Abstract
The innate immune system is the first line of defense against invading pathogens. One important feature of innate immune recognition is self versus nonself discrimination. The selectivity for microbial ligands is achieved through substrate motif specificity, spatial compartmentalization, and functions of negative regulators. Loss-of-function mutations in negative regulators or gain-of-function mutations in drivers of innate immune signaling have been associated with autoimmune diseases such as lupus, rheumatoid arthritis, inflammatory vasculopathy, and a variety of interferonopathies. This review will focus on TREX1 and STING, which are opposing regulators of the cytosolic DNA-sensing pathway. Tremendous effort over the past decade among academic and clinical research groups has elucidated molecular mechanisms underlying immune diseases associated with TREX1 and STING dysfunction. We have also witnessed rapid therapeutic translation of the molecular findings. Several targeted treatment options or druggable candidates are now available for these once incurable diseases. With great enthusiasm from both academia and industry partners, we look forward to seeing the remaining scientific questions answered and, more importantly, the affected patients benefited from these discoveries.
Keywords: : TREX1, STING, cytosolic DNA sensing, Aicardi–Goutières syndrome, retinal vasculopathy with cerebral leukodystrophy, STING-associated vasculopathy with onset in infancy
Introduction
Innate immune recognition of microbial pathogens is crucial for the host response to infections. Our cells have evolved an extensive panel of pattern recognition receptors (PRRs), which recognize specific components of microbes, or pathogen-associated molecular pattern (PAMPs). Once a PRR recognizes a ligand, it triggers a signaling cascade that usually involves an adaptor protein, a protein kinase, and phosphorylation of transcription factors that activate the expression of immune genes. Self versus nonself discrimination is an important feature of most PRRs that allows defense against infections, while avoiding attacks against self. The selectivity for microbial ligand recognition is achieved either through substrate motif specificity (e.g., cytosolic RNA sensor RIG-I recognizes viral 5′ ppp-RNA, but not 5′ capped mRNA from the host) or through spatial compartmentalization [e.g., Toll-like receptor (TLR) 3, 7/8 and 9 in the endosomes, and DNA sensor cGAS in the cytosol]. As an additional layer of security, negative regulators of PRR signaling pathways are in place to prevent erroneous accumulation of host ligands that may mimic microbial PAMPs or gain access to PRRs with no substrate motif specificity. Constitutive activation of PRR signaling can have severe pathological consequences. Loss-of-function mutations in negative regulators or gain-of-function mutations in drivers of PRR signaling have been associated with autoimmune diseases such as lupus, rheumatoid arthritis (RA), inflammatory vasculopathy, and a variety of interferonopathies. This review will focus on TREX1 and STING, which are opposing regulators of the cytosolic DNA-sensing pathway.
Cytosolic DNA Sensing: Drivers and Negative Regulators
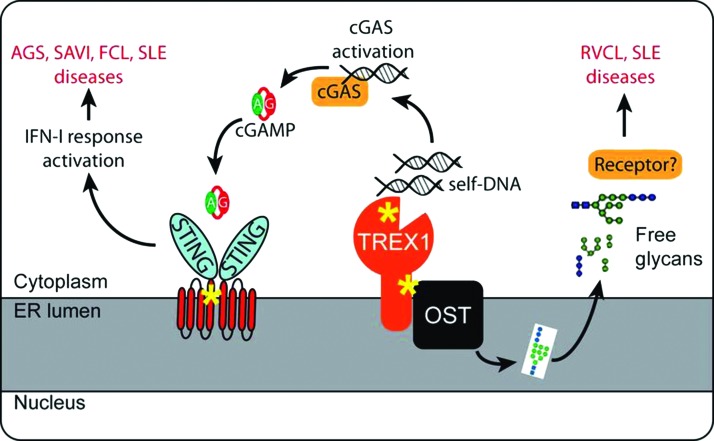
The cytosolic DNA-sensing pathway is initiated when the DNA sensor cGAS binds double-stranded DNA. Activated cGAS converts AMP and GMP into 2′3′ cyclic GMP-AMP (cGAMP), which acts as a second messenger and binds the adaptor protein STING on the endoplasmic reticulum (ER) (Sun and others 2013; Wu and others 2013) (Fig. 1). After cGAMP binding, STING translocates from the ER to the ER-Golgi intermediate compartment (ERGIC), and then to the Golgi, during which time it recruits TBK1 and IRF3 and activates downstream type I IFN signaling (Barber 2011; Dobbs and others 2015). The cGAS-STING pathway is critical for sensing a variety of microbial pathogens, including DNA viruses such as herpes simplex virus 1 (HSV-1), bacterial pathogens such as Listeria monocytogenes, Shigella flexneri, and Mycobacterium tuberculosis, and retroviruses such as human immunodeficiency virus 1 (HIV-1) (Stetson and Medzhitov 2006; Gao and others 2013; Li and others 2013; Collins and others 2015; Dobbs and others 2015; Waßermann and others 2015; Watson and others 2015).
FIG. 1.
An overall model of innate immune signaling involved in TREX1- and STING-associated immune diseases. Yellow asterisks indicate location of disease-associated mutation. AGS, Aicardi–Goutières syndrome; FCL, familial chilblain lupus; RVCL, retinal vasculopathy with cerebral leukodystrophy; SAVI, STING-associated vasculopathy with onset in infancy; SLE, systemic lupus erythematosus. Color images available online at www.liebertpub.com/jir
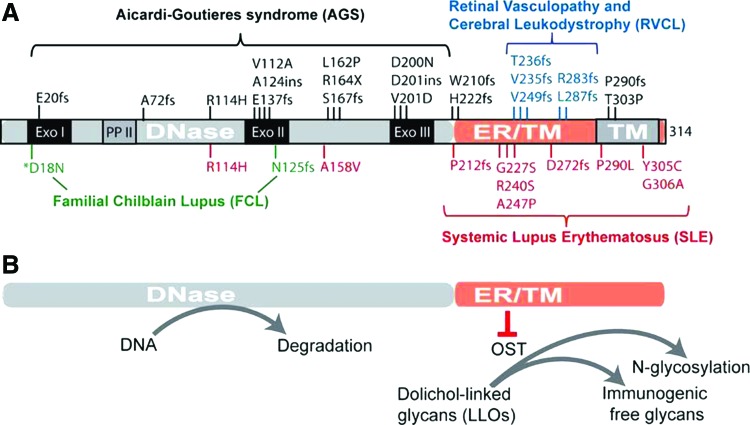
cGAS has no sequence specificity for DNA. Avoidance of cGAS recognizing self-DNA largely relies upon spatial separation and host DNases. Host DNA is mostly found in the nucleus and mitochondria, whereas the cytoplasm contains RNA and proteins. This spatial separation provides the basic mechanism for cGAS to avoid self-DNA, but it does not prevent occasional leakage of nuclear or mitochondrial DNA into the cytosol or uptake of extracellular DNA by macrophages. As a second layer of protection, mammalian cells have TREX1/DNase III, which is an ER tail-anchored exonuclease facing the cytosol, and DNase II, which is in lysosomes. Loss-of-function mutations in human TREX1 are associated with a broad range of autoimmune and inflammatory diseases, including Aicardi–Goutières syndrome (AGS), familial chilblain lupus (FCL), systemic lupus erythematosus (SLE), and retinal vasculopathy with cerebral leukodystrophy (RVCL) (Crow and Rehwinkel 2009) (Fig. 2). Trex1−/− mice also develop systemic inflammation and have an average survival of 8–10 weeks (Morita and others 2004; Stetson and others 2008). Dnase II−/− mice accumulate undigested DNA in lysosomes of macrophages and are embryonic lethal (Nagata and Kawane 2011); when crossed to Ifnar1−/−, DnaseII−/−Ifnar1−/− mice survive to adulthood, but develop chronic polyarthritis resembling RA (Kawane and others 2006). The inflammation and mortality of both Trex1−/− or DnaseII−/− mice can be completely rescued by cGas−/− or Sting−/−, providing strong evidence that the cytosolic DNA-sensing pathway is the main driver of disease (Ahn and others 2012; Gao and others 2015). These human and mouse genetic studies underscore the importance of host DNases such as TREX1 and DNase II as safeguards that maintain immune tolerance to self-DNA.
FIG. 2.
TREX1 mutations and associated immune diseases. (A) A diagram of TREX1 protein and mutations associated various autoimmune and inflammatory diseases. (B) A diagram of 2 distinct functions of TREX1. TM, transmembrane domain. Color images available online at www.liebertpub.com/jir
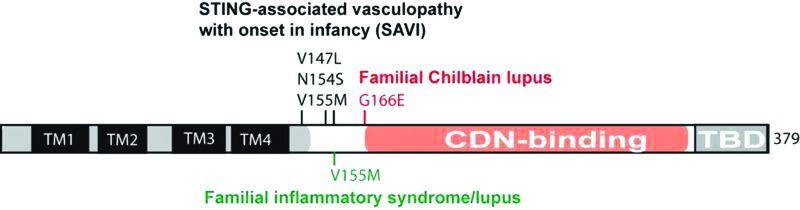
Fewer human disease mutations have been described for genes involved in driving the cytosolic DNA-sensing pathway. cGas−/− or Sting−/− mice are healthy when kept in a pathogen-free environment, although these mice are more susceptible to viral infections (Ishikawa and others 2009; Li and others 2013; Schoggins and others 2014). Interestingly, Sting−/− appears to aggravate autoimmune disease phenotypes of several TLR-driven lupus mouse models, indicating an unknown function of STING in inhibiting TLR-dependent autoimmunity (Sharma and others 2015). In humans, several mutations in TMEM173 encoding STING have been reported in STING-associated vasculopathy with onset in infancy (SAVI), patients with SLE-like syndromes or FCL (Jeremiah and others 2014; Liu and others 2014; König and others 2016). These STING mutations constitutively activate STING and type I IFN signaling and are associated with high childhood morbidity and mortality (Fig. 3).
FIG. 3.
STING mutations and associated immune diseases. TM, transmembrane domain. CDN, cyclic dinucleotide; TBD, TBK1 binding domain. Color images available online at www.liebertpub.com/jir
TREX1 and Associated Diseases
TREX1 structures and functions
TREX1 was discovered from a biochemical quest for the most abundant 3′→5′ exonuclease from the adult rabbit liver and also from calf thymus and human myoblasts (Mazur and Perrino 1999; Robins and others 1999). Human TREX1 is a single exon gene encoding a protein with an exonuclease domain on the N-terminus and a single pass transmembrane helix on the extreme C-terminus connected with a linker region (Fig. 2). The exonuclease domain shares sequence homology with the E. coli DnaQ/MutD proofreading subunit of the DNA Pol III holoenzyme and is well conserved throughout evolution. The C-terminus of TREX1 is only present in mammals and is important for TREX1 localization to the ER (Lindahl and others 2009). The TREX1 C-terminus is also subjected to the protein's only known posttranslational modification, monoubiquitination of multiple lysins by Ubiqulin 1, which regulates its ER localization (Orebaugh and others 2013). TREX1 is ubiquitously expressed in most tissues and cell types. A closely related gene, TREX2, encodes for a similar exonuclease, but lacks the C-terminal transmembrane helix, and localizes to the nucleus. TREX2 expression is restricted to the skin and keratinocytes (Parra and others 2009; Manils and others 2016). The crystal structure of the murine TREX1 N-terminal exonuclease domain reveals extensive contacts between the homodimer and a structural basis for 3′ nucleotide specificity (de Silva and others 2007; Brucet and others 2008). More recent structures of the human TREX1 D18N mutant (DNase-dead) in complex with dsDNA depict a novel DNA-unwinding mechanism by which TREX1 separates the 2 strands of dsDNA for ssDNA loading into the active site (Grieves and others 2015). This separation mechanism indicates that the endogenous substrates for TREX1 may be nicked dsDNA duplexes. These structural and functional studies define the 2 major functions of TREX1, namely the exonuclease activity on the N-terminus and the ER localization on the C-terminus, which are critical in understanding why different mutations in the same gene can cause distinct clinical diseases.
TREX1 DNase function in autoimmune and inflammatory diseases
The most notable function of TREX1 is its exonuclease activity. TREX1 was originally proposed to be a candidate proofreading enzyme for DNA polymerases α and β, which lack intrinsic 3′ exonuclease activities. Surprisingly, Trex1−/− mice did not show excessive DNA damage; instead, they exhibited profound systemic inflammation affecting multiple organs, elevated autoantibody production, and inflammatory myocarditis early in age (Morita and others 2004; Stetson and others 2008). This autoimmune phenotype was puzzling at first, but shortly after TREX1 mutations were discovered in human autoimmune and inflammatory diseases (Crow and others 2006; Lee-Kirsch and others 2007), it quickly became clear that the major function of TREX1 is to maintain host innate immune tolerance to self-DNA.
Studies from the past decade have established a clear mechanism by which TREX1 maintains immune tolerance to cytosolic self-DNA (Fig. 1). Mutations disrupting the TREX1 DNase activity lead to accumulation of self-DNA in the cytosol that activates the cGAS-STING-mediated type I IFN response and systemic inflammation. The inflammation and mortality of Trex1−/− mice can be genetically rescued by cGas−/−, Sting−/−, Ifnar1−/−, or Irf3−/− (Stetson and others 2008; Gao and others 2015; Gray and others 2015). Pharmacological inhibition of TBK1, a key serine/threonine kinase that phosphorylates STING and IRF3, alleviates autoimmune disease phenotypes and increases overall survival of Trex1−/− mice (Hasan and others 2015a). These studies delineate a singular innate immune signaling pathway that directly causes disease associated with TREX1 mutants with a defective DNase activity. More importantly, they also pave a clear path forward for therapeutic treatment of affected patients.
Remaining questions of TREX1 DNase disease pathogenesis
Although the main mechanistic framework for DNA-mediated inflammation and autoimmunity in Trex1−/− mice is well established, there remain several molecular details yet to be fully understood. First, Trex1−/− mice do not develop any pathology in the brain, whereas neurological inflammation and calcification are the 2 most defining clinical features of TREX1-AGS. The reason for these discrepancies is unclear. One possibility is that embryonic development or TREX1 tissue expression patterns may differ between mice and humans, thus manifesting different disease presentations. Another possibility is that the null allele used in most mouse studies may affect multiple functions of TREX1, whereas missense mutations mostly found in human patients would likely only affect 1 function.
Second, the identity and source of self-DNA ligands in Trex1−/− cells remain unclear. The 2 main possibilities are endogenous retroelements and nuclear DNA leakage. These 2 possibilities are not mutually exclusive. Since endogenous retroelements constitute almost half of the mammalian genome, DNA leaked from the nucleus could be enriched in retroelement sequences. DNA damage also induces transcription of retroelements (Wylie and others 2016), and retroelement insertions could cause DNA damage. Supporting evidence for the “retroelements theory” includes enrichment of retroelement sequences through direct sequencing of cytosolic DNA isolated from Trex1−/− cells (Stetson and others 2008) or TREX1 patient fibroblasts (Lim and others 2015), and TREX1 inhibits retroelement replication in vitro (Stetson and others 2008). Convincing evidence of retroelement reactivation in TREX1 patient fibroblasts is, however, lacking (Lim and others 2015). Supporting evidence for the “DNA leakage theory” includes evidence of DNA damage in Trex1−/− cells (Yang and others 2007) and genome-wide DNA hypomethylation in TREX1 patient fibroblasts (Lim and others 2015). A possible mechanism of DNA damage is through exhaustion of RPA and Rad51 by the accumulating ssDNA (Wolf and others 2016).
Third, the identity of cell types contributing to autoimmune phenotypes caused by Trex1 deficiency is controversial. Classical bone marrow (BM) chimera experiments by Ahn and others (2014) demonstrated that chimeric Trex1−/− mice, which received wild-type BM, showed improved disease and reduced mortality, while chimeric wild-type mice, which received Trex1−/− BM, developed disease. A different set of BM chimera experiments (Gall and others 2012) using Trex1−/−Rag2−/− mice as recipients (no disease, but still produce IFN) found that wild-type BM (Ifnar1-sufficient), but not Ifnar1−/− BM, reduced overall survival of recipient mice. Genetic marking of cells with activated ISG promoter using the Rosa26-YFP/Mx-Cre system identified nonhematopoietic cells at the apex of the heart becoming positive as early as day 3 postbirth (Gall and others 2012), suggesting a role for IFN-producing nonhematopoietic cells in Trex1 disease pathogenesis. Conditional Trex1 knockout studies show that loss of Trex1 in dendritic cells, but not B cells, cardiomyocytes, neurons, and astrocytes, is sufficient to cause autoimmune phenotypes (Peschke and others 2016). In addition, Trex1-deficient keratinocytes and microglia show increased ISG expression, but do not induce inflammation or pathology (Peschke and others 2016). Trex1−/− MEFs and TREX1 patient fibroblasts also express cell-intrinsic ISG signatures, independent of the IFNAR1 receptor (Hasan and others 2013). Collectively, an emerging conclusion from these studies is that both hematopoietic and nonhematopoietic cells contribute to TREX1 disease. Loss of tolerance in DCs may be the initial driver that can cause disease to some degree on its own; cell-intrinsic activation of IFN and ISGs in nonhematopoietic cells or tissues is also critical for lowering the tolerance threshold or enhancing locally the activity of hematopoietic cells. Chronic activation of both cell types could contribute to the systemic spread of inflammation and pathology.
Despite tremendous success in using the Trex1-null mouse model to establish the mechanism for the human disease, the majority of TREX1-AGS mutations are missense mutations. To better mimic the human disease, Perrino and colleagues recently generated the TREX1-D18N knock-in mouse (Grieves and others 2015). TREX1-D18N is considered DNase-dead (D18N reduces TREX1 exonuclease activity by 160,000-fold in vitro (Fye and others 2011)). TREX1-D18N homozygous mice develop many similar autoimmune disease phenotypes as Trex1−/− mice, including inflammation in multiple tissues and elevated autoantibodies. Interestingly, the overall disease burden in TREX1-D18N mice appears to be less severe compared to Trex1−/− mice; the average survival of TREX1-D18N homozygous mice is over 1 year (compared to 8–10 weeks for Trex1−/− mice). These observations raise the possibility that other DNase-independent functions of TREX1 may exist.
TREX1 DNase function in the innate immune response to retrovirus infection
TREX1 also plays an important role in preventing the innate immune response to retroviruses such as HIV-1 (Yan and others 2010; Hasan and Yan 2014). HIV-1 infection produces ssDNA and dsDNA through reverse transcription (RT) in the cytosol. Few copies of the full-length dsDNA HIV-1 genome are packaged in the preintegration complex, which subsequently enter the nucleus and integrate into the host genome. The remaining partial length HIV-1 DNA species, mostly from abortive RT (HIV-1 reverse transcriptase is error prone), need to be cleared by TREX1. In the absence of TREX1, HIV-1 RT DNA accumulates in the cytosol and gets sensed by the cGAS-STING pathway, leading to type I IFN expression (Gao and others 2013). cGAS can also sense RT DNA produced by multiple other retroviruses, including HIV-2, MuLV, and SIV (Gao and others 2013). It remains unclear how TREX1 gains access to HIV-1 RT DNA and whether TREX1 targets specific structural features of viral DNA, although the earliest single-stranded HIV-1 DNA products do appear to be suitable substrates for the TREX1 3′–5′ exonuclease activity. Interestingly, a recent study found a sequence-specific stem-loop structure, or Y-form DNA, in HIV-1 strong-stop ssDNA (the earliest DNA product during infection), to be highly stimulatory and specifically activates cGAS (Herzner and others 2015). Thus, TREX1 and cGAS could be competing for these early HIV-1 DNA products to prevent or stimulate innate immune response, respectively.
TREX1 non-DNase function in inflammatory vasculopathy and lupus
As a single exon gene, TREX1 mutations are associated with a broad spectrum of autoimmune and inflammatory diseases, including AGS, FCL, SLE, and RVCL. Recessive missense mutations that disrupt the TREX1 DNase activity are predominately associated with AGS, which is likely caused by self-DNA-mediated inflammation. In contrast, dominant frame-shift mutations that truncate the C-terminus remain DNase active, and are largely associated with RVCL and in some cases SLE. Clinical presentations of RVCL are very different from AGS. RVCL patients have late onset (in 40's or 50's) with mostly vasculopathy, whereas AGS patients have very early onset in infancy and childhood with predominant neurological inflammation. The discordance between the genetics and clinical etiologies of AGS versus RVCL suggests a novel function of TREX1 that is associated with the C-terminus and independent of DNase activity.
We recently showed that TREX1 interacts with the oligosaccharyltransferase (OST) complex on the ER through the C-terminus (Hasan and others 2015b). The OST is required for transferring lipid-linked oligosaccharide (LLO) to asparaginyl residues (also known as N-glycosylation) and hydrolysis of LLOs to produce free oligosaccharides. Trex1 deficiency or C-terminal truncation mutants cause dysregulation of OST activity, shifting toward increased hydrolysis of LLOs and the production of a large amount of free glycans. The free glycans from Trex1−/− cells appear to be immunogenic and structurally distinct from that of wild-type cells (Hasan and others 2015b). Importantly, an FDA drug approved for cancer therapy, aclacinomycin, which has been previously found to inhibit an OST activity (Bennett and others 2013), effectively suppresses free glycan release and immune activation in Trex1−/− mice and TREX1-V235fs patient lymphoblasts.
To better model the TREX1-RVCL disease, the TREX1-V235fs knock-in mouse was generated (Hasan and others 2015b). Although TREX1-V235fs mice do not develop retinal disease as in human patients, they do produce a broad panel of elevated autoantibodies in the serum and present similar molecular defects such as an elevated ISG signature and accumulation of free glycans as in Trex1−/− mice. TREX1-V235fs-truncated protein expressed in mice remains DNase active and the autoantibodies produced by the TREX1-V235fs mice are mostly against cytoplasmic, but not nuclear, antigens (T.S., N.Y. unpublished observations). Both of these observations are consistent with a non-DNase function associated with TREX1 C-terminus. Glycans are 1 of the 4 fundamental macromolecular components of all cells. Foreign glycan structures from bacteria are well-known PAMPs that can be recognized by the innate immune system (Osorio and Reis e Sousa 2011). Future studies will need to better define the structure(s) of immunogenic self-glycans in TREX1 truncation mutants and the corresponding immune pathways activated by these glycans. Structurally related protein-linked glycans of the host have also been implicated in autoimmune disease in the α-Mannosidase-II deficiency mouse model that presents SLE-like phenotypes (Chui and others 2001; Green and others 2007).
One remaining mystery is why the TREX1 C-terminus only exists in mammals, while the N-terminal DNase domain is well conserved throughout evolution. Could this be coevolution of innate immunity and the emergence of the IFN response in mammals? Or would this be a coincidence with the expansion of OST from a single enzyme in yeast and bacteria to a complex with 8 subunits and 2 alternative enzymes in mammals? TREX1 regulation of OST may shed new lights on OST biology and its associated diseases. Mutations in several OST subunits in humans are associated with congenital disorder of glycosylation (CDG) (Kelleher and Gilmore 2006). Six of the 8 OST subunits (DDOST, STT3A, STT3B, TUSC3, MAGT1, and RPN2) have been associated with CDG, but mutations in only 3 of the genes (DDOST, STT3A, and STT3B) have documented defects in glycosylation (Vleugels and others 2009; Shrimal and others 2013). It will be interesting to explore the possibility of immune defects that might be associated with OST-associated CDG and other aspects of immunoglycobiology that may be affected by TREX1.
STING and Associated Diseases
STING activation and diseases associated with gain-of-function mutations
STING is an essential adaptor protein for the cytosolic DNA-mediated type I IFN response (Barber 2014). Many proposed upstream DNA sensors signal through STING, although increasing evidence is pointing to cGAS as the main DNA sensor with clear genetic evidence and a plausible biochemical and structural mechanism (Vance 2016). STING is activated by a variety of cyclic dinucleotides (CDN), including the mammalian 2′3′-cGAMP (produced by cGAS upon activation by DNA), bacterial second messenger c-di-AMP, and c-di-GMP. Mammalian cGAMP has the highest affinity to STING (Kd is about 4 nM), which is 300 times stronger than bacterial c-di-GMP (Zhang and others 2013). Upon CDN binding, STING translocates from the ER to the ERGIC and the Golgi along the secretory pathway, then to cytoplasmic vesicles (Ishikawa and others 2009; Dobbs and others 2015). STING turns on downstream signaling as soon as it exits the ER by recruiting TBK1 and IRF3, then quickly turns it off after it reaches the vesicles, possibly through degradation of the protein itself (Konno and others 2013; Dobbs and others 2015). It remains enigmatic how STING remains on the ER at steady state and how CDN binding triggers ER exit and trafficking.
A recent study revealed several missense mutations of STING (V147L, N154S, and V155M) that are associated with a pulmonary and autoinflammatory disease called SAVI. The V155M mutation was also found in patients with familial lupus-like phenotypes (Jeremiah and others 2014). Another mutation, G166E, was found in patients with FCL (König and others 2016). The molecular mechanisms underlying these diseases are not clear, but they appear to have at least one thing in common, which is constitutive activation of type I IFN signaling. Interestingly, all of these mutations cluster at the “neck” region connecting the N-terminal transmembrane domain and C-terminal CDN binding domain. N154S and V155M mutants cause STING to constitutively translocate from the ER to the ERGIC and the Golgi, where it recruits TBK1 and activates type I IFN signaling (Jeremiah and others 2014; Dobbs and others 2015). The G166E mutant may enhance STING dimerization, and activates STING signaling to a lesser degree compared to N154S (König and others 2016). In addition, the N154S and V155M mutants constitutively activate the STING-mediated IFN response independently of cGAMP binding in vitro (Dobbs and others 2015). These gain-of-function mutations collectively reveal an important aspect of STING biology, which is ligand-independent activation. This alternative mode of STING activation likely involves ER exit and TBK1 recruitment to STING, but molecular details remain scarce. Crystal structures of the STING cytosolic domain with and without cGAMP binding suggest an uplifting motion of the STING dimer away from the ER membrane (Zhang and others 2013), which indicates that STING needs to break free from a potential ER retaining force. This proposed ER retention mechanism could be the basis for ligand-independent activation of STING (Dobbs and others 2015).
The gain-of-function STING mutations also raise an important therapeutic question on how to best block STING signaling activated independent of ligand binding. Small molecule allosteric inhibitors aimed at the cGAMP binding pocket would likely be ineffective for gain-of-function mutations. Also, competing for cGAMP binding to STING would be challenging, because of the high affinity between cGAMP and STING (Zhang and others 2013). More likely, we will have to target downstream events, such as STING's ER exiting, TBK1 kinase activity, or the type I IFN and Janus Kinase (JAK)/STAT pathway. For example, Brefeldin A, which blocks all vesicle trafficking from the ER to the Golgi, potently blocks STING ER exit and downstream IFN signaling. STING trafficking is also antagonized by the cytosolic bacteria S. flexneri, which utilize a Type 3 Secretion System (T3SS) to inject an effector protein IpaJ to inhibit ER to Golgi vesicle trafficking (Burnaevskiy and others 2013). IpaJ inhibits STING activation by blocking its translocation from the ER to the ERGIC (Dobbs and others 2015). Interestingly, the constitutive ER to ERGIC localization by STING disease mutations can be reversed by IpaJ expression, as can the associated type I IFN response in vitro (Dobbs and others 2015). JAK1/2 inhibitors have also been shown to inhibit STING signaling in vitro and in several patients carrying gain-of-function STING mutations (Liu and others 2014; Frémond and others 2016; König and others 2016).
STING activation and antitumor immunotherapy
A recent study found that STING expression and signaling are suppressed in a wide variety of cancers, including colorectal carcinoma (Xia and others 2015). Loss of STING signaling impedes the DNA damage response and antitumor T cell priming (Xia and others 2015). Sting−/− mice do not develop spontaneous tumors, but do show impaired control of xenograted tumor cells (Woo and others 2014). Population studies in larger cancer patient cohorts are still needed to establish STING as an “immune tumor suppressor.”
On the other hand, a lot of excitement is quickly mounting from studies using STING agonists for cancer therapy (Corrales and others 2016). STING signaling elicits a strong antitumor response by boosting host immune recognition of tumor antigens (Li and others 2013; Corrales and others 2015; Fu and others 2015; Woo and others 2015). Intratumoral injection of STING agonists such as cGAMP has shown a remarkable therapeutic activity in mouse models. One CDN from Aduro Biotech, ADU-S100, has recently entered phase I clinical testing on patients with cutaneous accessible metastatic solid tumors or lymphomas (ClinicalTrials.gov identifier: NCT02675439).
Therapeutic Treatment of TREX1 and STING Diseases
TREX1 AGS
Molecular understanding of TREX1 and STING disease mechanisms establishes the basis for therapeutic development. Many of these patients are minimally responsive to a broad spectrum of immunosuppressive therapies. TREX1-AGS patients with a defective DNase activity have a constitutively active cGAS-STING-TBK1-IFN signaling cascade. Mutations in RNASEH2 genes (RNASEH2A, 2B, and 2C), which are also associated with AGS, trigger the same signaling pathway (Pokatayev and others 2016). These AGS patients will likely respond positively to inhibitors against key molecules of this pathway, such as cGAS, TBK1, IFNα/β, and IFNAR1 receptor or JAK 1/2. FDA-approved drugs are available for targeting IFNα/β or the IFNAR1 receptor (e.g., anti-IFNα antibody sifalimumab and anti-IFNAR1 antibody anifrolumab) and JAK1/2 (e.g., ruxolitinib and tofacitinib). These drugs have been tested in clinical trials with SLE patients with some success (Crow and others 2015). One important consideration is that blocking downstream type I IFN signaling would only temper IFNα/β target cell function and have little or no effect on IFN-producing cells, thus allowing the endogenous stimulus, in this case self-DNA, to continue inducing IFN and ISGs. In contrast, upstream targets such as cGAS and TBK1 have the clear advantage of blocking IFN signaling from the very beginning. Many academic and industry groups are actively developing inhibitors against cGAS. TBK1 inhibitors are available from studies of its function in cell proliferation and cancer; several compounds are highly effective at inhibiting TBK1 at a subnanomolar range (Hasan and Yan 2016). A combination of drugs that targets both up- and downstream signaling molecules for treating TREX1 AGS and other autoimmune disease will be an exciting possibility.
TREX1 RVCL
TREX1 RVCL mutations affect the OST enzymatic activity, likely by altering its substrate preference, resulting in overproduction of free glycans. The OST inhibitor aclacinomycin is effective in reducing free glycans and immune activation in Trex1−/− mouse models (Hasan and others 2015b). A clinical trial is currently being set up to evaluate aclacinomycin in RVCL patients (ClinicalTrials.gov identifier: NCT02723448). Other potential inhibitors against the OST are available and should be tested in TREX1 RVCL disease models such as the TREX1-V235fs mouse. In addition, a better molecular understanding of the glycan and glycosylation defects caused by TREX1 frameshift mutations is also imperative for therapeutic development.
STING-mediated diseases
STING disease mutations trigger constitutive activation of trafficking and downstream type I IFN signaling. Similar to TREX1 AGS, inhibitors against TBK1, IFNα/β, IFNAR1 receptor, and JAK1/2 should be effective for treating STING-mediated disease. JAK1/2 inhibitors suppressed the IFN gene signature in SAVI patient cells in vitro (Liu and others 2014). A recent clinical study evaluated JAK1/2 inhibitor ruxolitinib on 3 children with SAVI, and the treatment showed marked positive effects, including improved pulmonary function, almost complete resolution of cutaneous lesions, and reduced febrile episodes (Frémond and others 2016). JAK1/2 inhibitor tofacitinib also inhibited IFN signature in 2 individuals with chilblain lupus caused by the STING G166E mutation (König and others 2016). Based on the current understanding of the STING-mediated disease mechanism, potential compounds that block STING ER exiting or trafficking, mimicking the actions of BFA or IpaJ, should also be considered an alternative category of inhibitors for STING signaling.
Concluding Remarks
The original observations that cytosolic DNA can trigger type I IFN response were made 10 years ago (Ishii and others 2006; Stetson and Medzhitov 2006). Over the past decade, efforts from many laboratories have discovered components of the cytosolic DNA-sensing pathway and defined their essential functions in immune defense against microbial infections. The endogenous functions of this pathway come into the spotlight after clinical studies that uncovered immune diseases when this pathway is dysregulated. We have witnessed a remarkable concerted effort among academic and clinical research groups that has propagated our understanding of this pathway and the development of targeted therapeutics. We now have fairly clear mechanisms for autoimmune and inflammatory diseases associated with loss-of-function mutations in TREX1 or gain-of-function mutations in STING. There is also a lot of enthusiasm from the industry on developing therapeutics for these diseases. There are several immediate treatment options depending on the molecular defect, such as JAK1/2 inhibitors for TREX1 AGS and STING diseases and aclacinomycin for TREX1 RVCL. We also have potential druggable candidates, such as cGAS and TBK1. Many questions still remain, such as what is the endogenous source of TREX1 DNase substrate? How does TREX1 C-terminus regulate the OST? How do STING gain-of-function mutations activate signaling? Why do clinical presentations of TREX1 loss-of-function patients differ from STING gain-of-function patients? We look forward to continued collaboration between clinicians and academic researchers to answer these questions and, more importantly, to rapidly translate these findings into better treatments for affected patients.
Acknowledgments
I thank Vladislav Pokatayev for proofreading this article. I apologize for not being able to cite all relevant original research and review articles due to space limitations. Research in Nan Yan's laboratory is supported by the Rita C. and William P. Clements, Jr. Endowed Scholar Award from UT Southwestern, the U.S. National Institute of Health (AI98569, AR067135), Alliance for Lupus Foundation, Welch Foundation (I-1831), and Burroughs Wellcome Fund.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahn J, Gutman D, Saijo S, Barber GN. 2012. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A 109:19386–19391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Ruiz P, Barber GN. 2014. Intrinsic self-DNA triggers inflammatory disease dependent on STING. J Immunol 193:4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. 2011. STING-dependent signaling. Nat Immunol 12:929–930 [DOI] [PubMed] [Google Scholar]
- Barber GN. 2014. STING-dependent cytosolic DNA sensing pathways. Trends Immunol 35:88–93 [DOI] [PubMed] [Google Scholar]
- Bennett DC, Charest J, Sebolt K, Lehrman M, Rehemtulla A, Contessa JN. 2013. High-throughput screening identifies aclacinomycin as a radiosensitizer of EGFR-mutant non-small cell lung cancer. Transl Oncol 6:382–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucet M, Querol-Audí J, Bertlik K, Lloberas J, Fita I, Celada A. 2008. Structural and biochemical studies of TREX1 inhibition by metals. Identification of a new active histidine conserved in DEDDh exonucleases. Protein Sci 17:2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnaevskiy N, Fox TG, Plymire DA, Ertelt JM, Weigele BA, Selyunin AS, Way SS, Patrie SM, Alto NM. 2013. Proteolytic elimination of N-myristoyl modifications by the Shigella virulence factor IpaJ. Nature 496:106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui D, Sellakumar G, Green R, Sutton-Smith M, McQuistan T, Marek K, Morris H, Dell A, Marth J. 2001. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc Natl Acad Sci U S A 98:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Cai H, Li T, Franco LH, Li X-D, Nair VR, Scharn CR, Stamm CE, Levine B, Chen ZJ, et al. 2015. Cyclic GMP-AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe 17:820–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo S-R, Lemmens E, Banda T, Leong JJ, et al. 2015. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 11:1018–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, McWhirter SM, Dubensky TW, Gajewski TF. 2016. The host STING pathway at the interface of cancer and immunity. J Clin Invest 126:2404–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MK, Olferiev M, Kirou KA. 2015. Targeting of type I interferon in systemic autoimmune diseases. Transl Res 165:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow YJ, Hayward B, Parmar R, Robins P, Leitch A, Ali M, Black D, van Bokhoven H, Brunner H, Hamel B, et al. 2006. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet 38:917–920 [DOI] [PubMed] [Google Scholar]
- Crow YJ, Rehwinkel J. 2009. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum Mol Genet 18:R130–R136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva U, Choudhury S, Bailey SL, Harvey S, Perrino FW, Hollis T. 2007. The crystal structure of TREX1 explains the 3′ nucleotide specificity and reveals a polyproline II helix for protein partnering. J Biol Chem 282:10537–10543 [DOI] [PubMed] [Google Scholar]
- Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. 2015. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe 18:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frémond M-L, Rodero MP, Jeremiah N, Belot A, Jeziorski E, Duffy D, Bessis D, Cros G, Rice GI, Charbit B, et al. 2016. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in three children. J Allergy Clin Immunol 138:1752–1755 [DOI] [PubMed] [Google Scholar]
- Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, et al. 2015. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7:283ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fye JM, Orebaugh CD, Coffin SR, Hollis T, Perrino FW. 2011. TREX1 dominant mutations in lupus and Aicardi-Goutieres syndrome. J Biol Chem 286:32373–32382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall A, Treuting P, Elkon KB, Loo Y-M, Gale M, Barber GN, Stetson DB. 2012. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent Autoimmune Disease. Immunity 36:120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Li T, Li X-D, Chen X, Li Q-Z, Wight-Carter M, Chen ZJ. 2015. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci U S A 112:E5699–E5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Wu J, Wu Y-T, Du F, Aroh C, Yan N, Sun L, Chen ZJ. 2013. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341:903–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Treuting PM, Woodward JJ, Stetson DB. 2015. Cutting edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi-Goutières syndrome. J Immunol 195:1939–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RS, Stone EL, Tenno M, Lehtonen E, Farquhar MG, Marth JD. 2007. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity 27:308–320 [DOI] [PubMed] [Google Scholar]
- Grieves JL, Fye JM, Harvey S, Grayson JM, Hollis T, Perrino FW. 2015. Exonuclease TREX1 degrades double-stranded DNA to prevent spontaneous lupus-like inflammatory disease. Proc Natl Acad Sci U S A 112:5117–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Dobbs N, Khan S, White MA, Wakeland EK, Li Q-Z, Yan N. 2015a. Cutting edge: inhibiting TBK1 by Compound II Ameliorates Autoimmune Disease in mice. J Immunol 195:4573–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Fermaintt CS, Gao N, Sakai T, Miyazaki T, Jiang S, Li Q-Z, Atkinson JP, Morse HC, III, Lehrman MA, et al. 2015b. Cytosolic nuclease TREX1 regulates oligosaccharyltransferase activity independent of nuclease activity to suppress immune activation. Immunity 43:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Koch J, Rakheja D, Pattnaik AK, Brugarolas J, Dozmorov I, Levine B, Wakeland EK, Lee-Kirsch MA, Yan N. 2013. Trex1 regulates lysosomal biogenesis and interferon-independent activation of antiviral genes. Nat Immunol 14:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Yan N. 2014. Safeguard against DNA sensing: the role of TREX1 in HIV-1 infection and autoimmune diseases. Front Microbiol 5:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Yan N. 2016. Therapeutic potential of targeting TBK1 in autoimmune diseases and interferonopathies. Pharmacol Res 111:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzner A-M, Hagmann CA, Goldeck M, Wolter S, Kübler K, Wittmann S, Gramberg T, Andreeva L, Hopfner K-P, Mertens C, et al. 2015. Sequence-specific activation of the DNA sensor cGAS by Y-form DNA structures as found in primary HIV-1 cDNA. Nat Immunol 16:1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, et al. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol 7:40–48 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg M-C, Goudin N, Frémond M-L, Nitschke P, Molina TJ, et al. 2014. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest 124:5516–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. 2006. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443:998–1002 [DOI] [PubMed] [Google Scholar]
- Kelleher DJ, Gilmore R. 2006. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology 16:47R–62R [DOI] [PubMed] [Google Scholar]
- König N, Fiehn C, Wolf C, Schuster M, Costa EC, Tüngler V, Alvarez HA, Chara O, Engel K, Goldbach-Mansky R, et al. 2017. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis 76:468–472 [DOI] [PubMed] [Google Scholar]
- Konno H, Konno K, Barber GN. 2013. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell 155:688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee Y-A, de Silva U, Bailey SL, Witte T, Vyse TJ, et al. 2007. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet 39:1065–1067 [DOI] [PubMed] [Google Scholar]
- Li X-D, Wu J, Gao D, Wang H, Sun L, Chen ZJ. 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341:1390–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YW, Sanz LA, Xu X, Hartono SR, Chedin F. 2015. Genome-wide DNA hypomethylation and RNA:DNA hybrid accumulation in Aicardi-Goutières syndrome. Elife 2015;4:e08007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T, Barnes DE, Yang Y-G, Robins P. 2009. Biochemical properties of mammalian TREX1 and its association with DNA replication and inherited inflammatory disease. Biochem Soc Trans 37:535–538 [DOI] [PubMed] [Google Scholar]
- Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, Tenbrock K, Wittkowski H, Jones OY, Kuehn HS, et al. 2014. Activated STING in a vascular and pulmonary syndrome. N Engl J Med 371:507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manils J, Casas E, Viña-Vilaseca A, López-Cano M, Díez-Villanueva A, Gómez D, Marruecos L, Ferran M, Benito C, Perrino FW, et al. 2016. The exonuclease Trex2 shapes psoriatic phenotype. J Invest Dermatol 136:2345–2355 [DOI] [PubMed] [Google Scholar]
- Mazur DJ, Perrino FW. 1999. Identification and expression of the TREX1 and TREX2 cDNA sequences encoding mammalian 3′→5′ exonucleases. J Biol Chem 274:19655–19660 [DOI] [PubMed] [Google Scholar]
- Morita M, Stamp G, Robins P, Dulic A, Rosewell I, Hrivnak G, Daly G, Lindahl T, Barnes DE. 2004. Gene-targeted mice lacking the Trex1 (DNase III) 3′→5′ DNA exonuclease develop inflammatory myocarditis. Mol Cell Biol 24:6719–6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Kawane K. 2011. Autoinflammation by endogenous DNA. Adv Immunol 110:139–161 [DOI] [PubMed] [Google Scholar]
- Orebaugh CD, Fye JM, Harvey S, Hollis T, Wilkinson JC, Perrino FW. 2013. The TREX1 C-terminal region controls cellular localization through ubiquitination. J Biol Chem 288:28881–28892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio F, Reis e Sousa C. 2011. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity 34:651–664 [DOI] [PubMed] [Google Scholar]
- Parra D, Manils J, Castellana B, Viña-Vilaseca A, Morán-Salvador E, Vázquez-Villoldo N, Tarancón G, Borràs M, Sancho S, Benito C, et al. 2009. Increased susceptibility to skin carcinogenesis in TREX2 knockout mice. Cancer Res 69:6676–6684 [DOI] [PubMed] [Google Scholar]
- Peschke K, Achleitner M, Frenzel K, Gerbaulet A, Ada SR, Zeller N, Lienenklaus S, Lesche M, Poulet C, Naumann R, et al. 2016. Loss of Trex1 in dendritic cells is sufficient to trigger systemic autoimmunity. J Immunol 197:2157–2166 [DOI] [PubMed] [Google Scholar]
- Pokatayev V, Hasin N, Chon H, Cerritelli SM, Sakhuja K, Ward JM, Morris HD, Yan N, Crouch RJ. 2016. RNase H2 catalytic core Aicardi-Goutières syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice. J Exp Med 213:329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins P, Naven TJ, Pappin DJ, Lindahl T. 1999. A human DNA editing enzyme homologous to the Escherichia coli DnaQ/MutD protein. EMBO J 18:3868–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Macduff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, et al. 2014. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505:691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Campbell AM, Chan J, Schattgen SA, Orlowski GM, Nayar R, Huyler AH, Nündel K, Mohan C, Berg LJ, et al. 2015. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci U S A 112:E710–E717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrimal S, Ng BG, Losfeld M-E, Gilmore R, Freeze HH. 2013. Mutations in STT3A and STT3B cause two congenital disorders of glycosylation. Hum Mol Genet 22:4638–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Ko JS, Heidmann T, Medzhitov R. 2008. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134:587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93–103 [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE. 2016. Cytosolic DNA sensing: the field narrows. Immunity 45:227–228 [DOI] [PubMed] [Google Scholar]
- Vleugels W, Schollen E, Foulquier F, Matthijs G. 2009. Screening for OST deficiencies in unsolved CDG-I patients. Biochem Biophys Res Commun 390:769–774 [DOI] [PubMed] [Google Scholar]
- Waßermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, Schmid-Burgk JL, Schmidt T, Hornung V, Cole ST, et al. 2015. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe 17:799–810 [DOI] [PubMed] [Google Scholar]
- Watson RO, Bell SL, Macduff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. 2015. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17:811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, Rapp A, Berndt N, Staroske W, Schuster M, Dobrick-Mattheuer M, Kretschmer S, König N, Kurth T, Wieczorek D, et al. 2016. RPA and Rad51 constitute a cell intrinsic mechanism to protect the cytosol from self DNA. Nat Commun 7:11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-R, Corrales L, Gajewski TF. 2015. Innate immune recognition of cancer. Annu Rev Immunol 33:445–474 [DOI] [PubMed] [Google Scholar]
- Woo S-R, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MYK, Duggan R, Wang Y, Barber GN, Fitzgerald KA, et al. 2014. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41:830–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. 2013. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339:826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie A, Jones AE, D'Brot A, Lu W-J, Kurtz P, Moran JV, Rakheja D, Chen KS, Hammer RE, Comerford SA, et al. 2016. p53 genes function to restrain mobile elements. Genes Dev 30:64–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Konno H, Ahn J, Barber GN. 2015. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep 14:282–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. 2010. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol 11:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lindahl T, Barnes DE. 2007. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131:873–886 [DOI] [PubMed] [Google Scholar]
- Zhang X, Shi H, Wu J, Zhang X, Sun L, Chen C, Chen ZJ. 2013. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell 51:226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]