillance Summaries 1993–2006; CDC 2006c). We apply this percentage to the 9.2 million cases of foodborne illness from Norwalk virus estimated by Mead et al. (1999) and divide by an underreporting ratio of 38 (as reported by Mead et al. (1999) for mild illnesses) to get an active surveillance estimate.
Once we adjust the number of outbreak or passive surveillance cases to reflect the total reported seafood borne cases, we apply an underreporting ratio to calculate a more probable incidence of disease. This ratio accounts for unreported cases due to a failure of medical practitioners to report the illness to public health authorities, limitations in laboratory practices (i.e. failing to perform the necessary diagnostic test, the physician does not obtain a specimen) and/or the ill person’s decision not to seek medical help. For seafood borne illness we assign one of three underreporting ratios to the first two categories (lost productivity and physician visit) depending on illness severity: 38 times the reported number for mild cases, 20 times the reported number for moderately severe cases, and 2 times the reported number for very severe cases (Mead et al. 1999). For pathogens with no known underreporting ratio, we apply one of these estimates depending on the type and duration of the disease symptoms. We apply a weighted average underreporting ratio of 67.5 for CFP in this study (based on underreporting in Hawaii and Florida (Hoagland et al. 2002)) and an underreporting ratio of 10 is used for PSP and NSP based on an estimate from Todd (1989).
Hospitalizations and deaths are also underreported for several reasons (Mead 1999), although to a lesser degree. We apply one underreporting ratio to all pathogens and marine toxins in the two higher severities. Because there is little published information on the number of seafood borne illnesses that lead to hospitalization or premature death, our estimate is based on a study of notifiable infectious diseases that found only 79% of AIDS, tuberculosis and sexually transmitted disease cases were reported (Doyle et al. 2002). Given the severity of these illnesses compared to marine borne diseases, it is likely that the fraction of reported cases in our study is substantially lower. However, in the absence of specific data for the more severe foodborne diseases, we apply an underreporting estimate of 25% to each of these categories across all pathogens.
Direct exposure
The number of illnesses from infection of Vibrio vulnificus, Vibrio parahaemolyticus and Vibrio alginolyticus due to direct exposure is determined from COVIS (CDC 1999–2006) by counting the isolates from wound infections and from the CDC’s Surveillance for Waterborne Disease and Outbreaks Associated with Recreation from 2003 to 2006 (CDC 2008b; CDC 2006a). We estimate the total number of respiratory illnesses from aerosolized K. brevis exposure by assuming that the annual predicted 218 emergency room visits (Hoagland et al. 2009) accounts for 1% of the total number of cases, a proportion found for other mild illness such as Salmonella (ERS 2010). No scaling ratio or underreporting ratio is applied to cases from direct exposure (table 1), mainly due to a lack of information on the degree of underreporting.
Aside from the three Vibrio species and K. brevis, there is not enough surveillance data on other pathogens or marine toxins to produce a reliable estimate of illnesses from direct exposure. Therefore, we use a model to estimate the annual number of illnesses from beach exposure in the United States. Specifically, we extrapolate the 1,500,000 excess cases of gastroenteritis found in a study of beach exposure in Southern California (Given et al. 2006) to the rest of the country. Using the Environmental Protection Agency’s beach and swimming advisory data (EPA 2006) we determine the percentage of beach days (number of beaches multiplied by the number of swimming days in a year) for each state that had at least one notifiable action (either a closure or a swimming advisory) due to high levels of indicator bacteria. We multiply this by the total number of swimming participation days from national marine-recreation participation data (Leeworthy and Wiley 2001) to estimate exposure at the state level. Using the proportion of excess cases of GI illness to our exposure estimate (in days) in California we estimate excess GI cases in every other state.
Estimating cost
Once an incidence estimate is determined, we assign a cost to each of the four severity categories. We use the proportion of illnesses that fall into each category to determine a total cost for each pathogen and marine toxin, and exposure to contaminated beach water.
Proportions
To estimate the proportion of cases that falls into each category, we used several data sources and follow the method for categorization that was used by ERS (table 2). Each case is included in only one of the four categories. If the reported hospitalizations include cases that also died, we exclude 90% of the deaths from the hospitalized proportion because it has been estimated that 90% of patients who died were hospitalized first (Frenzen et al. 1999). Except for the marine toxins, the proportion of cases requiring no medical care is determined by subtracting the sum of the last three categories’ proportions from one. To determine the number of cases in each category from aerosolized K. brevis, we assign the estimated number of emergency room visits (Hoagland et al 2009) to the physician visit category and, using proportions similar to those of a low severity illness, extrapolate to estimate the total cases in the other three categories.
Table 2.
Severity category proportion of illnesses and corresponding cost estimates by causative agent
| Proportions | Cost per year (in millions) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Severity categories | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Norwalk | .898a | .100b. | .0023c | .00001c | 8.26 | 9.20 | 0.14 | 0.42 | 18.02 0 |
| Hepatis A | .791a | .186d | .0216c | .00096c | 0.0027 | 0.0063 | 0.0064 | 0.14 | 0.16 |
| Campylobacter jejuni | .941a | .054f | .0051c | .00008c | 3.24 | 1.86 | 0.13 | 0.59 | 5.82 |
| Clostridium botulism | .0h | .138g | .7931c | .06900c | 0 | 0.00083 | 0.062 | 2.69 | 2.75 |
| Clostridium perfringens | .887a | .113e | .0002c | .00003c | 0.330 | 0.42 | 0.00042 | 0.035 | 0.78 |
| Plesiomonas | .887a | .113e | .0k | .0k | 0.034 | 0.043 | 0 | 0 | 0.077 |
| Salmonella | .875a | .113e | .0120c | .0004c | 1.220 | 1.57 | 0.12 | 1.90 | 4.81 |
| Shigella | .800a | .186d | .0139c | .00002c | 0.640 | 1.48 | 0.15 | 0.083 | 2.35 |
| Staphylococcus aureus | .886a | .113e | .0010c | .00001c | 0.028 | 0.035 | 0.00022 | 0.0012 | 0.065 |
| V. alginolyticus | .604a | .186d | .2000c | .01020c | 0.0048 | 0.015 | 0.021 | 0.53 | 0.57 |
| V. cholera O1 | .334a | .186d | .4568i | .02273i | 0.00020 | 0.0011 | 0.036 | 0.187 | 0.92 |
| V. cholerae non-O1 | .503a | .113e | .3363i | .04737i | 0.036 | 0.081 | 0.32 | 22.17 | 22.6 |
| V. fluvialis | .403a | .186d | .3867i | .02463i | 0.0150 | 0.071 | 0.19 | 6.08 | 6.36 |
| V. hollisae | .217a | .186d | .5968i | .00000i | 0.0030 | 0.026 | 0.11 | 0 | 0.14 |
| V. mimicus | .466a | .186d | .3030i | .04478i | 0.0056 | 0.022 | 0.047 | 3.50 | 3.57 |
| V. parahaemolyticus | .694a | .113e | .1837i | .00886i | 0.23 | 0.38 | 0.80 | 19.22 | 20.63 |
| V. vulnificus | .0h | .118 g | .5687i | .31333j,i | 0 | 0.013 | 0.84 | 232.18 | 233.04 |
| PSP | .300q | .3375p | .3375s | .02500n,o,p | 0.011 | 0.13 | 0.33 | 12.58 | 13.50 |
| CFP | .300q | .4490p | .2500t,u,v,w,x | .00100n,o,p,r | 0.65 | 9.79 | 2.11 | 4.23 | 16.78 |
| K. brevis (NSP) | .300q | .3400p | .3600 s | .0k | 0.0067 | .075 | .206 | 0 | .29 |
| Seafood Subtotal | 14.69 | 25.22 | 5.63 | 306.78 | 352.30 | ||||
| V. alginolyticus | .850 a | .0 | .1369l,m | .00971l,m | .0012 | 0 | .035 | 1.24 | 1.27 |
| V. parahaemolyticus | .600a | .0 | .3896l,m | .01493l,m | .00057 | 0 | .074 | 1.42 | 1.49 |
| V. vulnificus | .170a | .0 | .65l,m | .1842l,m | .00025 | 0 | .20 | 28.09 | 28.29 |
| K. brevis (respiratory) | .899z | .10y | .001y | .0y | .62 | .69 | .14 | 0 | 1.45 |
| Contaminated beach water | .9899A | .01z | .0001z | .0z | 255.4 | 25.8 | 5.16 | 0 | 286.36 |
| Direct exposure subtotal | 179.42 | 26.49 | 5.61 | 30.75 | 242.24 | ||||
| OVERALL TOTAL | 194.11 | 51.71 | 11.24 | 337.53 | 594.54 | ||||
1-sum of proportions for categories 2, 3, and 4
ERS, Online Cost Calculator for Estimating the Economic Cost of Illness (E. Coli)
ERS, Online Cost Calculator for Estimating the Economic Cost of Illness (Salmonella)
1-sum of proportions for categories 1, 3 and 4
Given the severity of the illness and the high proportion of hospitalized cases we make the assumption that all cases seek some level of medical care
No record of death or hospitalization in the United States (CDC)
.7 (Lawrence 1980)- sum of proportions for categories 3,4
our estimates, assumed similar to low severity/salmonella
our estimates, based on typically minor symptoms
For the majority of the pathogens, the proportion of cases in the physician visit category was unavailable. For these pathogens, we assume that for mild illnesses the proportions are similar to those published by ERS for Salmonella, and for more severe illnesses the rates published for E.Coli. An illness was deemed either mild or severe based on the type and duration of symptoms. The proportions for PSP, NSP and CFP are based on studies that reported approximately 30% of CFP cases have only gastrointestinal symptoms () and 70% have neurological symptoms (de Fouw et al. 2001; Lawrence 1980;, Luber et al. unpublished), although these proportions vary by geographic location and specific outbreak. We assume that some medical help would be sought for neurological symptoms. The proportions used for illnesses from contaminated beach water are based on the typically mild symptoms experienced.
Cost
While some cost estimates include intangible factors such as impaired quality of life or non-medical health costs (Scott et al. 2000; Todd 1995; Scharff et al. 2009), we estimate a cost for each category that only includes lost wages, physician and hospital services, and the statistical cost of a premature death. The cost estimates do not include chronic effects, pain and suffering, inconvenience, and time lost from recreational activities. In a broader study of foodborne illness, Salmonella cases not requiring a physician’s visit were found to cost (in 2008 dollars) $52 while cases requiring a physician’s visit cost $536 (Frenzen et al. 1999; ERS 2010). Mild cases of E. Coli infection had similar costs, with $30 per case with no physician visit and $540 per case for those with a physician visit. Each case of Salmonella that required hospitalization cost over $11,000; each E. Coli case with hospitalization cost about $7,400. Based on these findings, we assume in our calculations that an illness that does not require a physician visit costs $50, a physician visit costs $500, and each hospitalization costs $10,000. We use the same category costs for illness from exposure to contaminated waters.
Although rare, premature deaths due to Salmonella and E. coli were estimated to cost roughly $5 million (ERS 2010). This estimate is based on two widely cited labor market studies (Fisher et al. 1989; Viscusi 1993) that use the Hedonic-Wage approach or “willingness to pay”. An alternative is the ‘human capital’ approach developed by Landefeld and Seskin (1982), which takes into account the age of disease onset, but we lack sufficient data to quantify the cost of premature death by this method. Estimates of the value of a statistical life (VSL) ranges considerably depending on the approach used but several studies have suggested a reasonable estimate is about $5 million with a range of uncertainty of about 3.2 million (EPA 1997; Fisher et al. 1989; Viscusi (1992,1993); Desvousges et al. 1998).
Sensitivity analysis
Our model includes several parameters that are difficult to measure accurately. In several instances, we had to make assumptions to estimate these parameters in our model and, as a result, our cost estimates are characterized by varying degrees of uncertainty. To quantify this uncertainty we conduct a sensitivity analysis for seafood borne illness costs and pathogen specific illness from direct exposure. While there is also uncertainty in the model estimates for illness from beach contamination and from unknown etiology, there were two few parameters to conduct a detailed sensitivity analysis. The estimates of illness from beach contamination and from unknown etiology should be considered order of magnitude estimates.
For seafood borne and pathogen specific illness estimates, we perturb the scaling ratio, the underreporting ratio and the proportion by 20%. (Two pathogens have scaling ratios of one, so they are not varied since a scaling ratio cannot be less than one.) Because it is necessary to maintain the unit sum across the severity category proportions, we modified the proportions in the adjacent categories when perturbing these proportions based on the rationale that uncertainty in a proportion would likely be reflected in the adjacent category. For example, while is it possible that 20% of the people who did not seek medical care (category 1) did in fact see a doctor (category 2), it is less likely that a person who was incorrectly determined to have not sought medical care (category 1) in fact died (category 4).
Results and Discussion
We calculate the annual health costs of seafood borne diseases to be $360 million and the costs of illnesses from direct exposure to Vibrio vulnificus, Vibrio parahaemolyticus, Vibrio alginolyticus and aerosolized Karenia brevis to be over $30 million. We estimate the cost of the 5 million cases of gastrointestinal illness from beach exposure to be almost $300 million. Combined, this suggests that marine borne diseases with known etiology in the United States have annual health costs on the order of $600 million (graph 1).
Graph 1.
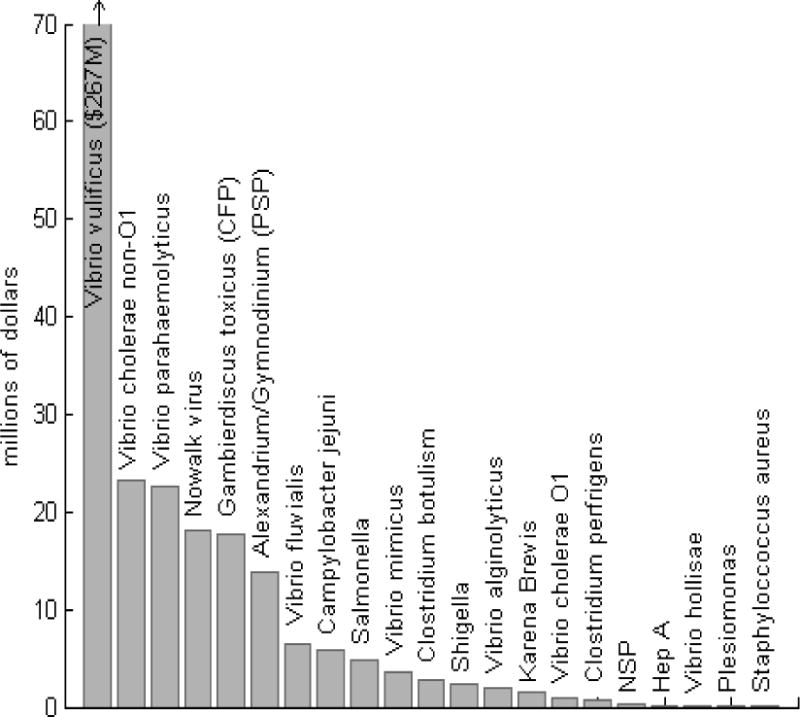
Overall cost of pathogen specific marine borne illnesses
Our estimate of pathogen-specific seafood borne illness cost excludes cases with unknown pathogenic etiology, including those that were misdiagnosed or could not be identified as marine borne and therefore is likely anunderestimate of the total cost of seafood borne disease. Over 80% of the estimated 76 million annual cases of foodborne disease in the United States have an unknown etiology (Mead et al. 1999). Assuming that 5% of the 62 million cases with unknown etiology are from seafood (CSPI 2007; Huss et al. 2004), the number of additional seafood borne cases could be over 3 million with costs of almost $300 million. (We assume here that these cases have severity proportions similar to those of Norwalk virus.) Including the cost of seafood borne illnesses with unknown etiology almost doubles the total cost of seafood borne illness.
Cost of seafood borne illness
Our results indicate that premature deaths contribute most to the total costs of seafood borne illness ($315 million) (table 2), with the remainder due to medical care ($25 million for physician visits and $6 million for hospitalizations) and lost productivity ($15 million). Vibrio Vulnificus is the most costly marine borne pathogen, accounting for about a third of the total seafood borne costs. and over 85% of the costs of Vibrio infections from direct exposure (graph 1). This is primarily a result of the high rate of premature death among Vibrio vulnificus cases. With a death rate of 31% for seafood born infection and 18% for infections from direct exposure, the cost from premature death ($238 million) accounts for 99% of the total Vibrio vulnificus health costs and 75% of the total cost of premature death.
Following Vibrio vulnificus, the five most costly pathogens result in health costs between $15 and $20 million annually. Vibrio parahaemolyticus and Vibrio cholera Non-O1 have an annual cost on the order of $20 million, while Norwalk virus and CFP rank next with costs of $18 and $17 million, respectively. PSP costs are slightly lower, around $13 million. Campylobacter and Vibrio fluvius have costs between $5 and $10 million while Vibrio mimicus, Shigella, Clostridium botulism and Vibrio Cholera all have costs between about $4 million and $1 million. The rest of the pathogens have individual annual costs less than $500,000.
Cost of illness from direct or recreational exposure
The health costs from direct exposure are more difficult to assess; but results from the limited surveillance data and the extrapolation analyses suggest that they are on the order of $240 million/year (table 2). Using incidence data from COVIS, it is estimated that Vibrio vulnificus infections cost over $28 million while Vibrio alginolyticus cost over $1 million. Vibrio parahaemolyticus infections are less common from direct exposure and have costs of about $1.5 million compared to the $21 million from ingestion of seafood. Respiratory illness from K. brevis corresponds to a cost of over $2 million. The health costs from contaminated beach water are the largest component of direct exposure health costs. We estimate a total of over five million cases of excess gastrointestinal illness due to beach exposure, corresponding to almost $300 million/year.
Sensitivity analysis
To evaluate cost model sensitivity, we perturbed the model variables by 20% in various combinations to yield 243 different cost estimates for all of the seafood borne pathogens except Norwalk virus, which had only 81 cost combinations because no scaling ratio was used. Pathogens causing illness from direct contact had only 9 cost combinations because no scaling ratio or underreporting ratio was used. In the sensitivity analysis, the total cost for pathogen specific illness ranged from $166 million to $967 million. However, random sampling of the sensitivity results indicates that when the lower and upper 5% of the possible combinations are excluded, the total cost range is approximately $300 million to $550 million, considerably closer to the point estimate.
Figure 1 includes error bars showing the 2.5% and 97.5% percentiles of the costs for each pathogen in the sensitivity analysis. The pathogens with the greatest cost range are V. vulnificus, V. parahaemolyticus and CFP, while plesmonias, staphylococcus and Hepatitis A have the narrowest ranges. Pathogens with a high uncertainty relative to their estimated cost, such as CFP and vibrio hollisae, tend to have a high hospitalization rates (category 3) and low death rates (category 4). The sensitivity analysis perturbed adjacent categories by 20% to maintain the same total number of cases. For pathogens with high hospitalization rates, these perturbations transferred cases to the death category, resulting in a broader range of cost outcomes due to the high cost of death.
Figure 1.
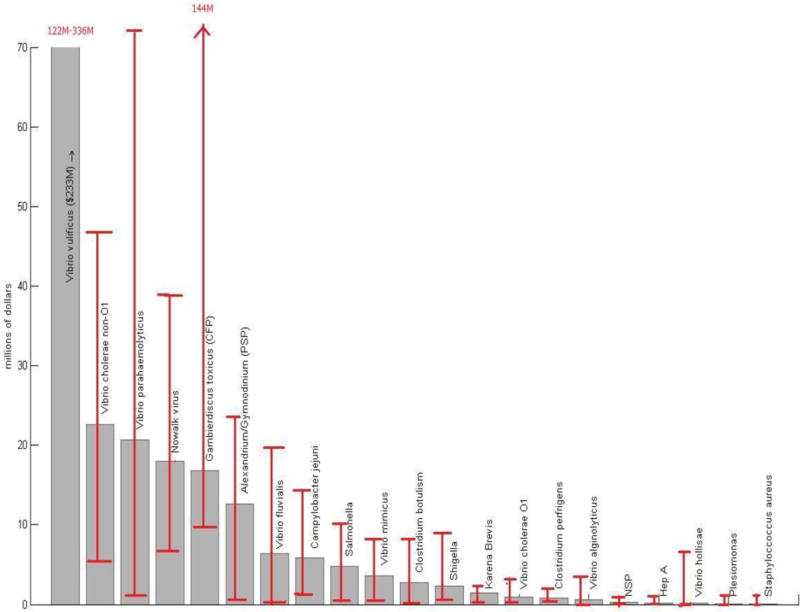
We examined the impact of death on the cost uncertainty because it accounts for a large fraction of the total cost. To assess whether the high cost of death or a high death rate among a few pathogens was driving the total cost uncertainty, we also ran the sensitivity analysis both with an altered cost of a premature death and by removing the cost of death from the estimate. Although the cost range without death decreased under the different scenarios, the upper and lower bounds of the cost estimates remained proportionally similar to the point cost estimate, suggesting that death alone does not primarily drive the range of uncertainty.
One goal of this study was to identify the pathogens and marine toxins that prevention and monitoring efforts should target to produce the most significant economic benefits. Vibrio vulnificus is the most costly pathogen in our study with an annual cost of illness ten times higher than any other pathogen; it makes up 66% of the seafood borne illness health costs and 26% of the total health costs. The high costs are primarily driven by high death rates, underscoring the public health importance of this illness. Despite efforts by government agencies to address the problem, deaths due V. vulnificus have not declined over recent years (CDC 1999–2006; FDA 2009).
While V. vulnificus is the most significant contributor to the total health cost estimate, other naturally occurring pathogens also have relatively high health costs. For example, V. parahaemolyticus had an annual health cost of over $20 million, and recent evidence suggests that illness from this pathogen may be increasing. In 2006 a total of 177 cases were reported in New York, Washington, and Oregon, substantially more than in the previous five years (CDC 2006d). Ciguatera Fish Poisoning (CFP) had health costs approaching $17 million. Due to changing environmental conditions, such as warming ocean temperatures and coral bleaching, we may see an increase in the source of the toxin Gambierdiscus toxicus in the future. Milder, self-limiting illnesses from both seafood consumption and direct or recreational exposure also have a significant economic impact. Although Norwalk virus rarely results in death, the costs of the estimated 184,000 cases annually approach $18 million. Similarly, the total cost of gastrointestinal illness from beach exposure is significant because of the large number of cases (estimated 5 million), even though most do not seek medical care.
The cost estimates for seafood borne illness and from beach contamination require assumptions for the model parameters. Most of the parameters based on published data and our assumptions are conservative, but uncertainty remains in our estimate. For example, the estimated cost of a premature death is an important factor in the results. Without the cost of death included, the total health cost from illness with known etiology is $257 million – about 40% of the overall cost. The cost of premature death accounts for almost 90% of the total seafood borne health costs. The cost model assumes that the value of a statistical life (VSL) is $5 million. Although VSL in the literature can range from $.5 million to $21 million (Viscusi and Aldy 2003), the commonly used range is substantially narrower, from about $5.5 million to $7.5 million (Kniesner et al 2007). Among federal agencies, the FDA estimates a VSL to be about $8 million, the EPA has a slightly lower estimate of $7 million, and the ERS/USDA estimate is about $5.5 million. We have used the lower end of this range, and increases in the VSL would substantially increase the total health cost estimates.
In addition to the conservative parameter estimates, thecost estimate for marine borne illness may represent a lower bound of the true costs because we do not include costs that difficult to measure chronic effects and pain and suffering. A recent study that used a cost-of-illness model developed by the FDA that included a measure of chronic pain and suffering and functional disability found the total cost of food borne illness to be $152 billion (Scharff et al 2009; Scharff 2010). The productivity losses associated with pain and suffering are also likely important, but assigning reliable cost estimates introduces additional uncertainly. We used a lost productivity estimate that only included lost days of work and foregone compensation, so our cost per case is about $1300 versus a cost of about $1800 per case in the alternative cost model (Scharff 2010).
Similarly, chronic health effects from marine borne illnesses may introduce substantially higher costs than we have assumed. While there are few data from which to quantify the prevalence of long-term symptoms, it has been estimated that chronic sequelae may occur in 2%–3% of food-borne illnesses generally, and that the costs of these health consequences could be greater than the costs of the acute symptoms (Lindsay 1997). For example, the estimate cost of chronic effects from ten cases of E.Coli in 2006 was $7,363,814 (2007 dollars) in medical care expenses alone (ERS 2010). The cost estimate here does not address marine agents that are thought to be particularly associated with chronic effects (REF), such as such as heavy metals or persistent organic pollutant (REF). Similarly, we do not incorporate low cost but high frequency costs often associated with mild illness such as over-the-counter medication.
The health cost estimates depend in part on disease incidence. Exposure and incidence rates reflect patterns of human behavior around the oceans and seafood. For example, there are differences in incidence rates among certain subgroups, such as those with compromised immune systems or island/coastal populations who are high seafood consumers. It has been shown that Asian and Pacific Island communities in California and tribal communities in the Pacific Northwest have an increased risk of seafood borne disease because of their greater seafood consumption (NEJACM, 2002), and immunocompromised individuals have the greatest risk of death from exposure to vibrio vulnficsu (REF). Future research should include an evaluation of the costs for specific communities.
Disease incidence can be affected by monitoring and warning systems that are in place. For example, shellfish are routinely monitored for HAB toxins, and harvesting is restricted when toxins are detected. This monitoring is widely credited with limiting illness from HAB toxins in shellfish. To reduce risks from direct exposure to aerosolized brevetoxins, a real-time reporting system was recently implemented on several public beaches in Florida (Kirkpatrick et al. 2008). A useful extension of the results reported here would be an evaluation of the costs of monitoring and warning programs relative to the residual health costs to determine where additional investments in monitoring may be warranted.
In the absence of a model designed specifically to estimate the cost of seafood borne illness, we use a model that was developed to estimate the cost of food borne illness in general. There may be differences, however, between the average American and the average seafood consumer. The average American consumes less seafood than other meat and poultry. In 2004, Americans consumed four times more beef than seafood and more than five times the amount of chicken (NOAA 2004; Davis and Lin 2005). Seafood consumers also tend to be more educated (He et al. 2003) and have a higher household income (Hicks et al.2008) – two factors that may be associated with a greater loss of productivity. Similar distinctions may be important for direct exposure risks at beaches, as recreational beach participation rates increase with both education level and income (Leeworthy 2005). Despite these possible limitations this model allows us to estimate the health costs of seafood borne illness that can be used as a foundation for future research to establish a more precise estimate.
Conclusion
We estimate the acute health cost of marine borne disease in the United States to be close to a billion dollars annually, with seafood borne disease making up almost three-fourths the cost, and illness from direct exposure to the marine environment accounting for the rest. We identify several pathogens that contribute substantially to these costs, notably the Vibrio species, CFP, and Norwalk virus. Incomplete reporting of marine borne illnesses, unknown pathogenic etiology of many foodborne cases, and limitations in identifying specific pathogens in beach recreation cases introduce considerable uncertainty into the overall estimate. Future research should focus on resolving these uncertainties, on extending the estimate to include the cost of chronic health effects and pain and suffering and determining an economically optimal policy response to preventing marine borne illness.
Relative to the heath costs of other illnesses, such as cancer, HIV/AIDS and diabetes, the cost of marine borne illness is small. Annual direct medical costs are estimated to be $74 billion for cancer (Meropol and Shulman 2007), over $32 billion for AIDS/HIV (Schackman et al 2006; CDC 2008a) and about $116 billion for diabetes (ADA 2008). The costs of marine borne disease are low in part because public health and coastal and marine resources departments in the United States have been effective at limiting the threats to human health. In developing countries, where public health infrastructure is often less robust, disease associated with marine environments is suspected to have a greater prevalence (Todd 2006).
One goal of estimating the cost of marine-borne illness is to determine the economically optimal policy response to managing marine health. If the cost of preventing marine borne illness is significantly outside the range of cost uncertainty, then from a policy point of view, a more precise estimate may not be necessary. Future research should focus on determining the appropriate policy for preventing marine borne illness based on the optimal economic response and compare it to other public health problems.
Acknowledgments
Funding for this work was provided by the NSF-NIEHS Woods Hole Center for Oceans and Human Health and by the WHOI Marine Policy Center; grant numbers NIEHS P50 ES012742 and NSF OCE-043072. The authors would like to thank Laura Fleming for helpful suggestions on an early draft of the manuscript.
Abbreviations
- ADA
American Diabetes Association
- ASP
Amnesiac Shellfish Poisoning
- CDC
Centers for Disease Control and Prevention
- CFP
Ciguatera Fish Poisoning
- CSPI
Center for Science in the Public Interest
- COVIS
The Cholera and Other Vibrio Illness Surveillance System
- EPA
Environmental Protection Agency
- ERS
Economic Research Service
- ERSD
End-stage Renal Disease
- FoodNET
Foodborne Disease Active Surveillance
- HAB
Harmful Algal Blooms
- HUS
Hemolytic Uremic Syndrome
- NNDSS
National Notifiable Disease Surveillance System
- NRC
National Research Council
- NSP
Neurotoxic Shellfish Poisoning
- PSP
Paralytic Shellfish Poisoning
- USDA
United States Department of Agriculture
References
- Ahmed FE, editor. Seafood Safety. Washington, D.C: National Academy Press; 1991. [PubMed] [Google Scholar]
- ADA (American Diabetes Association) Economic cost of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- Boehm A. Enterococci concentrations in diverse coastal environments exhibit extreme variability. Environmental Science and Technology. 2007;41(24):8227–8232. doi: 10.1021/es071807v. [DOI] [PubMed] [Google Scholar]
- Buzby JC, Roberts T, Jordan Lin CT, MacDonald JM. Bacterial foodborne disease: Medical costs and productivity losses. USDA, Economic Research Service; 1996. (AER-741). [Google Scholar]
- Cabelli VJ, Dufour AP, McCabe LJ, Levine MA. Swimming-associated gastroenteritis and water quality. American Journal of Epidemiology. 1982;115:606–16. doi: 10.1093/oxfordjournals.aje.a113342. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Epidemiologic notes and reports ciguatera fish poisoning—Bahamas, Miami. MMWR. 1982;31(28):391–2. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Foodborne Disease Outbreaks - Annual Summaries 1973–1987. USDHHS Publ. Center Disease Control; 1988. [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Ciguatera Fish Poisoning, Florida –1991. MMWR. 1993;42(21):417–8. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Outbreak Surveillance Summaries, 1993–2006. 1993–2006 Available: http://www.cdc.gov/foodborneoutbreaks/outbreak_data.htm[accessed August 23, 2008]
- CDC (Centers for Disease Control and Prevention) Surveillance for foodborne disease outbreaks - United States, 1988–1992. MMWR. 1996;45(SS-5):1–55. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Ciguatera Fish Poisoning, Texas –1997. MMWR. 1998;47(33):692–4. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Summaries of human Vibrio Isolates reported to CDC, Cholera and Other Vibrio Illness Surveillance System (COVIS) 1999–2006 Available: http://www.cdc.gov/nationalsurveillance/cholera_vibrio_surveillance.html [accessed October 21, 2008]
- CDC (Centers for Disease Control and Prevention) Surveillance for food borne disease outbreaks – United States, 1993–1997. MMWR. 2000;49(SS01):1–51. [Google Scholar]
- CDC (Centers for Disease Control and Prevention) FoodNet (Foodborne Diseases Active Surveillance Network) annual reports. 2000–2004 Available: http://www.cdc.gov/foodnet/index.htm [accessed March 18, 2009]
- CDC (Centers for Disease Control and Prevention) Nationally Notifiable Diseases Surveillance System. 2001–2005 Available: http://www.cdc.gov/ncphi/disss/nndss/nndsshis.htm [accessed August 5, 2008]
- CDC (Centers for Disease Control and Prevention) National Botulism Surveillance Summaries. 2001–2007 Available http://www.cdc.gov/nationalsurveillance/botulism_surveillance.htm [accessed 27 February 2009]
- CDC (Centers for Disease Control and Prevention) Surveillance for Waterborne Disease and Outbreaks associated with recreational water – United States, 2003–2004. MMWR. 2006a;55(S12):1–24. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Ciguatera Fish Poisoning, Texas –1998, South Carolina –2004. MMWR. 2006b;55(34):935–7. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Surveillance for foodborne disease outbreaks – United States, 1998–2002. MMWR. 2006c;55(SS10):1–34. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Vibrio parahaemolyticus infections associated with consumption of raw shellfish - Three states, 2006. MMWR. 2006d;55(31):854–856. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) HIV Surveillance Report: Diagnoses of HIV infection and AIDS in the United States and Dependent Areas, 2008. 2008a Available: http://www.cdc.gov/hiv/surveillance/resources/reports/2008report/
- CDC (Centers for Disease Control and Prevention) Surveillance for Waterborne Disease and Outbreaks associated with recreational water use and other aquatic facility-associated health events – United States, 2005–2006. MMWR. 2008b;57(S S09):1–29. [PubMed] [Google Scholar]
- CSPI (Center for Science in the Public Interest) Outbreak Alert: Closing the Gaps in our Federal Food-Safety Net. 2007 Available: http://www.cspinet.org/foodsafety/outbreak_alert.pdf [accessed August 5, 2008]
- Davis CG, Lin B. Factors affecting US beef consumption. U.S. Department of Agriculture, Economic Research Service; Oct, 2005. (LDP-M-135-02). http://www.ers.usda.gov/publications/ldp/Oct05/ldpm13502/ldpm13502.pdf [January 5, 2010] [Google Scholar]
- Desvousges WH, Johnson FR, Banzhaf HS. Environmental Policy Analysis with Limited Information, Principles and Applications of the Transfer Method. Edward Elgar Publishing Limited; Northhampton: 1998. [Google Scholar]
- de Fouw JC, van Egmond HP, Speijers GJA. (RIVM Report No.388802021).Ciguatera Fish Poisoning: A review. 2001 Available: www.rivm.nl/bibliotheek/rapporten/388802021.html [accessed January 12, 2011]
- Doyle TJ, Glynn KM, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: An analytical literature review. American Journal of Epidemiology. 2002;155(9):866–874. doi: 10.1093/aje/155.9.866. [DOI] [PubMed] [Google Scholar]
- ERS (Economic Research Service) Online Cost Calculator for Estimating the Economic Cost of Illness. 2010 Available: http://www.ers.usda.gov/Data/FoodborneIllness [accessed March 19, 2010]
- (EPA) U.S. Environmental Protection Agency. The benefits and costs of the Clean Air Act. 1970–1990. 1997 410-R-97-002. [Google Scholar]
- (EPA) U.S. Environmental Protection Agency. EPA’s Beach Report: 2006. 2006 swimming season. Available: http://www.epa.gov/waterscience/beaches/seasons/2006/ [accessedJuly 15 2008]
- Feldhusen F. The role of seafood in bacterial foodborne diseases. Microbes and Infection. 2000;2:1651–1660. doi: 10.1016/s1286-4579(00)01321-6. [DOI] [PubMed] [Google Scholar]
- Fisher A, Chestnut LG, Violette DM. The Value of Reducing Risks of Death: A Note on New Empirical Evidence. Journal of Policy Analysis and Management. 1989;8(1):88–100. [Google Scholar]
- Fleisher JM, Kay D, Salmon RL, Jones F, Wyer MD, Godfree AF. Marine waters contaminated with domestic sewage: nonenteric illnesses associated with bather exposure in the United Kingdom. American Journal of Public Health. 1996;86:1228–34. doi: 10.2105/ajph.86.9.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher JM, Kay D, Wyer MD, Godfree AF. Estimates of the severity of illnesses associated with bathing in marine recreational waters contaminated with domestic sewage. International Journal of Epidemiology. 1998;27:722–726. doi: 10.1093/ije/27.4.722. [DOI] [PubMed] [Google Scholar]
- Fleisher JM, Fleming LE, Solo Gabriele HM, Kish JK, Sinigalliano CD, Plano L, Elmir SM, Wang J, Withum K, Shibata T, Gidley ML, Abdelzaher A, He GG, Ortega C, Zhu X, Wright M, Hollenbeck J, Backer LC. The BEACHES Study: health effects and exposures from non-point source microbial contaminants in subtropical recreational marine waters. International J Microbiology. 2010:1–8. doi: 10.1093/ije/dyq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (FDA) Food and Drug Administration. Taylor, Michael, Senior Advisor to the commissioner; ISSC Biennial meeting; Manchester NH. October 2009; Available: http://www.fda.gov/NewsEvents/Speeches/ucm187012.htm [accessed December 1, 2009] [Google Scholar]
- (FDH) Florida Department of Health. Ciguatera outbreak associated with consumption of Barracuda. 2006 August 2006. Epi Update, 25 August. Available: http://www.doh.state.fl.us/Disease_Ctrl/epi/Epi_Updates/Epi_Weekly/Epi_Update.htm [accessed August 23, 2008]
- Frenzen PD, Drake A, Angulo FJ, Emerging Infections Program FoodNet Working Group Journal of Food Protection. 2005;68(12):2623–2630. doi: 10.4315/0362-028x-68.12.2623. [DOI] [PubMed] [Google Scholar]
- Frenzen PD, Riggs TL, Buzby JC, Breuer T, Roberts T, Voetsch D, et al. Salmonella Cost Estimate Updated Using FoodNet Data. Food Review. 1999;22(2):10–15. [Google Scholar]
- Given S, Pendleton LH, Boehm AB. Regional public health cost estimates of contaminated coastal waters: A case study of gastroenteritis at southern California beaches. Environmental Science and Technology. 2006;40(16):4851–67. doi: 10.1021/es060679s. [DOI] [PubMed] [Google Scholar]
- Haile RW, Witte JS, Gold M, Cressey R, McGee C, Millikan RC, et al. The health effects of swimming in ocean water contaminated by storm drain runoff. Epidemiology. 1999;10(4):355–363. [PubMed] [Google Scholar]
- He S, Fletcher S, Rimal A. Identifying factors influencing beef, poultry and seafood consumption. Journal of Food Distribution Research. 2003:51–55. [Google Scholar]
- Hicks D, Pivarnik L, McDermott R. Consumer perceptions about seafood – an Internet survey. Journal of Food Service. 2008;19:213–226. [Google Scholar]
- Hoagland P, Anderson DM, Kaoru Y, White AW. The Economic Effects of Harmful Algal Blooms in the United States: Estimates, Assessment Issues and Information Needs. Estuaries. 2002;25(4b):819–837. [Google Scholar]
- Hoagland P, Jin D, Polansky LY, Kirkpatrick B, Kirkpatrick G, Fleming LE, et al. The costs of respiratory illnesses arising from Florida Gulf Coast Karenia brevis blooms. Environmental Health Perspectives. 2009;117(8):1239–43. doi: 10.1289/ehp.0900645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss HH, Ababouch L, Gram L. Assessment and management of Seafood Safety and Quality. Food and Agriculture Organization of the United Nations; Rome: 2004. (In FAO Fisheries Technical Paper No. 444). Available: ftp://ftp.fao.org/docrep/fao/006/y4743e/y4743e00.pdf [accessed August 5, 2008] [Google Scholar]
- Kirkpatrick B, Currier R, Nierenberg K, Reich A, Backer L, Stumpf R, Fleming L, Kirkpatrick G. Florida red tide and human health: A pilot beach conditions reporting system to minimize human exposure. Science of the Total Environment. 2008;402(10):1–8. doi: 10.1016/j.scitotenv.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Backer LC, Bean JA, Tamer R, Kirkpatrick G, et al. Environmental exposures to Florida red tides: Effects on emergency room respiratory diagnosis visits. Harmful Algae. 2006;5:526–533. doi: 10.1016/j.hal.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E, Smith DL, Laxminarayan R. Hospitalizations and Deaths caused by methicilin-resistant Staphylococcus aureus, United States, 1999–2005. Emerging Infectious Diseases. 2007;13(12):1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniesner TJ, Viscusi WK, Woock C, Ziliak JP. Pinning down the value of a statistical life. (Discussion paper no. 3107).IZA (Institute for the Study of Labor) 2007 Oct;:1–32. [Google Scholar]
- Landefeld JS, Seskin EP. The economic value of life: Linking theory to practice. American Journal of Public Health. 1982;6:555–66. doi: 10.2105/ajph.72.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DN, Enriquez MB, Lumish RM, Maceo A. Ciguatera fish poisoning in Miami. JAMA. 1980;244:254–258. [PubMed] [Google Scholar]
- Lees D. Viruses and bivalve shellfish. International Journal of Food Microbiology. 2000;59:81–116. doi: 10.1016/s0168-1605(00)00248-8. [DOI] [PubMed] [Google Scholar]
- Leeworthy VR, Bowker JM, Hospital JD, Stone EA. US Department of Commerce. Silver Springs, Maryland: National Oceanographic Atmospheric Administration; 2005. Projected participation in marine recreation: 2005 & 2010. [Google Scholar]
- Leeworthy VR, Wiley PC. National survey on marine recreation and the environment 2000: Current participation patterns in marine recreation, a report to the US Department of Commerce. Silver Springs, Maryland: National Oceanographic Atmospheric Administration; 2001. [Google Scholar]
- Lindsay JA. Chronic sequelae of foodborne disease. Emerging Infectious Diseases. 1997;3(4):443–452. doi: 10.3201/eid0304.970405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber G, Azziz-Baumgartner E, Latka R, Monteihl C, Conklin L, Backer L. Surveillance for Ciguatera Fish Poisoning in Culebra. Puerto Rico: Unpublished. [Google Scholar]
- McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus–associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12:1715–23. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Sharpiro C, et al. Food-related illness and death in the United States. Emerging Infectious Diseases. 1999;5(5):607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meropol HJ, Schulman KA. Cost of cancer care: issues and complications. Journal of Clinical Oncology. 2007;25(2):180–186. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]; Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, EMERGEncy ID Net Study Group Methicillin-resistant S. aureus infections among patients in the emergency department. New England Journal of Medicine. 2006;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- NOAA (National Oceanic and Atmospheric Administration) Seafood consumption statistics from “Fisheries of the United States, 2004. 2004 Available: http://www.nmfs.noaa.gov/docs/2004%20Seafood%20Consumption%20Statistics.pdf [accessed January 5, 2010]
- NRC (National Research Council) From Monsoons to Microbes: Understanding the Oceans Role in Human Health. National Academy Press; Washington, DC: 1999. [PubMed] [Google Scholar]
- Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, Weinstein MC, Seage GR, Moore RD, Freedberg KA. The Lifetime cost of current immunodeficiency virus care in the United States. Medical Care. 2006;44(11):990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- Scharff RL, McDowell J, Medeiros L. Economic cost of foodborne illness in Ohio. Journal of Food Protection. 2009;72(1):128–136. doi: 10.4315/0362-028x-72.1.128. [DOI] [PubMed] [Google Scholar]
- Scharff RL. Health-related costs from foodborne illness in the United States. The Produce Safety Project at Georgetown University. 2010 Available: http://www.producesafetyproject.org/admin/assets/files/Health-Related-Foodborne-Illness-Costs-Report.pdf-1.pdf [accessed March 18, 2010]
- Scott WG, Scott HM, Baker MG. Economic cost to New Zealand of foodborne infectious disease. New Zealand Medical Journal. 2000;113(1113):281–284. [PubMed] [Google Scholar]
- Shapiro RL, Altekruse S, Hutwagner L, Bishop R, Hammond R, Wilson S, et al. The role of gulf coast oysters harvested in warmer months in the Vibrio vulnificus infections in the United States, 1988–1996. Journal of Infectious Diseases. 1998;178:752–759. doi: 10.1086/515367. [DOI] [PubMed] [Google Scholar]
- Thompson JR, Marcelino LA, Polz MF. Diversity, Sources and Detection of Human Bacterial Pathogens in the Marine Environment. In: Belkin S, Colwell RR, editors. Oceans and Human Health: Pathogens in the Marine Environment. New York: Springer; 2005. pp. 29–68. [Google Scholar]
- Todd ECD. Preliminary estimates of costs of foodborne disease in the United States. Journal of Food Protection. 1989;52(8):595–601. doi: 10.4315/0362-028X-52.8.595. [DOI] [PubMed] [Google Scholar]
- Todd ECD. Estimated costs of paralytic shellfish, diarrhetic shellfish and ciguatera fish poisoning in Canada. In: Lassus P, Arzul G, Erard E, et al., editors. Harmful Algal Blooms. Paris: Lavoisier; 1995. pp. 831–834. [Google Scholar]
- Todd ECD. Challenges to global surveillance of disease patterns. Marine Pollution Bulletin. 2006;53:569–578. doi: 10.1016/j.marpolbul.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Turbow D, Osgood N, Jiang S. Evaluation of recreational health risk in coastal waters based on Enterococccus densities and bathing patterns. Environmental Health Perspectives. 2003;111(4):598–603. doi: 10.1289/ehp.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi WK. The value of risks to life and health. Journal of Economic Literature. 1993;31:1912–46. [Google Scholar]
- Viscusi WK. Fatal Tradeoffs: Public & Private Responsibilities for Risk. New York: Oxford University Press; 1992. [Google Scholar]
- Viscusi WK, Aldy J. The Value of a Statistical Life: A Critical Review of Market Estimates throughout the World. Journal of Risk and Uncertainty. 2003;27(1):5–76. [Google Scholar]
- Widdowson M, Sulka A, Bulens SN, Beard RS, Chaves SS, Hammond R, et al. Norovirus and foodborne disease, United States, 1991–2000. Emerging Infectious Disease. 2005;11(1):95–102. doi: 10.3201/eid1101.040426. [DOI] [PMC free article] [PubMed] [Google Scholar]


