Summary
Type 1 diabetes is a T-cell-mediated autoimmune disease against pancreatic beta cells. T cells target various antigens such as insulin, chromogranin A, glutamic acid decarboxylase and islet-specific glucose-6-phosphatase catalytic subunit-related protein. Elimination of insulin dramatically prevents diabetes in the non-obese diabetic (NOD) mouse model and response to insulin occurs prior to that to other antigens. These findings suggest that insulin is a target antigen at the early stage of the disease and is likely to be essential to cause anti-islet autoimmunity in NOD mice. In this review, we discuss whether insulin is truly essential and is only the single essential autoantigen for NOD mice and potentially for man. Although the ultimate principle is still being addressed, it is certain that T-cell response to insulin is a major check point to develop type 1 diabetes in NOD mice. Given multiple similarities between diabetes of NOD mice and man, targeting insulin and insulin-reactive T cells may provide opportunities to develop robust immunotherapies.
Keywords: insulin, autoantigen, type 1 diabetes, autoimmune
Introduction
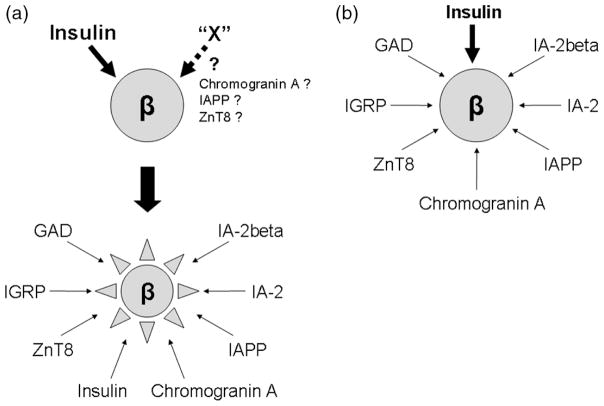
Recent studies by Vignali and Unanue separately showed that only T cells recognizing islet antigens (IAs) but not bystander T cells exist in the pancreatic islets [1,2]. Given evidence that multiple T-cell clones isolated from non-obese diabetic (NOD) islets (e.g. BDC-2.5 [3], 12-4.1 [4], NY8.3 [5]) are capable of inducing diabetes, it is clear that T cells recognizing various antigens infiltrate pancreatic islets and contribute to the beta-cell destruction in animal models such as NOD mice. Among those multiple islet-derived antigens, a fundamental question is whether there are essential antigens that are required to trigger and exaggerate islet inflammation, and if there are essential antigens, is only a single antigen or multiple antigens required? Two proposed models illustrate how T cells might initiate targeting beta cells (Figure 1). Here, we discuss the two models with the focus on insulin as an essential autoantigen.
Figure 1.
Models for the initiation of beta-cell destruction by individual antigen-specific autoreactive T cells. (a) T cells reacting with the limited number of antigens including insulin initiate beta-cell destruction. Once the destruction is initiated, T cells reacting with other antigens are involved in the further destruction. The subsequent destruction does not occur without the initial event. It is unknown whether another initial antigen (‘X’) exists. (b) T cells reacting with multiple antigens simultaneously initiate and expand beta-cell destruction. Contribution of response to a fraction of antigens such as insulin is essential to develop diabetes
Insulin as an essential autoantigen for the NOD mouse model
Using NOD mice, both knockout and transgenic mouse models that eliminate several autoantigens and T cells that react to those antigens have been generated to elucidate if the antigens are essential to cause type 1 diabetes. Among those antigens, including glutamic acid decarboxylase [6,7], islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) [8,9] and IA-2/IA-2β [10,11], only the elimination of insulin and insulin-reactive T cells prevents diabetes of NOD mice thus far [12–14]. In particular, expression of insulin B chain amino acid 9–23 (insulin B:9–23) in pancreatic islets is required for NOD mice to develop anti-islet autoimmunity [15]. This does not necessarily imply that the insulin B:9–23 peptide is truly the first antigen that triggers anti-islet autoimmunity; however, it is very likely that response to this peptide is one of the essential ‘checkpoints’ in the initiation of diabetes. Kay and colleagues showed that insulin-reactive T cells are still detected in the absence of IGRP-reactive T cells, whereas IGRP-reactive T cells are undetectable in NOD mice when insulin-reactive T cells are eliminated [8]. Thus, insulin is an upstream autoantigen of IGRP that is required to cause anti-islet autoimmunity. In NOD mice, it is likely that T cells reacting with limited antigens such as the insulin B:9–23 peptide initiate targeting beta cells (Figure 1a) rather than the simultaneous attack by those reacting with various antigens (Figure 1b). Of importance, blocking or modifying the response to insulin provides us high chance of preventing subsequent destruction of beta cells.
Is the insulin B:9–23 peptide truly essential?
The double insulin-knockout NOD mice have a transgene encoding a mutated preproinsulin at insulin B chain position 16 (tyrosine → alanine) to supply hormonally functioning insulin (B16:A insulin) [12]. Provision of native insulin B:9–23 peptide or replacement of the preproinsulin transgene with the native tyrosine restores the development of anti-islet autoimmunity in insulin-knockout NOD mice [15]. Thus, the presence of the native insulin B:9–23 sequence is required for the development of anti-islet autoimmunity in the insulin-knockout NOD mice.
The development of anti-islet autoimmunity in B:16A insulin-knockout mice is extremely suppressed, but long-term observation found that the prevention is not perfect. In greater than 800 B16:A insulin-knockout mice produced in the Eisenbarth laboratory between 2003 and 2008, two mice developed diabetes with typical insulitis (1 out of 375 B16:A preproinsulin transgenic founder strain B and 1 out of 444 strain F). The ‘leak’ from the complete suppression might be because the alanine substitution is not sufficient to completely conceal the insulin B:9–23 from T cells [16]. Indeed, insulin B:9–23 with the B16:A mutation is presented by I-Ag7 and stimulates a fraction of B:9–23-reactive T-cell receptors (unpublished data by Nakayama). T cells expressing such T cell receptors may cause anti-islet autoimmunity in response to the mutated B16:A insulin B:9–23 peptide. However, we cannot completely rule out the possibility that there exists a side-pathway that does not require insulin B:9–23-reactive T cells (arrow ‘X’ in Figure 1a) and that the insulin B:9–23 is not absolutely essential. Likewise, diabetes of the transgenic mouse model in which insulin 2-reactive T cells are eliminated by the overexpression of preproinsulin 2 is dramatically but not perfectly suppressed [14]. The incomplete suppression might be due to undetectable levels of T cells reacting with the insulin 1-derived insulin B:9–23 peptide, but it is possible that insulin is not completely essential. Further studies to identify a mutation that completely abrogates binding of B:9–23 to I-Ag7 in the right register (see below) and to generate insulin-knockout mice with the preproinsulin transgene with the mutation will clarify if the insulin B:9–23 is truly essential for anti-islet autoimmunity in NOD mice.
Is insulin the only single antigen that is essential to cause type 1 diabetes in the NOD mouse model?
Are there any other essential antigens that determine the initiation of anti-islet autoimmunity other than the insulin B:9–23 peptide (arrow ‘X’ in Figure 1a)? Insulin is exclusively expressed in pancreatic beta cells, whereas other autoantigens such as zinc transporter 8 [17], IA-2 and even chromogranin A [18] are expressed in multiple endocrine cells including pancreatic alpha cells. If T-cell response to these antigens were an essential determinant of the subsequent autoimmune destruction, other endocrine cells should also be targeted. Glucagon-secreting pancreatic alpha cells are preserved even after the diabetes onset when most of beta cells are destroyed, suggesting that T cells recognizing these antigens can clearly distinguish beta cells from alpha cells despite the fact that both cells express their target molecules. Nevertheless, it is still possible that antigens expressed in multiple endocrine cells are essential to initiate the autoimmunity to beta cells. The amount of expression and/or the processing of antigens differ by cell types and may influence their antigenicity. For example, it is still unknown if a novel antigen chromogranin A, which the highly diabetogenic BDC-2.5 and BDC-10.1 CD4 T cell clones recognize [18,19], is required to develop NOD diabetes. It will be interesting to see whether elimination of chromogranin A will prevent NOD diabetes. I believe that it is important to confirm necessity of individual autoantigens and that accumulation of those findings will address our question if there are any other essential antigens.
What makes the insulin B:9–23 peptide autoantigenic?
Although it is not conclusive yet if the insulin B:9–23 is truly the single essential autoantigen, there is no doubt that response to this peptide is a major key point for the development of type 1 diabetes in the NOD mouse model. Why is the insulin B:9–23 peptide recognized and targeted by T cells and how distinct is it from conventional peptides? Unanue and colleagues recently discovered that insulin B:9–23-reactive T cell receptors that were directly isolated from pancreatic islets react with only the peptide presented by islet-derived antigen presenting cells but not by those in the thymus and spleen [20]. Another study performed by Kappler and colleagues demonstrated that the peptide register recognized by insulin B:9–23-reactive T cells, called ‘register 3’, shows extremely low affinity to the I-Ag7 major histocompatibility complex (MHC) class II molecule [21]. Furthermore, Wong and colleagues showed that an insulin B:15–23 motif recognized by an islet-derived CD8 T-cell clone binds to the Kd MHC class I molecule with low avidity [22]. These novel findings support a hypothesis that B:9–23-reactive T cells escape thymic selection due to poor epitope recognition but when they encounter the large volume of antigens processed in the islets in the proper fashion, it results in the dramatic reaction to beta cells seen in type 1 diabetes.
Is insulin critical for human type 1 diabetes?
In human type 1 diabetes, T-cell responses to multiple antigens including insulin are detected in peripheral blood [23] and T cell clones derived from peripheral blood and pancreatic lymph nodes of patients have been shown to react with preproinsulin, insulin, and glutamic acid decarboxylase peptides [24,25]. In addition, Peakman and colleagues demonstrated that CD8 T cell clones reacting with preproinsulin can destroy islet cells [26]. Thus, it is clear that insulin/preproinsulin peptides contribute to the development of human type 1 diabetes.
Compared to mouse models, humans have far more genetic variability and environmental exposures which complicate the identification of essential antigens. Nevertheless, evidence that insulin autoantibodies are often detected prior to the development of antibodies to glutamic acid decarboxylase, IA-2/IA-2β and zinc transporter 8 may suggest that human type 1 diabetes occur by limited antigens and is exaggerated in response to multiple antigens (Figure 1a) [27,28]. A genetic risk associated with thymic insulin gene expression also suggests the involvement of insulin-reactive T cells in controlling the development of anti-islet autoimmunity [29,30]. In addition, similarity between human type 1 diabetes and the NOD mouse model has been reported. Approximately 50% of type 1 diabetic patients have the DQ8 MHC class II molecule and the structure of DQ8 is similar to that of NOD I-Ag7 [31]. Especially, both class II molecules lack a common amino acid residue, aspartic acid, at position 57 of the DQ β chain, which shapes one of the peptide-binding grooves (pocket 9) [32]. It has been shown that I-Ag7 and DQ8 present a certain number of identical peptides [33]. Finally, insulin peptide (insulin A:1–15)-reactive CD4 T cell clones derived from pancreatic lymph nodes of patients with type 1 diabetes require an extremely large amount of peptides for stimulation, suggesting that these T-cell receptors may bind to the peptide–MHC complex weakly [24]. Taken together, these cumulative findings suggest that type 1 diabetes of a subset of patients may develop in a similar fashion to that of animal models and that insulin peptides may play a central role to induce anti-islet autoimmunity in humans. Studies using human islet-reactive T cells will provide further insight.
Antigen-specific therapies utilizing insulin
Given successful treatment targeting insulin in NOD mice, multiple trials using insulin and insulin peptides to prevent human type 1 diabetes are underway. The initial oral insulin treatment tested by the Diabetes Prevention Trial of Type 1 (DPT-1) did not result in a remarkable preventive effect overall [34]. However, it was capable of reducing and delaying diabetes development in a group with the highest levels of insulin autoantibodies [35]. It will be important to identify the population for whom insulin immunotherapies is effective and the appropriate route, site, dose and timing to deliver insulin and insulin peptides. Animal and virtual computer models would be helpful to solve these issues [36,37]. Bresson and colleagues have shown that a combination therapy with anti-CD3 antibody and nasal proinsulin prevents diabetes more efficiently than the anti-CD3 antibody treatment alone in mouse models [38]. DNA vaccine encoding insulin may be another way to alter autoreactive T-cell response [39].
Conclusions
It is still inconclusive if insulin is the only single essential antigen to initiate anti-islet autoimmunity in mouse or man. Nevertheless, it should be emphasized that subsequent events can be dramatically averted if response to insulin is abrogated. Targeting insulin either by deletion or immunoregulating therapies may provide robust opportunities to prevent type 1 diabetes.
Acknowledgments
This work is supported by the National Institutes of Diabetes & Digestive & Kidney Diseases (R00 DK080885 and P30 DK057516), National Institutes of Health Autoimmune Prevention Center (U19 AI050864) and the Juvenile Diabetes Foundation (4-2007-1056 and 25-2010-624).
Footnotes
Conflict of interest
The author declares no conflict of interest.
References
- 1.Lennon GP, Bettini M, Burton AR, et al. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity. 2009;31:643–653. doi: 10.1016/j.immuni.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calderon B, Carrero JA, Miller MJ, Unanue ER. Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans. Proc Natl Acad Sci U S A. 2011;108:1561–1566. doi: 10.1073/pnas.1018973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 4.Jasinski JM, Yu L, Nakayama M, et al. Transgenic insulin (B:9–23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes. 2006;55:1978–1984. doi: 10.2337/db06-0058. [DOI] [PubMed] [Google Scholar]
- 5.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeckel E, Klein L, Martin-Orozco N, von Boehmer H. Normal incidence of diabetes in NOD mice tolerant to glutamic acid decarboxylase. J Exp Med. 2003;197:1635–1644. doi: 10.1084/jem.20030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto T, Yamato E, Tashiro F, et al. Development of autoimmune diabetes in glutamic acid decarboxylase 65 (GAD65) knockout NOD mice. Diabetologia. 2004;47:221–224. doi: 10.1007/s00125-003-1296-0. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman SM, Evans AM, Han B, et al. Identification of the {beta} cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamurthy B, Dudek NL, McKenzie MD, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest. 2006;116:3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubosaki A, Miura J, Notkins AL. IA-2 is not required for the development of diabetes in NOD mice. Diabetologia. 2004;47:149–150. doi: 10.1007/s00125-003-1252-z. [DOI] [PubMed] [Google Scholar]
- 11.Kubosaki A, Nakamura S, Notkins AL. Dense core vesicle proteins IA-2 and IA-2{beta}: metabolic alterations in double knockout mice. Diabetes. 2005;54(Suppl 2):S46–S51. doi: 10.2337/diabetes.54.suppl_2.s46. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French MB, Allison J, Cram DS, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1996;46:34–39. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 14.Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol. 2004;5:1028–1035. doi: 10.1038/ni1120. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama M, Beilke JN, Jasinski JM, et al. Priming and effector dependence on insulin B:9–23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117:1835–1843. doi: 10.1172/JCI31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alleva DG, Gaur A, Jin L, et al. Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9–23) peptide. Diabetes. 2002;51:2126–2134. doi: 10.2337/diabetes.51.7.2126. [DOI] [PubMed] [Google Scholar]
- 17.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stadinski BD, Delong T, Reisdorph N, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol. 2005;87(123-62):123–162. doi: 10.1016/S0065-2776(05)87004-X. [DOI] [PubMed] [Google Scholar]
- 20.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci U S A. 2010;107:10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong FS, Siew LK, Scott G, et al. Activation of insulin-reactive CD8 T-cells for development of autoimmune diabetes. Diabetes. 2009;58:1156–1164. doi: 10.2337/db08-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velthuis JH, Unger WW, Abreu JR, et al. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes. 2010;59:1721–1730. doi: 10.2337/db09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent SC, Chen Y, Bregoli L, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435:224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 25.Gebe JA, Unrath KA, Yue BB, Miyake T, Falk BA, Nepom GT. Autoreactive human T-cell receptor initiates insulitis and impaired glucose tolerance in HLA DR4 transgenic mice. J Autoimmun. 2008;30:197–206. doi: 10.1016/j.jaut.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skowera A, Ellis RJ, Varela-Calvino R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated pre-proinsulin epitope. J Clin Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114:589–597. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugliese A, Zeller M, Fernandez A, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type I diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 30.Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 31.Corper AL, Stratmann T, Apostolopoulos V, et al. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science. 2000;288:505–511. doi: 10.1126/science.288.5465.505. [DOI] [PubMed] [Google Scholar]
- 32.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 33.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest. 2005;115:2268–2276. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skyler JS, Brown D, Chase HP, et al. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685B–1691B. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 35.Skyler JS. Update on worldwide efforts to prevent type 1 diabetes. Ann N Y Acad Sci. 2008;1150:190–196. doi: 10.1196/annals.1447.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fousteri G, Chan JR, Zheng Y, et al. Virtual optimization of nasal insulin therapy predicts immunization frequency to be crucial for diabetes protection. Diabetes. 2010;59:3148–3158. doi: 10.2337/db10-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brehm MA, Shultz LD, Greiner DL. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17:120–125. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bresson D, Togher L, Rodrigo E, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solvason N, Lou YP, Peters W, et al. Improved efficacy of a tolerizing DNA vaccine for reversal of hyperglycemia through enhancement of gene expression and localization to intracellular sites. J Immunol. 2008;181:8298–8307. doi: 10.4049/jimmunol.181.12.8298. [DOI] [PubMed] [Google Scholar]