Abstract
In 1973, Singleton and Merten described a new syndrome in 2 female probands with aortic and cardiac valve calcifications, early loss of secondary dentition, and widened medullary cavities of the phalanges. In 1984, Aicardi and Goutières defined a phenotype resembling congenital viral infection with basal ganglia calcification and increased protein content in the cerebrospinal fluid. Between 2006 and 2012, mutations in 6 different genes were described to be associated with Aicardi–Goutières syndrome, specifically—TREX1, RNASEH2A, RNASEH2B, RNASEH2C, ADAR, and SAMHD1. More recently, mutations in IFIH1 were reported in a variety of neuroimmunological phenotypes, including Aicardi–Goutières syndrome, while a specific Arg822Gln mutation in IFIH1 was described in 3 discrete families with Singleton–Merten syndrome (SMS). IFIH1 encodes for melanoma differentiation-associated gene 5 (MDA5), and all mutations identified to date have been associated with an enhanced interferon response in affected individuals. In this study, we present a male child demonstrating recurrent febrile episodes, spasticity, and basal ganglia calcification suggestive of Aicardi–Goutières syndrome, who carries the same Arg822Gln mutation in IFIH1 previously associated with SMS. We conclude that both diseases are part of the interferonopathy grouping and that the Arg822Gln mutation in IFIH1 can cause a spectrum of disease, including neurological involvement.
Keywords: : Singleton–Merten syndrome, Aicardi–Goutières syndrome, IFIH1
Introduction
In 1973, Singleton and Merten described 2 females with early loss of secondary teeth, osteoporosis of the distal limbs, and extensive calcification of the aortic arch and valve (Singleton and Merten 1973). After their initial report, 9 additional cases appeared in the literature up to 2013 (McLoughlin and others 1974; Gay and Kuhn 1976; Feigenbaum and others 1988, 2013; Rutsch and others 2005; Valverde and others 2010). It has thus emerged that the main features, although variably expressed, of this rare syndrome include: a delay in primary tooth exfoliation and permanent tooth eruption, as well as truncated root formation and root and alveolar bone resorption; premature calcification of the ascending aorta and the aortic and mitral valve; and acroosteolysis, widened medullary cavities of the distal limbs and scoliosis. Psoriasis, muscular weakness, and glaucoma represent less frequently observed additional features (Feigenbaum and others 2013; Rutsch and others 2015). Studying a large pedigree of Canadian ancestry, Feigenbaum and others (1988) determined that the Singleton–Merten syndrome (SMS) is inherited as an autosomal dominant trait, and more recently, we reported the specific gain of function mutation p.Arg822Gln in IFIH1, encoding for melanoma differentiation-associated gene 5 (MDA5) as causing SMS in 3 families (Rutsch and others 2015). Interestingly, in 2014, Rice and others (2014) described different monoallelic gain-of-function mutations in IFIH1 in a group of patients demonstrating a spectrum of neuroimmunological phenotypes, including Aicardi–Goutières syndrome. Aicardi–Goutières syndrome (OMIM, No. 225750), initially described in 1984 by Jean Francois Aicardi and Francoise Goutières is typically characterized by bilateral spasticity and dystonia, abnormal cerebrospinal fluid (CSF) protein content, and basal ganglia calcification (Aicardi and Goutières 1984). Up to 2014, mutations in 6 genes had been found associated with Aicardi–Goutières syndrome, including TREX1 (3 prime repair exonuclease 1), genes encoding for the ribonuclease H2 (RNASEH2) subunits RNASEH2A, RNASEH2B, and RNASEH2C, ADAR1 (double-stranded RNA [dsRNA]-specific adenosine deaminase), and SAMHD1, encoding for ribonuclease SAM domain and HD domain 1 (Crow and others 2006a, 2006b; Rice and others 2009, 2012; Thiele and others 2010; Ramesh and others 2010). Accordingly, the Aicardi–Goutières phenotype caused by IFIH1 mutations was coined as Aicardi–Goutières type 7 (OMIM No. 615846). Since the original report of mutations in IFIH1 in individuals with SMS, it has been a matter of debate if there might be a continuum of features between Aicardi–Goutières and SMS, or if the p.Arg822Gln mutation in IFIH1 results in a specific alteration of MDA5 function discretely leading to SMS (Rutsch and others 2015). In favor of the former possibility, Bursztejn and others (2015) reported 3 patients from a 2-generation family exhibiting an overlap between Aicardi–Goutières syndrome and SMS, in the context of a distinct c.1465G>A, p.Ala489Thr mutation in IFIH1. In this review, we present a case report providing evidence for a phenotypic continuum between these 2 clinical entities.
Case Report: Male Proband with Features of Aicardi–Goutières Syndrome Carrying the c.2654G>A (p.R822Q) Mutation in IFIH1
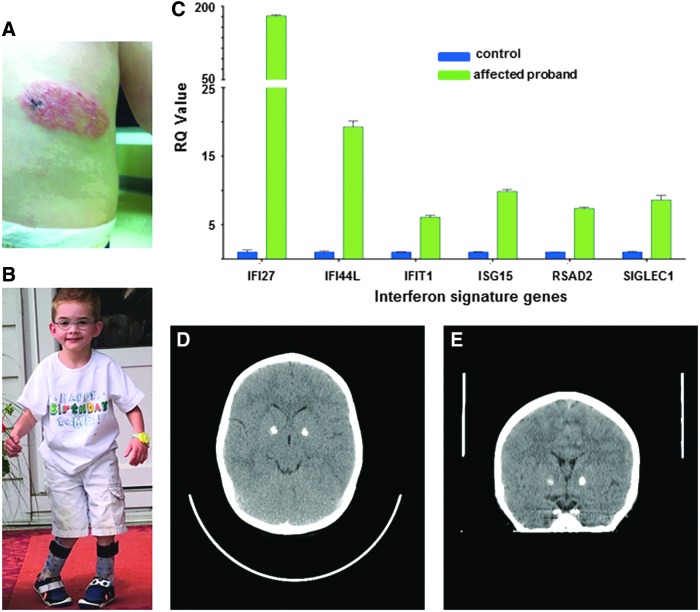
The boy was born to a 34-year-old Caucasian G5P3 mother at 39 weeks of gestation. Both parents are healthy. Birth weight was 3310 g. At the age of 4 weeks, he was noted to have a right facial droop, which was initially thought to be secondary to birth trauma and resolved spontaneously during follow-up. In the first 6 months of life, he was seen multiple times for an oral mucositis. He also suffered from recurrent ear infections requiring tympanostomy tubes at the age of 10 months. At the age of 6 months, he developed an itching, well-defined erythematous skin lesion on the right flank, which slowly progressed in size (Fig. 1A). A biopsy of this lesion revealed histological changes consistent with an inflammatory linear verrucous epidermal nevus. Bilateral nephrocalcinosis was found on ultrasound of the abdomen. Developmental delay was noted at the age of 6 months, at which time he was not rolling or sitting and did not bear weight on his legs. At the age of 12 months, the child was able to pull and stand, but these gross motor milestones were lost at 15 months of age, at which point general muscular hypotonia, a worsening of the right facial palsy, and a flare of the erythematous rash on the right flank was noted. The boy demonstrated poor weight gain from 6 months of age, but starting at 9 months of age, he lost weight and required significant caloric supplementation. Within the second and third year of life, he developed intermittent and recurrent joint pain and stiffness, which was worse in the morning. During this time, he also suffered from recurrent upper respiratory tract infections, including streptococcal angina, so that he underwent tonsillectomy and adenoidectomy. Over the following several years, he developed a spastic gait and contractures of the knee joints and elbows (Fig. 1B). Extensive laboratory investigations, including screening for metabolic disorders, revealed mostly normal results, as were a complete blood count, serum electrolytes, serum immunoglobulins, serum C3 and C4-complement, serum C-reactive protein, creatine kinase, urinalysis, and urine protein analysis. Protective antibodies against tetanus, H. influenza, and pneumococci were also normal. A brain magnetic resonance imaging (MRI) study yielded normal results at the age of 2 years. Abnormal results included elevated aspartate aminotransferase (AST) (116 U/l, normal: 10–40 U/l) and alanine aminotransferase (ALT) levels (105 U/L, normal: 7–56 U/L) at the age of 2 years and a low level of insulin-like growth factor 1 (<25 U/L).
FIG. 1.
Clinical features of the proband carrying the Arg822Gln mutation in IFIH1. (A) Well-defined scaly erythematous skin lesion on the left flank at the age of 12 months. (B) Spastic gait and mild right facial palsy at the age of 5 years. (C) Quantitative analysis of interferon stimulated genes (ISGs) in peripheral blood mononuclear cells (PBMCs) of the proband. The expression of IFI27, IFI44L, IFIT1, ISG15, RSAD2, and SIGLEC1 was analyzed by quantitative PCR in 3 technical replicates. The relative expression of each gene in PBMCs from the proband was normalized to controls and represented as a mean ± standard deviation. (D+E) Sagittal and coronal image showing calcification of the globi pallidi bilaterally. PCR, polymerase chain reaction. (Written parental consent obtained to reproduce photo.) Color images available online at www.liebertpub.com/jir
At the age of 6 years, whole-exome sequencing was performed, which identified a heterozygous c.2654G>A (p.Arg822Gln) mutation in IFIH1. The mutation was not present in the parental DNA samples and thus was considered to have arisen de novo. An interferon (IFN) signature pattern based on whole blood RNA from the proband revealed upregulation of IFN-induced gene expression (Fig. 1C), and a cranial computed tomography scan performed at the age of 6 years showed calcification of the basal ganglia bilaterally (Fig. 1D, E).
Discussion: Aicardi–Goutières Type 7 and SMS: A Disease Continuum
Aicardi–Goutières syndrome type 7
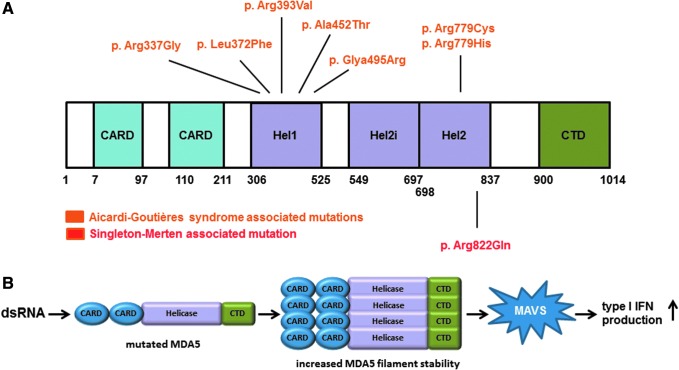
Aicardi–Goutières syndrome type 7 is an autosomal dominant inflammatory disorder encompassing a variety of neurological features, including delayed psychomotor development, spasticity, basal ganglia calcification, cerebral atrophy, and abnormalities of the deep white matter (Rice and others 2014). In 2014, Rice and others identified 6 either de novo or transmitted variants in the IFIH1 gene (c.2159G>A; p.Arg720Gln, c.2336G>A; p.Arg779His, c.1009A>G; p.Arg337Gly, c.2335C>T; p.Arg779Cys, c.1483G>A; p.Gly495Arg, c.1178A>T; p.Asp393Val) as the cause of this neuroinflammatory phenotype (Fig. 2A). In addition, 3 IFIH1 variants (c.1354G>A; p.Ala452Thr, c.1114C>T; p.Leu372Phe, and c.2336G>A; p.Arg779His) were reported in 3 unrelated Japanese patients, demonstrating a phenotype typical of classical Aicardi–Goutières syndrome (Oda and others 2014, Fig. 2A). IFIH1 encodes for the MDA5 protein, a member of the retinoic acid inducible gene-I (RIG-I) receptor family, which includes RIG-I, MDA5, and LGP2 (Barral and others 2009; del Toro Duany and others 2015). Proteins of the RIG-I family are sensors for viral dsRNA and are composed of a C-terminal domain (CTD) and a DExD/H motif helicase domain followed by a caspase activation recruitment domain (2CARD) at the N-terminus (Barral and others 2009). Recognition of cytoplasmic viral dsRNA by MDA5 induces adenosine triphosphate (ATP)-dependent filament assembling along the dsRNA axis. The helicase domains and CTD constitute an RNA recognition unit, whereas the CTD facilitates MDA5 filaments formation and the oligomerization of several 2CARD (Peisley and others 2011, 2012; Berke and Modis 2012a; Berke and others 2012b; Wu and others 2013). The interaction of the 2CARD oligomers with mitochondrial antiviral-signaling protein (MAVS) leads to an increased synthesis of type I IFN, and the subsequent induction of IFN stimulated genes (ISGs) as well as proinflammatory cytokines through the activation of different signaling pathways such as IRF3 (interferon regulatory factor 3), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), and AP-1 (activator protein 1) (Andrejeva and others 2004; Peisley and others 2011, 2012; Berke and Modis 2012a; Berke and others 2012b; Shrivastav and Niewold 2013; Wu and others 2013).
FIG. 2.
Localization and effect of Aicardi–Goutières syndrome- and Singleton–Merten syndrome (SMS)-associated MDA5 variants. (A) MDA5 protein structure and the position of the identified Aicardi–Goutières syndrome and SMS causing variants. (B) Disease-causing mutations in IFIH1 leading to increased MDA5 filament stability and enhanced type I interferon production. MDA5, melanoma differentiation-associated gene 5. Color images available online at www.liebertpub.com/jir
All identified Aicardi–Goutières type 7-associated IFIH1 variants are monoallelic missense mutations (Oda and others 2014; Rice and others 2014). Structurally, the IFIH1 variants are localized in the highly conserved MDA5 helicase domain or on the surface of the RNA-binding site (Rice and others 2014). Interestingly, no alteration of ATP hydrolysis activity was detectable in in vitro studies. However, an enhancement of baseline type I IFN signaling and an increased induction of IFN signaling in response to dsRNA could be shown in HEK293T cells transfected with mutated IFIH1 compared with controls (Rice and others 2014). In addition, Oda and others (2014) confirmed a type I IFN signature in blood cells of their Aicardi–Goutières type 7 patients, as well as increased type I IFN production after transfection of mutated IFIH1 into a hepatoma cell line. These results indicate that the IFIH1 variants associated with Aicardi–Goutières type 7 lead to increased MDA5 filament stability and a gain-of-function of the protein (Fig. 2B).
SMS type 1 and type 2
The clinical characteristics of SMS include a multiplicity of interfamilial and intrafamilial phenotypes with a broad range of signs and symptoms. Core features include severe calcification of the aortic and mitral valves as well as the ascending aorta, a delay in primary tooth exfoliation and an early loss of secondary teeth. Less frequent observations include psoriasis, glaucoma, muscular weakness, scoliosis, and an unusual face (Feigenbaum and others 2013; Rutsch and others 2015; Buers and others 2016).
A specific missense mutation c.2465G>A; p.Arg822Gln in IFIH1 was identified in 3 families conforming to the SMS type 1 phenotype (Fig. 2A, Rutsch and others 2015). This mutation is localized in the MDA5 helicase domain and possibly results in enhanced MDA5 filament stability due to conformational changes (Fig. 2B, Rutsch and others 2015, Hall and Matson 1999). The finding of an upregulation of IFN-induced gene transcripts in blood samples of SMS individuals lead to the suggestion that, as in patients with neuroimmunological disease due to mutations in IFIH1, the MDA5 conformational changes consequent upon the SMS-related p.Arg822Gln mutation also confer a gain of MDA5 activity and enhanced type I IFN signaling.
Funabiki and others (2014) published a mouse model with an MDA5 p.G821S variant. Heterozygous mutant mice demonstrated growth retardation, calcifications of the liver, and enhanced cytokine and chemokine levels. In addition, the expression of IFN-β and RIG-I-like receptors was elevated in these animals. Conformational changes of mutated MDA5 caused a hyperactivity of the protein in a dsRNA-independent manner (Funabiki and others 2014). Thus, this mouse model supports the idea that hyperactivity of MDA5 is caused by gain-of-function mutations in MDA5.
In contrast to SMS type 1, SMS type 2 has been reported to be caused by mutations (c.1118A>C; p.Glu373Ala or c.803G>T; p.Cys268Phe) in the Dead-box polypeptide 58 gene (DDX58, OMIM No. 609631, Jang and others 2015). Patients carrying either 1 of these mutations presented with glaucoma, aortic calcification, and skeletal abnormalities and an absence of any dental anomalies. DDX58 encodes the RIG-I protein, which is structurally similar to MDA5. Similar to MDA5, RIG-I consists of a helicase domain, a C-terminal repressor domain, including the CTD, and the N-terminal 2CARD (Takahasi and others 2008; Lässig and others 2015). In its inactive form, the CTD of RIG-I masks the 2CARD. Binding of short dsRNA to the CTD causes a conformational change of the RIG-I protein, leading to an exposed 2CARD and therefore to an active RIG-I protein (Takahasi and others 2008; Barral and others 2009). The activation of RIG-I allows the assembly of RIG-I filaments and the oligomerization of their 2CARD, which then activates MAVS proteins in the mitochondrial membrane resulting in a type I IFN immune response mediated through the NF-κB, IRF3, and AP-1 signaling pathways (Barral and others 2009; Buers and others 2016).
SMS-associated RIG-I mutations cause an enhanced NF-κB and PRDIII-I reporter gene activity as well as elevated IFNB1 and ISG15 expression patterns (Jang and others 2015). In addition, SMS-associated mutations in RIG-I enhance the interaction of RIG-I and endogenous RNA (Jang and others 2015). Lässig and others (2015) speculated that the ATPase of RIG-I confers specificity to viral RNA by preventing signaling by the abundant background of self-RNA. In summary, the identified SMS-associated RIG-I mutations lead to a gain-of-function of the RIG-I protein associated with an enhanced IFN-dependent immune response.
The interferonopathy spectrum of disease associated with mutations in IFIH1
Aicardi–Goutières syndrome was initially defined by the presence of bilateral spasticity and dystonia, abnormal CSF protein content, and basal ganglia calcifications, resembling congenital viral infection (Aicardi and Goutières 1984). Over time, further delineation of the syndrome led to the identification of additional associated features, including glaucoma, which is also seen in the context of SMS (Table 1).
Table 1.
Features Associated with Mutations in IFIH1
| Feature | SMS | AGS | Overlap |
|---|---|---|---|
| Short dental roots/early loss of permanent teeth | + | ||
| Muscular weakness | + | ||
| Aortic calcification | + | ||
| Unusual face | + | ||
| Psoriasis | + | ||
| Hallux valgus | + | ||
| Osteopenia of hands/feet | + | ||
| Wide medullary cavities of the phalanges | + | ||
| Subluxation of joints | + | ||
| Thick neurocranium | + | ||
| Congenital microcephaly | + | ||
| Calcification of white matter | + | ||
| Periventricular calcification | + | ||
| Quadriplegia/rigidity of legs | + | ||
| Irritability | + | ||
| Hepatosplenomegaly | + | ||
| Lymphadenopathy | + | ||
| Thrombocytopenia | + | ||
| High signal in the periventricular and deep white matter on T2 weighted imaging | + | ||
| Cerebral atrophy | + | ||
| Hypertonia | + | ||
| Regression | + | ||
| Developmental delay | + | + | + |
| Hypotonia | + | + | + |
| Glaucoma | + | + | + |
| Elevated levels of interferon signature genes | + | + | + |
| Chilblains | + | + | |
| Basal ganglia calcification | + | + | + |
AGS, Aicardi–Goutieres Syndrome; SMS, Singleton–Merten syndrome.
Since basal ganglia calcification, developmental delay, and spasticity had not been reported in patients with SMS, there was reason to believe that these syndromes were distinct and that the specificity of the later phenotype might be explained by the discrete Arg822Gln mutation in IFIH1 identified in all 3 families published in 2015. More recently, a study by Bursztejn and others (2015) showed that a different mutation (p.Ala489Thr) in IFIH1 could also be associated with features of SMS. Of particular note, the father in this 2-generation family demonstrated the typical dental abnormalities and joint involvement of SMS, as well as basal ganglia calcification in the absence of overt neurological disease. Until now, however, it has remained unclear if the specific MDA5 p.Arg822Gln mutation described by Rutsch and others (2015) could also be associated with a neurological phenotype. The case presented in this study illustrates that this is the case, the proband demonstrating bilateral spasticity, developmental delay, and basal ganglia calcification in association with the MDA5 p.Arg822Gln substitution. In this case, the diagnosis of an interferonopathy was not considered until exome sequencing revealed the presence of the p.Arg822Gln variant in IFIH1. In our proband, no clear abnormality was seen on cerebral MRI scanning performed at the age of 2 years. This delay in diagnosis might be related to the limited capability of routine MRI to detect intracranial calcification, which can be more easily seen on computed tomography (and which investigation was only performed after the finding of the causative mutation using a whole-exome sequencing approach in the case described in this study).
The discovery that both the Singleton–Merten and neuroinflammatory phenotypes represent allelic conditions highlights the pathological overlap of these disorders and the possible role of enhanced type I IFN signaling in their causation (Rodero and Crow 2016).
Acknowledgments
I.B. and F.R. are supported by a grant from Innovative Medical Research, Münster University Hospital and by a grant from Interdisciplinary Clinical Research (IZKF), Münster University. Y.J.C. acknowledges funding from the European Research Council (GA 309449: Fellowship to Y.J.C.), and a state subsidy managed by the National Research Agency (France) under the “Investments for the Future” (ANR-10-IAHU-01).
Author Disclosure Statement
No competing financial interests exist.
References
- Aicardi J, Goutières F. 1984. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol 15:49–54 [DOI] [PubMed] [Google Scholar]
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A 101:17264–17269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral PM, Sarkar D, Su ZZ, Barber GN, DeSalle R, Racaniello VR, Fisher PB. 2009. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol Ther 124:219–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke IC, Modis Y. 2012a. MDA5 cooperatively forms dimers and ATP-sensitive filaments upon binding double-stranded RNA. EMBO J 31:1714–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke IC, Yu X, Modis Y, Egelman EH. 2012b. MDA5 assembles into a polar helical filament on dsRNA. Proc Natl Acad Sci U S A 109:18437–18441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buers I, Nitschke Y, Rutsch F. 2016. Novel interferonopathies associated with mutations in RIG-I like receptors. Cytokine Growth Factor Rev 29:101–117 [DOI] [PubMed] [Google Scholar]
- Bursztejn AC, Briggs TA, Del Toro Duany Y, Anderson BH, O'Sullivan J, Williams SG, Bodemer C, Fraitag S, Gebhard F, Leheup B, Lemelle I, Oojageer A, Raffo E, Schmitt E, Rice GI, Hur S, Crow YJ. 2015. Unusual cutaneous features associated with a heterozygous gain-of-function mutation in IFIH1: overlap between Aicardi-Goutières and Singleton-Merten syndromes. Br J Dermatol 173:1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, van Bokhoven H, Brunner HG, Hamel BC, Corry PC, Cowan FM, Frints SG, Klepper J, Livingston JH, Lynch SA, Massey RF, Meritet JF, Michaud JL, Ponsot G, Voit T, Lebon P, Bonthron DT, Jackson AP, Barnes DE, Lindahl T. 2006a. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat Genet 38:917–920 [DOI] [PubMed] [Google Scholar]
- Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, Ali M, Semple C, Aicardi J, Babul-Hirji R, Baumann C, Baxter P, Bertini E, Chandler KE, Chitayat D, Cau D, Déry C, Fazzi E, Goizet C, King MD, Klepper J, Lacombe D, Lanzi G, Lyall H, Martínez-Frías ML, Mathieu M, McKeown C, Monier A, Oade Y, Quarrell OW, Rittey CD, Rogers RC, Sanchis A, Stephenson JB, Tacke U, Till M, Tolmie JL, Tomlin P, Voit T, Weschke B, Woods CG, Lebon P, Bonthron DT, Ponting CP, Jackson AP. 2006b. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection. Nat Genet 38:910–916 [DOI] [PubMed] [Google Scholar]
- del Toro Duany Y, Wu B, Hur S. 2015. MDA5-filament, dynamics and disease. Curr Opin Virol 12:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbaum A, Kumar A, Weksberg R. 1988. Singleton-Merten (S-M) syndrome: autosomal dominant transmission with variable expression. Am J Hum Genet 43:A48 [Google Scholar]
- Feigenbaum A, Müller C, Yale C, Kleinheinz J, Jezewski P, Kehl HG, MacDougall M, Rutsch F, Hennekam RC. 2013. Singleton-Merten syndrome: an autosomal dominant disorder with variable expression. Am J Med Genet A 161:360–370 [DOI] [PubMed] [Google Scholar]
- Funabiki M, Kato H, Miyachi Y, Toki H, Motegi H, Inoue M, Minowa O, Yoshida A, Deguchi K, Sato H, Ito S, Shiroishi T, Takeyasu K, Noda T, Fujita T. 2014. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 40:199–212 [DOI] [PubMed] [Google Scholar]
- Gay BB, Kuhn JP. 1976. A syndrome of widened medullary cavities of bone, aortic calcification, abnormal dentition, and muscular weakness (the Singleton-Merten syndrome). Radiology 118(2):389–395 [DOI] [PubMed] [Google Scholar]
- Hall MC, Matson SW. 1999. Helicase motifs: the engine that powers DNA unwinding. Mol Microbiol 34:867–877 [DOI] [PubMed] [Google Scholar]
- Jang MA, Kim EK, Now H, Nguyen NT, Kim WJ, Yoo JY, Lee J, Jeong YM, Kim CH, Kim OH, Sohn S, Nam SH, Hong Y, Lee YS, Chang SA, Jang SY, Kim JW, Lee MS, Lim SY, Sung KS, Park KT, Kim BJ, Lee JH, Kim DK, Kee C, Ki CS. 2015. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet 96:266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lässig C, Matheisl S, Sparrer KM, de Oliveira Mann CC, Moldt M, Patel JR, Goldeck M, Hartmann G, García-Sastre A, Hornung V, Conzelmann KK, Beckmann R, Hopfner KP. 2015. ATP hydrolysis by the viral RNA sensor RIG-I prevents unintentional recognition of self-RNA. Elife 4:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin MG, Pasternac A, Morch J, Wigle ED. 1974. Idiopathic calcification of the ascending aorta and aortic arch in two young women. Br Heart J 36:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Nakagawa K, Abe J, Awaya T, Funabiki M, Hijikata A, Nishikomori R, Funatsuka M, Ohshima Y, Sugawara Y, Yasumi T, Kato H, Shirai T, Ohara O, Fujita T, Heike T. 2014. Aicardi-Goutières syndrome is caused by IFIH1 mutations. Am J Hum Genet 95:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Jo MH, Lin C, Wu B, Orme-Johnson M, Walz T, Hohng S, Hur S. 2012. Kinetic mechanism for viral dsRNA length discrimination by MDA5 filaments. Proc Natl Acad Sci U S A 109:E3340–E3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A, Lin C, Wu B, Orme-Johnson M, Liu M, Walz T, Hur S. 2011. Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc Natl Acad Sci U S A 108:21010–21015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh V, Bernardi B, Stafa A, Garone C, Franzoni E, Abinun M, Mitchell P, Mitra D, Friswell M, Nelson J, Shalev SA, Rice GI, Gornall H, Szynkiewicz M, Aymard F, Ganesan V, Prendiville J, Livingston JH, Crow YJ. 2010. Intracerebral large artery disease in Aicardi-Goutières syndrome implicates SAMHD1 in vascular homeostasis. Dev Med Child Neurol 52(8):725–732 [DOI] [PubMed] [Google Scholar]
- Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, Ali M, Gornall H, Couthard LR, Aeby A, Attard-Montalto SP, Bertini E, Bodemer C, Brockmann K, Brueton LA, Corry PC, Desguerre I, Fazzi E, Cazorla AG, Gener B, Hamel BC, Heiberg A, Hunter M, van der Knaap MS, Kumar R, Lagae L, Landrieu PG, Lourenco CM, Marom D, McDermott MF, van der Merwe W, Orcesi S, Prendiville JS, Rasmussen M, Shalev SA, Soler DM, Shinawi M, Spiegel R, Tan TY, Vanderver A, Wakeling EL, Wassmer E, Whittaker E, Lebon P, Stetson DB, Bonthron DT, Crow YJ. 2009. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet 41:829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, Bader-Meunier B, Baildam EM, Battini R, Beresford MW, Casarano M, Chouchane M, Cimaz R, Collins AE, Cordeiro NJ, Dale RC, Davidson JE, De Waele L, Desguerre I, Faivre L, Fazzi E, Isidor B, Lagae L, Latchman AR, Lebon P, Li C, Livingston JH, Lourenço CM, Mancardi MM, Masurel-Paulet A, McInnes IB, Menezes MP, Mignot C, O'Sullivan J, Orcesi S, Picco PP, Riva E, Robinson EA, Rodriguez D, Salvatici E, Scott C, Szybowska M, Tolmie JL, Vanderver A, Vanhulle C, Vieira JP, Webb K, Whitney RN, Williams SG, Wolfe LA, Zuberi SM, Hur S, Crow YJ. 2014. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 46:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Kasher PR, Forte GMA, Mannion NM, Greenwood SM, Szynkiewicz M, Dickerson JE, Bhaskar SS, Zampini M, Briggs TA, Jenkinson EM, Bacino CA, and 42 others. 2012. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nature Genet 44:1243–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodero MP, Crow YJ. 2016. Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J Exp Med 213:2527–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Kehl HG, Ruf N, Vogt J, Kleinheinz J, Rauch F, Hofbauer LC, Rehder H, Arslan-Kirchner M, Nuernberg P. 2005. Singleton-Merten syndrome: evidence of autosomal dominant inheritance in the first European family. Eur J Hum Genet 13:112(P0154) [Google Scholar]
- Rutsch F, MacDougall M, Lu C, Buers I, Mamaeva O, Nitschke Y, Rice GI, Erlandsen H, Kehl HG, Thiele H, Nürnberg P, Höhne W, Crow YJ, Feigenbaum A, Hennekam RC. 2015. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am J Hum Genet 96:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastav M, Niewold TB. 2013. Nucleic acid sensors and type I interferon production in systemic lupus erythematosus. Front Immunol 4:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton EB, Merten DF. 1973. An unusual syndrome of widened medullary cavities of the metacarpals and phalanges, aortic calcification and abnormal dentition. Pediatr Radiol 1:2–7 [DOI] [PubMed] [Google Scholar]
- Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R, Gale M, Jr., Inagaki F, Fujita T. 2008. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell 29:428–440 [DOI] [PubMed] [Google Scholar]
- Thiele H, du Moulin M, Barczyk K, George C, Schwindt W, Nürnberg G, Frosch M, Kurlemann G, Roth J, Nürnberg P, Rutsch F. 2010. Cerebral arterial stenoses and stroke: novel features of Aicardi-Goutières syndrome caused by the Arg164X mutation in SAMHD1 are associated with altered cytokine expression. Hum Mutat 31:E1836–E1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde I, Rosenthal E, Tzifa A, Desai P, Bell A, Pushparajah K, Qureshi S, Beerbaum P, Simpson J. 2010. Singleton-Merten syndrome and impaired cardiac function. J Am Coll Cardiol 56:1760. [DOI] [PubMed] [Google Scholar]
- Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. 2013. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152:276–289 [DOI] [PubMed] [Google Scholar]