Abstract
IN BRIEF Epidemiological studies have found a lower prevalence of type 2 diabetes among vegetarians compared to nonvegetarians. This reduced risk is likely a function of improved weight status, higher intake of dietary fiber, and the absence of animal protein and heme iron in the diet. Interventional studies have shown that vegetarian diets, especially a vegan diet, are effective tools in glycemic control and that these diets control plasma glucose to a greater level than do control diets, including diets traditionally recommended for patients with diabetes (e.g., diets based on carbohydrate counting). Vegetarian diets are associated with improvement in secondary outcomes such as weight reduction, serum lipid profile, and blood pressure. Studies indicate that vegetarian diets can be universally used in type 2 diabetes prevention and as tools to improve blood glucose management.
Diet plays an important role in diabetes prevention and management. According to the American Diabetes Association (ADA), a variety of eating patterns are acceptable for the management of diabetes (1). The impacts of vegetarian eating patterns on the risk of type 2 diabetes, glycemic control, and prevention of diabetes comorbidities have been the focus of several recent research studies.
Vegetarian diets encompass several diet types, including semi-vegetarian (flexitarian), pesco-vegetarian, lacto-vegetarian, ovo-vegetarian, lacto-ovo-vegetarian, vegan, and raw-food vegan diets. Semi-vegetarians include small amounts of meat, mainly from fish and poultry. Pesco-vegetarians ingest some fish, in addition to foods of animal and plant origin. Milk and dairy products are ingested by lacto-vegetarians; ovo-vegetarians include eggs; and lacto-ovo-vegetarians ingest both dairy products, including milk, and eggs. Individuals who adhere to vegan diets exclude all meats and animal products.
There are additional variations within each of the above categories. For example, some individuals who consider themselves vegans do not eat honey or other bee products, whereas others just limit their dietary exclusions to dairy products and eggs. In addition, plant-based diets are an eating pattern that mainly includes unrefined foods of plant origin but may include small amounts of meats, mainly white meat.
Vegetarian diets have been associated with improvements in many modifiable heart disease risk factors, including serum lipid profile, serum glucose concentration, and systolic and diastolic blood pressure (2–5). Consequently, vegetarians have been shown to have a lower risk of hospitalization or death from ischemic heart disease (6). Furthermore, vegetarian diets have been shown to regress arterial stenosis among heart disease patients (7). These diets are also associated with reduced risk of other health conditions, including type 2 diabetes, some types of cancer, diverticular disease, and cataracts (8–11).
This manuscript is a review of the impact of vegetarian diets on diabetes. The specific objectives included: 1) assessment of the incidence of type 2 diabetes among vegetarians in comparison to nonvegetarians, 2) review of the impact vegetarian diets have on type 2 diabetes management/blood glucose control and treatment, and 3) evaluation of the influence of vegetarian diets on diabetes comorbidities.
Vegetarian Diets and Incidence of Type 2 Diabetes
The effect of vegetarian diets on the risk of developing type 2 diabetes has been assessed in a few large studies with individuals of different sexes, ethnic backgrounds, and geographical locations. In a recent study, Agrawal et al. (12) reported an association between the consumption of a vegetarian diet and the occurrence of type 2 diabetes in a nationally representative sample of 156,317 East Indian participants who were 20–49 years of age. Individuals who adhered to any type of vegetarian diet except for vegan had statistically significant lower odds of diabetes (odds ratio [OR] 0.67 [95% CI 0.58–0.76], P <0.01 for lacto-vegetarians; OR 0.70 [95% CI 0.51–0.96], P = 0.03 for lacto-ovo-vegetarians; and OR 0.77 [95% CI 0.60–0.98], P = 0.03 for semi-vegetarians). The association for vegans was not statistically significant (OR 0.91 [95% CI 0.61–1.36], P = 0.643).
The results described by Agrawal et al. are consistent with findings from another Asian study from Taiwan (13). This study included 4,384 individuals who were Buddhist volunteers. The reported analyses were sex and age specific. Vegetarian men had statistically significant lower odds for both diabetes (OR 0.49 [95% CI 0.28–0.89]) and impaired fasting glucose (IFG; OR 0.66 [95% CI 0.46–0.95]). A similar pattern was observed for postmenopausal women (OR 0.25 [95% CI 0.15–0.42] for diabetes and OR 0.73 [95% CI 0.56–0.95] for IFG). The odds for premenopausal women were not statistically significant for either diabetes (OR 0.26 [95% CI 0.06–1.21]) or IFG (OR 0.60 [95% CI 0.35–1.04]).
Vegetarians living in North America also have lower odds of being diagnosed with type 2 diabetes, according to results from the Adventist Health Study 2 (8), which included 15,200 men and 26,187 women. Compared to nonvegetarians, vegans had the lowest risk (OR 0.381 [95% CI 0.236–0.617]), followed by semi-vegetarians (OR 0.486 [95% CI 0.312–0.755]) and lacto-ovo-vegetarians (OR 0.618 [95% CI 0.503–0.760]). This trend was similar among both black and nonblack participants.
Vegetarian Diets in Type 2 Diabetes Management and Blood Glucose Control and Treatment
Results of a meta-analysis (14) that assessed the impact of vegetarian diets on plasma glucose suggested that such diets are effective in blood glucose management. The analyses were based on six interventional studies involving 255 individuals. Five of the studies assessed the effect of a vegan diet, whereas the sixth assessed a lacto-ovo-vegetarian diet. The study periods varied from 4 to 74 weeks (average 23.7 weeks). Adhering to a vegan or vegetarian diet resulted in a statistically significant lower mean A1C (–0.39 percentage points [95% CI –0.62 to –0.15], P = 0.001) compared to A1C among participants from control diets. No heterogeneity was observed among study results (P = 0.389 for heterogeneity).
Since the meta-analysis described above was published, Lee et al. (15) published results of a study in which they assessed the impact of a brown rice–based vegan diet on glycemic control among 46 Korean patients and 47 control participants who used a diet recommended by the Korean Diabetes Association. The trial lasted for 12 weeks. Although A1C significantly decreased in both groups, the improvement was greater among participants in the vegan group (–0.5%, P <0.01 vs. –0.2%, P <0.05). This difference became greater when the analysis was restricted to patients with the highest rate of compliance to the respective diets (–0.9% in the vegan group and –0.3% in the conventional group, P = 0.010).
The beneficial effects of vegetarian diets described above indicate that these diets might be effective in diabetes management and treatment. This conclusion is supported by findings reported by Barnard et al. (16), in a study with 99 individuals with type 2 diabetes. Participants were divided into group 1, with 49 people between 33 and 82 years of age who were assigned to a vegan diet, and group 2, with 50 people between 27 to 80 years of age who were assigned to follow a diet recommended by the ADA based on carbohydrate counting. The first group was advised to consume vegetables, fruits, whole-grain cereals, and beans. The amounts of calories and carbohydrates consumed were not limited. Carbohydrates constituted ∼75% of consumed calories, with the remainder provided by fat (10%) and protein (15%).
Participants in both groups reduced their caloric intake from 1,759 ± 468 to 1,425 ± 427 kcal/day (P <0.0001) in the vegan group and from 1,846 ± 597 to 1,391 ± 382 kcal/day (P <0.0001) in the control group. Protein intake among vegans dropped from 77 ± 27 to 51 ± 16 g/day (P <0.0001), whereas in the control group it decreased from 85 ± 27 to 73 ± 23 g/day (P <0.002). Similarly, fat intake fell in both groups from 72 ± 28 to 30 ± 19 g/day (P <0.0001) in the experimental group and from 73 ± 35 to 52 ± 21 g/day (P <0.0001) in the control group. Conversely, carbohydrate intake increased from 205 ± 69 to 251 ± 70 g/day (P <0.0001) in the vegan group and from 213 ± 70 to 165 ± 51 g/day (P <0.0001) in the control group.
After 22 weeks, individuals as- signed to consume the high-carbo- hydrate vegan diet lowered their A1C from an average of 8.0 to 7.1% (12.6%). A1C in the control group dropped from 7.9 to 7.4% (6.8%) in the same time period. Moreover, 21 of the 49 participants (43%) in the vegan group reduced their doses of medication prescribed for blood glucose control, compared to 26% of individuals in the control group.
Vegetarian Diets and Diabetes Comorbidities
Vegetarian diets have been associated with reduced risk of several diabetes comorbidities. In one of the most recent studies, Bunner et al. (17) showed that individuals consuming a vegan diet supplemented with vitamin B12 (1,000 µg of methylcobalamin/day) had a reduced risk of diabetes-related neuropathy pain. At the end of the 20-week intervention with the vegan diet and vitamin B12 supplement, participants experienced a decrease of 9.1 points on a pain questionnaire (from 22.6 at baseline to 13.5 at 20 weeks). In contrast, the decrease among participants in the control group, who also received a vitamin B12 supplement but did not consume a vegan diet, was 0.9 points during the same time period.
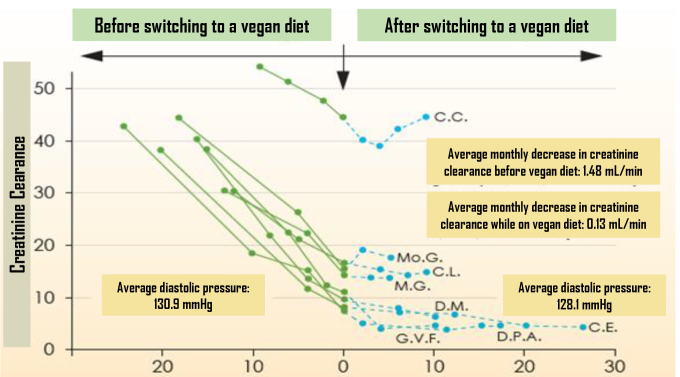
Intriguing findings were reported by Barsotti et al. (18) in a study involving patients diagnosed with diabetic neuropathy and renal failure (varying in degree from mild to significant). After the adoption of a strict vegan diet supplemented with protein, the progression of kidney insufficiency was halted. The average ingested protein intake before the experiment was 1.2 g/kg/day. After switching to a vegan diet, protein intake decreased to 0.3 g/kg/day. Within 15 months prior to switching to the vegan diet, creatinine clearance decreased from 40.9 to 15.6 mL/min. After ∼1 year on a meatless diet, creatinine clearance was 13.6 mL/min. The average monthly decrease in creatinine clearance before adoption of the vegan diet was 1.48 mL/min. After switching to a vegan diet, the decrease in creatinine clearance markedly decreased to 0.13 mL/min. Urine protein levels decreased from 5.2 to 2.8 g/day, mean cholesterol decreased from 254 to 165 mg/dL, and average blood glucose decreased from 166 to 131 mg/dL.
The findings of Barsotti et al. (18) were confirmed in a newer study involving 25 vegan Buddhist monks and 25 nonvegetarian control subjects in Thailand (19). All assessed parameters of renal function, including blood urea nitrogen (BUN), BUN-to-creatinine ratio, urinary protein content, and serum creatinine, were improved among the vegan participants compared to their nonvegetarian counterparts. For example, mean urinary protein among vegans was 1.4 mg/dL, compared to 5.2 mg/dL among control subjects. Figure 1 illustrates creatinine clearance before and after adoption of the vegan diet. Results of the study by Barnard et al. (16) showed that a vegan diet improved traditional cardiovascular disease (CVD) risk factors (Table 1). In addition to the improvement in CVD risk factors, participants in this study had a reduction in proteinuria which, consistent with results of Barsotti et al. (18), indicated an improvement in kidney function.
FIGURE 1.
Creatinine clearance before and after adoption of an amino acid– supplemented vegan diet. Reprinted from ref. 18 with permission of S. Karger AG, Basel.
TABLE 1.
Effect of a Vegan Diet on Traditional CVD Risk Factors.
| Risk Factor | Baseline | After 22 Weeks of Vegan Diet |
|---|---|---|
| Body weight (kg) | 97 | 91.1 |
| BMI (kg/m2) | 33.9 | 31.8 |
| Systolic blood pressure (mmHg) | 123.8 | 120 |
| Diastolic blood pressure (mmHg) | 77.9 | 72.8 |
| Total cholesterol (mg/dL) | 187 | 159.3 |
| LDL cholesterol (mg/dL) | 104.4 | 88 |
| Triglycerides (mg/dL) | 148.1 | 119.7 |
Adapted from ref. 16.
Discussion
The prevalence of diabetes is on the rise, both in the United States and worldwide. According to the International Diabetes Federation (20), ∼415 million individuals worldwide have diabetes. This number is projected to increase to 642 million by 2040. In the United States, the number of individuals with diabetes increased from ∼20.8 million in 2005 to ∼29.1 million in 2012 (21). Epidemiological studies (5,12,13) comparing the prevalence of type 2 diabetes among vegetarians and nonvegetarians have shown that vegetarians have a lower risk. Thus, it is logical to conclude that promotion and adoption of a vegetarian diet may help in controlling the diabetes epidemic.
The lower risk of type 2 diabetes among vegetarians may be explained in part by improved weight status (i.e., lower BMI) (8). However, the lower risk also may be explained by higher amounts of ingested dietary fiber and plant protein, the absence of meat- and egg-derived protein and heme iron, and lower intake of saturated fat (22–27). Most studies report the lowest risk of type 2 diabetes among individuals who adhere to vegan diets. This may be explained by the fact that vegans, in contrast to ovo- and lacto-ovo-vegetarians, do not ingest eggs. Two separate meta-analyses (28,29) linked egg consumption with a higher risk of type 2 diabetes.
The studies included in the meta-analysis that assessed the impact of vegetarian diets on plasma glucose showed that vegetarian diets not only improved glycemic control, they did so to a greater level than did control diets, including diets based on carbohydrate counting (14). Also, these diets were associated with an improvement of secondary outcomes, such as weight reduction, serum lipid profile, and systolic and diastolic blood pressure. Furthermore, when supplemented with vitamin B12, a vegan diet used in a study by Bunner et al. (17) showed that it may be helpful in reducing neuropathy pain among diabetes patients. The respective studies were conducted in Brazil, Czech Republic, and the United States (14). The newest study that assessed a brown rice–based vegetarian diet on glucose control was conducted in South Korea (15). The unidirectional outcomes suggest that vegetarian diets can improve blood glucose control regardless of cultural food choices or product availability due to geographical locations.
The improvement in glycemic control found in some individuals assigned to vegetarian/vegan diets have resulted in decreases in diabetes medications and insulin doses. For example, Barnard et al. (16) reported that up to 43% of their experimental group altered their medication. In comparison, 26% of the control group assigned to a carbohydrate counting–based dietary method altered their medications. Similarly, in a study by Kahleova et al. (30), 43% of participants in the experimental group and 5% of participants in the control group reduced their diabetes medications (P <0.001).
The findings reported by Kahleova et al. (30) deserve special consideration. In this study, 74 participants were randomly assigned to either an experimental or a control diet group. The former consisted of a vegetarian diet, whereas the latter was a conventional diabetic diet. Although the two diets were isocaloric with 500 kcal restriction, individuals from the experimental group lost more body weight (mean –6.2 kg [95% CI –6.6 to –5.3] vs. mean –3.2 kg [95% CI –3.7 to –2.5) and had greater improvements in insulin sensitivity (30% [95% CI 24.5–39] vs. 20% [95% CI 14–25], P = 0.04). Furthermore, compared to baseline, A1C was statistically significantly lower only in the experimental group (–0.65 ± 1%, P = 0.002 vs. –0.21 ± 1.1%). Also, markers of oxidative stress improved in the experimental group. For example, superoxide dismutase increased in the experimental group by 49% (95% CI 44.7–57.4, P <0.001), compared to baseline, but it decreased in the control group by 30% (95% CI –50 to –14, P <0.001). These findings highlight several benefits of following a vegetarian diet for patients with diabetes, from improvement in glycemic control to amendment of factors important in preventing diabetes complications.
No statistically significant reduction in risk of type 2 diabetes was found for vegan participants from the study by Agrawal et al. (12). The reason why these findings differed from the results of other studies is not clear, although the authors suggested a few possible explanations. First, vegan diets in India might differ from vegan diets in Western countries. For example, Indian vegans may eat greater amounts of butter or ghee (clarified butter). Also, only 26 participants from the vegan group were diagnosed with type 2 diabetes. Thus, the study may not have had adequate statistical power.
In addition to the studies that assessed the impact of vegetarian diets on the risk of type 2 diabetes and glycemic control, several studies assessed the impact of plant-based diets on risk of type 2 diabetes. Plant-based diets are mainly composed of plant foods and may or may not contain small amounts of meat. For example, analyses based on a combined sample of >200,000 participants, of which 16,162 developed type 2 diabetes, from the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study showed a 34% risk reduction among participants in extreme deciles of a healthful plant-based diet index (HR [adjusted for BMI] 0.66 [95% CI 0.61–0.72, P <0.001 for the trend) (25).
Vegetarians have been shown to have a high prevalence of vitamin B12 deficiency (31). In addition, metformin therapy increases the risk of vitamin B12 deficiency (32,33). Poor vitamin B12 status is associated with hyperhomocysteinemia (34), which in turn has been shown to increase risk of several diabetes complications, including retinopathy, neuropathy, bone fractures, and CVD (34–37). Thus, it would be prudent to routinely monitor vitamin B12 status and to prescribe supplementation as needed for patients with diabetes who adopt a vegetarian lifestyle.
Vegetarian diets also increase risk of a deficiency of a few additional nutrients, such as iron (38). Vegetarians also have lower intake and status of the long-chain omega-3 fatty acids EPA and DHA than their nonvegetarian counterparts (39). Some studies also have documented low vitamin D levels and low intake of calcium (mainly among vegans) (40–42). The potential impact of compromised vitamin D, iron, and calcium levels on diabetes risk, diabetes management, and the development of diabetes comorbidities is not clear. However, adequate intake of the long-chain omega-3 fatty acids EPA and DHA has been associated with some benefits for patients with diabetes, including reduction of albuminuria, improvement in renal function, and risk reduction of diabetic retinopathy (43–45). These fatty acids also have been associated with a lower risk of CVD incidence and mortality among patients with diabetes (46). Algal EPA and DHA supplements (oils derived from algae) are available in health foods stores. These supplements are suitable for both vegetarians and vegans. Considering the risk of these nutrient deficiencies, patients should be referred to a registered dietitian nutritionist (RDN) for dietary consultation to discuss effective ways to maintain adequate status of these nutrients.
The studies reviewed above indicate that vegetarian diets can be used universally in type 2 diabetes prevention and as tools to improve blood glucose management. In addition to the published studies, substantial unpublished anecdotal evidence is available in support of the effectiveness of vegetarian diets in diabetes management. In many cases, adopting vegetarian, and especially vegan, diets resulted in discontinuation of medication or insulin therapy. Given that vegetarian diets result in better plasma glucose control than a diet based on carbohydrate counting, patients find them easier to adhere to, and they are associated with reduction in risk of developing diabetes comorbidities, recommending these diets to patients with diabetes may result in better dietary adherence and better health outcomes. The ADA now endorses vegetarian diets as one option for patients with diabetes (1). In addition, the Canadian Diabetes Association has issued a statement in support of the use of plant-based diets for the management of type 2 diabetes (47). Because vegetarian diets improve glycemic control, patients adopting such a diet should have their medication and insulin doses adjusted to prevent hypoglycemia.
It is important to note that patients with diabetes find it easier to adhere to a vegetarian diet, mainly a vegan diet, than to a diet based on carbohydrate counting and that there is a higher degree of compliance with vegetarian diets than with alternative diets. Such findings have been reported in more than one study. For example, in the study by Lee et al. (15) the mean compliance score (maximum score of 10) during the intervention period was 9.2 ± 1.6 in the vegan group and 8.2 ± 1.5 in the conventional diet group (P = 0.002). These findings are important in light of the common assumption that patients will be unwilling to adopt a vegan or vegetarian diet. Although this assumption may hold true for some patients, anecdotal evidence indicates that even many patients who are advanced in age are willing to adopt such diets when advised by their physician.
It is equally important to point out that, in most of the studies that assessed the impact of vegetarian or vegan diets on glycemic control in patients with diabetes, participants were not provided ready-to-eat meals. Rather, these participants were living in their respective communities and may only have received basic instruction regarding vegetarian/vegan meal planning from dietetic or other health professionals. Similarly, plant-based diets had generally good adherence during worksite interventional management programs for type 2 diabetes (48). These findings indicate some patients with diabetes may find vegetarian or plant-based diets easier to accept than many clinicians may fear.
A healthy, vegetarian diet is composed of a variety of unrefined foods, including grain products such as bread, cereal, and pasta; fruits, including berries (e.g., blueberries and raspberries) and avocados; vegetables, especially legumes and green leafy vegetables; and nuts and seeds. These plant foods, with few exceptions (e.g., potatoes and dates), tend to improve glycemic control and reduce the risk of diabetes complications (49–51). Traditional meat-containing dishes can easily be made vegetarian with the use of meat analogues such as soy meat substitutes. Vegetarian substitutes for burgers, sausages, hot dogs, crumbles, strips, “meatballs,” and a variety of other foods are available in regular grocery stores. Patients willing to adopt a vegan diet can substitute dairy products with soy products, including soymilk, tofu, and tempeh.
Patients also can be referred to existing resources to guide them in adopting a healthy, vegetarian diet. Such resources may include online information from the ADA, the USDA, and the Vegetarian Research Group (52–54). Patients can also find online search engines that list vegetarian restaurants (55,56). In addition to meal-planning guides and websites that list vegetarian restaurants, many ethnic restaurants, especially Middle Eastern and Mexican restaurants, offer a variety of meatless dishes. Again, to successfully implement a healthy vegetarian eating plan, patients can be referred to an RDN, who can assist them in meal planning to minimize risk of nutrient deficiencies, maximize glycemic control, and address their individual dietary preferences.
More research is needed to assess the impact of vegetarian diets on the prevention and management of diabetes complications. Similarly, no research to date has assessed how poor vitamin B12 status among vegetarians may affect their risk of diabetes complications and whether improving the status of this nutrient may exhibit an additional benefit to patients with diabetes who adopted such diets. Also, future research should assess barriers faced by health care providers in recommending vegetarian, and especially vegan, diets to their patients. Barriers faced by patients with diabetes in adopting a vegetarian diet also need to be identified and addressed.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Evert AB, Boucher JL, Cypress M, et al.; American Diabetes Association . Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36:3821–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferdowsian HR, Barnard ND. Effects of plant-based diets on plasma lipids. Am J Cardiol 2009;104:947–956 [DOI] [PubMed] [Google Scholar]
- 3.Pettersen BJ, Anousheh R, Fan J, Jaceldo-Siegl K, Fraser GE. Vegetarian diets and blood pressure among white subjects: results from the Adventist Health Study-2 (AHS-2). Public Health Nutr 2012;15:1909–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnard ND, Katcher HI, Jenkins DJ, Cohen J, Turner-McGrievy G. Vegetarian and vegan diets in type 2 diabetes management. Nutr Rev 2009;67:255–263 [DOI] [PubMed] [Google Scholar]
- 5.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis 2013;23:292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowe FL, Appleby PN, Travis RC, Key TJ. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr 2013;97:597–603 [DOI] [PubMed] [Google Scholar]
- 7.Gupta SK, Sawhney RC, Rai L, et al. Regression of coronary atherosclerosis through healthy lifestyle in coronary artery disease patients: Mount Abu Open Heart Trial. Indian Heart J 2011;63:461–469 [PubMed] [Google Scholar]
- 8.Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009;32:791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G. Vegetarian diets and the incidence of cancer in low risk population. Cancer Epidemiol Biomarker Prev 2013;22:286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appleby PN, Allen NE, Key TJ. Diet, vegetarianism, and cataract risk. Am J Clin Nutr 2011;93:1128–1135 [DOI] [PubMed] [Google Scholar]
- 11.Crowe FL, Appleby PN, Allen NE, Key TJ. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): prospective study of British vegetarians and non-vegetarians. BMJ 2011;343:d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal S, Millett CJ, Dhillon PK, Subramanian SV, Ebrahim S. Type of vegetarian diet, obesity and diabetes in adult Indian population. Nutr J 2014;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu TH, Huang HY, Chiu YF, et al. Taiwanese vegetarians and omnivores: dietary composition, prevalence of diabetes and IFG. PLoS One 2014;9:e88547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoyama Y, Barnard ND, Levin SM, Watanabe M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther 2014;4:373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YM, Kim SA, Lee IK, et al. Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: a 12-week randomized clinical trial. PLoS One 2016;11:e0155918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnard N, Cohen C, Jenkins D, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006;29:1777–1783 [DOI] [PubMed] [Google Scholar]
- 17.Bunner AE, Wells CL, Gonzales J, Agarwal U, Bayat E, Barnard ND. A dietary intervention for chronic diabetic neuropathy pain: a randomized controlled pilot study. Nutr Diab 2015;5:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barsotti G, Navalesi R, Giampietro O, et al. Effects of a vegetarian, supplemented diet on renal function, proteinuria, and glucose metabolism in patients with ‘overt’ diabetic nephropathy and renal insufficiency. Contrib Nephrol 1988;65:87–94 [DOI] [PubMed] [Google Scholar]
- 19.Wiwanitkit V. Renal function parameters of Thai vegans compared with non-vegans. Ren Fail 2007;29:219–220 [DOI] [PubMed] [Google Scholar]
- 20.International Diabetes Federation IDF Diabetes Atlas. 6th ed. Available from http://www.diabetesatlas.org. Accessed 25 August 2016
- 21.Centers for Disease Control and Prevention National diabetes statistics report, 2014. Available from http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html. Accessed 25 August 2016
- 22.Yao B, Fang H, Xu W, et al. Dietary fiber intake and risk of type 2 diabetes: a dose-response analysis of prospective studies. Eur J Epidemiol 2014;29:79–88 [DOI] [PubMed] [Google Scholar]
- 23.Sluijs I, Beulens JW, van der ADL, Spijkerman AM, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 2010;33:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White DL, Collinson A. Red meat, dietary heme iron, and risk of type 2 diabetes: the involvement of advanced lipoxidation endproducts. Adv Nutr 2013;4:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satija A, Bhupathiraju SN, Rimm EB, et al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comerford KB, Pasin G. Emerging evidence for the importance of dietary protein source on glucoregulatory markers and type 2 diabetes: different effects of dairy, meat, fish, egg, and plant protein foods. Nutrients 2016;8:pii:E446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viguiliouk E, Stewart SE, Jayalath VH, et al. Effect of replacing animal protein with plant protein on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2015;7:9804–9824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Zhou C, Zhou X, Li L. Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis 2013;229:524–530 [DOI] [PubMed] [Google Scholar]
- 30.Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med 2011;28:549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D. How prevalent is vitamin B12 among vegetarians? Nutr Rev 2013;71:110–117 [DOI] [PubMed] [Google Scholar]
- 32.Niafar M, Hai F, Porhomayon J, Nader ND. The role of metformin on vitamin B12 deficiency: a meta-analysis review. Intern Emerg Med 2015;10:93–102 [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Li S, Quan H, Li J. Vitamin B12 status in metformin treated patients: systematic review. PLoS One 2014;9:e100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawlak R. Is vitamin B12 deficiency a risk factor for cardiovascular disease in vegetarians? Am J Prev Med 2015;48:e11–e26 [DOI] [PubMed] [Google Scholar]
- 35.Xu C, Wu Y, Liu G, Liu X, Wang F, Yu J. Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol 2014;9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jianbo L, Yuche C, Ming S, et al. Association of homocysteine with peripheral neuropathy in Chinese patients with type 2 diabetes. Diabetes Res Clin Pract 2011;93:38–42 [DOI] [PubMed] [Google Scholar]
- 37.van Wijngaarden JP, Doets EL, Szczecińska A, et al. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: a systematic review with meta-analyses. J Nutr Metab 2013;2013:486186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawlak R, Berger J, Hines I. Iron status of vegetarian adults: a literature review. Am J Life Med. In press [DOI] [PMC free article] [PubMed]
- 39.Sanders T. DHA status of vegetarians. Prostaglandins Leukot Essent Fatty Acids 2009;81:137–141 [DOI] [PubMed] [Google Scholar]
- 40.Chan J, Jaceldo−Siegl K, Fraser G. Serum 25−hydroxyvitamin D status of vegetarians, partial vegetarians, and nonvegetarians: the Adventist Health Study−2. Am J Clin Nutr 2009;89(Suppl.):1686S–1692S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appleby P, Roddam A, Allen N, Key T. Comparative fracture risk in vegetarians and nonvegetarians in EPIC−Oxford. Eur J Clin Nutr 2007;61:1400−1406 [DOI] [PubMed] [Google Scholar]
- 42.Kohlenberg-Mueller K, Raschka L. Calcium balance in young adults on a vegan and lactovegetarian diet. J Bone Miner Metab 2003;21:28–33 [DOI] [PubMed] [Google Scholar]
- 43.Han E, Yun Y, Kim G, et al. Effects of omega-3 fatty acid supplementation on diabetic nephropathy progression in patients with diabetes and hypertriglyceridemia. PLoS One 2016;11:e0154683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H, Park S, Yang H, Choi YJ, Huh KB, Chang N. Association between fish and shellfish, and omega-3 PUFAs intake and CVD risk factors in middle-aged female patients with type 2 diabetes. Nutr Res Pract 2015;9:496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sala-Vila A, Díaz-López A, Valls-Pedret C, et al. ; Prevención con Dieta Mediterránea (PREDIMED) Investigators. Dietary marine ω-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol 2016;134:1142–1149 [DOI] [PubMed] [Google Scholar]
- 46.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 2003;107:1852–1857 [DOI] [PubMed] [Google Scholar]
- 47.Rinaldi S, Campbell EE, Fournier J, O’Connor C, Madill J. A comprehensive review of the literature supporting recommendations from the Canadian Diabetes Association for the use of a plant-based diet for management of type 2 diabetes. Can J Diabetes 2016;40:471–477 [DOI] [PubMed] [Google Scholar]
- 48.Lee V, McKay T, Ardern CI. Awareness and perception of plant-based diets for the treatment and management of type 2 diabetes in a community education clinic: a pilot study. J Nutr Metab 2015;2015:236234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li M, Fan Y, Zhang X, Hou W, Tang Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open 2014;4:e005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Miao S, Huang Y, et al. Fruit intake decreases risk of incident type 2 diabetes: an updated meta-analysis. Endocrine 2015;48:454–460 [DOI] [PubMed] [Google Scholar]
- 51.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol 2013;28:845–858 [DOI] [PubMed] [Google Scholar]
- 52.American Diabetes Association Meal planning for vegetarian diets [Internet]. Available from http://www.diabetes.org/food-and-fitness/food/planning-meals/meal-planning-for-vegetarians. Accessed 8 November 2016
- 53.U.S. Department of Agriculture Healthy eating for vegetarians [Internet]. Available from https://www.nutrition.gov/smart-nutrition-101/healthy-eating/eating-vegetarian. Accessed 8 November 2016
- 54.Vegetarian Resource Group Vegan diet for people with diabetes [Internet]. Available from https://www.vrg.org/journal/vj2003issue2/2003_issue2_diabetes.php. Accessed 8 November 2016
- 55.Vegetarian Resource Group Vegetarian Journal’s guide to vegetarian and vegan restaurants in the U.S. and Canada [Internet]. Available from http://www.vrg.org/restaurant/index.php. Accessed 8 November 2016
- 56.Happycow. Vegan options near you [Internet]. Available from https://www.happycow.net. Accessed 8 November 2016 [Google Scholar]