Abstract
Synaptic dysfunction may represent an early and crucial pathophysiology in Alzheimer’s disease (AD). Recent studies implicate a connection between synaptic plasticity deficits and compromised capacity of de novo protein synthesis in AD. The mRNA translational factor eukaryotic elongation factor 1A (eEF1A) is critically involved in several forms of long-lasting synaptic plasticity. By examining postmortem human brain samples, a transgenic mouse model, and application of synthetic human Aβ42 on mouse hippocampal slices, we demonstrated that eEF1A protein levels were significantly decreased in AD, particularly in the hippocampus. In contrast, brain levels of eukaryotic elongation factor 2 were unaltered in AD. Further, upregulation of eEF1A expression by the adenylyl cyclase activator forskolin, which induces long-lasting synaptic plasticity, was blunted in hippocampal slices derived from Tg2576 AD model mice. Finally, Aβ-induced hippocampal long-term potentiation defects were alleviated by upregulation of eEF1A signaling via brain-specific knockdown of the gene encoding tuberous sclerosis 2. In summary, our findings suggest a strong correlation between the dysregulation of eEF1A synthesis and AD-associated synaptic failure. These findings provide insights into the understanding of molecular mechanisms underlying AD etiology and may aid in identification of novel biomarkers and therapeutic targets.
Keywords: Alzheimer’s disease, elongation factor, long-term potentiation, mTOR, protein synthesis, synaptic plasticity
INTRODUCTION
The molecular mechanisms underlying the etiology of Alzheimer’s disease (AD) remain unclear. Mounting evidence indicates that the pathology of AD, the most common dementia syndrome in the elderly, involves impairments of hippocampal synaptic efficacy/plasticity, i.e., the ability of synapses to strengthen or weaken over time. Studies from multiple AD animal models also demonstrate that long-term potentiation (LTP), a major form of synaptic plasticity and a cellular model for memory, is impaired before either the onset of behavioral deficits or the development of amyloid-beta (Aβ) plaques in the brain [1–6]. Therefore, investigation of the detailed molecular mechanisms underlying synaptic dysfunction in AD could result in the identification of novel diagnostic/prognostic biomarkers and potential therapeutic targets. Here we propose that one related mechanism might involve eukaryotic elongation factor 1A (eEF1A) and its associated signaling pathway.
That de novo protein synthesis is indispensable for maintaining synaptic plasticity and memory has been demonstrated in many studies [7–11]. Consistently, defects in the capacity for de novo protein synthesis are linked to cognitive and neuronal plasticity dysfunction in neurodegenerative diseases such as AD, prion disease, and frontotemporal dementia [12–15]. As one of the most abundant mRNA translational factors involved in controlling protein synthesis, a role of eEF1A has been well documented in long lasting forms of synaptic plasticity. For example, either electrical or chemical synaptic stimulation that induces long-term synaptic plasticity results in rapid and significant increases in the neuronal expression of eEF1A. Importantly, synaptic plasticity is not maintained under conditions where eEF1A upregulation is blocked [16–18].
Along with several other components of translational machinery, eEF1A synthesis is known to be controlled by signaling pathways associated with mammalian target of rapamycin complex 1 (mTORC1) [19]. The mRNAs encoding these proteins of translational machinery are collectively termed as TOP mRNAs, due to the common structural hallmark of terminal oligopyrimidine (TOP) in their 5′ untranslated regions (UTRs) [19]. Further, dysregulation of mTORC1 signaling has been implicated in AD, although inconsistent results have been reported [5, 20, 21]. In the current study we found reduction of basal eEF1A protein levels in AD. Additionally, increased expression of eEF1A in response to stimulation that induces synaptic plasticity was disrupted in hippocampal slices from a mouse model of AD. Furthermore, Aβ-induced LTP failure is mitigated via manipulating the signaling pathway that leads to eEF1A upregulation. In short, eEF1A dysregulation may represent a previously unknown molecular mechanism underlying AD-associated neuronal plasticity damage. Our findings are also congruent with recent evidence that compromised protein synthesis capacity contributes to cognitive defects in neurodegenerative diseases [12, 13, 15]. These findings also provide insights into our mechanistic understanding of the pathophysiology in neurodegenerative diseases and the identification of potential novel therapeutic targets.
MATERIALS AND METHODS
Mice
All mice were housed in the Transgenic Mouse Facility of New York University and the Wake Forest School of Medicine Animal Facility. Mice were kept in compliance with the NIH Guide for Care and Use of Laboratory Animals. Both facilities kept a 12 h light/dark cycle with regular feeding, cage cleaning, and 24 h water access. Both male and female mice aged 3–4 months were used for experiments. Tg2576 AD model mice (C57BL/6) were purchased from Taconic. Forebrain-specific TSC2+/− mice (C57B1, 129/SvJae) were generated as previously described [22]. All genotyping was determined by PCR.
Postmortem tissue samples
Postmortem human tissue samples were obtained from the University of Washington School of Medicine brain bank. Samples were collected in accordance with approved Institutional Review Board protocols. Studies were performed using tissue from patients clinically diagnosed with AD (n = 9) and age-matched controls (n = 9). Diagnoses were based on cognitive testing and postmortem Braak (AD stages V–VI) and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores. Mean age of death was 89.6 years. The postmortem interval (PMI) ranged between 2 and 10 h with a mean of 5.3 h. Additional experiments were performed with postmortem brain tissue samples from patients with mild cognitive impairment (MCI; n = 5), Lewy body dementia (LBD; n = 5), and their respective age-matched controls (n = 5 for both MCI and LBD experiments). For MCI subjects, mean age of death was 93.8 years and PMI ranged between 3 and 13 h (mean 5.6 h). Mean age of death for LBD subjects was 85.2 years with a PMI between 3 and 9 h (mean 5.5 h).
Hippocampal slice preparation and electrophysiology
Acute 400 μm transverse hippocampal slices were prepared using a Leica VT1200S vibratome as described previously [23]. Slices were maintained before experimentation at room temperature for at least 2 h in artificial cerebrospinal fluid (ACSF) containing (in mM) 118 NaCl, 3.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 24 NaHCO3, 1.25 NaH2PO4, and 15 glucose, bubbled with 95% O2/5% CO2. For electrophysiology, monophasic, constant-current stimuli (100 μs) were delivered with a bipolar silver electrode placed in the stratum radiatum of area CA3. Field excitatory postsynaptic potentials (fEPSPs) were recorded using a glass microelectrode from the stratum radiatum of area CA1. LTP was induced using high-frequency stimulation (HFS) consisting of two 1-s 100 Hz trains separated by 60 s, each delivered at 70–80% of the intensity that evoked spiked fEPSPs.
Western blots for mouse experiments
Mouse hippocampal slices were flash-frozen on dry ice and sonicated as previously described [23]. Samples containing equal amounts of protein lysate were loaded on 4–12% Tris-glycine SDS-PAGE (Invitrogen) gels for standard gel electrophoresis. Following transfer, nitrocellulose membranes were blocked for 30 min in blocking buffer composed of 5% nonfat dry milk in TBS containing 0.1% Tween 20 (TBS-T). All primary and secondary antibodies were diluted in blocking buffer. Blots were probed with primary antibodies for eEF1A (1: 1000; EMD Millipore), TSC2 (1: 1000, Cell Signaling), GAPDH (1: 10,000, Cell Signaling), and actin (1: 10000; Sigma Aldrich). Densitometric analysis was performed using Scion Image Software. Data for eEF1A were normalized to actin.
Immunofluorescence and confocal microscopy
Ice-cold 4% paraformaldyhyde was used to fix 400 μm mouse hippocampal slices overnight. Post-fixation slices were further cut into 30 μm sections. Free-floating sections were blocked with 10% normal goat serum, 1% BSA, and 0.1% sodium azide in PBS for 2 h. Sections were incubated overnight with eEF1A primary antibody (1: 250; EMD Millipore) at 4ºC followed by an Alexa 488 goat anti-mouse (1: 250; Invitrogen) secondary antibody at room temperature. The stained sections were mounted, coverslipped, and imaged used a Leica TCS SP5 confocal microscope. All parameters were held constant across all sections from the same experiment.
Western blots for postmortem human brain samples
Human tissue samples were collected on dry ice and sonicated in lysis buffer as previously described [24]. Homogenates were then centrifuged in 4°C for 10 min at 21,000 g. The supernatant was collected for protein quantification using the Pierce™ BCA Protein Assay Kit (Thermo Scientific). Samples containing 20 μg protein were loaded into pre-cast Mini-Protean® TGX gels (4–10%; Biorad) and resolved by standard gel electrophoresis. Following transfer, nitrocellulose membranes were blocked for 1 h in 5% milk/TBST blocking buffer. All primary and secondary antibodies were diluted in blocking buffer. Blots were probed with primary antibodies for eEF1A (1: 5000, EMD Millipore), eEF2 (1: 1000, Cell Signaling), GAPDH (1: 10,000, Cell Signaling), and βIII- tubulin (1: 10,000, Sigma-Aldrich). Protein bands were visualized using chemiluminescence (Clarity™ ECL; Biorad) and the Biorad ChemiDoc™ MP Imaging System. Densitometric analysis was performed using ImageJ. Data for eEF1A were normalized to GAPDH unless otherwise specified.
Immunohistochemistry
Postmortem tissue sections used in immunohistochemistry were prepared at the University of Washington. Brains were fixed in 10% neutral buffered formalin and various anatomic regions, including middle frontal gyrus, hippocampus, and cerebellar cortex, were dissected, processed, embedded in paraffin sectioned at 5 μm thickness. Sections were mounted on positively charged microscope slides and baked for 30 min at 60°C. For staining, sections were deparaffinized in xylene and rehydrated through a graded alcohol series. Antigen retrieval utilized citrate buffer (pH 6.0) in a standard 15 min microwave procedure. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide for 25 min. Slides were incubated in a humid chamber in primary antibody for eEF1A (1: 1000; EMD Millipore) overnight at 4°C. Sections were then incubated in biotinylated α-mouse secondary antibody (1: 200; Vector Labs, Burlingame, CA) for 30 min at room temperature followed by Vectastain® Elite ABC reagent (Vector Labs) for another 30 min. Primary and secondary antibodies and ABC reagent were diluted in 1% BSA/PBS. Diaminobenzidine (DAB) was diluted in Tris buffer (pH 7.7) and 3% hydrogen peroxidase in a working DAB solution. Sections were developed in DAB for 10 min in a 42°C water bath. Slides were counterstained using Mayer’s hematoxylin and blued with 0.2% lithium carbonate. Negative controls were incubated in 1% BSA with mouse IgG as the primary antibody. Sections were dehydrated in an alcohol series and cleared with xylene, coverslipped, and dried overnight. Imaging was done using brightfield microscopy on a Zeiss Axioplan 2 Epiflourescent Microscope (Oberkochen, Germany) using Zen 2012 Imaging Software.
Drug treatment
Drugs were prepared as stock solutions in either DMSO or dH2O and diluted into ACSF to final concentration before experiments. For hippocampal slices, drug incubation was performed at 30–32ºC in a submersion maintenance chamber containing ACSF saturated with bubbling 95% O2 and 5% CO2. The final concentration and sources were as follows: forskolin (50 μM, Sigma), rapamycin (1 μM; Calbiochem/Millipore). Aβ42 stock was prepared as described to yield ample oligomers [5].
Data analyses
Data are presented as mean ± SEM. Summary data are presented as group means with SE bars. Values are normalized to either wildtype or wildtype vehicle-treated slices depending on the experiment. For comparisons between two groups, a two-tailed independent Student’s t test was performed using Prism 6 statistics software (GraphPad Software, San Diego, CA). Error probabilities of p < 0.05 were considered statistically significant.
RESULTS
Levels of eEF1A in hippocampus are decreased by Aβ and are decreased in AD model mice
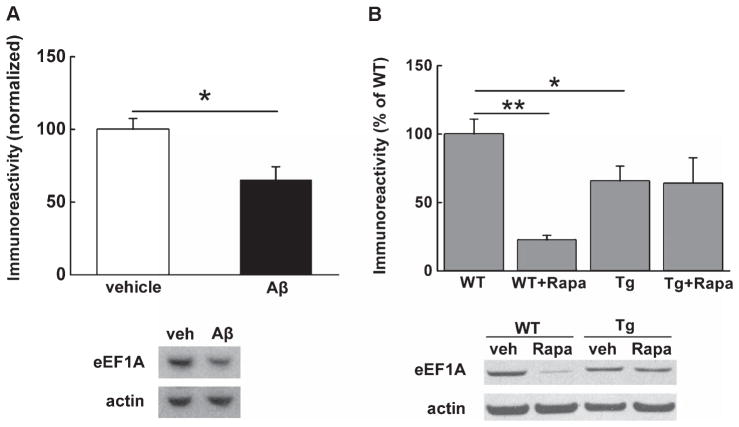
We set out to determine whether basal levels of eEF1A in brain regions important for learning and memory are altered in AD. First, we treated acute hippocampal slices from wild type adult mice with synthetic human Aβ42 (500 nM) for 1 h and harvested slices for western blot experiments. As shown in Fig. 1A, protein levels of eEF1A were significantly decreased by Aβtreatment, compared to the vehicle-treated controls. In addition, western blot experiments on hippocampal slices from 10- to 12-month-old Tg2576 AD model mice demonstrated that eEF1A expression was downregulated, compared to the wild-type littermates (Fig. 1B). Of note, treatment of slices with mTORC1 inhibitor rapamycin (Rapa, 1 μM, for 1 h) caused robust reduction in eEF1A levels in wild-type mice, but not in AD model mice (Fig. 2B). Collectively, these findings suggest that hippocampal eEF1A protein levels are abnormally downregulated in response to Aβ and in AD model mice.
Fig. 1.
Levels of eEF1A in hippocampus are decreased by Aβ and in an AD model mice. (A) Western blot experiments on WT hippocampal slices reveal decreased expression of eEF1A following treatment with 500 nM synthetic Aβ. Cumulative data are shown in the bar graph accompanied by representative blots. n = 8 for vehicle, n = 10 for Aβ. Unpaired independent t test. *p < 0.05. (B) Hippocampi from Tg2576 AD model mice (Tg) also exhibit decreased levels of eEF1A compared to WT littermates. Moreover, 1 μM rapamycin (Rapa) treatment results in downregulation of eEF1A levels in WT slices, with no effects on slices from Tg mice. Cumulative data are shown in bar graphs accompanied by representative blots. n = 9 for WT, n = 3 for WT+Rapa, n = 10 for Tg, n = 5 for Tg+Rapa. Unpaired independent t test. *p < 0.05, **p < 0.01
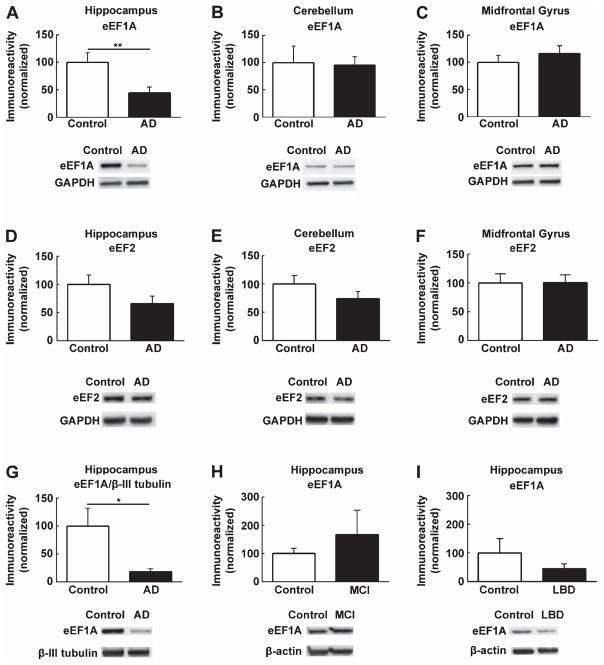
Fig. 2.
Hippocampal eEF1A expression is diminished in postmortem human AD patients. (A) Western blot analysis of postmortem human hippocampal tissue showed reduced eEF1A expression in AD patients compared to age-matched controls. n = 9, unpaired independent t-test; p < 0.01. No significant difference in eEF1A levels between AD and control groups was observed in tissue from (B) cerebellum (n = 7, p = 0.89) or (C) midfrontal gyrus (n = 7, p = 0.40). Moreover, protein levels of eEF2 were not altered in AD in (D) hippocampus (n = 8, p = 0.11), (E) cerebellum (n = 6, p = 0.18), and (F) midfrontal gyrus (n = 6, p = 0.97). (G) When eEF1A was normalized to a neuron-specific marker, βIII-tubulin, AD patients still showed significantly decreased levels of hippocampal eEF1A compared to controls (n = 9, p < 0.05). In comparison, eEF1A expression was not significantly altered in patients with (H) mild cognitive impairment (MCI; n = 5, p = 0.47) and (I) Lewy body dementia (LBD; n = 5, p = 0.33), compared to their respective age-matched controls. Cumulative data are shown in bar graphs normalized to WT with representative blots; unpaired independent t-test; **p < 0.01, *p < 0.05.
Hippocampal eEF1A expression is diminished in postmortem human AD patients
Furthermore, we examined regulation of eEF1A protein levels in brain samples from postmortem human AD patients (see methods for details). Three brain regions were investigated: hippocampus, mid-frontal gyrus, and cerebellum. Hippocampal eEF1A levels (normalized to either GAPDH or βIII-tubulin, a neuronal marker) were significantly reduced in AD patients, compared to age-matched controls (Fig. 2A, 2G). In contrast, no significant differences in eEF1A protein levels were observed in either cerebellum or midfrontal gyrus of AD samples, compared to controls (Fig. 2B, 2C).
We next investigated whether there was an AD-related alteration in the levels of eukaryotic elongation factor 2 (eEF2), another mRNA translational factor that also belongs to the “TOP” protein family [19]. Western blot experiments showed no significant changes in eEF2 protein levels in any of the three brain areas (hippocampus, cerebellum, and mid-frontal gyrus) (Fig. 2D–F). However, there was a trend toward decreased levels in hippocampi of AD patients, in comparison with age-matched controls (Fig. 2D, p = 0.11). We further examined eEF1A expression in postmortem brain tissue from patients with MCI and LBD. Contrasting our AD findings, hippocampal eEF1A expression in both MCI and LBD tissues was not significantly different from age-matched control tissue (Fig. 2H, I).
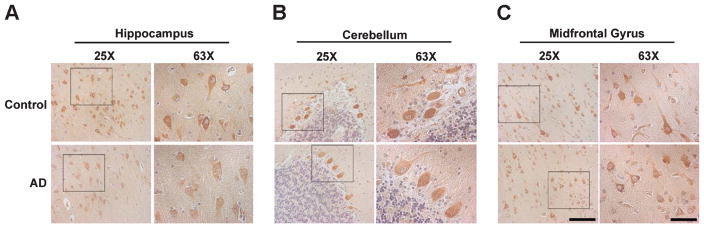
To gain insight into the cellular distribution of AD-associated eEF1A dysregulation, we performed immunohistochemistry experiments on formalin-fixed, paraffin-embedded (FFPE) sections from human brain tissues. In agreement with the results from biochemical assays, neuronal eEF1A levels were decreased in hippocampal sections, but not altered in either cerebellum or midfrontal gyrus sections (Fig. 3A–C). These data are consistent with the findings from the animal models (Fig. 1), and indicate dysregulation of eEF1A expression in AD.
Fig. 3.
Hippocampal neuronal eEF1A levels are decreased in human AD. (A) DAB staining showed decreases in hippocampal eEF1A in AD compared to age-matched controls. In contrast, there were no histological differences between AD and control groups in eEF1A expression in (B) cerebellum or (C) midfrontal gyrus. Representative images (from at least three independent experiments) with 25X and 63X magnification (inset) are shown. Scale bars: 100 μm for 50x and 50 μm for 63x.
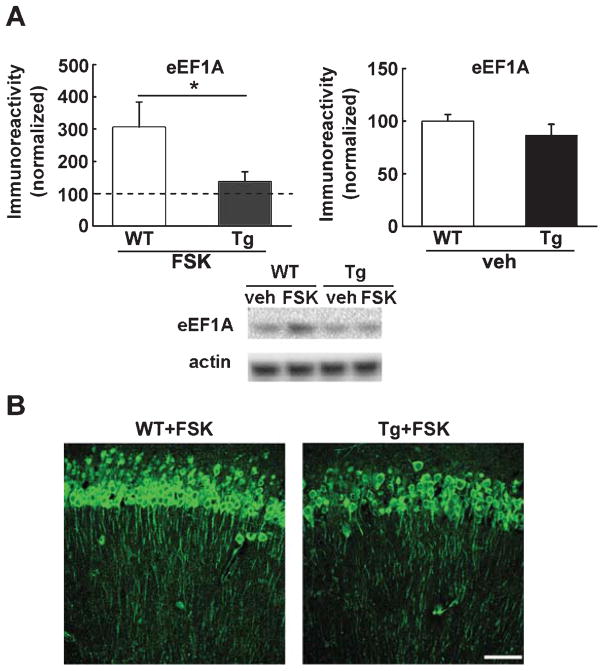
Forskolin-induced upregulation of eEF1A is blunted in AD model mice
To determine whether neuronal plasticity-associated eEF1A regulation is altered in AD, we treated hippocampal slices derived from either Tg2576 mice or wild-type controls with the adenylyl cyclase activator forskolin (FSK, 50 μM for 1 h), which is known to induce long-lasting, protein synthesis-dependent LTP [25]. In response to FSK treatment, wild type slices demonstrated a more robust increase in eEF1A levels (more than two-folds on average), compared to slices from Tg2576 AD model mice (Fig. 4A). In addition to western blot experiments, FSK-treated slices were fixed and subject to immunofluorescence/confocal imaging. In agreement with the biochemical findings, a more dramatic eEF1A upregulation was observed in both the soma and dendrites of the stratum radiatum in area CA1 of wild type hippocampal slices compared with Tg2576 slices (Fig. 4B). Together, these data indicate that increased expression of eEF1A following exposure to forskolin is inhibited in AD model mice.
Fig. 4.
Forskolin-induced upregulation of eEF1A is blunted in AD model mice. (A) Western blot experiments show that treatment of slices with FSK induces significantly higher levels of eEF1A in WT mice compared to Tg mice. Cumulative data are shown in bar graphs with representative blots. Dash line indicates the vehicle-treated group to which the FSK-treated group is normalized. n = 11 for WT+FSK, n = 12 for Tg+FSK. Unpaired independent t-test; *p < 0.05. (B) Immunofluorescence confocal microscopy reveals that FSK-induced eEF1A expression in hippocampal CA1 area is lowered in Tg mice compared to WT mice. Scale bar: 50 μm.
Aβ-induced hippocampal LTP impairments are alleviated by brain-specific knockdown of TSC2
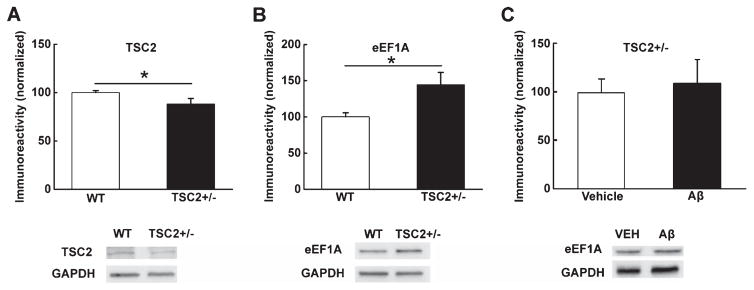
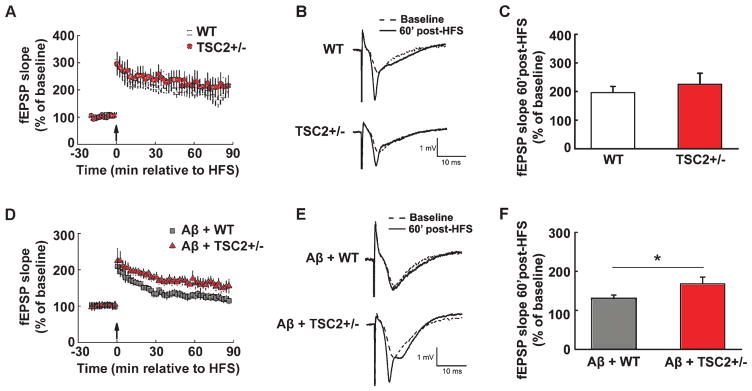
To further elucidate the role of eEF1A dysregulation in AD pathophysiology, we investigated whether Aβ-induced synaptic plasticity failure can be compensated through eEF1A upregulation. We took advantage of a line of transgenic mice in which the TSC2 gene is knocked down specifically in excitatory neurons in forebrain areas (TSC2+/−) [22]. TSC2 functions as the primary upstream negative regulator for mTORC1. Consequently, mTORC1 signaling is upregulated in TSC2+/− mice, leading to increased synthesis of TOP proteins including eEF1A [22]. We first confirmed that there was a reduction in total TSC2 protein levels that was associated with an elevation in eEF1A expression in hippocampi of TSC2+/− mice, compared to wild type littermates (Fig. 5A, B). In addition, Aβ-induced decreases in hippocampal eEF1A expression were blunted in TSC2+/− mice (Fig. 5C). Next, we demonstrated that hippocampal LTP was normally induced in slices from TSC2+/− mice, compared to wild-type control mice (Fig. 6A–C). Moreover, the application of Aβ42 to slices from wild-type mice caused LTP decline (Fig. 6D–F), as previously reported [26]. Markedly, in slices derived from TSC2+/− mice, Aβ-induced LTP defects were significantly improved (Fig. 6D–F). These findings provided further evidence that eEF1A dysregulation is strongly correlated with impaired synaptic plasticity in AD.
Fig. 5.
Conditional TSC2 knockout mice. Forebrain-specific TSC2 knockout mice (TSC2+/−) demonstrated (A) reduction of TSC2 protein levels (n = 4) and (B) elevated eEF1A protein levels (n = 10 for WT, n = 7 for TSC2+/−) in hippocampal tissue. (C) Aβ-induced decreases in eEF1A expression are blunted in TSC2+/− hippocampal slices (n = 3 for vehicle, n = 5 for Aβ). Cumulative data are shown in bar graphs with representative blots; unpaired independent t-test; *p < 0.05.
Fig. 6.
Aβ-induced hippocampal LTP impairments are alleviated by brain-specific deletion of TSC2. (A) HFS induced similar LTP in WT (open squares) and TSC2+/− (filled circles) hippocampal slices. Arrow indicates the time point at which HFS was delivered. (B) Representative fEPSP traces before and after HFS for LTP experiments shown in A. (C) Cumulative data showing mean fEPSP slopes 60 min after HFS based on the experiments shown in A. n = 7 for WT, n = 8 for TSC2+/−. unpaired independent t-test. p = 0.52. (D) Aβ-induced LTP impairments are mitigated in hippocampal slices derived from TSC2+/− mice (filled triangles) compared to WT slices (gray squares). Arrow indicates the time point at which HFS was delivered. (E) Representative fEPSP traces before and after HFS for the LTP experiments shown in D. (F) Cumulative data showing mean fEPSP slopes 60 min after HFS based on experiments shown in D. n = 9 for A+WT, n = 8 for Aβ+TSC2+/−. Unpaired independent t-test. *p < 0.05.
DISCUSSION
Mounting evidence suggests that synapses are key early targets for abnormally accumulated brain Aβ, leading to neurodegeneration in AD [1, 6, 27]. A better understanding of the molecular and cellular mechanisms related to AD-associated synaptic dysfunction is urgently needed to develop potential novel therapeutic strategies and diagnostic biomarkers for this devastating disease. In the present study we observed a significant reduction of the mRNA translational factor eEF1A in hippocampi of both AD model mice and postmortem human AD patients. Importantly, upregulation of eEF1A signaling via brain-specific genetic knockdown of the TSC2 gene alleviated the Aβ-induced synaptic plasticity (LTP) failure. These findings would help provide insights into understanding of molecular mechanisms underlying AD-associated synaptic dysfunction and memory loss.
Examination of the human brain samples in the current study revealed that the most significant reduction of eEF1A levels happens in hippocampal area CA1. It is worth mentioning that the pathology stage of control brain samples from aged-matched non-dementia people was Braak stage II–III, which implicates involvement of CA1 and subiculum by neurofibrillary tangles mainly composed of tau proteins [28]. In contrast, we confirmed that Aβ pathology was absent in hippocampi from control cases but frequently present in AD cases (data not shown). It would be intriguing in the future to investigate whether Aβ plays a more important role than tau does in causing eEF1A dysregulation and the correlated impairments in synaptic plasticity seen in AD.
Forskolin induces mTORC1-dependent activation of 5′TOP mRNA translation (including eEF1A), which is critical for the stabilization of long-lasting synaptic plasticity [25]. In agreement, we observed blunted eEF1A expression in the presence of forskolin in hippocampi of Tg2576 mice, which may contribute to the synaptic dysfunction in AD. Further, the findings that hippocampal slices from AD model mice do not react to rapamycin treatment on eEF1A expression are striking. These results indicate the loss of ability to regulate (up or down) protein synthesis capacity, which might be associated with the failure of hippocampal plasticity in AD. A substantial body of evidence demonstrates that maintenance of long-term synaptic plasticity and memory is dependent on de novo protein synthesis [9, 10]. Each stage (initiation, elongation, and termination) of protein synthesis (mRNA translation) requires action of multiple translational protein factors to facilitate the process. Although it is generally thought that translational control at the initiation phase is the most important, regulation at the elongation stage is particularly useful in two situations: (1) when mRNA translation has already begun but remains quiescent until needed, thus accelerating synthesis of new proteins by bypassing the initiation step which happens relatively slowly [29]; or (2) in cellular environments such as neuronal dendrites where the translational capacity is low [30]. Thus both initiation and elongation processes need to be upregulated to fulfill the substantial requirements of new protein synthesis associated with synaptic plasticity. Indeed, multiple lines of evidence indicate a robust, early phase of protein synthesis during learning, and consistently it was reported that upregulation of eEF1A levels happens almost immediately upon synaptic stimulation that induces long-lasting neuronal plasticity [9, 16, 17, 31]. Of note, growing evidence indicates that eEF1A has several non-canonical functions such as viral-host interaction and subcellular organizations [32]. The possibility is not excluded that the role of eEF1A in AD pathophysiology is connected to some of these functions. In addition, the rescuing effects of Aβ-induced LTP failure in TSC2 mice (Fig. 6) need to be explained with caution. TSC2 is the primary upstream negative regulator of mTORC1, which controls synthesis of multiple proteins including eEF1A [10]. We focused on eEF1A in the current study because of its established role in synaptic plasticity [17], and its significant dysregulation in AD (Figs. 1–3). To the best of our knowledge, currently there is no genetic or pharmacological approach available to directly “upregulate” eEF1A levels in mouse brain. The association between eEF1A upregulation and synaptic failure in AD is a topic worth of further investigation when appropriate tools are available in the future.
As described above, eEF1A belongs to transcripts of the TOP mRNAs family, which encodes mainly components of the translational machinery including ribosomal proteins and elongation factors [19]. Thus, a reduction of translational machinery in AD would lower the ability of synapses to synthesize proteins that are important for maintenance of long-term memory and synaptic plasticity [31]. We did not observe in AD brains significant decreases of eEF2, another translational elongation factor and TOP protein, suggesting specificity of eEF1A dysregulation in AD pathophysiology. Further, eEF1A synthesis is known to be controlled by the mTORC1 signaling pathway. The role of mTORC1 signaling in AD is a topic under considerable debate. Conflicting results have been reported on how the mTORC1 signaling pathway is regulated in AD, which could be attributed to factors such as disease stage, mouse line, or brain areas, among others [5, 6, 21]. Notably, none of these studies have examined how eEF1A is regulated.
In summary, our data indicate that eEF1A dysregulation in specific brain areas might be a previously unknown molecular mechanism that contributes to AD pathogenesis. Our data also suggest that eEF1A could be a novel biomarker and therapeutic target in AD. These findings are also congruent with recent reports that cellular stress-induced impairments of protein synthesis ability are involved in cognitive deficits of neurodegenerative diseases [12, 13, 15]. Future studies of eEF1A in other models of neurodegenerative diseases can help determine how specific eEF1A alterations are to AD.
Acknowledgments
This work was supported by National Institutes of Health grants K99 AG044469 and R00 AG044469 (T.M.), R01 AG031892 and P50 AG05136 (C.D.K.), and a grant from the BrightFocus Foundation (T.M.). We thank Ms. Allison Beller for administrative support and Ms. Samantha Rice for technical support. We thank Karen Klein, MA, ELS (Biomedical Research Services Administration, Wake Forest University Health Sciences) for editing the manuscript.
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0036r2).
References
- 1.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 2.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen JS, Wu C-C, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, Ishibashi K, Teraoka R, Sakama N, Yamashita T, Nishitsuji K, Ito K, Shimada H, Lambert MP, Klein WL, Mori H. A mouse model of amyloid beta oligomers: Their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, Tampellini D, Klann E, Blitzer RD, Gouras GK. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e12845. doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma T, Klann E. Amyloid β: Linking synaptic plasticity failure to memory disruption in Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):140–148. doi: 10.1111/j.1471-4159.2011.07506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- 8.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberini C. The role of protein synthesis during the labile phases of memory: Revisiting the skepticism. Neurobiol Learn Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg T, Gal-Ben-Ari S, Dieterich DC, Kreutz MR, Ziv NE, Gundelfinger ED, Rosenblum K. The roles of protein expression in synaptic plasticity and memory consolidation. Front Mol Neurosci. 2014;7:86. doi: 10.3389/fnmol.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, Cavener DR, Klann E. Suppression of eIF2α kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat Neurosci. 2013;16:1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG, Halliday M, Morgan J, Dinsdale D, Ortori CA, Barrett DA, Tsaytler P, Bertolotti A, Willis AE, Bushell M, Mallucci GR. Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature. 2012;485:507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, Mallucci GR. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med. 2013;5:206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- 15.Radford H, Moreno JA, Verity N, Halliday M, Mallucci GR. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol. 2015;130:633–642. doi: 10.1007/s00401-015-1487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong C, Bach SV, Haynes KA, Hegde AN. Protea-some modulates positive and negative translational regulators in long-term synaptic plasticity. J Neurosci. 2014;34:3171–3182. doi: 10.1523/JNEUROSCI.3291-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giustetto M, Hegde AN, Si K, Casadio A, Inokuchi K, Pei W, Kandel ER, Schwartz JH. Axonal transport of eukaryotic translation elongation factor 1alpha mRNA couples transcription in the nucleus to long-term facilitation at the synapse. Proc Natl Acad Sci U S A. 2003;100:13680–13685. doi: 10.1073/pnas.1835674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- 20.Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R, Pradier L, Hugon J. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J Neurochem. 2005;94:215–225. doi: 10.1111/j.1471-4159.2005.03187.x. [DOI] [PubMed] [Google Scholar]
- 21.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: Effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chévere-Torres I, Kaphzan H, Bhattacharya A, Kang A, Maki JM, Gambello MJ, Arbiser JL, Santini E, Klann E. Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the ΔRG mouse model of tuberous sclerosis complex. Neurobiol Dis. 2012;45:1101–1110. doi: 10.1016/j.nbd.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma T, Du X, Pick JE, Sui G, Brownlee M, Klann E. Glucagon-like peptide-1 cleavage product GLP-1 (9–36) amide rescues synaptic plasticity and memory deficits in Alzheimer’s disease model mice. J Neurosci. 2012;32:13701–13708. doi: 10.1523/JNEUROSCI.2107-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Maram-baud P, Klann E. Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid β. J Neurosci. 2014;34:12230–12238. doi: 10.1523/JNEUROSCI.1694-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gobert D, Topolnik L, Azzi M, Huang L, Badeaux F, Des-Groseillers L, Sossin WS, Lacaille J-C. Forskolin induction of late-LTP and up-regulation of 5′ TOP mRNAs translation via mTOR, ERK, and PI3K in hippocampal pyramidal cells. J Neurochem. 2008;106:1160–1174. doi: 10.1111/j.1471-4159.2008.05470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, Murphy MP, Pautler RG, Klann E. Amyloid β-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J Neurosci. 2011;31:5589–5595. doi: 10.1523/JNEUROSCI.6566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh DM, Selkoe DJ. A beta oligomers–a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal K, Alonso AdC, Chen S, Chohan MO, El-Akkad E, Gong C-X, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- 30.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mateyak MK, Kinzy TG. eEF1A: Thinking outside the ribosome. J Biol Chem. 2010;285:21209–21213. doi: 10.1074/jbc.R110.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]