Abstract
Recent studies have linked antibody Fc-mediated effector functions with control of human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus infections. Interestingly, the presence of antibodies with potent antibody-dependent cellular cytotoxicity (ADCC) activity in RV144 vaccine trial participants correlated inversely with HIV-1 acquisition risk. These antibodies were recently found to recognize epitopes on the HIV-1 envelope (Env) glycoprotein exposed upon Env-CD4 binding. Accordingly, small-molecule CD4 mimetics (CD4mc) that induce Env to sample the CD4-bound conformation were shown to sensitize HIV-1-infected cells to ADCC mediated by sera from HIV-1-infected individuals. However, it remains unknown whether antibodies elicited through immunization can also mediate CD4mc-induced ADCC. In this study, we tested the capacity of CD4mc to sensitize HIV-1-infected cells to ADCC by sera from Env-vaccinated nonhuman primates using a FACS-based ADCC assay. In parallel, we evaluated the ability of CD4mc to sensitize HIV-1 viral particles to neutralization by sera from these immunized animals. We found that the vaccine-induced antibodies were able to mediate ADCC and viral neutralization in the presence, but not the absence, of CD4mc. Thus, CD4mc are capable of sensitizing HIV-1-infected cells to ADCC and infectious viral particles to neutralization by easy-to-elicit antibodies that are otherwise unable to mediate these activities.
Keywords: : HIV-1, CD4 mimetics, Env, ADCC, nonneutralizing antibodies, neutralization
The CD4-bound conformation of human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins (Env) has been shown to represent a major target of antibody-dependent cellular cytotoxicity (ADCC)-mediating antibodies present in sera from HIV-1-infected individuals.1 HIV-1 minimizes the exposure of this ADCC-susceptible Env conformation through Nef- and Vpu-mediated CD4 downregulation.1–3 Thus, forcing Envs to sample this conformation with small CD4-mimetic compounds (CD4mc) results in sensitization of HIV-1-infected cells to ADCC responses mediated by HIV+ sera.4,5 CD4mc also sensitize infectious viral particles to neutralization by CD4-induced (CD4i), but otherwise nonneutralizing antibodies.6 Interestingly, previous studies showed that antibodies with the capacity to neutralize viral particles in the presence of subinhibitory concentrations of CD4mc could be elicited by multiple Env immunogens both in humans and nonhuman primates (NHP).6
In this study, we tested whether these Env immunogens elicited antibodies able to mediate ADCC responses.
Individual plasma from five groups of immunized monkeys previously shown to neutralize HIV-1 viral particles in the presence of CD4mc6 were studied for their capacity to mediate ADCC against primary CD4+ T cells infected with a transmitted founder (TF) virus (CH58 TF). In NHP #36.1, rhesus macaques were primed with an empty ALVAC vector followed by three boosts with a combination of the same ALVAC vector and a mixture of two gp120 glycoproteins from clade B and clade E HIV-1 strains. In NHP #36.2, rhesus macaques were primed with an ALVAC vector that encodes HIV-1 Gag, Pol, and Env proteins, followed by three boosts like those in NHP #36.1. In NHP #54.1, rhesus macaques were immunized with gp140 Envs corresponding to Envs that were sequentially isolated from a human infected with a clade C HIV-1, who developed broadly neutralizing antibody responses (CAP206).7 In NHP #54.2, rhesus macaques were immunized with a mixture of gp140 Envs corresponding in sequence to the swarm of viruses observed in the CAP206 individual,7 and in NHP #62.1, rhesus macaques were immunized with gp120 and 140 glycoproteins from different TF and primary HIV-1.7
Primary CD4+ T cells were isolated by negative selection (EasySep human CD4+ T cell enrichment kit, STEMCELL) from peripheral blood mononuclear cells (PBMCs) from three healthy HIV-1-negative individuals and infected with CH58 TF virus for 48 h before staining with sera from the five groups of NHP described above. Infected cells were identified by intracellular p24 staining (KC57-RD1, Beckman Coulter) and analyzed on an LSRII cytometer (BD Biosciences, Mississauga, ON, Canada); data analysis was performed using FlowJo vX.0.7 (Tree Star, Ashland, OR).
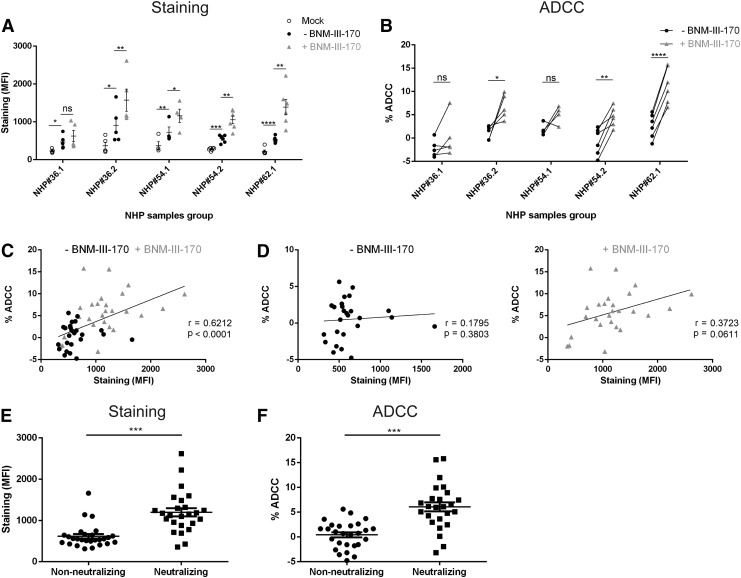
As shown in Figure 1A, individual sera from the five groups of immunized rhesus macaques specifically, but weakly, recognized Env on the surface of infected cells. We then evaluated whether “forcing” Env to sample the CD4-bound conformation on the surface of infected cells impacted this recognition. Small CD4mc compounds engage gp120 within the Phe43 cavity8 and can act as CD4 agonists, inducing thermodynamic changes in the Env trimer similar to those observed upon CD4 binding.6,8 The synthesis and chemical characterization of the small-molecule CD4mc (+)(R,R) BNM-III-170 (BNM-III-170) used in this study were previously described.8 Addition of BNM-III-170 enhanced the binding of plasma from four out of the five groups of immunized macaques (Fig. 1A), but plasma from the NHP group #36.1 did not respond to BNM-III-170 with increased Env recognition. Interestingly, the only difference with group #36.2, which exhibited a robust enhancement in recognition of HIV-1-infected cells by the CD4mc, was the priming step of the immunization. While both groups received three gp120 boosts, group #36.1 was primed with an empty ALVAC vector and group #36.2 with ALVAC VPC. Thus, our data indicate that immune priming is critical for the elicitation of CD4mc-responsive Env binding antibodies in this vaccination regimen.
FIG. 1.
The CD4mc BNM-III-170 sensitizes human immunodeficiency virus type 1 (HIV-1)-infected cells to antibody-dependent cellular cytotoxicity (ADCC) mediated by antibodies elicited by multiple envelope glycoprotein (Env) immunogens in rhesus macaques. Primary CD4+ T cells were isolated by negative selection (EasySep human CD4+ T cell enrichment kit; STEMCELL) from resting peripheral blood mononuclear cells (PBMCs) from three healthy HIV-1-negative individuals and were activated as described.4 Cells were then infected with CH58 T/F virus for 48 h before performing staining and ADCC. (A) Infected cells were identified by intracellular p24 staining (KC57-RD1; Beckman Coulter). Surface staining of HIV-1-infected cells was done with 1:1,000 dilutions of plasma from rhesus macaque immunization groups NHP#36.1, NHP#36.2, NHP#54.1, NHP#54.2, and NHP#62.1. Plasma was added in the presence of 50 μM BNM-III-170 or an equivalent volume of DMSO (negative control) at 37°C for 1 h. Goat anti-human antibody (AF-647) was used as secondary antibody and the mean fluorescence intensity (MFI) of Fluor647 was read to determine the level of staining on Aqua Vivid (Invitrogen) negative cells. (B) For ADCC, infected primary CD4+ T cells were incubated with autologous PBMC (Effector: Target ratio of 10:1) from the same donor for 4–6 h at 37°C in the presence of a 1:1,000 dilution of plasma and 50 μM BNM-III-170 or equivalent volume of DMSO. The percentage of ADCC killing was determined with the following formula: [(% of p24+ cells in Targets plus Effectors)—(% of p24+ cells in Targets plus Effectors plus serum)]/(% of p24+ cells in Targets) by gating infected (p24+) live (Aqua Vivid negative) target cells, as described previously.4 For (A, B), data shown are the average of at least four independent experiments. Statistical significance was tested by paired t-test. (*, p < .05; **, p < .01; ***, p < .001; ****, p < .0001; ns, not significant). Correlations between staining (MFI) and ADCC (C, D). Plasma samples used in a recent study6 to neutralize viral particles bearing the Tier-2 primary HIV-1JRFL Env were separated into two categories: those unable to neutralize viral particles at any given dilution (nonneutralizing) and those exhibiting a titer that neutralized 50% at a dilution equal to or below 1:80 (neutralizing). Staining (MFI) (E) and ADCC (%) (F) were compared with the Mann–Whitney U-tests for pair-wise comparisons between nonneutralizing and neutralizing plasma samples reported in6; ***p < .001. NHP, nonhuman primates.
To evaluate the ability of the elicited antibodies to mediate ADCC against CH58 TF virus-infected cells, we used a previously described FACS-based ADCC assay.4,9 Briefly, primary CD4+ T cells infected for 48 h were incubated with autologous PBMC (Effector: Target ratio of 10:1) in the presence of NHP sera (1:1,000) and either the CD4mc BNM-III-170 or an equivalent volume of DMSO. Of note, NHP sera dilution of 1:1,000 was selected based on previous work, which identified this dilution as being efficient for ADCC responses.1The percentage of cytotoxicity was calculated as described.4 In agreement with the poor recognition of infected cells, plasma from group #36.1 did not mediate ADCC either in the absence or presence of BNM-III-170; ALVAC VPC priming modified this response, with measurable ADCC responses mediated by plasma from group #36.2 upon BNM-III-170 addition (Fig. 1B). BNM-III-170 also enhanced ADCC by plasma from the remaining groups, but to different degrees. For example, plasma from both #54.1 and #54.2 NHP groups exhibited an enhanced ADCC activity upon BNM-III-170 addition, but only in the samples from #54.2 was the enhancement statistically significant. Plasma samples from group #62.1 exhibited the most significant ADCC response to BNM-III-170 (Fig. 1B).
It was recently reported that the ability of a given antibody to recognize Env on the surface of HIV-1-infected cells did not necessarily translate into an ADCC activity.3 Although the reasons for this are not clear, the ability of a given antibody to expose efficiently the Fc portion to allow interaction with the Fcγ receptor on the surface of effector cells (NK, monocytes, etc.) has been suggested as a potential mechanism.3 Therefore, we evaluated whether recognition of infected cells in the absence or presence of BNM-III-170 correlated with the ADCC activity. Figure 1C indicates that Env recognition (mean fluorescence intensity) by NHP plasma positively correlated with the ADCC activity. However, this correlation was mainly driven by samples tested in the presence of the CD4mc, where a positive trend was observed (p = .0611) (Fig. 1D, right panel). No trend or correlation between ADCC and staining was observed in the absence of BNM-III-170 (Fig. 1D, left panel).
The lack of ADCC activity observed in the absence of CD4mc is akin to the recently reported inability of NHP-immunized plasma to neutralize infectious viral particles bearing the Tier-2 primary HIV-1JRFL Env.6 However, addition of the same CD4mc used in this study, BNM-III-170, induced these otherwise nonneutralizing plasmas to neutralize a Tier-2 virus, which mimics the enhanced ADCC activity observed upon CD4mc addition reported here (Fig. 1B). Since in this study we used the same NHP plasma samples as those reported in the work reported by Madani et al.,6 we wished to determine if the samples able to neutralize viral particles upon CD4mc addition were the same as those that mediated ADCC in the presence of CD4mc. Therefore, we separated the plasma samples into two categories: those unable to neutralize viral particles at any tested dilution (nonneutralizing), and those that neutralized 50% of the virus at a dilution below 1:80 (neutralizing). Plasma unable to neutralize viral particles was tested in the absence of CD4mc and exhibited lower recognition of HIV-1-infected cells (Fig. 1E) and a lower ADCC activity (Fig. 1F). These results highlight the dual effect of these CD4mc, which render HIV-1 viral particles sensitive to neutralization as well as infected cells sensitive to ADCC by CD4i antibodies.
In summary, the capacity of small-molecule CD4mc to enhance the viral neutralization and ADCC activities of antibodies elicited in NHP by several different Env immunogens suggests that combining a vaccine with a small-molecule CD4mc, administered orally or in a microbicide formulation, might be useful as a prophylactic strategy against HIV-1 transmission.
Acknowledgments
The authors thank Dominique Gauchat from the CRCHUM Flow Cytometry Platform and Nathalie Brassard for technical assistance. The authors also thank Daniel Kaufmann and Jonathan Richard for helpful discussions. This work was supported by a CIHR foundation grant #352417 to A.F. A.F. is the recipient of a Canada Research Chair on Retroviral Entry. This study was also supported by AI100645 Center for HIV/AIDS Vaccine Immunology and Immunogen Design (CHAVI-ID), by the National Institutes of Health grant #GM56550, the late William F. McCarty-Cooper. Our funding sources had no role in data collection, analysis, or interpretation, and were not involved in the writing of this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Veillette M, Coutu M, Richard J, et al. : The HIV-1 gp120 CD4-Bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 2015;89:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veillette M, Desormeaux A, Medjahed H, et al. : Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 2014;88:2633–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding S, Veillette M, Coutu M, et al. : A highly conserved residue of the HIV-1 gp120 inner domain is important for antibody-dependent cellular cytotoxicity responses mediated by anti-cluster A antibodies. J Virol 2016;90:2127–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richard J, Veillette M, Brassard N, et al. : CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 2015;112:E2687–E2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard J, Veillette M, Ding S, et al. : Small CD4 mimetics prevent HIV-1 uninfected bystander CD4 + T cell killing mediated by antibody-dependent cell-mediated cytotoxicity. EBioMedicine 2016;3:122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madani N, Princiotto AM, Easterhoff D, et al. : Antibodies elicited by multiple envelope glycoprotein immunogens in primates neutralize primary human immunodeficiency viruses (HIV-1) sensitized by CD4-mimetic compounds. J Virol 2016;90:5031–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiehe K, Easterhoff D, Luo K, et al. : Antibody light-chain-restricted recognition of the site of immune pressure in the RV144 HIV-1 vaccine trial is phylogenetically conserved. Immunity 2014;41:909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melillo B, Liang S, Park J, et al. : Small-molecule CD4-mimics: Structure-based optimization of HIV-1 entry inhibition. ACS Med Chem Lett 2016;7:330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard J, Veillette M, Batraville LA, et al. : Flow cytometry-based assay to study HIV-1 gp120 specific antibody-dependent cellular cytotoxicity responses. J Virol Methods 2014;208:107–114 [DOI] [PubMed] [Google Scholar]