The burden of diabetes is complex and growing in epidemic proportions. According to the American Diabetes Association (ADA), ∼29.1 million Americans (9.3% of the population) were diagnosed with diabetes as of 2015, and >1 million new cases of diabetes are diagnosed in people ≥20 years of age in the United States each year (1). Diabetes is also on the rise globally. The International Diabetes Federation has predicted that the number of people with diabetes worldwide will rise from the present 415 million to 642 million in 2040 (2).
Diabetes is the seventh leading cause of death in the United States and kills more people every year than AIDS and breast cancer combined (1). Patients with diabetes often have comorbid conditions of hypertension, dyslipidemia, retinopathy, neuropathy, kidney failure, lower limb amputations, and cardiovascular disease (3). Alarmingly, in the past decade, research has shown an increased risk for pancreatic, liver, colorectal, endometrial, and breast cancer among people living with diabetes (4). Although additional studies are needed to determine the causes of this risk, cancer screening is a necessary standard of diabetes care.
Increased diabetes incidence in the United States is attributed to an aging population, increased diabetes risk among expanding minority groups (i.e., non-Hispanic blacks, Asians, Hawaiians/Pacific Islanders, and Native Americans/Alaska Natives), sedentary lifestyle, and high rates of obesity (5). In 2012, the ADA reported that diabetes accounted for $245 billion in public costs, including $176 billion in direct costs and $69 billion in indirect costs (i.e., disability, work loss, and premature mortality). In the absence of an improved diabetes management strategy, escalating costs will create an enormous economic burden for the already strained U.S. health care system.
The prevalence of diabetes is 17% higher in adults living in rural areas than in the population as a whole (6). Rural populations are at increased risk for diabetes complications because of several barriers to care, including lower income levels, lower levels of educational attainment, limited health insurance, physician shortages, and distance to health clinics. Also, primary care providers (PCPs) delivering services in rural areas face challenges with regard to a lack of health care resources and limited access to diabetes education centers (7).
A study by Hale et al. (6) found a disparity in diabetes care for rural versus urban patients. These researchers performed a cross-sectional analysis of data from the 2006 Behavioral Risk Factor Surveillance System and reviewed responses of patients ≥18 years of age with a self-reported diagnosis of diabetes (n = 29,501). Rural patients were defined as living in a nonmetropolitan county with a population of <50,000 people. Patients living in rural areas reported having fewer dilated eye exams (69.1% rural vs. 72.4% urban, P = 0.005) and fewer foot exams (70.6 vs. 73.7%, P = 0.007). Rural patients also reported having diabetic retinopathy and problems with slow-healing foot wounds more often than their urban counterparts (25.8 vs. 22.0%, P = 0.007 and 13.2 vs. 11.2%, P = 0.036, respectively). Furthermore, there was less participation in diabetes self-management education (DSME) in rural areas than in urban areas (52% rural vs. 55.9% urban, P = 0.003).
National data from the 2007 Medical Expenditure Panel Survey (MEPS) confirmed the disparity regarding access to diabetes education in rural areas. Brown-Guion et al. (8) examined MEPS data on 1,747 adults with type 2 diabetes to evaluate the likelihood of receiving diabetes education in relation to race, urban/rural location, and region. The demographics of the study cohort were as follows: 49.3% were male, and 50.6% were female; 65.6% were white, 15% were black, and 19.4% were of other racial groups; 46.9% were <64 years of age; 39.8% had more than a high school education; 34.1% were from low-income households; 35.1% were in the middle-income range, and 30.8% were in the high-income range; 39.5% lived in the South, and 80.6% lived in rural areas. More than half of all subjects (63.7%) did not receive any type 2 diabetes education. Patients living in the rural areas of the South were least likely to receive DSME (67.5% received no formal education). Logistic regression also demonstrated that being black (odds ratio [OR] 1.38 [95% CI 1.03–1.84]) and living in an urban area (OR 1.4 [95% CI 1.00–1.97]) were associated with a higher likelihood of receiving diabetes education.
The Healthy People 2020 (9) diabetes goal is to reduce the disease and its economic burden, as well as to improve the quality of life of people with or at risk for diabetes. Innovative diabetes management and education models are needed to achieve this goal and reduce health disparities.
Telehealth and telemedicine may be an option for improving access to cost-effective quality care and reducing risks of diabetes complications. The American Telemedicine Association defines telemedicine as “the use of medical information exchanged from one site to another via electronic communications to improve a patient’s clinical health status” (10). Telemedicine includes varied applications of two-way video, sometimes using high-definition cameras, email, smartphones, wireless tools, and other telecommunications technologies. Telehealth falls under a broader definition of remote health care and does not always involve clinical services.
This article will review the literature on the use of interactive telehealth models to provide diabetes specialty care and DSME. A focus on video conferencing telehealth models is presented to discuss its application in rural primary care practice. Adapting these innovative health care delivery models may be a cost-effective and reliable alternative for improving diabetes care in rural communities.
Methods
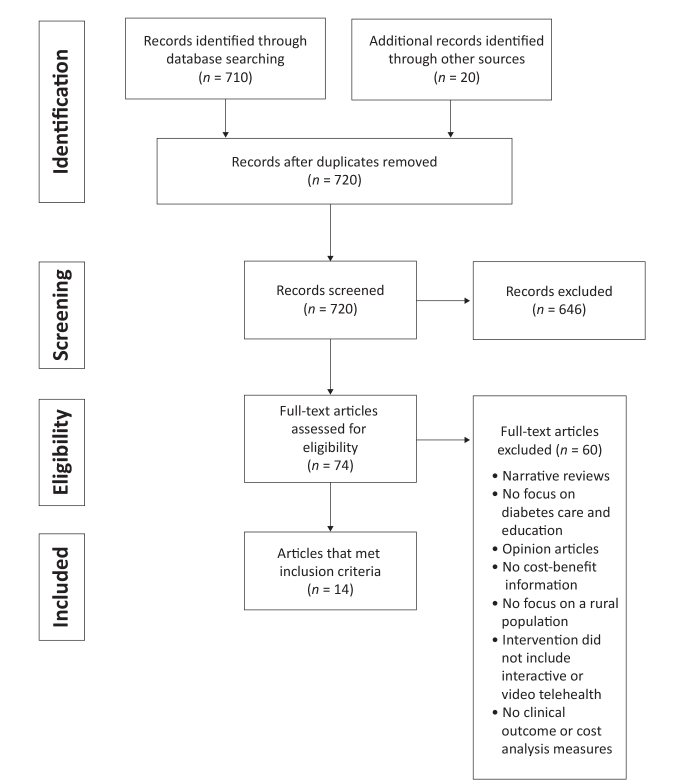
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines regarding eligibility criteria, information sources, search methods, and study selection were followed in conducting the systematic review (11). The objective was to appraise current research on the effectiveness and cost-benefit considerations of interactive video telehealth models. The search focused on how these models may be used for linkages to endocrinology consultations, clinical care management, and DSME for patients living in rural areas. The CINAHL Plus, ProQuest Nursing Journals, PubMed, Joanna Briggs Institute, and OVID databases were searched for English-language articles published between 2010 and 2016. Keywords included diabetes, telehealth, rural, cost-benefit, endocrinologists, and telemedicine. Secondary searches were performed by identifying studies in full-text articles screened in the review process. Inclusion criteria concentrated on systematic reviews of studies and original research that included DSME and endocrinology consultations by telehealth technology and cost analyses. Descriptive studies, retrospective studies, clinical trials, and observational and pilot studies emphasizing video telehealth models were included in the review. Narrative reviews and opinion articles were excluded. Additional literature exclusion details are shown in Figure 1.
FIGURE 1.
PRISMA flow diagram.
Results
Fourteen original research articles were selected; these articles analyzed specific outcomes related to the provision of telehealth for endocrinology consultations, clinical care, and/or DSME and included cost-benefit analyses of interactive telehealth models. Articles reviewed varied in study design and number of participants. None of the studies performed a blinded outcome assessment. The smallest study was a descriptive quality improvement project involving 14 participants, with limitations on its generalizability. The largest study was a retrospective cost analysis review of 1,767 control participants and 1,767 intervention participants. All of the studies concentrated on services provided for participants living in rural or remote areas (Tables 1 and 2).
TABLE 1.
Interactive Video Telehealth Models
| Source | Design | Sample | Intervention | Results | Implications |
|---|---|---|---|---|---|
| Holloway et al., 2011 (7) | Intervention project; nurse practitioner–led multidisciplinary team to enhance diabetes care through DSME using pre-/postintervention evaluation | Nonrandom purposive sample (n = 118) from five rural Montana clinics | Telehealth video conferencing for diabetes care and DSME | Most (97%) of program staff felt that telehealth was a useful tool for patient management and education; patients adapted quickly to technology and shared sensitive issues openly; after 1 year, patients reported improvement in diabetes care of 30–200% post-intervention compared to baseline | Model had a positive impact on diabetes self-management and patient satisfaction by bringing a multidisciplinary team into a rural setting to work in partnership with local PCPs |
| Levin et al., 2013 (12) | Retrospective study | Convenience sample (n = 73) of patients treated in telemedicine program on Aeroe Island, Denmark; mainland treatment center location Svendborg, Denmark | Synchronous and asynchronous telehealth interaction with patients and providers for diabetes clinical care and management, including: | Intervention group compared with data from the Danish National Diabetes Registry: | Major cost savings for patients using telemedicine; estimated savings was $60–70 per patient per visit; overall program cost savings were $9,430–11,170 compared with usual care |
| Post-intervention: | |||||
|
|
||||
| Six-month telemedicine intervention; two visits and two A1C values | Patient satisfaction was related to major reduction in transportation costs | ||||
| Siminerio et al., 2014 (17) | Baseline and post-program behavioral and psychosocial survey; satisfaction survey post-program | Convenience sample (n = 35); patients referred by a PCP if A1C >7% and needed improved glycemic management | Video telehealth for DSME, diabetes self-care empowerment; diabetes team consisting of endocrinologist in urban setting and diabetes nurse educator in rural setting; DES-SF tool used for empowerment assessment | Significant improvement in patient empowerment and self-care when patients received telehealth DSME (DES-SF score 3.8 vs. 4.5, P <0.01); patients’ adherence to diet and glucose monitoring recommendations improved (3.8 ± 2.3 vs. 5.2 ± 1.8; P = 0.01) | Alternative care model for diabetes education and specialty care management in rural community |
| Toledo et al., 2014 (18) | Clinical trial | Convenience sample (n = 31); patients referred by rural PCP and patient volunteers with A1C >7%; location in rural setting with linkage to endocrinologist in urban academic hospital setting | Videoconferencing-based telemedicine with endocrinologists for 1-year follow-up study | Statistically improved glycemic control in the intervention group; baseline A1C 8.6 ± 0.3 in the telemedicine group vs. 8.9 ± 0.4% in usual care; completion A1C 6.6 ± 0.2 in the telemedicine group vs. 8.1 ± 0.2% in usual care, P = 0.02; 93% of intervention group adhered to SMBG recommendation, and 84% of intervention patients received intensified treatment | Videoconferencing-based telemedicine consultants offer potential to overcome geographical barriers to care in rural communities; this model had a significant impact in improving A1C outcomes |
| Davis et al., 2010 (19) | Clinical trial | Random sample (n = 165); patients attended one of three rural community health centers in northeastern South Carolina | Interactive video conferencing for DSME and eye exams | Significant improvement in A1C in the intervention group (baseline 9.2 ± 0.4, 6-month 8.3 ± 0.5; and 12-month 7.4 ± 0.5%) compared with usual care (baseline 8.7 ± 0.4, 6-month 8.6 ± 0.4, and 12-month 8.1 ± 0.4%); P = 0.05 for baseline to 6 months and 0.004 for 12 months; clinical improvement in eye exams (51.2% having exams in the intervention group vs. 46.3% usual care; P = 0.29) | The model used retinal imaging to provide eye exams; digital retinal imaging was electronically transferred to a network ophthalmologist, and abnormal findings were linked to care |
| Fatehi et al., 2013 (21) | Descriptive study with post-program questionnaire | Convenience sample (n = 56); two participating endocrinologists | Medical interventions; reviewed endocrinologists’ opinions on the use of telehealth for specialty care | Fifty-six consultations were provided from a tertiary teaching hospital; after consultations, the physicians interviewed indicated that 34% of the cases seen could have made a better decision if there had been an in-person physical exam; 12 patients required an in-person exam | Endocrinology specialty care can be performed through telehealth; most needed exams can be performed by local provider or, if necessary, in-person follow-up after a telehealth consultation |
| Fatehi et al., 2015 (20) | Cross-sectional observational survey | Questionnaires mailed to 62 participants enrolled in telemedicine program in Australia | Questionnaire with 15 multiple-choice questions and 1 open-ended question was developed for assessing patient satisfaction with video conferencing for specialty care | Questionnaire items showed strong internal consistency (Cronbach’s χ = 0.90); 34% response rate; four dimension assessment:
|
Patients with diabetes who were seen remotely by endocrinologists via video conferencing were satisfied with remote consultation |
| Toledo et al., 2012 (22) | Descriptive pilot study | Convenience sample (n = 25); PCPs (n = 7) | Telemedicine endocrinology consultants; PCPs in rural, medically underserved community in Pennsylvania referred patients with poorly controlled diabetes for consultation through telehealth | Mean A1C improved from 9.6 ± 0.4 to 8.5 ± 0.4% (P <0.001, paired t test); 75% of patients experienced absolute decrease in A1C of ≥0.5% from baseline | High levels of satisfaction reported by patients and providers; telehealth model offers improved access to specialty care in rural setting |
| Young et al., 2012 (23) | Randomized experimental study (control group vs. intervention group) | Random sample (n = 121); rural participants living with diabetes | 2-hour orientation at a rural clinic followed by a series of five phone or video contacts ∼2 weeks apart; English- and Spanish-speaking nurses provided coaching; Diabetes Empowerment Scale-Short Form and Diabetes History Form from the Michigan Diabetes Research and Training Center were used as participant assessment tools | From baseline to 9 months post-enrollment, intervention was associated with gains in five of eight indicators of self-efficacy (P <0.05) relative to the control group; intervention group had increased levels of satisfaction with their diabetes care from baseline to 16 weeks and from baseline to 9 months (P <0.05) relative to the control group | Significant improvement in participant self-efficacy in nurse coaching intervention group indicates that this telehealth technology may be an innovative way to empower individuals to work on goals for diabetes self-management, especially in rural areas |
| Watts et al., 2015 (24) | Retrospective study | Purposive sample (n = 35) | Telehealth video conference with diabetes specialists at an urban VHA hospital trained two PCPs at a rural VHA community clinic | After training, PCPs implemented two diabetes mini-clinics over 15 months; patients’ mean A1C improved from 10.2 ± 1.4 to 8.4 ± 1.8% (P <0.001) over average follow-up of 5 months | Telehealth models can be used for rural PCP professional training and have potential for future quality improvement projects for diabetes care/management |
TABLE 2.
Telehealth Models: Cost-Benefit Analysis
| Source | Design | Sample | Intervention | Results | Implications |
|---|---|---|---|---|---|
| Verbosky et al., 2016 (13) | Pilot descriptive study to evaluate cost savings and patient satisfaction with CVT technology | Convenience sample (n = 14); enrolled patients with diabetes attending a rural VHA clinic | CVT using real-time video and audio transmission | Average baseline A1C was 9%; 19 CVT encounters were performed; average distance from patients’ home to medical center was 59.4 miles; average distance to clinic was 11.6 miles; total travel distance averted was 1,795 miles; patients averted 88 miles for each telehealth visit; cost savings ranged from $182 to $300 over 5 months; patient satisfaction rate with telehealth was 91%, and 85% of veterans said they would recommend CVT to others | This small pilot showed promise in improving access to care and reducing costs, time, and risks associated with travel for patients living with diabetes in a rural area |
| Palmas et al., 2010 (14) | Cost analysis for the IDEATel program offering home telemedicine monitoring and education | Medicare claims data of IDEATel participants (n = 1,665) | Cost analysis of a diabetes case management telemedicine program; study evaluated reductions in health care expenditures and assessed costs for implementing the telehealth program in the future | Over a 6-year timeframe, $622 per participant per month was spent; mean (SE) annual Medicare payments were similar in the usual care and telemedicine groups, $9,040 (SE $386) and $9,669 (SE $443) per participant (P >0.05); IDEATel was not associated with a reduction in Medicare claims; IDEATel costs were high, and lower technology expense would be needed for future implementation | Integrated remote monitoring technology for high-risk patients with diabetes did not prove cost effective; reduction in technology expense is needed to make this model a viable option in health care |
| Baker et al., 2011 (15) | Cost analysis of Health Buddy program to evaluate whether program influenced health care spending | Medicare claims data of patients enrolled in Health Buddy program (intervention n = 1,767; control group n = 1,767); patients enrolled had at least one of three diagnoses: congestive heart failure, chronic obstructive pulmonary disease, or diabetes | Health Buddy program developed to improve care management; study examined whether spending patterns changed for the intervention group over first 2 years the program was offered; telemedicine group expenses compared to a control group that did not receive the intervention | Spending reduction in telemedicine group was significant; reductions of $312–542 per intervention patient per quarter; program expense of $128 per patient per month; net savings 7.7–13.3% per person per quarter | Carefully designed and implemented care management using low-cost telemedicine technology can help reduce health care spending |
| Kesavadev et al., 2012 (16) | Retrospective cohort study to evaluate glycemic control and costs | Convenience sample (n = 1,000); analyzed EMRs of patients with type 2 diabetes enrolled in DTMS program | Evaluation of participants enrolled in a telemedicine program who received follow-up by phone, email, or secure website; patients reported SMBG values and obtained treatment advice without a physical visit to the clinic; patients had access to a multidisciplinary team of physicians, diabetes educators, dietitians, nurses, pharmacists, and psychologists for 24-hour care 7 days per week | Patients showed a significant reduction in A1C from baseline at 3 and 6 months; mean A1C at baseline was 8.5 ± 1.4%. At 6-month visit, A1C was 6.3 ± 0.6% showing statistically significant improvement of 2.25% (P <0.001); 66,745 SMBG values reported via phone or email; 84% reported no hypoglycemic events; mean direct cost involved in SMBG was $6.34 per patient per month, together with $3.25 per month for telemedicine, resulted in recurring direct costs of $9.66 per patient per month for intensive management compared to the usual care cost of $5–15 per physical visit with a provider | Telemedicine intervention empowering patients to make decisions about goals, therapeutic options, and self-care behaviors are effective in achieving glycemic control and decreasing health care costs; despite costs of 24-hour care, improved health outcomes may decrease overall health care system costs |
Limited research was identified that provided detailed cost analysis of video conferencing telehealth models. Four articles were included for review of cost analysis that used clinical video conferencing and telehealth monitoring with assisted care management because these models may be a consideration for rural primary care practice. These research articles also provided information on health care expenditures, as well as assessment of telehealth implementation costs.
Review Articles
Telehealth can be used as an instrument to address the challenges of diabetes management and education (12). Improved access to quality care and reductions in health care utilization expenses are additional benefits of telehealth technology. Three of the four telehealth cost analysis reviews demonstrated promising benefits with regard to reducing treatment costs and complications for patients living in rural areas (13–16). Varied telehealth models are available as an alternative to in-person medical and educational encounters.
Video Conferencing Telehealth Models as a Training Tool
Six telehealth models reviewed used an endocrinologist at an urban site working in partnership with a diabetes educator, nurse, or registered dietitian (RD) in a rural location to provide focused DSME (7,16–20). Siminerio et al. (17) performed behavioral and psychosocial surveys and monitored A1C measures at baseline and after telehealth programs over a 6-month timeframe in the Telemedicine for Reach, Education, Access, and Treatment (TREAT) model. Participant satisfaction surveys were completed after the program. This study included a convenience sample of 35 participants referred by area PCPs. The eight-item Diabetes Empowerment Scale–Short Form (DES-SF) was used to evaluate participants’ ability to assess readiness to change, set/reach goals, overcome barriers, cope with emotions, manage stress, obtain support, remain motivated, and make cost/benefit decisions. Study findings revealed significant improvement in patient empowerment and self-care for patients who received DSME through video telehealth (DES-SF score 3.8 vs. 4.5, P <0.01). Patients’ self-reported adherence to diet and meter download evaluation of self-monitoring also improved (DES-SF scores 3.8 ± 2.3 vs. 5.2 ± 1.3, P <0.01 and 4.6 ± 2.6 vs. 5.8 ± 1.8, P = 0.01, respectively) (17).
Endocrinology consultations through video conferencing also allowed for medication adjustments and laboratory monitoring within the TREAT model design. Patients demonstrated improvement in glycemic control after one video consultation and continued to show progress in lowering A1C levels after completion of the telehealth program from baseline to 6 months of follow-up. A comparison of telehealth and usual care indicated statistically significant A1C change (baseline 8.6 ± 0.3% in the telemedicine group vs. 8.9 ± 0.4 with usual care; completion 6.6 ± 0.2 in the telemedicine group vs. 8.1 ± 0.2% with usual care, P = 0.02) (18).
A randomized clinical trial performed with 165 patients in rural South Carolina found improvement in glycemic control using video telehealth technology. A 1-year remote DSME program initiated with a nurse/certified diabetes educator (CDE) and RD showed statistically significant improvement in A1C in the telehealth intervention group compared with usual care from baseline to 6 and 12 months (19). Davis et al. (19) used a mixed linear regression model for repeated measures to show a significant reduction in A1C. The telehealth group had improvement in glycemic control from baseline to 6 and 12 months (A1C 9.2 ± 0.4, 8.3 ± 0.5, and 7.4 ± 0.5%, respectively) compared with usual care (8.7 ± 0.4, 8.6 ± 0.4, and 8.1 ± 0.4, respectively). P values (with <0.05 regarded significant) indicated a significant change from baseline to 6 (P = 0.05) and 12 months (P = 0.004). However, the study showed no differences in improvement of systolic blood pressure (135.3 ± 21.2 telehealth vs. 138.5 ± 19.9 mmHg with usual care, P = 0.33), diastolic blood pressure (76.2 ± 12.0 vs. 74.8 ± 10.4 mmHg, P = 0.42), BMI (37.1 ± 8.1 vs. 35.9 ± 7.6 kg/m2, P = 0.33), waist circumference (115.1 ± 15.7 vs. 112.5 ± 18.4 cm, P = 0.35) or albumin-to-creatinine ratio (91.1 ± 210.2 vs. 96.9 ± 236.0 mg/g, P = 0.87) between telehealth and usual care, respectively. This study also indicated clinical improvement in completion of annual eye exams since they incorporated a telemedicine retinal imaging exam for patients needing a resource for eye care (51.2% having eye exams in the intervention group vs. 46.3% with usual care, P = 0.29).
Video Conferencing Telehealth Models for Endocrinology Consultations
Diabetes care in rural communities suffers major challenges because of the barriers of physician shortages and lack of specialty care. Linkage to endocrinology consultations through video conferencing with patient interviews can substitute for a large proportion of specialty care (21). Fatehi et al. (21) performed a descriptive study using a convenience sample of 56 patients in Brisbane, Australia. Endocrinologists completed a questionnaire regarding patient care provided remotely by telehealth in eight consecutive clinic sessions over a 5-month period. Frequent recommendations in clinical treatments were laboratory test orders (75%), insulin dose adjustments (39%), and referrals to allied health professionals such as CDEs and RDs (13%). Of 56 consultations performed by the endocrinologists, 12 patients needed an exam that could not be completed by telehealth. However, this study did not use high-definition cameras or telemedicine equipment to allow for distance exams, which created limitations for physical assessments.
As a follow-up to the specialty provider evaluation of telehealth consultations, Fatehi et al. (20) performed an observational cross-sectional survey regarding patient satisfaction with video conferencing technology for diabetes care. A questionnaire with 15 multiple-choice questions and 1 open-ended question was developed for assessing patient satisfaction with videoconferencing for specialty consultation. Specifically, the survey assessed satisfaction with equipment/technical issues; communication and rapport; clinical assessment; and program evaluation. A total of 62 questionnaires were mailed to patients and 24 responses (39%) were completed and returned. Video quality received a 100% patient satisfaction rating. However, patients expressed concern (satisfaction rating of 21%) regarding the lack of physical contact. The patients did not report any problems in building rapport with their consultant using the video system. Despite the concern about the limitation of physical contact for clinical assessment, 96% of the patients surveyed reported that telemedicine improved their access to specialist care and that they would continue to use the technology.
Because diabetes care in rural communities suffers from a lack of medical providers, Toledo et al. (22) performed a pilot study using video telehealth. This pilot was performed before implementation of the TREAT model to determine whether diabetes telemedicine consultations would be an acceptable alternative for rural patients and PCPs. The authors used a convenience sample of 25 patients who received two-way video telehealth endocrinology consultations. Participating patients lived in a medically underserved area in rural Pennsylvania. Seven PCPs within the pilot community (95 miles from the nearest endocrinology center) referred patients with poorly controlled diabetes to the study. One-time consultations with an endocrinologist were provided via teleconsultation (45 minutes) and focused on management of hyperglycemia. The endocrinologist, located at a distant site, performed clinical assessments that incorporated medical interviews, laboratory test reviews, and treatment recommendations. A diabetes nurse educator located at the originating rural site operated the telehealth video equipment and reinforced all treatment recommendations and education. The treatment plan, which included medication adjustments, lifestyle modifications, glucose monitoring, and additional laboratory tests, was also shared with the PCP. The results of the study indicated that patients’ post-program (median 18 weeks) mean A1C improved from 9.6 ± 0.4% to 8.5 ± 0.4% (P <0.001, paired t test). Three-fourths of patients (75%) experienced an absolute decrease in A1C of ≥0.5% from baseline. This project showed potential to move forward with a later comparison-controlled study performed by the researchers in 2014.
Telehealth Models for Primary Care Support
An innovative nurse telehealth coaching program for people living with diabetes in rural communities in California revealed promising results for primary care application. Young et al. (23) used a randomized, experimental design to compare a control group of patients receiving usual care and an intervention group receiving nurse health coaching in six rural communities. The intervention included a 2-hour orientation at the base clinic, followed by a series of five phone or video contacts with participants ∼2 weeks apart. Depending on the participants’ choice of language preference, nurses provided counseling in English or Spanish. Self-efficacy, empowerment, and overall satisfaction with care were assessed using the DES-SF and Diabetes History Form from the Michigan Diabetes Research and Training Center. Assessments were performed at baseline and 16 weeks and 9 months after enrollment. Statistical analysis in this study used a multilevel modeling approach with generalized linear mixed models to estimate intervention effects.
Young et al. (23) used a sample of 121 participants, of which 44% were women. The mean age of participants was 59 years (SD 11.47 years), and the study included a diverse ethnic population. From baseline to 9 months after enrollment, the intervention was associated with gains in five of eight indicators of self-efficacy (P <0.05) relative to the control group. The intervention group had increased levels of satisfaction with their diabetes care from baseline to 16 weeks and from baseline to 9 months (P <0.05) relative to the control group. The study indicated a positive treatment effect in participants’ self-efficacy using telehealth nurse health coaching. This telehealth model may be a potential resource for improving health in rural communities because the program design empowered individuals to achieve goals to self-manage their diabetes.
Watts et al. (24) studied a video conferencing telehealth model that provided endocrinology specialty care training for PCPs working in a rural Veterans Health Administration (VHA) clinic. The physicians completed 1 year of Specialty Care Access Network–Extension for Community Healthcare Outcomes training with a multidisciplinary team of diabetes experts. After this extensive training, the physicians set up a quality improvement program implementing two diabetes mini-clinics to treat patients with poorly controlled diabetes. The researchers utilized results from the quality improvement program to develop a retrospective clinical study with a purposive sample of patients (n = 39). All of the study participants attended the rural VHA outpatient clinic that was part of the Cleveland VHA Hospital Network. Inclusion for enrollment required a patient A1C >9%. Patients were seen in the two PCP mini-clinics over a 15-month timeframe. Mean A1C improved from 10.2 ± 1.4% to 8.4 ± 1.8% (P <0.001) over an average follow-up period of 5 months.
Telehealth technology demonstrated high patient satisfaction and reduced patient costs as evidenced in studies by Levin et al. (12), Davis et al. (19), and Holloway et al. (7). Patients (n = 118) enrolled in an intervention study that provided DSME by telehealth in rural Montana adapted quickly to the new technology and openly shared personal or sensitive issues via the video conferencing system (7). High participant retention rates and cost savings on patients’ travel expenses and time away from work were major additional benefits using telehealth models (7,12,16,21).
A study performed in South India assessed the effectiveness and safety of the Diabetes Tele Management System (DTMS), which provided treatment, education, and care management for patients with type 2 diabetes. Kesavadev et al. (16) performed a retrospective cohort study using electronic health records (EHRs) of 1,000 patients with type 2 diabetes receiving services in their diabetes program with a 6-month follow-up timeframe. Telemedicine was offered to the patients through three options: phone, email, or secure website communication. Using one of these methods, patients reported self-monitoring of blood glucose (SMBG) values and obtained treatment advice without a physical visit to the provider clinic. Patients were able to communicate with DTMS teams that included physicians, dietitians, nurses, pharmacists, and diabetes educators. Specific services offered included modifying insulin and oral hypoglycemic medication doses, making diet and exercise recommendations, troubleshooting problems with devices such as insulin pens and glucose meters, and providing diabetes care during concomitant illnesses. Each DTMS session also offered an opportunity for DSME and counseling.
Records reviewed included patients with a mean age of 53.2 ± 9.8 years, 64% of whom were male. Results indicated that patients had an average of 17 ± 2 telemedicine follow-ups and reported 66,745 SMBG results in 6 months. Mean A1C value was 8.5 ± 1.4% at the initial visit and was reduced to 6.3 ± 0.6% at 6 months (P <0.001). The rate of SMBG values <70 mg/dL was ∼0.04 per patient per month, with 84% of patients reporting no hypoglycemia.
Cost-Benefit Review
There was limited research available on the cost of implementing video conferencing telehealth models or their impact on health care utilization. Four articles were identified that performed a cost analysis on telehealth technology. One telehealth video conferencing model and three home monitoring/care management telehealth models were included (Table 2). Three of the four studies reviewed offered positive findings on the costs versus benefits for patients receiving diabetes care through remote technology.
Clinical video telehealth (CVT) is used by the VHA to increase access to care and improve clinical outcomes. Telehealth is also used for reducing costs, time, and risks associated with traveling to a main medical center (13). A pilot quality improvement program to provide CVT pharmacotherapy clinics for patients with diabetes was implemented at the West Palm Beach Veterans Affairs Medical Center (WPBVA) from 1 October 2012 to 30 April 2013. Patients’ medications were managed by a clinical pharmacy specialist and supported by nurses and telehealth technicians. Fourteen patients enrolled in the pilot program and remained at their outpatient clinic to receive care. The average distance from patients’ homes to the WPBVA was 59.4 miles, and the average distance from patients’ homes to the outpatient clinic was 11.6 miles. Enrolled patients averted 1,795 miles, and individual patients averted an average of 88 miles for each telehealth visit. Cost savings ranged from $182 to $300 over 5 months, depending on patients’ travel reimbursement status. There was a 91% patient satisfaction rate with the telehealth sessions, and 88% of veterans said they would recommend telehealth services to other veterans. Because this was a small pilot study, its results may not be generalizable. However, CVT for pharmacological therapy appears to have the potential for reducing costs of and improving patient satisfaction with their health care access.
On a larger scale, the VHA introduced a national home telehealth program called Care Coordination/Home Telehealth (25). This program integrates home monitoring and health informatics to provide disease management for veterans. Since 2012, the VHA has offered remote home telemonitoring and video conferencing for 119,535 veterans, which has resulted in annual savings of $1,999 per patient. The VHA innovative program assisted 36% of enrolled patients in remaining independent at home. Also, compared to 2011 data, hospital admissions decreased by 38%, and the average inpatient length of stay decreased by 58% (25).
Palmas et al. (14) analyzed Medicare claims payments for 1,665 participants enrolled in a telehealth home monitoring program in New York City and upstate New York known as the Informatics for Diabetes Education and Telemedicine case management program (IDEATel). The purpose of their study was to determine whether a diabetes case management program provided through remote technology reduced health care expenditures in a medically underserved population. Over a 6-year timeframe (2000–2006), 28,821 telehealth intervention visits were delivered at an estimated cost of $622 per participant per month. Final claims analysis indicated that the telemedicine intervention did not reduce Medicare payments for combined inpatient or outpatient services compared to usual diabetes care. The researchers also determined the cost of implementing the telehealth intervention was high due to hardware and software expenses and that lower-cost technology was needed to expand the use of this model.
A telehealth case management model studied by Baker et al. (15) analyzed the Health Buddy program offered in Washington and Oregon. The study included 1,819 patients enrolled in the program for a 300-day study period. Participants used a small handheld monitoring system that connected to patients’ telephone lines and transmitted medical information to a care manager at a distant site. Medicare claims data indicated that this model resulted in a reduction in spending of ∼7.7–13.3% ($312–542) per person per quarter. The researchers concluded that a well-designed remote telehealth care management program could reduce health care utilization costs and deserved consideration in the future.
The studies performed by Kesavadev et al. (16,26) in India indicated that recurring costs to patients for DTMS was equivalent to $9.66 per month. The researchers concluded that, although there were extra costs involved in SMBG and teleconsultation staffing, the money and time saved in physical visits to the clinic that would have been needed in a traditional health care delivery model made up for the extra costs. Also, they concluded that better glycemic control is cost-effective long term because it may delay or prevent complications of diabetes.
Additionally, Levin et al. (12) indicated that their study demonstrated cost reductions primarily related to the avoidance of transportation costs and was estimated to save $60–70 per patient per visit. Approximately 30% of the patients who attended their telemedicine clinic had a full-time job. Working patients who had to travel to an urban clinic had to take a complete day off from work. The telehealth intervention reduced time needed away from work to only 1 hour.
Discussion
Benefits of Telehealth in Rural Areas
Telehealth technology may offer multiple benefits for rural patients and health professionals. Specifically, telehealth can improve local access to specialty health care and clinical management and lower the costs of travel and related costs for accommodations, childcare, food, and parking, as well as limit the inconvenience (and expense) of time away from work, home, and family (27). PCPs can benefit from improved quality of clinical services, access to continuing education and professional development, increased support from and access to specialists and their resources, improved continuity of care, and improved coordination and review management of patients who may need to transfer to a tertiary care center (27).
To address disparities in health care access for rural residents in the state of Mississippi, one academic medical center is leading the implementation of a large-scale telehealth program. In the past 10 years, the center has expanded an initial two-way video telehealth emergency program into a statewide telehealth network (28). Henderson et al. (28) reported that the telehealth network provides 30 specialties via telemedicine to >100 sites across the state. Recently, the network added the Mississippi Diabetes Telehealth Network, which will pilot a telehealth diabetes care program to an existing rural health clinic that does not offer any specialty services. Patients with poorly controlled diabetes are eligible for the program and will be provided a small computer tablet that will allow for real-time health sessions and coaching, as well as remote monitoring of vital signs and glucose levels. A rural community nurse practitioner (NP) or physician will have access to endocrinologists, ophthalmologists, specialty NPs, nurses, diabetes educators, pharmacists, and nutritionists to provide quality care and education on a regular schedule. The program will also offer education sessions to reinforce DSME.
Challenges of Telehealth
Incorporating telehealth technology into standard practice in rural areas may improve quality of care, reduce access barriers, and control health care costs. However, PCPs have concerns regarding the use of these relatively new remote models of care. A study performed by Davis et al. (29) explored PCPs’ interest in telehealth and what would be necessary to incorporate such technology into rural health practice. Interviewed providers expressed concern regarding the amount of data that would have to be evaluated, patient acceptance of and motivation to use the technology, and potential changes to patient-provider encounters. Also, adoption of telehealth would be difficult without changes to restrictive reimbursement policies.
There also may be risks associated with the use of the telehealth technology. Kesavadev et al. (26) indicated that communication errors and inefficiency in responding to patients’ questions might increase patient safety issues. Also, as with all strategies for providing care, rigorous and continuous training and supervision of multidisciplinary team members will be required to ensure quality of care. Additional time may also be required for professionally trained multidisciplinary teams to gain confidence in using the technology. Consensus guidelines should be developed for the diabetes care management provided via telemedicine to outline safe standards of care (26).
Telehealth implementation costs can also present a challenge. Program costs may differ depending on the clinical setting, type of vendor-supported technology needed (both hardware and software), available infrastructure for system networking, and local technical expertise (14). Many of these costs are related to initiating the technology and thus should be considered one-time program costs.
Reimbursement for telehealth services also creates multiple challenges for providers. The U.S. Department of Health and Human Services’ Centers for Medicare and Medicaid Services (CMS) indicates that not all telehealth costs are reimbursed (30). According to CMS, Medicare reimburses for telehealth services when the originating site (patient site) is in a Health Professional Shortage Area or in a county that is outside of any Metropolitan Statistical Area. The originating site must be a medical facility such as a rural health clinic, provider’s office, or hospital and may only receive a minimal facility fee. These restrictions do not apply to the distance site (provider site). Medicare will only reimburse for face-to-face interactive video consultation services with patients present. There is no standard reimbursement for telehealth services by private insurance companies. Payment for telehealth services also may require advance approval (30).
Implications
As the incidence of diabetes increases, improved access to diabetes care and education is necessary to reduce morbidity and mortality from this disease (6). PCPs face multiple challenges in caring for patients in rural areas. Efforts to develop and implement quality improvement programs to increase access to specialty care and DSME should incorporate telehealth models.
This technology can link providers and patients to endocrinology consultations, physical assessments, and retinal imaging for eye exams, as well as provide linkages to multidisciplinary care teams for nutrition and DSME services and to multiple specialty care providers. Rural PCPs may consider telehealth for professional training to update their knowledge on current diabetes standards of care (24). Telehealth technology may also improve PCPs’ ability to support chronic disease management through integrated remote systems of home monitoring (15,16,25,26). Additionally, rural health care systems may wish to evaluate the financial impact of interactive telehealth programs by monitoring their capacity to retain patients for care in the community. Improving access to specialty care has the potential to lower medical staffing costs by enabling the sharing of resources, providing medical services not readily available in the community, and decreasing hospital admissions and penalties for readmissions (31).
Despite the potential for cost savings from telehealth models, Medicare reimbursement of such care is restrictive, and there is no standard reimbursement policy among private insurance providers. National health care policy to support improved reimbursement for telehealth may encourage more rural PCPs to use this technology, which has demonstrated effectiveness in improving patient clinical outcomes, increasing satisfaction, and lowering health care costs.
Conclusion
Interactive video telehealth technology may improve glycemic control through improved access to quality care (7,12,13,18,22,24). Patient empowerment and self-care management through DSME also may be improved (17,23). Diabetes care provided through video telemedicine may offer laboratory monitoring, medication adjustment, and comprehensive physical exams through high-definition cameras and connective equipment for retinal imaging and other specialty care.
Various models using multidisciplinary teams of PCPs, endocrinologists, pharmacists, NPs, nurses, CDEs, and RDs may be implemented depending on the specific needs and resources of the rural practices and communities involved. Patients using telehealth in rural areas have expressed high satisfaction rates resulting from improved access to specialty care, reduced costs, and enhanced convenience (7,12,13 17,19,27). PCPs have also expressed satisfaction with the technology’s ability to provide resources for both professional development and patient care management (18,21,24,27).
Standard telehealth guidelines for safe, effective practice; appropriate reimbursement for various telehealth models; and information regarding costs and benefits of the technology for health care systems are evident gaps in the current literature. To advance the promotion and increase the acceptability of this technology, more research on these issues will be needed.
Acknowledgments
The author thanks Felecia Wood, PhD, RN, CNL, for supportive guidance on the writing of this manuscript and Nancy Stanley, DPT, and Milly Kennedy for their reviews and comments.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.American Diabetes Association Fast facts: data and statistics about diabetes [Internet]. Available from www.professional.diabetes.org/facts. Accessed 9 October 2015
- 2.International Diabetes Federation IDF Diabetes Atlas. 7th ed. Available from www.diabetesatlas.org.across-the-globe.html. Accessed 14 August 2016
- 3.Centers for Disease Control and Prevention National diabetes statistics report, 2014 [Internet]. Available from www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed 10 October 2015
- 4.Nathan D. Diabetes advances in diagnosis and treatment. JAMA 2015;314:1052–1062 [DOI] [PubMed] [Google Scholar]
- 5.Nichols GA, Schroeder EB, Karter AJ, et al. Trends in diabetes incidence among 7 million insured adults, 2006–2011: the SUPREME-DM project. Am J Epidemiol 2015;181:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hale NL, Bennett KJ, Probst JC. Diabetes care and outcomes: disparities across rural America. J Community Health 2010;35:365–374 [DOI] [PubMed] [Google Scholar]
- 7.Holloway B, Coon PJ, Kersten DW, Ciemins EL. Telehealth in rural Montana: promoting realistic independent self-management of diabetes. Diabetes Spectrum 2011;24:50–54 [Google Scholar]
- 8.Brown-Guion SY, Youngerman SM, Hernandez-Tejada MA, et al. Racial/ethnic, regional, and rural/urban differences in receipt of diabetes education. Diabetes Educ 2013;39:327–333 [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and human Services Diabetes. In Healthy People 2020 [Internet]. Available from www.healthypeople.gov/2020/topics-objectives/topic/diabetes. Accessed 20 October 2015
- 10.American Telemedicine Association What is telemedicine? [Internet]. Available from www.americantelemed.org/about-telemed.org/about-telemedicine/what-is-telemedicine#.VjAbkrerTIV. Accessed 25 October 2015
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG; the PRISMA Group. Preferred Reporting Items for Systematic Review and Meta-analysis: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin K, Madsen JR, Petersen I, Wanscher CE, Hangaard J. Telemedicine diabetes consultations are cost-effective, and effects on essential diabetes treatment parameters are similar to conventional treatment: 7-year results from the Svendborg Telemedicine Diabetes project. J Diabetes Sci Technol 2013;7:587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verbosky N, Beckey C, Lutfi N. Implementation and evaluation of diabetes management via clinical video telehealth. Diabetes Care 2016;39:e1–e2 [DOI] [PubMed] [Google Scholar]
- 14.Palmas W, Shea S, Starren J, et al. Medicare payments, healthcare service use, and telemedicine implementation costs in a randomized trial comparing telemedicine case management with usual care in medically underserved participants with diabetes mellitus (IDEATel). J Am Med Inform Assoc 2010;17:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker LC, Johnson SJ, Macaulay D, Birnbaum H. Integrated telehealth and care management program for Medicare beneficiaries with chronic disease linked to savings. Health Aff (Millwood) 2011;30:1689–1697 [DOI] [PubMed] [Google Scholar]
- 16.Kesavadev J, Shankar A, Pillai PBS, Krishnan G, Jothydev S. Cost-effective use of telemedicine and self-monitoring of blood glucose via Diabetes Tele Management System (DTMS) to achieve target glycosylated hemoglobin values without serious symptomatic hypoglycemia in 1,000 subjects with type 2 diabetes mellitus: a retrospective study. Diabetes Technol Ther 2012;14:772–776 [DOI] [PubMed] [Google Scholar]
- 17.Siminerio L, Ruppert K, Huber K, Toledo FGS. Telemedicine for Reach, Education, Access, and Treatment (TREAT): linking telemedicine with diabetes self-management education to improve care in rural communities. Diabetes Educ 2014;40:797–805 [DOI] [PubMed] [Google Scholar]
- 18.Toledo FGS, Ruppert K, Huber KA, Siminerio LM. Efficacy of the Telemedicine for Reach, Education, Access, and Treatment (TREAT) model for diabetes care. Diabetes Care 2014;37:e179–e178 [DOI] [PubMed] [Google Scholar]
- 19.Davis RM, Hitch AD, Salaam MM, Herman WH, Zimmer-Galler IE, Mayer-Davis EJ. TeleHealth improves diabetes self-management in an underserved community: Diabetes TeleCare. Diabetes Care 2010;33:1712–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatehi F, Martin-Khan M, Smith A, Russell AW, Gray LC. Patient satisfaction with video teleconsultation in a virtual diabetes outreach clinic. Diabetes Technol Ther 2015;17:43–48 [DOI] [PubMed] [Google Scholar]
- 21.Fatehi F, Gray LC, Russell AW. Telemedicine for clinical management of diabetes: a process analysis of video consultations. J Telemed Telecare 2013;19:379–382 [DOI] [PubMed] [Google Scholar]
- 22.Toledo FG, Triola A, Ruppert K, Siminerio LM. Telemedicine consultations: an alternative model to increase access to diabetes specialist care in underserved rural communities. JMIR Res Protoc 2012;1:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young HM, Miyamoto S, Ward D, Griffin E, Patmon F. Nurse telehealth coaching for rural diabetics: innovation in care. Commun Nurs Res 2012;45:188 [Google Scholar]
- 24.Watts SA, Roush L, Julius M, Sood A. Improved glycemic control in veterans with poorly controlled diabetes mellitus using a specialty care access network: extension for community healthcare outcomes model at primary care clinics. J Telemed Telecare 2016;22:221–224 [DOI] [PubMed] [Google Scholar]
- 25.Darkins A. Scaling-up telemedicine: VA telehealth programs. Presentation at the American Telemedicine Association Policy Summit, Washington, D.C, 27–28 June 2013 [Google Scholar]
- 26.Kesavadev J, Saboo B, Shankar A, Krishnan G, Jothydev S. Telemedicine for diabetes care: an Indian perspective: feasibility and efficacy. Indian J Endocrinol Metab 2015;19:764–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moffatt JJ, Eley DS. The reported benefits of telehealth for rural Australians. Aust Health Rev 2010;34:276–281 [DOI] [PubMed] [Google Scholar]
- 28.Henderson K, Davis TC, Smith M, King M. Nurse practitioners in telehealth: bridging the gaps in healthcare delivery. J Nurse Pract 2014;10:845–849 [Google Scholar]
- 29.Davis MM, Currey JM, Howk S, et al. A qualitative study of rural primary care clinician views on remote monitoring technologies. J Rural Health 2014;30:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Department of Health and Human Services Centers for Medicare and Medicaid Services Telehealth services [Internet]. Available from https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed 12 January 2016
- 31.Kvedar J, Coye MJ, Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff (Millwood) 2014;33:194–199 [DOI] [PubMed] [Google Scholar]