Abstract
Mdm2 and Mdmx are critical regulators of the p53 tumour suppressor and are overexpressed in many human malignancies. However, in recent years, their impact on genome instability was shown to be at least, in part, independent of p53. Both Mdm2 and Mdmx inhibit DNA break repair through their association with the Mre11/Rad50/Nbs1 DNA repair complex. Recent evidence indicates that harnessing Mdm2 and/or Mdmx-mediated inhibition of DNA break repair in cancer cells could provide a therapeutic opportunity, particularly for those malignancies that have lost functional p53.
Keywords: Mdm2, Mdmx, Nbs1, DNA repair
Introduction
Mdm2 and its family member, Mdmx, are overexpressed in numerous human cancers (Eischen and Lozano, 2014). Both proteins are considered oncogenes due to their tumour promoting activity and ability to cooperate with other oncogenes when overexpressed (Lundgren et al., 1997; Jones et al., 1998; Wang et al., 2008; Xiong et al., 2010). Genetic studies with knockout mice have firmly established that Mdm2 and Mdmx regulate the tumour suppressor p53 (Jones et al., 1995; Montes de Oca Luna et al., 1995; Parant et al., 2001; Finch et al., 2002; Migliorini et al., 2002). Mdm2 and Mdmx regulate the activity, stability, and localization of p53 (Eischen and Lozano, 2014).
Increased expression of Mdm2 or Mdmx dampens DNA damage signalling and the p53 response (Wang et al., 2008; Xiong et al., 2010). However, a series of reports starting in the late 1990s revealed that Mdm2 appeared to have effects that were independent of p53 (Melo and Eischen, 2012). For example, overexpression of Mdm2 in the presence of wild-type p53 often led to cell death or an inability to generate clones or mice (Kubbutat et al., 1997; Brown et al., 1998; Jones et al., 1998; Carroll et al., 1999). These results suggested that Mdm2 was more than just regulating p53, as Mdm2 overexpression should have led to a growth/survival advantage if it only inhibited p53. Genetic data also provided more evidence of the p53-independent role of Mdm2 in cancer. Mdm2 transgenic p53-null mice developed a different tumour spectrum compared with mice that were just p53-null. More sarcomas developed in the Mdm2 transgenic p53−/− mice than in the p53−/− mice, which preferentially developed lymphomas (Jones et al., 1998). Additionally, a haploinsufficiency in Mdm2, as in Mdm2 heterozygous p53-null mice, resulted in a different tumour spectrum and a slightly longer tumour latency (McDonnell et al., 1999; Eischen and Boyd, 2012). Multiple human and murine tumours have been identified that both overexpress Mdm2 and have inactivated p53 (Cordon-Cardo et al., 1994; Zou et al., 1995; Sigalas et al., 1996; Eischen et al., 1999; Peng et al., 2001; Ramos et al., 2001; Lu et al., 2002; Alt et al., 2003; Giglio et al., 2005). Finally, principal component analysis of microarray data comparing lymphomas overexpressing Mdm2 and harbouring wild-type p53, lymphomas overexpressing Mdm2 and lacking p53, and lymphomas with both indicated separation of these lymphomas based on differential mRNA expression (unpublished data). Together, the data indicate that Mdm2 has p53-independent effects that likely contribute to tumorigenesis.
Identification of a p53-independent function of Mdm2
The challenge was to identify the p53-independent function(s) of Mdm2 and the proteins that mediate the function(s). To identify proteins other than p53 that associate with Mdm2, we chose an unbiased approach by immunoprecipitating endogenous Mdm2 from HeLa cells and performing mass spectrometry on the associated proteins. We identified all three members of the Mre11/Rad50/Nbs1 (MRN) DNA repair complex (Alt et al., 2005). The MRN complex is activated in response to DNA breaks and facilitates DNA break repair by amplifying the DNA damage signal, holding the broken DNA ends together, and processing the ends (Shiloh and Ziv, 2013; Rein and Stracker, 2014). The MRN complex also aides in the activation of the DNA damage kinase ATM (Rein and Stracker, 2014). Extensive additional characterization of Mdm2 association with the MRN complex indicated their interaction occurred in primary, immortalized, and malignant cells from a variety of cellular origins (Alt et al., 2005; Bouska et al., 2008). Mdm2:MRN complex association was also independent of p53 status (wild-type, mutant, or deleted). Mdm2 co-localized with the MRN complex to sites of DNA damage (Alt et al., 2005). We determined Mdm2 bound directly to Nbs1 of the MRN complex and localized the binding domains of each protein to ~30 amino acids (Alt et al., 2005; Bouska et al., 2008). Therefore, there was a direct, specific association between Mdm2 and Nbs1 that occurred upon DNA damage and was independent of p53.
Evaluation of the effects of Mdm2 binding to Nbs1 was revealed in assays that measured DNA damage signalling and DNA breaks. Increased levels of Mdm2 led to reduced levels of DNA damage signalling and significant delays in DNA break repair (Alt et al., 2005; Bouska et al., 2008). Specifically, the levels of marks of ATM-mediated DNA damage signalling, such as phosphorylated H2AX (γH2AX) and phosphorylated serine/threonine glutamine (S/TQ) sites, were reduced shortly after DNA damage induced by gamma radiation in cells overexpressing Mdm2 (Bouska et al., 2008). Consequently, the repair of DNA breaks, as measured by comet assay, was delayed with increased levels of Mdm2. Neither the E3 ubiquitin ligase domain of Mdm2 nor its E3 ubiquitin ligase activity was required for Mdm2 to inhibit DNA repair (Alt et al., 2005). However, point mutations in the Nbs1-binding domain of Mdm2 were sufficient to block the inhibition of DNA break repair and the delay in DNA damage signalling. Additionally, DNA break repair and resolution of DNA damage foci were delayed with Mdm2 overexpression, but this did not occur in cells containing Nbs1 that could not bind to Mdm2 (Bouska et al., 2008). Elevated levels of Mdm2 in cells lacking p53 resulted in chromatid and chromosome breaks, and this was prevented with mutations in the Nbs1-binding domain of Mdm2. Another consequence of delayed DNA break repair was enhanced transformation. Increased levels of Mdm2 resulted in an increased transformation potential of p53-null fibroblasts, which required an intact Nbs1-binding domain (Bouska et al., 2008). Therefore, inhibition of DNA break repair by Mdm2 through its binding to Nbs1 was identified as a specific and novel function of Mdm2 that would contribute to genomic instability and tumorigenesis.
Mdmx also has a p53-independent function
Mdmx shares homology with Mdm2 and also regulates p53 (Shvarts et al., 1996). Mdm2 and Mdmx have overlapping functions in their regulation of p53, but differences also exist (Marine et al., 2007). Recently, we determined that similar to Mdm2, increased levels of Mdmx inhibit DNA break repair independent of p53 status. Mdmx associated with Nbs1 at chromatin following DNA damage. Mdmx delayed the DNA damage signalling response, resulting in DNA breaks persisting. Specifically, kinetic studies showed reduced levels of ATM-mediated DNA damage-induced phosphorylation events (γH2AX and phosphorylation of S/TQ sites) (Carrillo et al., 2015a). Notably, elevated levels of Mdmx in Mdm2/p53-null cells or Mdmx lacking its ring domain in cells containing Mdm2 resulted in a delay in DNA break repair (Carrillo et al., 2015a). These results indicate that the effects of Mdmx on DNA repair do not require Mdm2 and are independent of its association with Mdm2. Moreover, these data indicate that a conserved function of both Mdm2 and Mdmx is to inhibit DNA break repair.
Genetically altered mice also revealed a role for Mdmx in genome instability. Mice lacking Mdmx and p53 have an accelerated rate of tumorigenesis (Matijasevic et al., 2008b), whereas deletion of Mdm2 does not alter the rate of tumour development in a p53-null background (Jones et al., 1996). It was determined that Mdmx−/−p53−/− fibroblasts had multi-polar mitotic spindles and lost chromosomes, which was not evident in fibroblasts that lacked only p53 (Matijasevic et al., 2008a, b). It will be important in the future to determine whether the genome instability caused by loss of Mdmx is mediated by alterations in the regulation of the MRN complex or through a different mechanism. Nevertheless, together, the data highlight a p53-independent role for Mdmx in DNA break repair and genome instability.
Genome instability induced by Mdm2 and Mdmx
Both Mdm2 and Mdmx overexpression negatively affect genome stability. Evidence indicates that at least part of the explanation for this is through their interactions with the MRN complex, but many experiments never tested the effects of Mdm2 or Mdmx overexpression in the absence of p53. Mdm2 transgenic mice overexpressing Mdm2 in breast epithelial cells show the development of polyploid breast epithelial cells irrespective of p53 status (Lundgren et al., 1997). Increased levels of Mdm2 lead to increased DNA breaks, fusions, other structural chromosomal abnormalities, and aneuploidy in Mdm2 transgenic mice with Mdm2 driven by its native promoter (~3−4-fold increased expression) and in fibroblasts with ectopic overexpression (>5-fold) (Alt et al., 2005; Bouska et al., 2008; Wang et al., 2008; Lushnikova et al., 2011). The structural and numerical chromosome abnormalities increased with age in the Mdm2 transgenic mice and appeared prior to the development of tumours (Lushnikova et al., 2011). In non-transformed cell lines, elevated Mdm2 resulted in genome instability from centrosome amplification and aneuploidy (Carroll et al., 1999). Decreased levels of Mdm2 led to increased genome stability with Mdm2 heterozygous fibroblasts containing fewer breaks and fusions than Mdm2 wild-type fibroblasts (Wang et al., 2006). Overexpression of Mdm2 with point mutations in its Nbs1-binding domain was not able to induce chromosome instability, indicating Mdm2:Nb1 interactions are required (Bouska et al., 2008). Additionally, Nutlin3a (Nutlin), a compound that blocks Mdm2:p53 interactions also stabilizes Mdm2, leading to increased protein levels of Mdm2 regardless of p53 status (Vassilev et al., 2004; Li et al., 2012; Carrillo et al., 2015b). Exposure of multiple cell types lacking p53 to Nutlin results in increased DNA breaks and activation of the DNA damage response (Verma et al., 2010; Valentine et al., 2011; Carrillo et al., 2015b). Nutlin delays DNA break repair, which can be prevented with loss of Mdm2 (Carrillo et al., 2015b). Therefore, Mdm2 mediates Nutlin-induced inhibition of DNA break repair in cells lacking p53. Furthermore, elevated Mdmx levels in fibroblasts result in increased genome instability with increased DNA breaks, fusions, and aneuploidy (Carrillo et al., 2015a). These genomic alterations also occurred in cells lacking p53, indicating that the inhibition of p53 by Mdm2 or Mdmx was not responsible for these effects (Alt et al., 2005; Bouska et al., 2008; Carrillo et al., 2015a). Therefore, Mdm2 and Mdmx contribute to genome instability when overexpressed, which is mediated, in part, through their interaction with Nbs1.
Is modulation of DNA break repair an aberrant or normal Mdm2/Mdmx function?
The question arises as to whether the delay of DNA break repair by Mdm2 and Mdmx is aberrant and just something that cancer cells select for, or whether this is a normal function of these proteins. Experiments utilizing fibroblasts with mutant Nbs1 have indicated that this is a normal function of Mdm2. Specifically, primary murine fibroblasts lacking functional Nbs1 were reconstituted with Nbs1 containing point mutations in its Mdm2-binding domain that inhibit endogenous Mdm2 from binding, but not impact on its association with Mre11. In these cells, DNA repair occurred at a faster rate than cells reconstituted with wild-type Nbs1 (Bouska et al., 2008). These data indicate physiological levels of Mdm2 that exist in non-transformed cells regulate MRN-mediated DNA break repair. These results suggest that this function of Mdm2 is part of its normal activity. Cancer cells that have increased levels of Mdm2 and have inactivated p53 appear to have selected for this function of Mdm2. Although similar studies are needed for Mdmx, it is likely that analogous results will be obtained.
Why would normal cells have evolved this mechanism? DNA break repair is a precise process. Alterations in the rate or ability to repair DNA breaks can cause DNA aberrations and chromosome instability (Stracker et al., 2013; Mladenov et al., 2016). Therefore, since Mdm2 and Mdmx are able to modulate the speed of DNA repair, this may ensure that DNA is repaired properly or alternatively, that it is not repaired in a timely manner, depending on its levels and likely other signals in the cell. Cancer cells that have selected for a higher level of Mdm2 have either adjusted to the delays in DNA break repair or tolerated an increased amount of DNA breaks, as these can lead to translocations and other chromosome abnormalities that may confer survival and/or growth advantages to the malignant cell. Upon DNA damage or a variety of cellular stresses, Mdm2/Mdmx:p53 interactions are inhibited and p53 is free to transcribe genes needed for cell cycle inhibition and/or apoptosis (Eischen and Lozano, 2014). Therefore, if a cell has p53, Mdm2/Mdmx regulate it, but Mdm2/Mdmx also simultaneously regulate DNA break repair through the MRN complex (Figure 1). The two Mdm2/Mdmx functions are not mutually exclusive of each other, as DNA breaks lead to a DNA damage signal that activates p53. Moreover, when Mdm2/Mdmx inhibit DNA break repair, which leads to a delay in the resolution of the breaks, DNA damage signals persist (Bouska et al., 2008; Carrillo et al., 2015a), and this should lead to continued p53 activation in cells with p53. Therefore, in cells containing p53, increased levels of Mdm2/Mdmx inhibit MRN-mediated DNA break repair and suppress p53 activation, limiting the DNA damage response, which can lead to DNA breaks persisting. DNA breaks can induce apoptosis, but they also facilitate chromosomal abnormalities, such as translocations, fusions, ringed chromosomes, and other structural changes (Morgan et al., 1998; Bunting and Nussenzweig, 2013).
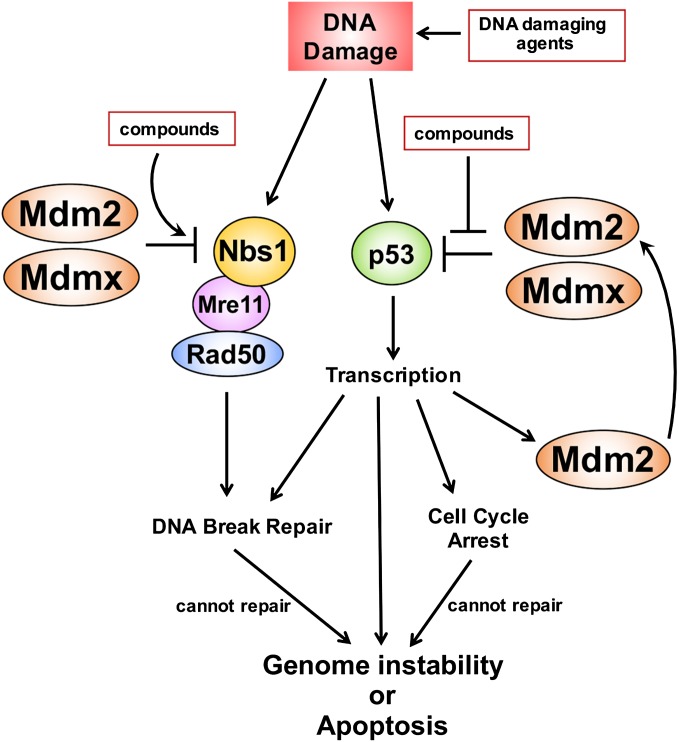
Figure 1.
Mdm2/Mdmx inhibit MRN-mediated DNA break repair and p53-mediated transcription. DNA damage activates the MRN complex to induce DNA break repair and p53-mediated transcription that facilitates DNA repair through multiple mechanisms. Both the MRN complex and p53 are inhibited by Mdm2/Mdmx. Failure to repair DNA breaks can result in genome instability and apoptosis. Compounds that inhibit Mdm2/Mdmx:p53 interactions and/or increase Mdm2 levels, resulting in increased inhibition of the MRN complex, impact DNA break repair and genome instability. These compounds combined with DNA damaging agents can lead to increased cancer cell apoptosis.
Currently, it is not entirely clear how a cell makes the decision to die instead of undergoing cell cycle arrest upon cellular stresses that result in damage (Carvajal and Manfredi, 2013). A large amount of cellular damage is more likely to lead to apoptosis, but how much damage is too much and what type of damage defaults to apoptosis remain unresolved. The possibility of Mdm2/Mdmx inhibiting DNA break repair evolving to facilitate p53-induced apoptosis of normal cells that have sustained too much or unrepairable damage is intriguing. It is known that following p53 activation, a transcriptional program is induced, which includes Mdm2 being transcriptionally upregulated by p53, leading to increased levels of Mdm2 protein (Barak et al., 1993). With elevated Mdm2 levels, DNA double-strand break repair that is occurring should be delayed (Alt et al., 2005; Bouska et al., 2008; Carrillo et al., 2015a). With breaks persisting and not getting repaired in a timely manner, apoptosis could ensue. Therefore, if p53 is attempting to induce apoptosis, an Mdm2 and/or Mdmx-mediated delay in DNA break repair may allow breaks to be maintained to facilitate apoptosis. However, evaluation of apoptosis in cultured primary fibroblasts overexpressing Mdm2 has not shown an overall increase in the levels of cell death in the population (Alt et al., 2005), but a small number of individual cells could be dying due to this mechanism. Additional research will be needed to further investigate the possibility of Mdm2/Mdmx inhibiting DNA break repair to facilitate apoptosis.
What does this function of Mdm2 and Mdmx mean for cancer treatment?
The identification of Mdm2 and Mdmx overexpression leading to inhibition of DNA break repair has opened up new therapeutic avenues for cancer therapy. Recently, therapeutic approaches have tested inhibiting DNA damage pathways/DNA repair in combination with DNA damaging agents to induce cancer cell death (O'Connor, 2015). This approach was slow in coming, possibly because there was fear that making a cancer cell more genomically unstable would result in a more difficult to treat cancer. Additionally, it is possible that knowledge gained from tumours, which contained wild-type p53 but went on to lose functional p53, resulting in the tumour becoming more genomically unstable, aggressive, and resistant to specific therapies, also led some to reject the idea of making genomes more unstable. However, data are emerging that such an approach may be beneficial and could include Mdm2 modulation. Approaches to combine Mdm2 modulation with DNA damaging agents in malignant cells that lack functional p53 have begun to be investigated. Specifically, prostate cancer cells with mutant or deleted p53 showed increased sensitivity to radiation in the presence of Nutlin (Supiot et al., 2008). In cutaneous T cell lymphoma and sarcoma that did or did not have functional p53, there was increased cell death with combined treatment with Nutlin and doxorubicin or cisplatin (Ohnstad et al., 2011; Manfe et al., 2012). Increased DNA replication stress induced by gemcitabine, together with the Mdm2 inhibitor MI-63, led to increased lymphoma cell death (Jones et al., 2011). Utilizing p53 deleted or mutated ovarian cancer cells, we showed that addition of Nutlin led to increased levels of Mdm2 protein and a delay in DNA break repair. A combination treatment with the DNA damaging agent cisplatin or etoposide together with Nutlin significantly increased ovarian cancer cell apoptosis. Notably, doses of cisplatin or etoposide that had little effect alone were able to kill ovarian cancer cells in the presence of Nutlin (Carrillo et al., 2015b). Similarly, treatment of breast cancer cells that harbour mutant p53 with Nutlin and carboplatin synergistically induced cell death. In addition, combined treatment of mice with Nutlin and carboplatin reduced breast cancer growth and metastasis in xenograft studies (Tonsing-Carter et al., 2015). Together, these studies are proof of principle that combining DNA damage with inhibition of DNA break repair through Mdm2 stabilization should have therapeutic potential in treating human cancers. This is particularly important for malignancies that have lost functional p53. Future studies should focus on further characterizing the effect of increased Mdm2 or Mdmx levels on DNA damage for therapeutic purposes.
Acknowledgements
I thank members of the Eischen lab who have worked on Mdm2/Mdmx project.
Funding
This work was supported by the grants NCI R01CA181204 and P30CA056036.
Conflict of interest: none declared.
References
- Alt J.R., Bouska A., Fernandez M.R., et al. (2005). Mdm2 binds to Nbs1 at sites of DNA damage and regulates double strand break repair. J. Biol. Chem. 280, 18771–18781. [DOI] [PubMed] [Google Scholar]
- Alt J.R., Greiner T.C., Cleveland J.L., et al. (2003). Mdm2 haplo-insufficiency profoundly inhibits Myc-induced lymphomagenesis. EMBO J. 22, 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y., Juven T., Haffner R., et al. (1993). Mdm2 expression is induced by wild type p53 activity. EMBO J. 12, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouska A., Lushnikova T., Plaza S., et al. (2008). Mdm2 promotes genetic instability and transformation independent of p53. Mol. Cell. Biol. 28, 4862–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.R., Thomas C.A., and Deb S.P (1998). The human oncoprotein MDM2 arrests the cell cycle: elimination of its cell-cycle-inhibitory function induces tumorigenesis. EMBO J. 17, 2513–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting S.F., and Nussenzweig A (2013). End-joining, translocations and cancer. Nat. Rev. Cancer 13, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo A.M., Bouska A., Arrate M.P., et al. (2015. a). Mdmx promotes genomic instability independent of p53 and Mdm2. Oncogene 34, 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo A.M., Hicks M., Khabele D., et al. (2015. b). Pharmacologically increasing Mdm2 inhibits DNA repair and cooperates with genotoxic agents to kill p53-inactivated ovarian cancer cells. Mol. Cancer Res. 13, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P.E., Okuda M., Horn H.F., et al. (1999). Centrosome hyperamplification in human cancer: chromosome instability induced by p53 mutation and/or Mdm2 overexpression. Oncogene 18, 1935–1944. [DOI] [PubMed] [Google Scholar]
- Carvajal L.A., and Manfredi J.J (2013). Another fork in the road—life or death decisions by the tumour suppressor p53. EMBO Rep. 14, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C., Latres E., Drobnjak M., et al. (1994). Molecular abnormalities of mdm2 and p53 genes in adult soft tissue sarcomas. Cancer Res. 54, 794–799. [PubMed] [Google Scholar]
- Eischen C.M., and Boyd K (2012). Decreased Mdm2 expression inhibits tumor development and extends survival independent of Arf and dependent on p53. PLoS One 7, e46148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen C.M., and Lozano G (2014). The Mdm network and its regulation of p53 activities: a rheostat of cancer risk. Hum. Mutat. 35, 728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen C.M., Weber J.D., Roussel M.F., et al. (1999). Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 13, 2658–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch R.A., Donoviel D.B., Potter D., et al. (2002). Mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 62, 3221–3225. [PubMed] [Google Scholar]
- Giglio S., Mancini F., Gentiletti F., et al. (2005). Identification of an aberrantly spliced form of HDMX in human tumors: a new mechanism for HDM2 stabilization. Cancer Res. 65, 9687–9694. [DOI] [PubMed] [Google Scholar]
- Jones R.J., Baladandayuthapani V., Neelapu S., et al. (2011). HDM-2 inhibition suppresses expression of ribonucleotide reductase subunit M2, and synergistically enhances gemcitabine-induced cytotoxicity in mantle cell lymphoma. Blood 118, 4140–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.N., Hancock A.R., Vogel H., et al. (1998). Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc. Natl Acad. Sci. USA 95, 15608–15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.N., Roe A.E., Donehower L.A., et al. (1995). Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378, 206–208. [DOI] [PubMed] [Google Scholar]
- Jones S.N., Sands A.T., Hancock A.R., et al. (1996). The tumorigenic potential and cell growth characteristics of p53- deficient cells are equivalent in the presence or absence of Mdm2. Proc. Natl Acad. Sci. USA 93, 14106–14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbutat M.H., Jones S.N., and Vousden K.H (1997). Regulation of p53 stability by Mdm2. Nature 387, 299–303. [DOI] [PubMed] [Google Scholar]
- Li X., Gilkes D., Li B., et al. (2012). Abnormal MDMX degradation in tumor cells due to ARF deficiency. Oncogene 31, 3721–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M.L., Wikman F., Orntoft T.F., et al. (2002). Impact of alterations affecting the p53 pathway in bladder cancer on clinical outcome, assessed by conventional and array-based methods. Clin. Cancer Res. 8, 171–179. [PubMed] [Google Scholar]
- Lundgren K., Montes de Oca Luna R., McNeill Y.B., et al. (1997). Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 11, 714–725. [DOI] [PubMed] [Google Scholar]
- Lushnikova T., Bouska A., Odvody J., et al. (2011). Aging mice have increased chromosome instability that is exacerbated by elevated Mdm2 expression. Oncogene 30, 4622–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfe V., Biskup E., Johansen P., et al. (2012). MDM2 inhibitor nutlin-3a induces apoptosis and senescence in cutaneous T-cell lymphoma: role of p53. J. Invest. Dermatol. 132, 1487–1496. [DOI] [PubMed] [Google Scholar]
- Marine J.C., Dyer M.A., and Jochemsen A.G (2007). MDMX: from bench to bedside. J. Cell Sci. 120, 371–378. [DOI] [PubMed] [Google Scholar]
- Matijasevic Z., Krzywicka-Racka A., Sluder G., et al. (2008. a). MdmX regulates transformation and chromosomal stability in p53-deficient cells. Cell Cycle 7, 2967–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matijasevic Z., Steinman H.A., Hoover K., et al. (2008. b). MdmX promotes bipolar mitosis to suppress transformation and tumorigenesis in p53-deficient cells and mice. Mol. Cell. Biol. 28, 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell T.J., Montes de Oca Luna R., Cho S., et al. (1999). Loss of one but not two mdm2 null alleles alters the tumour spectrum in p53 null mice. J. Pathol. 188, 322–328. [DOI] [PubMed] [Google Scholar]
- Melo A.N., and Eischen C.M (2012). Protecting the genome from mdm2 and mdmx. Genes Cancer 3, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini D., Lazzerini Denchi E., Danovi D., et al. (2002). Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol. Cell. Biol. 22, 5527–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenov E., Magin S., Soni A., et al. (2016). DNA double-strand-break repair in higher eukaryotes and its role in genomic instability and cancer: cell cycle and proliferation-dependent regulation. Semin. Cancer Biol. 37-38, 51–64. [DOI] [PubMed] [Google Scholar]
- Montes de Oca Luna R., Wagner D.S., and Lozano G (1995). Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378, 203–206. [DOI] [PubMed] [Google Scholar]
- Morgan W.F., Corcoran J., Hartmann A., et al. (1998). DNA double-strand breaks, chromosomal rearrangements, and genomic instability. Mutat. Res. 404, 125–128. [DOI] [PubMed] [Google Scholar]
- O'Connor M.J. (2015). Targeting the DNA damage response in cancer. Mol. Cell 60, 547–560. [DOI] [PubMed] [Google Scholar]
- Ohnstad H.O., Paulsen E.B., Noordhuis P., et al. (2011). MDM2 antagonist Nutlin-3a potentiates antitumour activity of cytotoxic drugs in sarcoma cell lines. BMC Cancer 11, 211: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant J., Chavez-Reyes A., Little N.A., et al. (2001). Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat. Genet. 29, 92–95. [DOI] [PubMed] [Google Scholar]
- Peng Y., Chen L., Li C., et al. (2001). Stabilization of the MDM2 oncoprotein by mutant p53. J. Biol. Chem. 276, 6874–6878. [DOI] [PubMed] [Google Scholar]
- Ramos Y.F., Stad R., Attema J., et al. (2001). Aberrant expression of HDMX proteins in tumor cells correlates with wild-type p53. Cancer Res. 61, 1839–1842. [PubMed] [Google Scholar]
- Rein K., and Stracker T.H (2014). The MRE11 complex: an important source of stress relief. Exp. Cell Res. 329, 162–169. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., and Ziv Y (2013). The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 14, 197–210. [PubMed] [Google Scholar]
- Shvarts A., Steegenga W.T., Riteco N., et al. (1996). MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 15, 5349–5357. [PMC free article] [PubMed] [Google Scholar]
- Sigalas I., Calvert A.H., Anderson J.J., et al. (1996). Alternatively spliced mdm2 transcripts with loss of p53 binding domain sequences: transforming ability and frequent detection in human cancer. Nat. Med. 2, 912–917. [DOI] [PubMed] [Google Scholar]
- Stracker T.H., Roig I., Knobel P.A., et al. (2013). The ATM signaling network in development and disease. Front. Genet. 4, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supiot S., Hill R.P., and Bristow R.G (2008). Nutlin-3 radiosensitizes hypoxic prostate cancer cells independent of p53. Mol. Cancer Ther. 7, 993–999. [DOI] [PubMed] [Google Scholar]
- Tonsing-Carter E., Bailey B.J., Saadatzadeh M.R., et al. (2015). Potentiation of carboplatin-mediated DNA damage by the Mdm2 modulator Nutlin-3a in a humanized orthotopic breast-to-lung metastatic model. Mol. Cancer Ther. 14, 2850–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J.M., Kumar S., and Moumen A (2011). A p53-independent role for the MDM2 antagonist Nutlin-3 in DNA damage response initiation. BMC Cancer 11, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L.T., Vu B.T., Graves B., et al. (2004). In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848. [DOI] [PubMed] [Google Scholar]
- Verma R., Rigatti M.J., Belinsky G.S., et al. (2010). DNA damage response to the Mdm2 inhibitor nutlin-3. Biochem. Pharmacol. 79, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Greiner T.C., Lushnikova T., et al. (2006). Decreased Mdm2 expression inhibits tumor development induced by loss of ARF. Oncogene 25, 3708–3718. [DOI] [PubMed] [Google Scholar]
- Wang P., Lushnikova T., Odvody J., et al. (2008). Elevated Mdm2 expression induces chromosomal instability and confers a survival and growth advantage to B cells. Oncogene 27, 1590–1598. [DOI] [PubMed] [Google Scholar]
- Xiong S., Pant V., Suh Y.A., et al. (2010). Spontaneous tumorigenesis in mice overexpressing the p53-negative regulator Mdm4. Cancer Res. 70, 7148–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M., Shi Y., al-Sedairy S., et al. (1995). The expression of the MDM2 gene, a p53 binding protein, in thyroid carcinogenesis. Cancer 76, 314–318. [DOI] [PubMed] [Google Scholar]