Abstract
Background
The aim of this study was to investigate the association between sex and speech-ABR in adults, and its relationship to sex hormone levels.
Material/Methods
Speech-ABR were elicited with the consonant-vowel syllable (/da/) in a total of 35 adults. Reproductive hormone levels were also measured.
Results
The transient response of the speech-ABR (waves V, A, and O) in females show a shorter latency (waves V, A and O) and a larger amplitude (waves V and A) than in males (P<0.05), except for the amplitude of peak O (P>0.05). The sustained response of females exhibited a larger amplitude (wave F, P<0.05) and a shorter latency (wave D, E, and F, P<0.05) than in males, except for the amplitude of peak D and E (P>0.05). The latencies of speech-ABR were positively correlated with testosterone level (P<0.05), and were negatively correlated with estradiol (E2) levels (P<0.05), except for wave E (P>0.05). The E2 showed a positive correlation with the absolute value of amplitude of the speech-ABR (P < 0.05). On the contrary, total testosterone showed a negative correlation with the absolute value of amplitude the speech-ABR (P<0.05), except for wave D and wave O (P>0.05).
Conclusions
Sex differences in speech-ABR are significant in adults. The latencies and amplitude of the speech-ABR waves were correlated with the E2 concentration and testosterone level. The sex hormones likely affect speech encoding in the brainstem.
MeSH Keywords: Audiometry, Speech; Evoked Potentials, Auditory, Brain Stem; Gonadal Steroid Hormones; Sex Characteristics; Young Adult
Background
Sex-related differences in cognitive abilities have been widely reported [1]. Many authors suggest that females outperform males in overall verbal ability. Strelnikov et al. [2] demonstrated that women are better speech readers than men when assessed based on single-word recognition. This difference was observed both in normal-hearing subjects and in deaf individuals. The relative superiority of women differed across groups and conditions. However, sex differences in verbal fluency have not consistently been found [3,4]. A critical review of sex differences in verbal abilities and in the language-related cortex suggested that sex differences in language proficiency do not exist in the normal population [5]. Even now, sex differences in speech ability continue to be a matter of debate. The majority of previous studies used behavioral tests to examine such differences [2,5,6], and varying test contents, test materials, and language tasks may have led to different results regarding speech and language processing abilities [7].
Recent work has established that the speech-evoked auditory brainstem response (speech-ABR) is elicited by speech sounds, such as vowels (/a/, /u/) and consonant-vowel syllables (/da/, /ba/). The speech-ABR can reliably reflect many acoustic properties of the speech signal in both the onset and frequency-following response (FFR) portions of the response, which could provide an objective biological index of auditory speech processing [8–10]. In the present study, the default /da/ stimulus of the BioMAP (Biological Marker of Auditory Processing) software was used to elicit the speech-ABR, as mentioned by Skoe and Kraus [11]. Speech ABRs elicited by speech sounds display high test-retest reliability [12]; as a result, this measure provides a powerful tool for investigating the brainstem encoding of speech. This type of investigation can uncover auditory processing deficits [13,14] and the experience-dependent enhancement of speech encoding in the brainstem [15,16], and it may provide further insight into sex differences in encoding. Krizman et al. [17] investigated such sex differences in young adults and found that males and females have measurably different neural responses to speech. Female responses to the fast-acoustic components of speech are generally earlier and more robust than male responses. Liu et al. [18] also showed that the females have shorter wave latency and larger amplitude of speech-ABR than males. Examining the sex differences in speech-ABR would help define accurate normative data for speech-ABR measures, which would aid in their clinical use for detecting/diagnosing peripheral and/or subcortical dysfunction.
Moreover, the mechanisms underlying the sex differences in brainstem speech encoding are not at all clear. Previous reports have suggested that head size may provide a basis for sex differences in the latency of click ABRs, which may be a reason for sex differences in speech ABRs. In addition, many authors have focused on the effect of hormones on ABRs. Ovarian hormones have been reported to affect click ABRs [19]. Sex differences in speech ability have been also suggested to be related to reproductive hormones [5]. The latest research further shows that differences in the levels of sex hormones in women during the menstrual cycle can affect the brainstem encoding of speech stimuli [20]. It is well known that the concentration of reproductive hormones fluctuates throughout different stages of life. Auditory function in women during the ovarian cycle has been previously investigated, with inconsistent findings because the studies lacked precise timing of the cycle [21]. This may be because it is difficult to obtain the exact correlation between hormonal levels and auditory function. The present study investigated sex differences in speech processing in young adults by comparing the speech ABRs of females and males. We evaluated the estradiol and testosterone concentrations on the same day after the conclusion of the speech-ABR test. We performed correlation analyses to identify the relationships between the latency and amplitude of the waves of the speech-ABR and the E2 concentration and total testosterone level, which more accurately reflects the relationship between sex hormones and speech-ABR.
Material and Methods
Participants
A total of 35 young adults participated in this study, including 18 males (24–34 years old, mean age=28.17 years, SD=2.50) and 17 females (24–31 years old, mean age=27.29 years, SD=1.83). All subjects were required to undergo a normal otoscopic examination and have normal 226 Hz tympanometry. Pure-tone air-conduction and bone-conduction threshold audiometry were performed in an audiometric room that complied with the acceptance criteria in China (GB/T16403-1996 and GB/T 16296-1996). All of the participants had air-conduction hearing thresholds of 20 dB HL or better across standard audiometric frequencies (250–8000 Hz) in both ears. Moreover, all of the adults had clear click-ABR responses using a click stimulus presented at 80 dB SPL, and a latency of wave V that was less than 6 ms. Wave V was identified using click stimulus presented at 30 dB SPL.
The adults who participated in the study were postgraduate students or young doctors from Beijing Chaoyang Hospital, Capital Medical University. All of the subjects were paid for their participation in this study. The recording procedures were approved by the Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University (2012-83). The study was also registered with the Chinese Clinical Trial Registry (ChiCTR-CCC-12002274).
Stimuli and recording parameters
The participants were tested in a sound-proof booth and were instructed to ignore the stimuli. Brainstem responses to both a click stimulus and a speech sound (/da/) were collected according to widely used procedures described in detail previously [8,11,13]. The Biologic Navigator Pro System (Biologic Systems Corporation, Natus Medical Inc., Mundelein, IL) was used to collect the physiological data. The Navigator’s BioMAP module was used to collect the /da/-evoked responses. BioMAP uses a Klatt-synthesized [22] 40-ms /da/ stimulus. The stimulus was composed of 5 formants that transitioned from the consonant /d/ to the vowel /a/. The fundamental frequency (F0) and the first 3 formants (F1, F2, and F3) changed linearly over the duration of the stimulus. F0 changed from 103 to 125 (0–35 ms) to 121.2 Hz (35–40 ms), F1 from 220 to 720 Hz, F2 from 1700 to 1240 Hz, and F3 from 2580 to 2500 Hz. F4 and F5 remained constant at 3600 and 4500 Hz, respectively [8,11]. The brainstem responses to speech and clicks were elicited by alternating polarities, and the brainstem responses to both stimuli were collected in the same manner and during the same recording session.
The test stimulus /da/ was delivered to the right ear through insert earphones (Bio-logic) at an intensity of 80 dB SPL in the recording of the speech-ABR. The left ear was not intervened. To ensure subject cooperation and to promote stillness, all subjects was required to watch videotaped programs, with the sound presented at a low level (<40 dB SPL) [23]. The subjects were required to attend to the video rather than to the stimulus. Reusable gold disc electrodes were placed on the right mastoid, on the forehead, and at Cz, acting as reference, ground, and active electrodes, respectively. The impedance values were 5 kΩ or better, and the impedance of all 3 electrodes was within 2 kΩ of each other. The impedance values were optimized at the beginning of each test session and checked periodically throughout the session to ensure that they remained stable.
Three blocks of 2000 no-artifact responses were collected at a rate of 13.3/s (click) and 10.9/s (/da/). For the click, the use of a 10.66-ms recording window (including an 0.8-ms pre-stimulus period) and the responses online filtering from 100 to 1500 Hz. For /da/, the use of a 74.67-ms recording window (including a 15-ms pre-stimulus period) [23]. The responses were sampled at 6857 Hz and bandpass filtered online from 100 to 2000 Hz. For both stimuli, trials with artifacts that measured in excess of ±35 μV were rejected from the averaged response. Trials in which more than 10% of sweeps were rejected as artifacts were repeated to obtain a cleaner response with less artifact contamination.
Evaluation of estradiol and testosterone concentration
Venous blood (3–5 ml) was collected from fasting subjects after the conclusion of the speech-ABR test (on the same day). The serum was separated, and the estradiol and testosterone concentrations were measured via chemiluminescence using the ARCHITECT i2000 chemiluminescence analyzer (Abbott US). The reproductive hormones were analyzed in strict accordance with the manufacturer’s instructions (Abbott Standard Interface RS-232 Manual). The chemiluminescent immunoassay reagent for the Abbott ARCHITECT i2000sr was imported from the Abbott Company, United States. The tests were completed by the laboratory center of the Capital Medical University-affiliated Beijing Chaoyang Hospital. In the original test results, the total testosterone concentration was reported in ng/ml, while the estradiol level was reported in pg/ml. The testosterone concentration was converted into units of pg/ml (=ng/ml ×1000) to enable direct comparison of the results.
Data analysis
The brainstem response to the speech sound /da/ has been described amply in previous reports [8,11,14] and is very reliable between and within subjects. The response measures included the peak latency and amplitude. Six prominent waves were identified for each waveform: V, A, D, E, F, and O (Figure 1). For each subject, the peak latency and amplitude were determined for the onset (waves V and A), offset (wave O), and frequency-following response (D, E, and F). A peak was deemed reliable if it was present in >85% of the total subject population [23]. Because peak C was deemed unreliable, statistical analysis was not performed for it. The V-A onset complex was further analyzed by computing the slope, which is a measure of neural synchrony for onset responders. The latency (ms) and magnitude (μV) values for each wave were calculated using BioMAP™ software.
Figure 1.
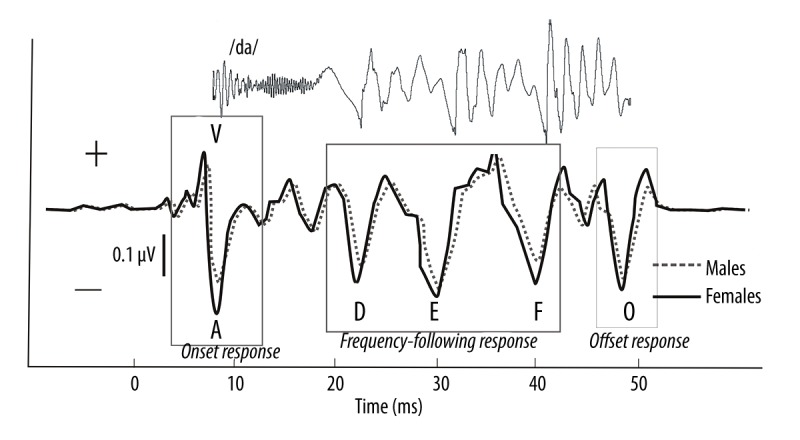
The grand average waveform for the females group (solid line) and the males group (dotted line) and a typical speech ABR with 6 characteristic peaks (V, A, D, E, F, O) elicited by the speech stimulus /da/. Waves V and A represent the response to the onset of sound. Waves D, E, and F indicate the frequency-following response (FFR). Wave O represents the offset response. The stimulus of the /da/ was time-shifted by ~6.8 ms to account for the time delay between the presentation of the stimulus and the generation of the response. The latency of each speech ABR response in the males was longer than in the females. The magnitude of the entire recorded wave was lower in the males than in the females.
Statistical analyses
The averaged data for the latency (ms) and magnitude (μV) of the speech ABRs, the latency of click-ABR, the E2 and testosterone concentrations are presented as the mean±standard deviation (SD). Sex differences in the speech ABRs of the young adults were evaluated using the analysis of variance (ANOVA), with significance defined as a P value of 0.05. The sex difference in the latency of wave V of click-ABR, E2, and testosterone concentrations between the young men and women were evaluated using the independent-samples t test, with significance defined as a P value of 0.05. The correlations between the reproductive hormone levels and the magnitude of the speech ABRs and between reproductive hormone levels and the latency of each speech-ABR were also analyzed using Spearman’s correlation coefficient. The reliability of Spearman’s correlation coefficient was tested. The correlations were considered statistically significant when the P value was less than 0.05. All statistical analyses were performed with the SPSS package, version 17 (SPSS, Chicago, IL, USA). Bar diagrams were drawn using GraphPad Prism V5.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Differences in speech-ABR between females and males
The present results show that the latency of wave V of click-ABR in males (5.66±0.19 ms) was significantly delayed compared to that in females (5.33±0.22 ms) (P<0.05). Consistent with sex differences of the click-ABR, the young adults showed obvious sex differences in speech-ABR (Figures 1–3). Significant differences in the latency of the transient response (waves V, A, and O) were found between the females and males (Table 1 and Figures 1, 2). The latency of the transient response of the speech-ABR (waves V, A, and O) was shorter in the females than in the males (P<0.05). In addition, the amplitude of peaks V and A in the females was also significantly larger than that in males (P<0.05) (Table 1 and Figure 3). However, no significant sex significance in the amplitude of peak O was found (P>0.05).
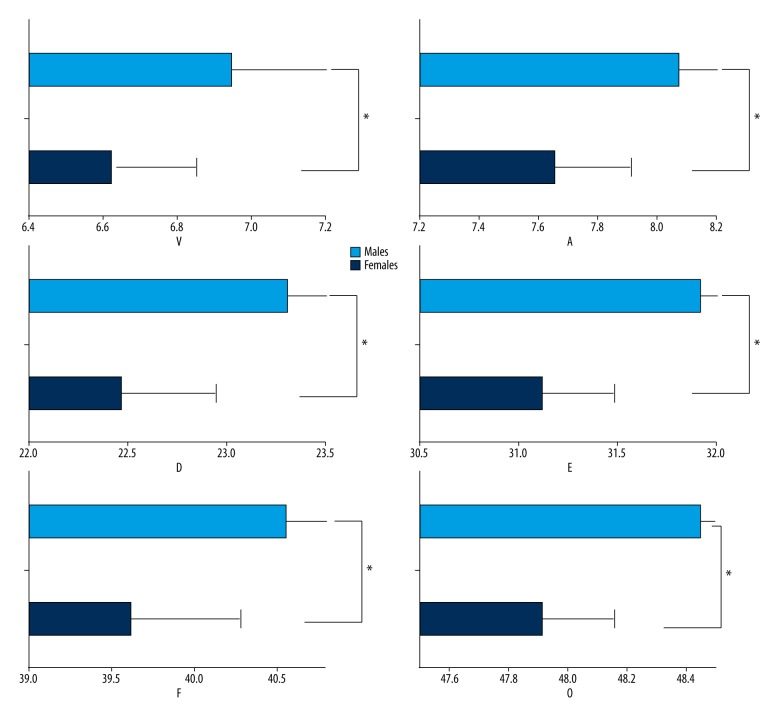
Figure 2.
The mean latencies of the 6 peaks of the speech-ABR are shown, with error bars representing+1 standard deviation of the mean. From these plots, it is clear that significantly longer latencies were observed for peaks V, A, D, E, F, and O in the young men compared with the young women (P<0.05). The asterisk (*) indicates a statistically significant difference (P<0.05).
Figure 3.
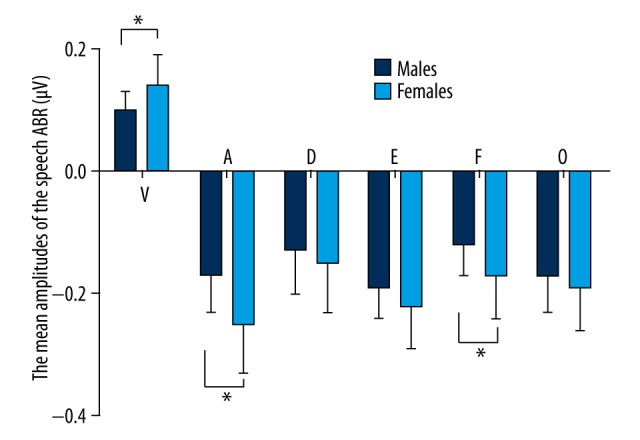
The mean amplitudes of the 6 peaks of the speech-ABR are shown, with error bars representing +1 standard deviation of the mean. From these plots, it is clear that the amplitudes of the peaks of the young men are smaller than those of the young women; this difference was statistically significant for waves V, A, and F (P<0.05), but the sex difference of the amplitude in waves D, E, and O were without statistical significance (P>0.05). The asterisk (*) indicates a statistically significant difference (P<0.05).
Table 1.
Sex differences in the speech ABR (means ±SD) in young adults.
| Women (n=17) | Men (n=18) | F value | P value | ||
|---|---|---|---|---|---|
| Latency (ms) | V | 6.62±0.23 | 6.94±0.37 | 9.691 | 0.004* |
| A | 7.65±0.26 | 8.07±0.34 | 17.590 | 0.000* | |
| D | 22.46±0.48 | 23.30±0.84 | 13.176 | 0.001* | |
| E | 31.11±0.37 | 31.92±0.75 | 16.443 | 0.000* | |
| F | 39.61±0.67 | 40.55±0.69 | 17.049 | 0.000* | |
| O | 47.88±0.28 | 48.38±0.46 | 14.659 | 0.001* | |
| V/A slope (μV/ms) | −0.40±0.14 | −0.24±0.08 | 19.840 | 0.000* | |
| Amplitude (μV) | V | 0.14±0.05 | 0.10±0.03 | 11.042 | 0.002* |
| A | −0.25±0.06 | −0.17±0.06 | 18.266 | 0.000* | |
| D | −0.15±0.08 | −0.13±0.06 | 1.154 | 0.290 | |
| E | −0.22±0.06 | −0.19±0.06 | 2.545 | 0.120 | |
| F | −0.17±0.08 | −0.12±0.04 | 6.827 | 0.013* | |
| O | −0.19±0.07 | −0.17±0.06 | 1.722 | 0.198 |
The analysis of ANOVA; significance level α=0.05; statistically significant differences are identified by asterisks:
P<0.05.
There was a statistically significant difference between the females and males in the sustained response (waves D, E and F); the response of females exhibited a larger amplitude (wave F, P<0.05) and a shorter latency (wave D, E and F, P<0.05 respectively) than that of the males, (Table 1 and Figures 1, 2). However, no sex significance in the amplitude of peak D and E was found statistically (P>0.05 respectively). The V/A slope in the females was also significantly steeper than that of the males (P<0.05).
Reproductive hormone levels in young adults
We found that the E2 levels of the females (118.77+102.66 pg/ml) were significantly higher than those of the males (52.91+14.77 pg/ml) (P<0.05), while the total testosterone concentration in the females (457.65+140.82 pg/ml) was significantly lower than that of the males (3677.37+1155.80 pg/ml) (P<0.05).
Correlations between speech ABRs and reproductive hormones in adults
A correlation analysis showed that the latencies of all reaction of the speech-ABR waves were negatively correlated with the E2 concentration (wave V r=−0.312; wave A r=−0.390; wave D r=−0.315; wave F r=−0.390, and wave O r=−0.396, P<0.05); the exception was wave E (r=−0.259, P>0.05). The correlation coefficient ranged between 0.259 and 0.396, indicating a weak correlation. In contrast, the total testosterone level and the latency of each peak of the speech-ABR were significantly positively correlated (wave V r=0.498; wave A r=0.592; wave D r=0.493; wave E r=0.613; wave F r=0.597, and wave O r=0.474, P<0.05) (Table 2). The correlation coefficient ranged between 0.474 and 0.613, indicating a moderate or strong correlation.
Table 2.
Correlations between the speech ABR and reproductive hormones in adults.
| Speech ABR (n=35) | E2 (r) | P value | Testosterone (r) | P value | ||
|---|---|---|---|---|---|---|
| Latency (ms) | V | 6.79±0.34 | −0.312 | 0.032* | 0.498 | 0.001* |
| A | 7.87±0.37 | −0.390 | 0.009* | 0.592 | 0.000* | |
| D | 22.90±0.81 | −0.315 | 0.031* | 0.493 | 0.001* | |
| E | 31.54±0.72 | −0.259 | 0.064 | 0.613 | 0.000* | |
| F | 40.11±0.82 | −0.390 | 0.009* | 0.597 | 0.000* | |
| O | 48.14±0.46 | −0.396 | 0.008* | 0.474 | 0.002* | |
| Amplitude (μV) | V | 0.12±0.05 | 0.480 | 0.002* | −0.384 | 0.010* |
| A | −0.21±0.07 | −0.383 | 0.011* | 0.489 | 0.001* | |
| D | −0.14±0.07 | −0.639 | 0.000* | 0.146 | 0.198 | |
| E | −0.20±0.06 | −0.415 | 0.006* | 0.360 | 0.016* | |
| F | −0.14±0.07 | −0.516 | 0.001* | 0.335 | 0.023* | |
| O | −0.18±0.06 | −0.294 | 0.041* | 0.133 | 0.219 | |
| V/A slope | −0.32±0.14 | −0.495 | 0.001* | 0.506 | 0.001* |
Significant correlations are indicated with asterisks:
P<0.05, l (1-tailed);
r: correlation coefficient.
The amplitude of wave V and the E2 concentration were positively correlated (r=0.480, P<0.05), while the E2 concentration was significantly negatively correlated with wave A r=−0.383; wave D r=−0.639; wave E r=−0.415; wave F r=−0.516; wave O r=−0.294, and the V/A slope r=−0.495, P<0.05 (Table 2). The correlation coefficient ranged between 0.383 and 0.639, indicating a moderate or strong correlation.
In contrast, the amplitude of the positive wave V was negatively correlated with the total testosterone concentration (r=−0.384; P<0.05). The amplitude of the negative waves A (r=0.489), E (r=0.360), and F (r =0.335), and the V/A slope (r=0.506), were positively correlated with the testosterone concentration (P<0.05) (Table 2). The correlation between the amplitudes of wave D and wave O and the total testosterone concentration was not statistically significant (wave D r=0.146; wave O r=0.133, P>0.05).
Discussion
In this study, significant sex differences in most of the response waves of the speech-ABR were found between 24- to 34-year-old females and males. All waves of the transient responses of the females showed a significantly shorter latency and larger amplitude than the corresponding responses of the males, except for the amplitude of peak O, D and E (P>0.05). The V/A slope was also significantly steeper in females than in males (P<0.05) (Table 1). Krizman et al. [17] found that females have significantly earlier peak latencies at peaks V and A, and a steeper V/A slope compared with men, in line with the present study. The V-A complex reflects a highly synchronized neural response to a stimulus onset. The observation of a shorter V-A complex latencies in females suggests that the neural response to the onset of a stimulus is more synchronous in females than in males. Furthermore, the sex difference was also found in the sustained portions of the speech-ABR (waves D, E, and F). Females showed a significantly shorter latency than males (waves D, E, and F, P<0.05). This result also suggests that the effect of sex on the outcome needs to be considered when speech ABRs are used as a diagnostic tool to assess speech processing in young adults. However, the amplitude of sustained portions of the speech-ABR (waves D, E, and F) was little affected by sex. Females only exhibited a larger amplitude in wave F (P<0.05) than that of the males (Table 1 and Figures 1, 3). However, no sex difference was found in the amplitude of peak D and E (P>0.05). This result suggests that the phase-locking ability of neurons in the brain stem to speech stimuli is little affected by sex.
Sex differences in the structure and function of the auditory system have been widely reported [24–27]. Females showed significantly lower hearing thresholds than males, especially at higher frequencies (3000 Hz and above), where the sex difference was approximately 4–5 dB [28]. Females also display significantly more spontaneous otoacoustic emissions (SOAEs), stronger SOAEs, and greater transient evoked otoacoustic emission (TEOAE) amplitudes than males in infancy [25,29], childhood [30], and adulthood [28]. Auditory processing, as assessed with the ABR, is more efficient in females than in males [31]. Wave III and wave V latencies and I–III and I–V interpeak latency (IPL) intervals are significantly shorter in females than in males [32], and women were found to have greater gray matter percentages (corrected for overall brain size and age) in language-related cortical regions than men in an fMRI study [33]. Don et al. [34] offered a convincing theory that attributes the shorter latency and larger amplitude of ABR peaks in females to the shorter cochlear length and greater basilar membrane stiffness gradient in female subjects. According to this theory, shorter latencies in females are caused by faster basilar membrane travel times, and greater amplitudes are caused by more neural activity per unit time. Moreover, previous reports have suggested that head size may provide a basis for sex differences in the latency of the ABR. Aoyagi et al. [32] found a significant positive correlation between head size and ABR wave latencies and IPL intervals, suggesting that head size may reflect brain size, which is an important factor in sex differences in ABR latencies. However, the present study did not measure head size, and therefore cannot totally rule out the effect of head size.
Many authors have focused on the effect of hormones on ABR. Increased ABR wave V peak latencies have been found to be associated with elevated levels of E2 or testosterone [35]. The latest research shows that differences in the levels of sex hormones throughout the menstrual cycle can affect women’s brainstem encoding of speech stimuli [20]. Effects of gonadal hormones, such as testosterone, progesterone, and E2, on language have also been suggested [36]. To determine the effect of reproductive hormone levels on the speech-ABR, we tested the reproductive hormone levels of 35 young adults (17 women and 18 men). We found that the E2 levels in females were significantly higher than those in males (P<0.05) and that the total testosterone concentration in females was significantly lower than in males (P<0.05). These differences may be related to the sex differences in speech-ABR in adults. To determine the effect of reproductive hormone levels on speech coding ability, we conducted a correlation analysis between reproductive hormone levels and the latency and amplitude of the speech-ABR in young adults. Our results show that the latency of all reaction waves except wave E was negatively correlated with the E2 concentration (r=0.259, P=0.064; Table 2). The latency of the speech-ABR decreased as the E2 level increased. The amplitude of wave V and the E2 concentration were positively correlated (r=0.480, P<0.05), while the E2 concentration was significantly negatively correlated with the amplitude of waves A (r=−0.383), D (r=−0.639), E (r=−0.415), F (r=−0.516), and O (r=−0.294). The amplitude of the speech-ABR increased as the E2 level increased. E2 impacts a wide variety of brain processes, including sex differentiation, mood, and learning. E2 also regulates the processing of acoustic signals in the vertebrate brain, specifically in the caudomedial nidopallium (NCM), which the songbird analog of the mammalian auditory association cortex [27]. High levels of circulating E2 enhance the synaptic responsiveness of medial vestibular nucleus neurons [37], and in (CA1) pyramidal cells in hippocampal slices, E2 can rapidly increase the amplitude of synaptically evoked local field potentials and enhance excitatory postsynaptic potentials (EPSPs) and excitatory postsynaptic currents (EPSCs). Both exogenous and locally generated E2 can increase auditory-evoked activity in the NCM. These results suggest that E2 may improve brainstem auditory neuron excitability and phase-locking ability for speech coding. Furthermore, the correlation coefficient between E2 and amplitude of speech-ABR was higher than that of E2 and the latency of speech-ABR (Table 2). This suggests that E2 is likely to affect the phase-locking of neurons (amplitude), but has little effect on the conduction velocity of neurons (latency). In particular, the correlation coefficient between E2 and the amplitude of the sustained response (wave D, r=−0.639; wave E, r=−0.415; wave F, r=−0.516) is greater than that of the transient response (wave V, r=0.480; wave A, r=−0.383; wave O, r=−0.294), suggesting that E2 is more closely related to the amplitude of sustained response than that of transient response.
In contrast, the present results also showed that the total testosterone level was significantly positively correlated with the latency of each wave of the speech-ABR (Table 2); the higher the total testosterone concentration, the greater the latency of the speech-ABR peaks. The latency of the speech-ABR increased as the total testosterone level increased. Previous results have also shown that circulating and neuronal testosterone can remarkably influence the synaptic responses of vestibular neurons in a manner that depends on its local conversion. At a physiological concentration, testosterone is often converted into 5α-dihydrotestosterone (DHT), which only mediates long-lasting depression [38]. Therefore, at a physiological level, testosterone may weaken the synchronization of neuronal activity; these effects may prolong the latency of the speech-ABR. Furthermore, the influences of androgens on behavior and speech have been widely investigated [39,40]. In a previous study of 22 female-to-male transsexuals, a battery of visuospatial and verbal ability tests was administered twice: shortly before and 3 months after the start of androgen treatment. Androgen administration had a deteriorating effect on verbal fluency tasks. This study offered preliminary evidence that androgens quickly and directly affect verbal cognitive performance in females [41]. Furthermore, in a study of the relation between verbal and non-verbal cognitive abilities and circulating androgen levels in healthy adult men, a negative correlation between androgen levels and measures of verbal production was found [42].
Furthermore, based on the present results, the effect of androgen on speech-ABR is different from that of estrogen. The correlation coefficient between testosterone and latency of speech-ABR was higher than that of testosterone and the amplitude of speech-ABR (Table 2). This suggests that testosterone is more likely to affect the synchronization of neural response, but has little effect on the phase-locking of neurons. In addition, animal experiments further confirmed that orchiectomy increased the synaptic transmission speed and excitation of the hippocampal mossy fiber pathway and that this phenomenon disappeared after testosterone was administered, further demonstrating the inhibitory effect of testosterone on nerve excitability and synaptic transmission [43]. Regarding amplitude of speech-ABR, the onset responses (Waves V, A, and V/A slope) were moderately correlated with the total testosterone concentration (Waves V r=−0.384; Waves A r=0.489, and V/A slope r=0.506, P<0.05). In contrast, regarding the sustained portions of the speech-ABR (waves D, E, and F), the correlation between the amplitude of wave D and the total testosterone concentration was not statistically significant (r=0.146, P>0.05), and the amplitude of waves E and F was only weakly correlated with the total testosterone concentration (wave E r=0.360, P<0.05; waves F, r=0.335, P<0.05). Taken together, these results suggest that testosterone has little effect on the FFR of the speech-ABR.
Conclusions
There were statistically significant sex differences in the latency of all waves of speech-ABR and the amplitude of the onset response of speech-ABR observed in adults. However, the amplitude of sustained portions of the speech-ABR (waves D, E, and F) was little affected by sex. This result suggests that synchronized neural response in the brain stem to speech stimuli could be significantly affected by sex; however, the phase-locking ability of neurons in the brain stem to speech stimuli is little affected by sex. We also found that the latency of the speech-ABR increased as the testosterone level increased and decreased as the E2 concentration increased, and the amplitude of the speech-ABR decreased as the testosterone concentration increased and increased as the E2 level increased. These results suggest that sex hormones likely affect speech encoding in the brainstem. Furthermore, our results suggest that E2 is likely to affect the phase-locking of neurons (amplitude), but has little effect on the conduction velocity of neurons (latency). E2 may improve brainstem auditory neuron excitability and phase-locking ability for speech coding. E2 is more closely related to the amplitude of sustained response (FFR). Moreover, the present results suggest that testosterone is more likely to affect the synchronization of neural response. These effects may prolong the latency of the speech-ABR, but testosterone has little effect on the phase-locking of neurons, especially on the amplitude of sustained response (FFR).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: The general work was supported by the Open Research Fund of Key Laboratory of Ministry of Education of Otorhinolaryngology Head and Neck Surgery (Capital Medical University) China (No. 2012EBYH02) and Beijing Natural Science Foundation (7162066) and (7162072)
References
- 1.Lohman DF, Lakin JM. Consistencies in sex differences on the Cognitive Abilities Test across countries, grades, test forms, and cohorts. Br J Educ Psychol. 2009;79(Pt 2):389–407. doi: 10.1348/000709908X354609. [DOI] [PubMed] [Google Scholar]
- 2.Strelnikov K, Rouger J, Lagleyre S, et al. Improvement in speech-reading ability by auditory training: Evidence from gender differences in normally hearing, deaf and cochlear implanted subjects. Neuropsychologia. 2009;47(4):972–79. doi: 10.1016/j.neuropsychologia.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Hyde JS, Linn MC. Gender differences in verbal ability: A meta-analysis. Psychological Bulletin. 1988;104(1):53–69. [Google Scholar]
- 4.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–77. [PubMed] [Google Scholar]
- 5.Wallentin M. Putative sex differences in verbal abilities and language cortex: A critical review. Brain Lang. 2009;108(3):175–83. doi: 10.1016/j.bandl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16(4):323–34. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- 7.Kitazawa S, Kansaku K. Sex difference in language lateralization may be task-dependent. Brain. 2005;128(Pt 5):E30. doi: 10.1093/brain/awh460. author reply E31. [DOI] [PubMed] [Google Scholar]
- 8.Johnson KL, Nicol T, Kraus N. The brainstem response to speech: A biological marker. Ear Hear. 2005;26(5):424–34. doi: 10.1097/01.aud.0000179687.71662.6e. [DOI] [PubMed] [Google Scholar]
- 9.Akhoun I, Gallégo S, Moulin A, et al. The temporal relationship between speech auditory brainstem responses and the acoustic pattern of the phoneme /ba/ in normal-hearing adults. Clin Neurophysiol. 2008;119(4):922–33. doi: 10.1016/j.clinph.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Liu JF, Wang NY, Fu X, et al. Comparison of the basic characters of speech-evoked auditory brainstem response between school-age children and young adults. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;47(8):646–50. [in Chinese] [PubMed] [Google Scholar]
- 11.Skoe E, Kraus N. Auditory brainstem response to complex sounds: A tutorial. Ear Hear. 2010;31(3):302–24. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song JH, Nicol T, Kraus N. Test–retest reliability of the speech-evoked auditory brainstem response. Clin Neurophysiol. 2011;122(2):346–55. doi: 10.1016/j.clinph.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JH, Banai K, Russo NM, et al. On the relationship between speech- and nonspeech-evoked auditory brainstem responses. Audiol Neurootol. 2006;11(4):233–41. doi: 10.1159/000093058. [DOI] [PubMed] [Google Scholar]
- 14.Banai K, Nicol T, Zecker SG, et al. Brainstem timing: Implications for cortical processing and literacy. J Neurosci. 2005;25(43):9850–57. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong PC, Skoe E, Russo NM, et al. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat Neurosci. 2007;10(4):420–22. doi: 10.1038/nn1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song JH, Skoe E, Wong PC, et al. Plasticity in the adult human auditory brainstem following short- term linguistic training. J Cogn Neurosci. 2008;20(10):1892–902. doi: 10.1162/jocn.2008.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krizman J, Skoe E, Kraus N. Sex differences in auditory subcortical function. Clin Neurophysiol. 2012;123(3):590–97. doi: 10.1016/j.clinph.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JF, Fu X, Wang D, et al. The sexual differential comparison of speech evoked auditory brainstem responses between children and young adults. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51(8):582–87. doi: 10.3760/cma.j.issn.1673-0860.2016.08.005. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 19.Upadhayay N, Paudel BH, Singh PN, et al. Pre- and postovulatory auditory brainstem response in normal women. Indian J Otolaryngol Head Neck Surg. 2014;66(Suppl 1):133–37. doi: 10.1007/s12070-011-0378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhu P, Banerjee N, Anil A, et al. Role of sex hormones produced during menstrual cycle on brainstem encoding of speech stimulus. Eur Arch Otorhinolaryngol. 2016;273(11):3647–50. doi: 10.1007/s00405-016-4009-2. [DOI] [PubMed] [Google Scholar]
- 21.Al-Mana D, Ceranic B, Djahanbakhch O, et al. Hormones and the auditory system: A review of physiology and pathophysiology. Neurosci. 2008;153(4):881–900. doi: 10.1016/j.neuroscience.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 22.Klatt DH. Software for a cascade/parallel formant synthesizer. J Acoust Soc Am. 1980;67:971–95. [Google Scholar]
- 23.Johnson KL, Nicol T, Zecker SG, et al. Developmental plasticity in the human auditory brainstem. J Neurosci. 2008;28(15):4000–7. doi: 10.1523/JNEUROSCI.0012-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Wang N, Li J, et al. Frequency distribution of synchronized spontaneous otoacoustic emissions showing sex-dependent differences and asymmetry between ears in 2- to 4-day-old neonates. Int J Pediatr Otorhinolaryngol. 2009;73(5):731–36. doi: 10.1016/j.ijporl.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Liu JF, Shi BY, Wang NY, et al. Characterization of spontaneous otoacoustic emissions in 2–4 day old neonates with respect to gender and ear. Neural Regen Res. 2009;4:67–71. [Google Scholar]
- 26.Canlon B, Frisina RD. Sex hormones and hearing: A pioneering area of enquiry. Hear Res. 2009;252(1–2):1–2. doi: 10.1016/j.heares.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29(18):5949–63. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snihur AW, Hampson E. Sex and ear differences in spontaneous and click-evoked otoacoustic emissions in young adults. Brain Cogn. 2011;77(1):40–47. doi: 10.1016/j.bandc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Kei J, Mcpherson B, Smyth V, et al. Transient evoked otoacoustic emissions in infants: Effects of gender, ear asymmetry and activity status. Audiology. 1997;36:61–71. doi: 10.3109/00206099709071961. [DOI] [PubMed] [Google Scholar]
- 30.Driscoll C, Kei J, McPherson B. Transient evoked otoacoustic emissions in 6-year-old school children a normative study. Scand Audiol. 2000;29:103–10. doi: 10.1080/010503900424516. [DOI] [PubMed] [Google Scholar]
- 31.Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol. 1980;106:387–91. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- 32.Aoyagi M, Kim Y, Yokoyama J, et al. Head size as a basis of gender difference in the latency of the brainstem auditory-evoked response. Audiology. 1990;29(2):107–12. doi: 10.3109/00206099009081652. [DOI] [PubMed] [Google Scholar]
- 33.Schlaepfer TE, Harris GJ, Tien AY, et al. Structural differences in the cerebral cortex of healthy female and male subjects: A magnetic resonance imaging study. Psychiatry Res. 1995;61(3):129–35. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- 34.Don M, Ponton CW, Eggermont JJ, et al. Gender differences in cochlear response time: An explanation for gender amplitude differences in the unmasked auditory brainstem response. J Acoust Soc Am. 1993;94:2135–48. doi: 10.1121/1.407485. [DOI] [PubMed] [Google Scholar]
- 35.Elkind-Hirsch KE, Wallace E, Malinak LR, et al. Sex hormones regulate ABR latency. Otolaryngol Head Neck Surg. 1994;110(1):46–52. doi: 10.1177/019459989411000105. [DOI] [PubMed] [Google Scholar]
- 36.Hines M, Golombok S, Rust J, et al. Testosterone during pregnancy and childhood gender role behavior: A longitudinal population study. Child Dev. 2002;3(6):1678–87. doi: 10.1111/1467-8624.00498. [DOI] [PubMed] [Google Scholar]
- 37.Grassi S, Frondaroli A, Scarduzio M, et al. Influence of sex and estrous cycle on synaptic responses of the medial vestibular nuclei in rats: Role of circulating 17β-estradiol. Brain Res Bull. 2012;87(2–3):319–27. doi: 10.1016/j.brainresbull.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Grassi S, Frondaroli A, Dieni C, et al. Effects of 17beta-estradiol on synaptic plasticity in the rat medial vestibular nuclei. Acta Otolaryngol. 2009;129(4):390–94. doi: 10.1080/00016480802566287. [DOI] [PubMed] [Google Scholar]
- 39.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Neufang S, Specht K, Hausmann M, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19(2):464–73. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- 41.Van Goozen SH, Cohen-Kettenis PT, Gooren LJ, et al. Activating effects of androgens on cognitive performance: Causal evidence in a group of female to-male transsexuals. Neuropsychologia. 1994;32(10):1153–57. doi: 10.1016/0028-3932(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 42.Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18(1):27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- 43.Atwi S, McMahon D, Scharfman H, et al. Androgen modulation of hippocampal structure and function. Neuroscientist. 2016;22(1):46–60. doi: 10.1177/1073858414558065. [DOI] [PMC free article] [PubMed] [Google Scholar]