Abstract
OBJECTIVE
We examined the association between the prevalence and incidence of electrocardiographic (ECG) abnormalities and the development of cardiovascular disease (CVD) in patients with type 1 diabetes, among whom these ECG abnormalities are common.
RESEARCH DESIGN AND METHODS
We conducted a longitudinal cohort study involving 1,306 patients with type 1 diabetes (mean age 35.5 ± 6.9 years; 47.7% female) from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. ECG abnormalities were defined by the Minnesota Code ECG classification as major, minor, or no abnormality. CVD events were defined as the first occurrence of myocardial infarction, stroke, confirmed angina, coronary artery revascularization, congestive heart failure, or death from any CVD.
RESULTS
During a median follow-up of 19 years, 155 participants (11.9%) developed CVD events. In multivariable Cox proportional hazard models adjusted for demographics and potential confounders, the presence of any major ECG abnormalities as a time-varying covariate was associated with a more than twofold increased risk of CVD events (hazard ratio [HR] 2.10 [95% CI 1.26, 3.48] vs. no abnormality/normal ECG, and 2.19 [1.46, 3.29] vs. no major abnormality). Also, each visit (year) at which the diagnosis of major ECG abnormality was retained was associated with a 30% increased risk of CVD (HR 1.30 [95% CI 1.14, 1.48]). The presence of minor ECG abnormalities was not associated with a significant increase in CVD risk.
CONCLUSIONS
The presence of major ECG abnormalities is associated with an increased risk of CVD in patients with type 1 diabetes. This suggests a potential role for ECG screening in patients with type 1 diabetes to identify individuals at risk for CVD.
Introduction
Type 1 diabetes is known to be associated with a higher incidence and prevalence of cardiovascular disease (CVD) (1), the primary cause of death in patients with type 1 diabetes (2–4). Increased CVD risk is not, however, uniform in all patients with type 1 diabetes; it varies according to individual characteristics and risk profile (1,5). Identifying these predictive characteristics and risk markers is necessary to improve our ability to identify patients with type 1 diabetes who are at a higher risk.
Electrocardiography (ECG) is the most widely used noninvasive tool for cardiac investigation. We recently showed that developing new ECG abnormalities is common in the course of type 1 diabetes; about three of every four participants in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study developed at least one new ECG abnormality, and about one of every six developed at least one new major ECG abnormality during 16 years of follow-up in EDIC (6). Prior reports have shown that the presence of these ECG abnormalities in populations without diabetes is associated with an increased risk of CVD events and all-cause mortality (7–11). Similarly, in a small study with a relatively short follow-up, the presence of ECG markers of myocardial ischemia in patients with type 1 diabetes was predictive of future coronary heart disease (12). However, no comprehensive reports have described the prognostic significance of ECG abnormalities in patients with type 1 diabetes, in whom CVD develops at least a decade sooner compared with the general population (1). Therefore, the purpose of this study was to examine the association between the presence of ECG abnormalities and incident CVD events in patients with type 1 diabetes enrolled in the EDIC Study, providing observational follow-up of the DCCT cohort.
Research Design and Methods
The EDIC observational component of the DCCT/EDIC Study started in 1994, after completion of the DCCT, which consisted of a comparison of the effects of intensive versus conventional diabetes therapy on long-term diabetes complications (13). During 1983–1989, DCCT enrolled 1,441 individuals aged 13–39 years old; 726 participants were assigned to the primary prevention cohort (diabetes duration 1–5 years, no retinopathy, and urinary albumin excretion rate [AER] <40 mg/day), and 715 to the secondary intervention cohort (diabetes duration 1–15 years, very mild to moderate nonproliferative retinopathy, and AER ≤200 mg/day). Intensive therapy (n = 711) aimed to achieve levels of glycemia as close to the nondiabetic range as safely possible, whereas conventional therapy (n = 730) aimed to maintain clinical well-being, with no specific glucose targets. At the end of the DCCT (1993), participants in the conventional treatment group were instructed in intensive diabetes therapy. In 1994, all surviving DCCT participants were invited to join the EDIC observational study. The study was approved by the institutional review board at each study site. All participants provided written informed consent.
For the purpose of this analysis, we included EDIC participants with a good-quality ECG at the EDIC year 1 visit (referred to as “baseline” in this article) and at least one follow-up visit thereafter. Patients who had CVD events before EDIC year 1 were eliminated from this analysis. Supplementary Fig. 1 shows the inclusion and exclusion criteria applied and how the analysis sample was achieved.
ECG
EDIC participants underwent resting 12-lead ECG recording annually. ECG tracings were centrally read at an ECG core facility, initially (EDIC years 1–11) at the University of Minnesota ECG Reading Center (Minneapolis, MN), then at the Epidemiological Cardiology Research Center (EPICARE) of Wake Forest School of Medicine (Winston-Salem, NC). The change in the ECG reading center did not affect the rate of developing new ECG abnormalities (interaction P values between the reading center and the DCCT treatment group for any ECG abnormalities and major ECG abnormalities were 0.98 and 0.65, respectively).
ECG abnormalities were classified as major and minor abnormalities using the standards of the Minnesota Code (MC) for ECG classification (14). Major ECG abnormalities included major ventricular conduction defect (complete left [MC 7.1] and right [MC 7.2] bundle branch block, major intraventricular conduction delay [MC 7.4], and bifascicular block [MC 7.8]), major Q-wave abnormalities (MC 1.1.x, MC 1.2.x), minor Q-wave abnormalities plus major ST/T-wave abnormalities (MC 1.3.x plus [MC 4.1.x, MC 4.2, MC 5.1, MC 5.2]), isolated major ST/T-wave abnormalities (MC 4.1.x, MC 4.2, MC 5.1, MC 5.2), left ventricular hypertrophy with strain pattern (MC 3.1 and [MC 4.1.x, MC 4.2, MC 5.1, MC 5.2]), atrial fibrillation/flutter (MC 8.3), major atrioventricular block (complete atrioventricular block [MC 6.1] and second-degree atrioventricular block [MC 6.2]), major QT prolongation (QT index >116%), electronic pacemaker (MC 6.8), and others (atrioventricular dissociation [MC 8.6], ventricular tachycardia [MC 8.2], and Wolf-Parkinson-White syndrome/pre-excitation [MC 6.4]). Minor ECG abnormalities included minor isolated Q-wave abnormalities (MC 1.3.x), minor isolated ST/T-wave abnormalities (MC 4.3, MC 4.4, MC 5.3, MC 5.4), high R waves/increased QRS voltage denoting left or right ventricular hypertrophy without strain pattern (MC 3.1, MC 3.2, MC 3.3, MC 3.4), nonischemic ST segment elevation (MC 9.2), incomplete bundle branch blocks (left [MC 7.6] and right [MC 7.3] bundle branch blocks and left anterior hemiblock [MC 7.7]), minor QT prolongation (QT index >112% but <116%), short PR interval (MC 6.5), axis deviation (left axis deviation [MC 2.1], right axis deviation [MC 2.2]), ventricular premature beats (MC 8.1.2, MC 8.1.3), and others (low QRS voltage [MC 9.1], premature atrial ectopic beats [MC 8.1.1], wandering atrial pacemaker [MC 8.1.4], abnormal P-wave amplitude [MC 9.3], prolonged PR interval/first-degree atrioventricular block [MC 6.3], marked sinus bradycardia [MC 8.8], and marked sinus tachycardia [MC 8.7]) (14).
Cardiovascular Events
All events were adjudicated by a mortality and morbidity review committee whose members were blinded to the DCCT treatment group and level of glycemia. CVD events used in this analysis occurred through December 2013 and were defined using methods described elsewhere (15). These events included the first occurrence of either a nonfatal myocardial infarction—including silent myocardial infarction, stroke, confirmed angina, coronary artery revascularization, and congestive heart failure—or death from any CVD. As part of the events adjudication process, death from CVD was further classified as sudden versus not sudden based on timing. Silent myocardial infarction was defined as the presence of significant serial Q-wave abnormalities denoting a new myocardial infarction, according to the standards of the MC ECG classification, in the absence of adjudicated clinical myocardial infarction. ECGs with major abnormalities that occurred on the same date as the silent myocardial infarction that is part of the CVD outcome were not included with the predictor ECGs.
Covariates
During EDIC, demographic variables (age and sex), smoking, use of lipid-lowering medications, and use of blood pressure–lowering medications were self-reported. Fasting lipid profile and albumin excretion were assessed biennially, in alternate years, whereas HbA1c, BMI, and blood pressure were measured annually. The mean values of BMI, blood pressure, lipids, and HbA1c over the combined duration of DCCT and EDIC were used as time-dependent covariates. The updated mean values were computed using weights proportional to the time interval between values because of the different visit schedules during DCCT and EDIC. A history of albuminuria was defined as any sustained AER ≥30 mg/day during at least two consecutive visits during DCCT/EDIC.
The presence of major ECG abnormalities (yes vs. no) was defined as the presence of such abnormalities on at least one ECG up to the time of each annual ECG. Likewise, the presence of any ECG abnormalities (yes vs. no) was defined as the presence of such abnormalities on at least one ECG up to the time of each annual ECG. The presence of a major abnormality alone up to the most recent ECG (yes vs. no) was used as a time-dependent covariate at the time of a CVD event, as was the presence of any abnormality (major or minor). The presence of a major abnormality or minor abnormality versus no abnormality was used as a three-category time-dependent covariate.
Statistical Analysis
Clinical characteristics at EDIC year 1 or the DCCT/EDIC weighted mean through EDIC year 1 of participants who developed CVD events during follow-up and those who did not were compared using the Wilcoxon rank sum test for quantitative variables and the χ2 test for categorical variables.
The association between ECG abnormalities and CVD abnormalities was examined using two approaches. First, Cox proportional hazards models were used to calculate the hazard ratios (HRs) and 95% CIs for the association between the presence (vs. absence) of ECG abnormalities (major and any, separately) as time-varying covariates and the risk of CVD events during EDIC follow-up. Second, using similar models, we also examined the CVD risk per number of visits (years), diagnosed separately as major ECG abnormality and any abnormality. Notably, when we initially examined the relationship between the number of visits (years) at which an ECG abnormality was diagnosed and the CVD risk by fitting a smoothing splines model (16), we noticed the risk did not increase beyond five visits (years). Therefore, any number of visits (years) exceeding five was set to five. The first approach investigates the association between ECG abnormalities and the risk of CVD per se, whereas the second approach investigates whether this association further depends on the amount of time since the development/diagnosis of ECG abnormality.
The minimally adjusted model (model 1) included age and sex at baseline (EDIC year 1), whereas the fully adjusted model (model 2) was further adjusted for DCCT cohort (primary prevention cohort vs. secondary intervention cohort) and the most significant time-varying traditional CVD risk factors. The traditional CVD risk factors that were initially considered included current smoking status and DCCT/EDIC weighted mean of systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, LDL cholesterol, non-HDL cholesterol, triglycerides, HbA1c, and albuminuria. Each of these CVD risk factors was added one at a time to the minimally adjusted model. Four CVD risk factors (HbA1c, albuminuria, systolic blood pressure, and non-HDL cholesterol) showed significant associations when added to the minimally adjusted model, and with the addition of DCCT cohort type (primary or secondary) and use of lipid-lowering and blood pressure–lowering medications, composed the fully adjusted model (model 2). Participants were censored at the time of an event, death, or 31 December 2013 (the end of follow-up), whichever occurred first.
Additional analysis examined the effect modification by sex and level of HbA1c. The proportional hazards assumption was tested by adding time-varying interaction terms between the covariates and log(time) (17). A two-sided P value ≤0.05 was considered statistically significant. All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC).
Results
This analysis included 1,306 participants (mean age 35.5 ± 6.9 years; 47.7% female; 96.3% white) from EDIC, who compose 92% of the surviving DCCT cohort. Table 1 shows the characteristics of the analysis sample at EDIC year 1 (baseline) stratified by the occurrence of CVD events during follow-up. As shown, study participants who developed CVD events during follow-up were more likely to be older, be current smokers, have a longer duration of diabetes, and have higher HbA1c, systolic blood pressure, diastolic blood pressure, non-HDL cholesterol, total cholesterol, triglycerides, and albuminuria at baseline.
Table 1.
Participant characteristics at EDIC baseline stratified by occurrence of a cardiovascular event during follow-up
| Characteristics | All participants (n = 1,306) | Cardiovascular event |
||
|---|---|---|---|---|
| Yes (n = 155) | No (n = 1,151) | P value* | ||
| Age (years) | 35.5 ± 6.9 | 39.1 ± 6.1 | 35.1 ± 6.9 | <0.001 |
| Female sex | 623 (47.7) | 80 (51.6) | 543 (47.2) | 0.30 |
| White race | 1,257 (96.3) | 149 (96.1) | 1,108 (96.3) | 0.91 |
| Intensive group | 655 (50.2) | 71 (45.8) | 584 (50.7) | 0.25 |
| Primary cohort | 652 (49.9) | 58 (37.4) | 594 (51.6) | <0.001 |
| Duration of diabetes (years) | 13.5 ± 4.9 | 14.5 ± 5.2 | 13.3 ± 4.8 | 0.013 |
| Current cigarette smokers | 245 (18.8) | 41 (26.5) | 204 (17.7) | 0.009 |
| Current use of blood pressure medications | 118 (9.0) | 24 (15.5) | 94 (8.2) | 0.003 |
| Current use of lipid-lowering medications | 29 (2.2) | 7.7 (12) | 1.5 (17) | <0.001 |
| Albuminuria (ever) | 226 (17.3) | 43 (27.7) | 183 (15.9) | <0.001 |
| Weighted means from DCCT | ||||
| BMI (kg/m2) | 25.1 ± 3.1 | 25.7 ± 3.6 | 25.1 ± 3.0 | 0.11 |
| Systolic blood pressure (mmHg) | 115 ± 8 | 118 ± 9 | 115 ± 8 | <0.001 |
| Diastolic blood pressure (mmHg) | 74 ± 6 | 76 ± 5 | 74 ± 5 | <0.001 |
| HbA1c (%) | 8.14 ± 1.34 | 8.46 ± 1.47 | 8.09 ± 1.32 | 0.005 |
| HDL cholesterol (mg/dL) | 52 ± 12 | 51 ± 12 | 52 ± 12 | 0.67 |
| Non-HDL cholesterol (mg/dL) | 130 ± 30 | 144 ± 30 | 128 ± 29 | <0.001 |
| LDL cholesterol (mg/dL) | 114 ± 26 | 126 ± 26 | 112 ± 26 | <0.001 |
| Total cholesterol (mg/dL) | 182 ± 29 | 195 ± 31 | 180 ± 29 | <0.001 |
| Triglyceride (mg/dL) | 81 ± 38 | 92 ± 43 | 79 ± 37 | <0.001 |
Data are n (%) for categorical variables and mean ± SD for continuous variables.
*P values are based on the χ2 test for categorical variables and the Wilcoxon rank sum test for continuous variables.
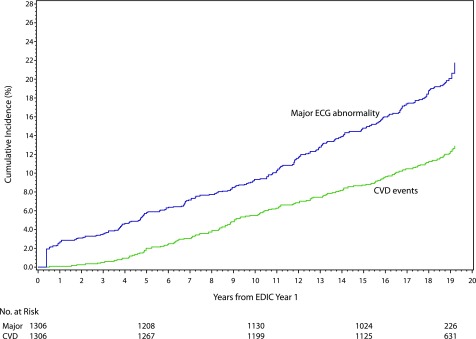
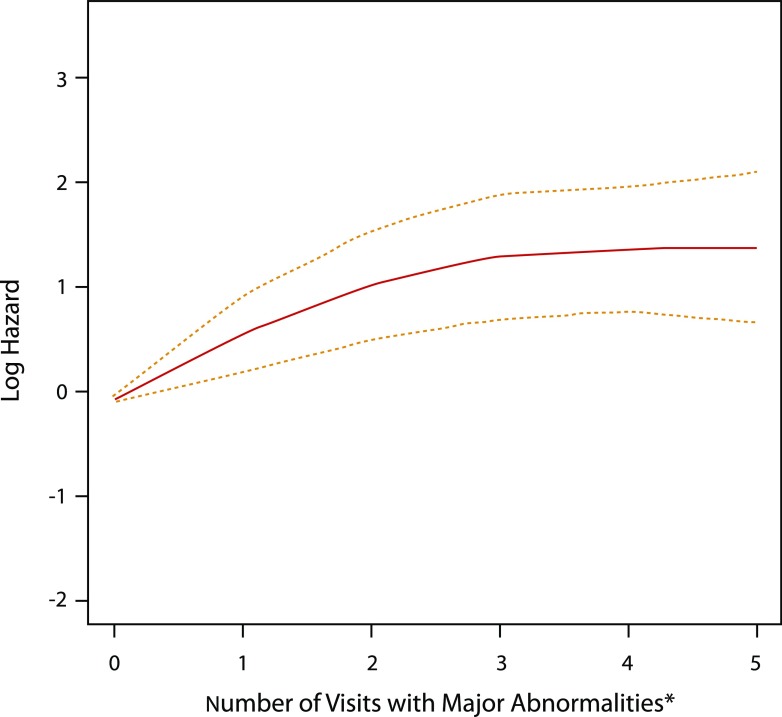
During a median follow-up of 19 years (mean 18 ± 4 years), 155 participants (11.9%) developed CVD events (incidence of 46.8 per 10,000 person-years). The CVD events were distributed as follows: 148 were nonfatal (34 with clinical myocardial infarction, 38 with silent myocardial infarction, 16 with confirmed angina pectoris, 39 with coronary revascularization, 3 with congestive heart failure, and 18 with stroke) and 7 were fatal (2 sudden deaths and 5 nonsudden deaths). In Cox proportional hazard models adjusted for demographic characteristics (age and sex), the presence of any major ECG abnormality as a time-varying covariate was associated with a more than 2.5-fold increased risk of a CVD event (HR 2.67 [95% CI 1.62, 4.40] vs. no abnormality/normal ECG, and 2.57 [1.72, 3.84] vs. no major abnormality). This association was slightly attenuated after further adjustment for potential confounders (HR 2.10 [95% CI 1.26, 3.48] vs. no abnormality/normal ECG, and 2.19 [1.46, 3.29] vs. no major ECG abnormality), and did not differ by sex or HbA1c (P for interaction = 0.63 and 0.16, respectively). Figure 1 shows that the cumulative incidence of a major ECG abnormality and of CVD events both increased in a parallel fashion throughout follow-up. Also, each visit (year) at which a diagnosis of a major ECG abnormality was retained was associated with a 30% increased risk of CVD (in the fully adjusted model: HR 1.30 [95% CI 1.14, 1.48]) (Table 2). As shown in Fig. 2, the log hazard rate for developing a CVD event was a strong linear function of the cumulative number of visits (years) with a diagnosis of a major ECG abnormality.
Figure 1.
Cumulative incidences of a major ECG abnormality and of CVD events.
Table 2.
Association between the presence of ECG abnormalities as time-dependent covariates and cardiovascular risk
| Major/any ECG abnormality (years 1–21)‡ | Minimally adjusted model* |
Fully adjusted model† |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| No abnormality/normal ECG | Reference | Reference | ||
| Minor abnormality | 1.05 (0.71, 1.57) | 0.80 | 0.94 (0.63, 1.41) | 0.78 |
| Major abnormality | 2.67 (1.62, 4.40) | 0.001 | 2.10 (1.26, 3.48) | 0.004 |
| No abnormality/normal ECG | Reference | Reference | ||
| Any abnormality (major or minor) | 1.23 (0.84, 1.80) | 0.29 | 1.08 (0.73, 1.59) | 0.70 |
| No major abnormality | Reference | Reference | ||
| Major abnormality | 2.57 (1.72, 3.84) | <0.001 | 2.19 (1.46, 3.29) | 0.001 |
| Visits/years with a major abnormality (per visit)@ | 1.37 (1.21, 1.55) | <0.001 | 1.30 (1.14, 1.48) | <0.001 |
| Visits/years with any abnormality (per visit)@ | 1.07 (0.98, 1.17) | 0.12 | 1.04 (0.95, 1.14) | 0.37 |
*Adjusted for sex and age at EDIC year 1.
†Adjusted for sex and age at year 1, primary vs. secondary cohort, weighted mean of systolic blood pressure, use of blood pressure–lowering medications, use of lipid-lowering medications, non-HDL cholesterol, HbA1c, and albuminuria. The following covariates were entered into each model as time-varying covariates: systolic blood pressure, use of lipid-lowering medications, use of blood pressure–lowering medications, non-HDL cholesterol, HbA1c, and albuminuria.
‡Time-varying covariates.
@Visits numbering more than five were set to five.
Figure 2.
Number of visits with a major ECG abnormality and risk of CVD. The red line represents the log hazard (y-axis) of CVD risk associated with the number of years with any major ECG abnormality. The yellow dashed lines represent 95% point-wise CIs for the log hazard (y-axis) of CVD risk associated with the number of years with any major ECG abnormality. *Visits numbering more than five were set to five.
The presence of any ECG abnormalities and the presence of minor ECG abnormalities were not associated with a significant increase in CVD risk in any of the models (Table 2). Supplementary Table 1 shows the individual ECG abnormalities by CVD status. As shown, the prevalence of individual major ECG abnormalities was generally higher in those who developed versus those who did not develop CVD, suggesting that the association between major ECG abnormalities with CVD events is not necessarily driven by a certain group of abnormalities.
Conclusions
In this analysis of the EDIC cohort, we examined the association between the presence and development of ECG abnormalities with incident CVD in patients with type 1 diabetes. We found that the presence of major ECG abnormalities is associated with a more than twofold increased risk of CVD events after adjusting for potential confounders, and that each additional visit (year) during which a diagnosis of major ECG abnormality was retained is associated with a 30% increased risk of a CVD event.
The potential mechanism by which major ECG abnormalities are predictive of outcome is not totally clear. However, they are mostly markers of subclinical cardiac disease, including subclinical myocardial injury/ischemia, myocardial dysfunction, and susceptibility to conduction defects and arrhythmias that could be fatal by themselves or could lead to myocardial dysfunction. Also, ECG abnormalities could be an indication of systemic disease negatively affecting the cardiovascular system, such as electrolyte imbalance resulting from kidney disease.
Our prior report from the EDIC study that showed a higher incidence of new major ECG abnormalities in patients with type 1 diabetes (6), and our findings from this analysis, which show that these abnormalities are predictive of poor CVD outcomes, emphasize the reported high CVD risk in patients with type 1 diabetes (1). Nevertheless, patients with type 1 diabetes vary in their individual risk profile and susceptibility to adverse outcomes (1,5). Hence, patients with type 1 diabetes must be stratified according to their clinical characteristics and risk factor profile for effective patient management. In our study, the association of a major ECG abnormality with CVD events persisted after controlling for several key clinical characteristics and risk factors, suggesting that ECG could be an additional tool for examining CVD risk in patients with type 1 diabetes.
The prognostic significance of ECG abnormalities as predictors of adverse outcomes in different populations is well established (7–11). Notably, however, the magnitude of risk associated with these ECG abnormalities varies across populations. These variations are further emphasized by the different levels of recommendations by the American Heart Association/American College of Cardiology Foundation regarding the utilization of ECG as a screening tool in asymptomatic adults (18). According to those guidelines, using resting ECG for cardiovascular risk assessment in asymptomatic adults with hypertension and type 2 diabetes is a class IIa recommendation. On the other hand, for those without hypertension or type 2 diabetes, it is a class IIb recommendation. The fact that the distribution and prognostic significance of ECG abnormalities vary across populations makes it inappropriate to extend evidence developed in one population to another without testing. This highlights the importance of our study as the first comprehensive report showing that ECG abnormalities are also predictive of poor outcomes in type 1 diabetes, which adds to the evidence of the potential benefit of ECG as a screening tool in high-risk populations. However, the ideal frequency and cost-effectiveness of using ECG to screen for CVD during the routine care of patients with type 1 diabetes need to be assessed.
Currently there are no widely used CVD risk prediction algorithms specific to patients with type 1 diabetes, despite some efforts to develop such algorithms (5,19,20). In the absence of data to the contrary, the current common approach to identifying CVD risk in patients with type 1 diabetes has been to apply the same risk assessment and diagnostic strategies used in the general population (1). Our conclusion that ECG abnormalities are predictive of poor outcomes in type 1 diabetes, which is in accordance with conclusions of studies of the general population, supports this approach and provides evidence that it is reasonable to presume that risk assessment tools for use with the general population could also work for individuals with type 1 diabetes. This does not, of course, obviate the need to refine a prediction score specific to type 1 diabetes, and ECG may be of help in this regard, given the magnitude of the association between major ECG abnormalities and CVD events we observed in patients with type 1 diabetes, which is stronger than the association observed in other populations who are even older and have more comorbidities. For example, the more than twofold association (HR 2.19) between major ECG abnormalities and CVD events in type 1 diabetes compares with only an 83% increased risk of CVD events (HR 1.83 [95% CI 1.12, 2.97]) in an HIV-infected population (age 43.5 ± 9.3 years) (10), a 47% increased risk of coronary heart disease events (1.47 [1.16, 1.86]) in a general population of elderly patients (age 73.5 ± 2.8 years) (8), and a 115% increased risk of CVD events (2.15 [1.56, 2.98]) in patients with chronic kidney disease who are older than 65 years (9). Needless to say, the differences in age, sex, and race among these populations, as well as the definitions of outcomes, make it difficult to appropriately compare the magnitude of risk between major ECG abnormalities with CVD.
Our study has some limitations. A significant association with an outcome is not always reflected as an improved prediction of that outcome. Hence, appropriate statistical methods that show the usefulness of major ECG abnormalities in improving CVD risk prediction are needed. However, all concordance and predictive measures in the context of time-to-event data, such as the area under the receiver operating characteristic curve, the integrated discrimination improvement, and the net reclassification improvement, are only defined for models with fixed covariates (21). This is not the case in our study, where ECG status and most of the covariates were evaluated longitudinally over time for each participant. Moreover, assessing the predictive power of a model typically requires a larger number of events than we have in our cohort at this time. Nevertheless, providing evidence for an association between major ECG abnormalities and CVD events in a comprehensive study like ours is a necessary first step to justify further efforts to examine the utility of such an association in improving prediction.
The majority of EDIC participants are Caucasian, which may limit the generalizability of our results to other races/ethnicities. However, the ethnic makeup of the DCCT/EDIC cohort is similar to the general population with type 1 diabetes, which is largely Caucasian.
We used global classification of ECG abnormalities (major, minor) rather than individual ECG abnormalities. Arguably, different individual ECG abnormalities might have different associations with CVD. However, our approach of using a global classification for ECG abnormalities is common, and several previous reports have shown its usefulness for both the assessment (22–25) and prediction (6–10) of CVD. The main reason to use a global classification of minor/major ECG abnormalities—in addition to its simplicity, which enhances comparability across studies—is that the approach overcomes statistical power concerns if ECG abnormalities are used individually.
Finally, clinical practice and care for type 1 diabetes have evolved since the start of the EDIC study in 1994. For example, HbA1c is monitored much more frequently in contemporary practice than before. If we had used time-fixed covariates, this could have raised concerns about the conclusions of our study. However, as stated in research design and methods, we used time-dependent covariates, which enabled our statistical models to capture any changes in key clinical factors resulting from changes in either practice or participant behavior during follow-up.
Despite these limitations, this is, to our knowledge, the first comprehensive report of the prognostic significance of ECG abnormalities in type 1 diabetes. The uniform collection of data, including centrally read ECG data, and the long-term follow-up of a cohort of patients with type 1 diabetes with extensive phenotyping are just a few of the many strengths of the EDIC study.
In conclusion, the presence of major ECG abnormalities during the course of type 1 diabetes is associated with an increased risk of CVD events. Identifying risk markers/predictors such as ECG abnormalities in type 1 diabetes could help guide future efforts toward the development of risk stratification tools to identify those who may benefit from closer follow-up and earlier, more aggressive risk factor management.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the data processing and technical assistance of Wanyu Hsu at the Biostatistics Center, The George Washington University, Rockville, MD, and Charles C. Campbell at the EPICARE ECG Center, Wake Forest School of Medicine, Winston-Salem, NC.
Funding. The DCCT/EDIC study has been supported by U01 Cooperative Agreement grants (1982-93, 2011–2016) and contracts (1982–2011) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grants U01 DK094176 and U01 DK094157), and through support from the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the Genetic Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006–present), Bethesda, MD.
The following industry contributors had no role in the DCCT/EDIC study but provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care Division, Animas, Bayer Diabetes Care, Becton Dickinson, Eli Lilly and Company, Extend Nutrition, Insulet Corporation, LifeScan, Medtronic Diabetes, Nipro Home Diagnostics, Nova Diabetes Care, Omron Corp., Perrigo Diabetes Care, Roche Diabetes Care, and Sanofi.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.Z.S. wrote the manuscript. E.Z.S., J.-Y.C.B., I.B., and J.M.L. interpreted the results. E.Z.S. and J.M.L. designed the study. J.-Y.C.B. and I.B. performed statistical analysis. J.-Y.C.B., I.B., T.J.O., B.Z., and J.M.L. critically revised the manuscript for content. J.M.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-2050/-/DC1.
A complete list of participants in the DCCT/EDIC Research Group can be found at http://www.nejm.org/doi/suppl/10.1056/NEJMoa1409463/suppl_file/nejmoa1409463_appendix.pdf.
References
- 1.de Ferranti SD, de Boer IH, Fonseca V, et al. . Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014;37:2843–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care 2015;38:316–322 [DOI] [PubMed] [Google Scholar]
- 3.Livingstone SJ, Looker HC, Hothersall EJ, et al. . Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 2012;9:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 2010;59:3216–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vistisen D, Andersen GS, Hansen CS, et al. . Prediction of first cardiovascular disease event in type 1 diabetes mellitus: the Steno Type 1 Risk Engine. Circulation 2016;133:1058–1066 [DOI] [PubMed] [Google Scholar]
- 6.Soliman EZ, Backlund JY, Bebu I, et al.; DCCT/EDIC Research Group . Progression of electrocardiographic abnormalities in type 1 diabetes during 16 years of follow-up: the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. J Am Heart Assoc 2016;5:e002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and minor ECG abnormalities in asymptomatic women and risk of cardiovascular events and mortality. JAMA 2007;297:978–985 [DOI] [PubMed] [Google Scholar]
- 8.Auer R, Bauer DC, Marques-Vidal P, et al.; Health ABC Study . Association of major and minor ECG abnormalities with coronary heart disease events. JAMA 2012;307:1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobre M, Brateanu A, Rashidi A, Rahman M. Electrocardiogram abnormalities and cardiovascular mortality in elderly patients with CKD. Clin J Am Soc Nephrol 2012;7:949–956 [DOI] [PubMed] [Google Scholar]
- 10.Soliman EZ, Prineas RJ, Roediger MP, et al. . Prevalence and prognostic significance of ECG abnormalities in HIV-infected patients: results from the Strategies for Management of Antiretroviral Therapy study. J Electrocardiol 2011;44:779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyer VA; U.S. Preventive Services Task Force . Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:512–518 [DOI] [PubMed] [Google Scholar]
- 12.Olson JC, Orchard TJ. Ischemic ECG changes predict coronary artery disease in type 1 diabetes. Int J Cardiol 2005;98:511. [DOI] [PubMed] [Google Scholar]
- 13.Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, et al. . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 14.Prineas RJ, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA, John Wright, 1982 [Google Scholar]
- 15.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 16.Therneau TM, Grambsch PM. Modeling survival data: Extending the Cox Model. Statistics for Biology and Health. New York, Springer-Verlag, 2000 [Google Scholar]
- 17.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. 2nd ed. New York, John Wiley & Sons, 2011 [Google Scholar]
- 18.Greenland P, Alpert JS, Beller GA, et al.; American College of Cardiology Foundation; American Heart Association . 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–e103 [DOI] [PubMed] [Google Scholar]
- 19.Zgibor JC, Ruppert K, Orchard TJ, et al. . Development of a coronary heart disease risk prediction model for type 1 diabetes: the Pittsburgh CHD in Type 1 Diabetes Risk Model. Diabetes Res Clin Pract 2010;88:314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cederholm J, Eeg-Olofsson K, Eliasson B, Zethelius B, Gudbjörnsdottir S; Swedish National Diabetes Register . A new model for 5-year risk of cardiovascular disease in type 1 diabetes; from the Swedish National Diabetes Register (NDR). Diabet Med 2011;28:1213–1220 [DOI] [PubMed] [Google Scholar]
- 21.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics 2005;61:92–105 [DOI] [PubMed] [Google Scholar]
- 22.Prineas RJ, Le A, Soliman EZ, et al.; Reasons for Geographic and Racial Differences in Stroke (REGARDS) Investigators . United States national prevalence of electrocardiographic abnormalities in black and white middle-age (45- to 64-year) and older (≥65-year) adults (from the Reasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol 2012;109:1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitelli LL, Crow RS, Shahar E, Hutchinson RG, Rautaharju PM, Folsom AR; Atherosclerosis Risk in Communities (ARIC) Study Investigators . Electrocardiographic findings in a healthy biracial population. Am J Cardiol 1998;81:453–459 [DOI] [PubMed] [Google Scholar]
- 24.Walsh JA 3rd, Prineas R, Daviglus ML, et al. . Prevalence of electrocardiographic abnormalities in a middle-aged, biracial population: Coronary Artery Risk Development in Young Adults study. J Electrocardiol 2010;43:385.e1–385.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denes P, Garside DB, Lloyd-Jones D, et al. . Major and minor electrocardiographic abnormalities and their association with underlying cardiovascular disease and risk factors in Hispanics/Latinos (from the Hispanic Community Health Study/Study of Latinos). Am J Cardiol 2013;112:1667–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.